Abstract
OBJECTIVE:
Childhood interstitial lung diseases (ILD) occur in a variety of clinical contexts. Advances in the understanding of disease pathogenesis and use of standardized terminology have facilitated increased case ascertainment. However, as all studies have been performed at specialized referral centers, the applicability of these findings to general pulmonary practice has been uncertain. The objective of this study was to determine the historical occurrence of childhood ILD to provide information reflecting general pediatric pulmonary practice patterns.
METHODS:
Childhood ILD cases seen at Vanderbilt Children’s Hospital from 1994 to 2011 were retrospectively reviewed and classified according to the current pediatric diffuse lung disease histopathologic classification system.
RESULTS:
A total of 93 cases were identified, of which 91.4% were classifiable. A total of 68.8% (64/93) of subjects underwent lung biopsy in their evaluations. The largest classification categories were disorders related to systemic disease processes (24.7%), disorders of the immunocompromised host (24.7%), and disorders more prevalent in infancy (22.6%). Eight cases of neuroendocrine cell hyperplasia of infancy (NEHI) were identified, including 5 that were previously unrecognized before this review.
CONCLUSIONS:
Our findings demonstrate the general scope of childhood ILD and that these cases present within a variety of pediatric subspecialties. Retrospective review was valuable in recognizing more recently described forms of childhood ILD. As a significant portion of cases were classifiable based on clinical, genetic, and/or radiographic criteria, we urge greater consideration to noninvasive diagnostic approaches and suggest modification to the current childhood ILD classification scheme to accommodate the increasing number of cases diagnosed without lung biopsy.
Keywords: childhood lung disease, interstitial lung disease, lung biopsy
What’s Known on This Subject:
Childhood interstitial lung diseases occur in a variety of clinical contexts and are associated with high morbidity and mortality. Advances in the understanding of disease pathogenesis and use of standardized terminology have facilitated increased case ascertainment.
What This Study Adds:
This study demonstrates that cases of newly described forms of childhood interstitial lung diseases likely occur at all children’s hospitals. With advances in genetic testing and recognition of imaging patterns, a significant portion of cases are identifiable with noninvasive evaluations.
Childhood interstitial lung diseases (ILD) are a heterogeneous group of diffuse lung diseases characterized by abnormal imaging findings and impaired gas exchange.1 These disorders occur in a variety of clinical contexts and are associated with high morbidity and mortality.2,3 Advances in the understanding of disease pathogenesis in addition to standardization of terminology and classification of childhood ILD have facilitated increased case ascertainment and further studies.4
Over the past 2 decades, the scope of childhood ILD was initially described through the experience of large single-site referral centers2,5,6 and more recently through national and international collaborative efforts.7–9 The incidence of childhood ILD has been reported as 3.6 cases per million in Ireland and the United Kingdom in 200210 and 1.32 cases per million in Germany in 2009.8 A 2004 European Respiratory Society task force identified 185 cases of ILD in immunocompetent children.7 In 2007, Deutsch et al reported 187 cases in children <2 years of age based solely on lung biopsy ascertainment from 11 pediatric centers over a 5-year time period.4 These lung biopsy cases were used to establish a histopathologic classification system of pediatric diffuse lung disease, an advancement that standardized terminology and emphasized forms of ILD unique to young children.
With recent advances in molecular genetics and imaging technology, some forms of childhood ILD are now diagnosed by a combination of clinical, genetic, and imaging features without lung biopsy and thus fit poorly into a classification scheme based largely on histopathologic criteria. These genetic diagnoses include surfactant metabolism defects, such as mutations in the genes encoding surfactant proteins B and C (SFTPB, SFTPC), the adenosine triphosphate binding cassette A3 (ABCA3), and the gene encoding the thyroid transcription factor 1 (NKX2.1/TTF-1).11–16 Furthermore, computed tomography (CT) scan patterns have been shown to be specific for several types of ILD,17,18 including neuroendocrine cell hyperplasia of infancy (NEHI), enabling diagnosis without lung biopsy in some cases.19 Thus, the routine use of lung biopsy to diagnose and characterize ILD in children may no longer be the standard of care, although the classification scheme is predicated upon this information.4
Recent studies of childhood ILD have been performed at specialized referral centers. As such, the applicability of these findings to general pulmonary practice has been uncertain. The objective of this study was to determine the historical occurrence of diffuse lung diseases at a moderately sized children’s hospital previously without a formal pediatric ILD referral center, to provide information about the scope of childhood ILD.
Methods
We conducted a comprehensive retrospective review of children younger than 18 years of age who were evaluated for ILD at Vanderbilt Children’s Hospital from 1994 to 2011. Cases were identified based on clinical and surgical billing queries using current International Classification of Diseases-9 and Current Procedural Terminology codes, enabling case ascertainment both by clinical diagnoses and by history of lung biopsy for diffuse lung disease. Cases received after formal establishment of an ILD clinic at our institution in 2012 were not included. This study was approved by the Vanderbilt University Institutional Review Board (IRB#120438).
Available clinical data, chest imaging, and lung biopsies were reviewed. External pathology over-read was performed for all available non-immunocompromised cases using the Aperio slide scanning system (Vista, CA) by 1 investigator (Dr Deutsch). The current classification scheme for diffuse lung disease in children was applied to the cases identified.4 The severity of illness scale introduced by Fan et al2 was used for classification of subject status. Previously performed genetic testing results were reviewed, but additional genetic investigations were not performed in the context of this retrospective study. Data are reported as median and interquartile range (IQR). Statistical analysis was performed by using GraphPad Prism (San Diego, CA).
Results
Study Subjects
A total of 93 cases of diffuse lung disease were identified at our institution during the time period ranging from 1994 to 2011. The majority of cases were identified from the second half of the study time period, with only 13 (20.3%) of the lung biopsies performed and 9 (34.5%) of non-biopsy cases initially presenting from 1994 to 2002. As a point of reference, our Cystic Fibrosis Center registered 165 pediatric patients in 2006 and 213 patients in 2011. The clinical characteristics of the ILD study cohort are displayed in Table 1. Subjects diagnosed at younger than 1 year of age had overall greater severity of illness at presentation compared with older subjects.
TABLE 1.
Clinical Features of the Study Population
| All Subjects | Diagnosis ≤1 Yr | Diagnosis >1 Yr | ||||
|---|---|---|---|---|---|---|
| N (%) | No. Reported (Max 93) | N (%) | No. Reported (Max 20)a | N (%) | No. Reported (Max 73)a | |
| Male sex | 49 (51.6) | 93 | 16 (80) | 20 | 32 (44.4) | 72 |
| Birth history | ||||||
| Premature birth (≤37 wk) | 12 (17.1) | 70 | 8 (42.1) | 19 | 4 (8) | 50 |
| O2 requirement at birth | 17 (23.9) | 71 | 12 (63.2) | 19 | 5 (9.6) | 52 |
| Intubation at birth | 9 (12.7) | 71 | 6 (31.6) | 19 | 3 (5.8) | 52 |
| Congenital heart disease | 2 (2.2) | 93 | 2 (10) | 20 | 0 (0) | 72 |
| Clinical presentation | ||||||
| Median age at diagnosis (mo) IQR1,3 | 90 (16, 47) | 92 | 2.5 (1, 5.5) | 20 | 120 (48, 156) | 72 |
| Severity of illness at presentationb | ||||||
| Asymptomatic | 7 (7.5) | 93 | 0 (0) | 20 | 7 (9.7) | 72 |
| Symptomatic, normal RA saturation | 28 (30.1) | 93 | 3 (15) | 20 | 25 (34.7) | 72 |
| Symptomatic, abnormal saturation (<90%) with sleep or exercise | 17 (18.3) | 93 | 3 (15) | 20 | 14 (19.4) | 72 |
| Symptomatic, abnormal saturation (<90%) at rest | 34 (36.6) | 93 | 11 (55) | 20 | 22 (30.6) | 72 |
| Symptomatic, pulmonary hypertension | 7 (7.5) | 93 | 3 (15) | 20 | 4 (5.6) | 72 |
| Diagnostic evaluations | ||||||
| Chest CT scan performed | 83 (89.2) | 93 | 16 (80) | 20 | 65 (90.2) | 72 |
| Lung biopsy performed | 64 (68.8) | 93 | 13 (65) | 20 | 50 (69.4) | 72 |
| Median age at biopsy (mo) IQR1,3 | 96 (23, 156) | 63 | 4 (2,5) | 13 | 126 (48, 165) | 49 |
Age at diagnosis was not documented for 1 subject.
The severity of illness scale used was originally reported by Fan et al.2
Use of Chest CT and Lung Biopsy in Diagnostic Evaluation
A total of 83 (89.2%) subjects underwent chest CT as part of clinical evaluation, and 64 (68.8%) underwent lung biopsy. In 25 (26.9%) subjects, therapeutic intervention was attempted before lung biopsy, including systemic corticosteroids (n = 14; 56%), other immunomodulatory therapies (n = 4; 16%), or both (n = 7; 28%). The use of CT scan and lung biopsy were similar in subjects ≤1 year of age compared with those >1 year of age at evaluation.
Five subjects underwent lung biopsy without previous chest CT, including 1 infant who was diagnosed with alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD-MPV) and another with severe lung growth abnormality. We also identified 5 subjects with childhood ILD based on clinical and/or genetic testing alone, without either chest CT or lung biopsy, including 1 with ABCA3 deficiency, 1 with hypersensitivity pneumonitis, 1 with granulomatosis with polyangiitis (Wegener), 1 with Goodpasture syndrome, and 1 with a family history of interstitial pneumonitis of unclear etiology whose case was deemed unclassifiable.
Diagnoses and Classification
As shown in Fig 1, 85/93 (91.4%) cases were classified using the structure of the current classification system, with the addition of a non-biopsy cohort. The majority (87.1%) of cases were assigned specific diagnoses within these categories (Supplemental Table 2). External pathology over-read resulted in identification of 1 previously unrecognized case of NEHI (previously considered follicular bronchiolitis and possible aspiration), diagnosis of lung growth abnormality in 1 case (previously considered non-diagnostic), and recognition of patchy pulmonary interstitial glycogenosis (PIG) in 1 case (previously diagnosed as only lung growth abnormality) (Fig 2). In addition, pathology re-review was confirmatory for 4 cases of NEHI retrospectively identified based on characteristic radiographic features. For the remaining cases, the pathology over-read was consistent with the classification assigned based on the original clinical pathology evaluation.
FIGURE 1.
Study cohort classification distribution according to the current classification system for childhood diffuse lung disease. The classification scheme was also applied to the cohort who did not undergo lung biopsy (termed “non-biopsy cohort”).
FIGURE 2.

Case of lung growth abnormality with previously unrecognized pulmonary interstitial glycogenosis. A, Chest radiograph at 3 weeks of age from a late preterm newborn with trisomy 21, pulmonary hypertension, and respiratory failure at birth. Bilateral diffuse reticular opacities and atelectasis are present. B, Lung biopsy at 3 weeks of age shows deficient alveolarization with enlarged simplified airspaces and limited secondary septation (×10, H&E). C, Patchy alveolar septal widening by immature round to oval mesenchymal cells is present, demonstrating findings of pulmonary interstitial glycogenosis (×40).
Disorders More Prevalent in Infancy
A total of 21 (22.6%) cases were classified as “disorders more prevalent in infancy.” There was 1 case of ACD-MPV, 4 cases of lung growth abnormality, 8 cases of NEHI, 1 case of PIG, and 7 cases of surfactant metabolism disorders. Most cases (n = 13; 61.9%) in this category were indeed diagnosed in children younger than 1 year of age. Six subjects (1 unknown surfactant mutation, 1 with ABCA3 mutations, and 4 NEHI) were 2 years of age or older at time of diagnosis, although all had symptoms in the first year of life.
Neuroendocrine Cell Hyperplasia of Infancy
From the historical clinical records, there were 3 cases of NEHI previously diagnosed at our institution based on lung biopsy. One of these subjects had relatively diffuse ground glass opacities on chest CT scan, which influenced the decision to pursue lung biopsy (Supplemental Fig 5). Through this retrospective study we identified an additional 5 subjects who met criteria for NEHI based on history and typical CT findings (Fig 3 and Supplemental Table 3). Four of these 5 subjects had undergone lung biopsy, with originally assigned pathologic descriptions of chronic bronchiolitis, follicular bronchiolitis, normal parenchyma with mild follicular bronchiolitis, and nonocclusive bronchiolitis obliterans with associated follicular bronchiolitis. Re-review of these 4 lung biopsies confirmed histologic criteria for NEHI. NEHI cases thus comprised 9.7% of our total study population.
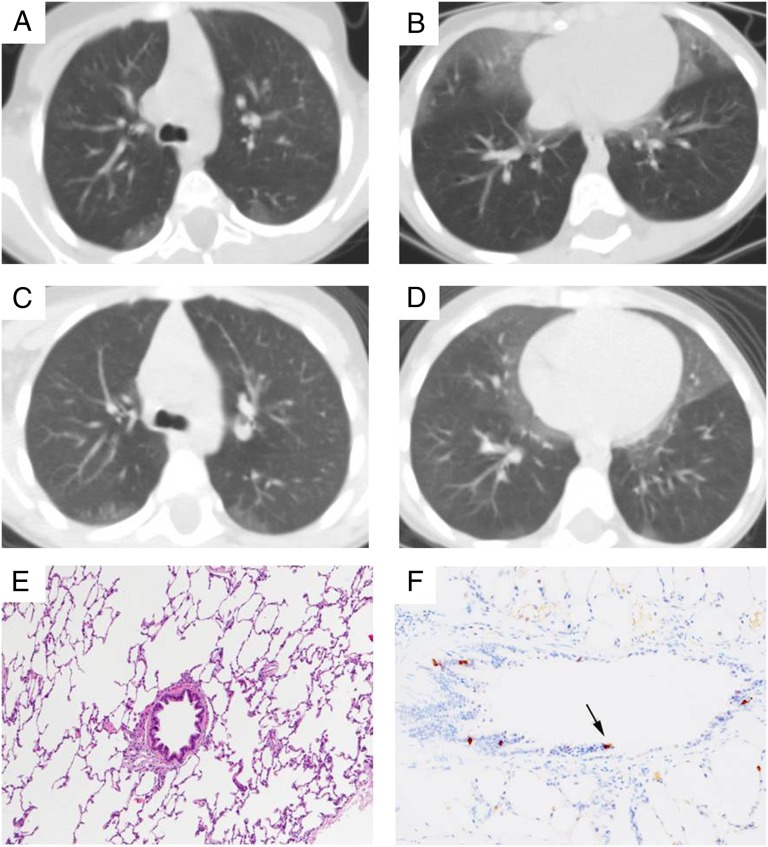
FIGURE 3.
Twins with NEHI identified through retrospective review. A–D, Chest CT images obtained at 3 years of age in identical twins (A and B, Twin A; C and D, Twin B) with history of respiratory symptoms since 2 months of age. The presence of ground glass opacities in the right middle lobe, lingula, and perihilar regions, without other abnormalities, demonstrates a pattern consistent with NEHI. E and F, Lung biopsy from Twin B performed at 4 years of age shows near normal architecture with minimal peribronchiolar fibrosis and no significant inflammation (E, H&E, ×10). Bombesin immunostaining (F, ×20) was performed after this case was retrospectively identified based on the CT scan. Neuroendocrine cells (black arrow) were prominent in distal bronchioles, providing histologic confirmation of the diagnosis of NEHI.
Surfactant Metabolism Disorders
Seven cases of surfactant metabolism disorders were identified (7.5% of total). A spectrum of radiographic findings was observed (Fig 4). Four cases had undergone lung biopsy and demonstrated findings characteristic of surfactant mutations, including type II pneumocyte hyperplasia with significant alveolar macrophage accumulation, cholesterol clefts, or some degree of alveolar proteinosis.4 There were 2 cases with confirmed disease-causing ABCA3 mutations and 3 cases with disease-causing SFTPC mutations (Supplemental Table 4). Two cases classified as disorders of surfactant metabolism based on consistent histology remain without an identified genetic etiology at this time. Figure 4 G, H, and I demonstrates 1 case that is highly suspicious for disease attributable to a NKX2.1 mutation based on the history of chronic, severe lung disease in the setting of congenital hypothyroidism with abnormal chest imaging and lung biopsy findings consistent with surfactant metabolism abnormality. The other undiagnosed case has clinical history, imaging, and lung histopathology consistent with a surfactant metabolism defect, although sequencing of ABCA3, SFTPC, and NKX2.1 were normal.
FIGURE 4.
ILD attributable to surfactant dysfunction disorders. A, Chest CT image of a 30-month-old child with persistent retractions, tachypnea, and hypoxemia showing diffuse ground glass opacities and reticular opacities. Birth history was notable for brief requirement for supplemental oxygen. B, The subject’s lung biopsy performed at 30 months of age shows type II pneumocyte hyperplasia and dense accumulation of alveolar macrophages (×40, H&E). The subject was later found to have 2 ABCA3 mutations (p.E292V/c.1742-9 G>A). C, A follow-up CT scan at age 18 years is remarkable for increased septal thickening and architectural distortion suggestive of fibrotic remodeling, although some ground-glass opacity persists. The subject died at age 19 years from respiratory failure. This subject’s genetic diagnosis was previously reported in the literature.25 D and E, Chest radiographs of a term, 8-month-old infant with chronic retractions and feeding difficulties. The subject’s birth history was remarkable for respiratory failure requiring intubation, mechanical ventilation, and surfactant administration. Findings include hyperexpansion and right upper and middle lobe as well as left upper lobe opacities. Chest CT and lung biopsy were not performed, as clinical suspicion led to genetic testing first. ABCA3 sequencing revealed homozygous E292V/E292V mutations. F, Chest CT revealing irregular focal areas of ground glass opacities and septal thickening from a 1-year-old child with an SFTPC mutation (L181V). This child had history of tachypnea starting at day of life 2, and several subsequent hospitalizations for evaluation of tachypnea and poor feeding with intermittent hypoxemia. A family history of pulmonary fibrosis in older individuals prompted the genetic testing. G, Chest CT image from a 2-month-old former 34-week preterm infant with congenital hypothyroidism, persistent tachypnea, and hypoxemia. Diffuse ground glass opacities and interstitial markings suggestive of a surfactant dysfunction disorder are noted. H, The subject’s lung biopsy performed at 2 months of age shows deficient alveolarization and interstitial thickening (×4, H&E). I, On higher magnification, findings include epithelial hyperplasia with hypercellular interstitial thickening and macrophage accumulation in the alveolar space consistent with surfactant metabolism defect (×40, H&E). Genetic sequencing for SFTPB, SFTPC, and ABCA3 showed no mutations. As this case was identified retrospectively and before current knowledge of NXK2-1 mutations as a cause of ILD and the availability of clinical genetic testing, this subject’s NKX2-1 mutation status is not currently known.
Disorders Related to Systemic Disease Processes
A total of 23 (24.7%) cases were classified as “disorders related to systemic disease processes.” Almost all of these cases (n = 22) had diffuse lung disease associated with rheumatologic or alveolar hemorrhage disorders (Supplemental Table 2; Supplemental Fig 6). Except for 1 patient with Gaucher disease, all presented for evaluation after 1 year of age. Only 8 subjects (34.8%) in this category underwent lung biopsy in their evaluations. Five cases (21.7%) were classified with contribution of a solid organ biopsy other than the lung (kidney, muscle), and the others were classified based on clinical history, serologic testing, and imaging.
Disorders of the Normal Host Presumed Immune-Intact
Thirteen (14%) cases were “disorders of the normal host.” Of these, 11 cases underwent lung biopsy. The 2 cases that were classified without lung biopsy were a case of hypersensitivity pneumonitis attributable to minocycline use and a case of bronchiolitis obliterans after documented adenovirus infection (Supplemental Fig 7).
Disorders of the Immunocompromised Host
A total of 23 (24.7%) cases were classified as “disorders of the immunocompromised host” based on history of malignancy for which they were receiving chemotherapy, a documented primary immunodeficiency, or history of bone marrow or stem cell transplant. Over half (12/23) of this population had infectious etiologies of their ILD (Supplemental Table 2). In both “normal” and immunocompromised hosts, infectious etiologies accounted for 18% of our cases of diffuse lung disease, a finding that is consistent with previous reports.4,20
Disorders Masquerading as ILD
Two cases (2.2%) were classified as “disorders masquerading as ILD.” One was a case of pulmonary venous stenosis in a 5-year-old, and the other was a case of pulmonary capillary hemangiomatosis in a teenager who presented with pulmonary hypertension and later required lung transplantation.
Unclassifiable
Eight (8.6%) cases were not classifiable. Three had undergone lung biopsy in their evaluations. One subject had histopathology with some features to suggest surfactant metabolism, such as the presence of type II pneumocyte hyperplasia, proteinosis, and prominent alveolar macrophages with cholesterol clefts. However, these features were patchy and lymphoid hyperplasia was prominent, rendering the histopathology atypical for a surfactant disorder. Genetic sequencing for SFTPB, SFTPC, ABCA3, and NKX2.1/TTF-1 yielded no abnormalities. One case had histopathology that was not specific for a definite etiology, and 1 case could not be interpreted owing to insufficient sampling. Five unclassifiable cases had diffuse lung disease on imaging but had not undergone lung biopsy and could not be confidently classified based on imaging patterns and clinical data. Supplemental Fig 8 displays a case that was deemed unclassifiable in the absence of lung biopsy or genetic diagnosis.
Outcomes
Duration of follow-up was highly variable (24 months; IQR 8.3 months, 72 months) in this study based on the retrospective design and the time period involved. A total of 75 (80.6%) of cases were alive without lung transplant at the time of follow-up. Three subjects (3.2%) had undergone lung transplantation with the following diagnoses: alveolar hypoplasia with patchy PIG, SFTPC mutation, and pulmonary hemangiomatosis and pulmonary hypertension. Of the 15 subjects who died, the cause of death was pulmonary failure related to disease in 10 cases, non-pulmonary causes in 3 cases, and not documented in 2 cases. Of those subjects who died of their pulmonary diseases, 4 had “disorders more prevalent in infancy,” including 2 with lung growth abnormalities, 1 with ACD-MPV, and 1 with ABCA3 mutations. Four deaths attributable to pulmonary disease occurred in subjects with “disorders of the immunocompromised host,” including cases of pulmonary fibrosis status-post therapy for lymphoblastoid leukemia, radiation fibrosis after treatment of rhabdomyosarcoma with pulmonary metastases, hemophagocytic lymphohistiocytosis and Cytomegalovirus pneumonitis, and disseminated aspergillosis after bone marrow transplantation for acute lymphoblastoid leukemia. Two subjects with “disorders related to systemic disease processes” died of pulmonary complications, including 1 with dermatomyositis whose lung biopsy showed capillaritis and follicular bronchiolitis, and 1 with scleroderma with pulmonary veno-occlusive disease and pulmonary hypertension.
Discussion
The primary goal of this study was to determine the historical occurrence of childhood ILD at our institution and analyze cases according to the current classification scheme. As our hospital was not a designated pediatric ILD referral center during the study period, our intention was to provide information about the scope of childhood ILD that would more closely reflect pulmonary practice patterns at most children’s hospitals. This study represents an approach to ILD investigation that has not previously been undertaken outside of major ILD referral centers or multicenter studies. Additionally, we aimed to determine the clinical utility of the current childhood ILD classification system. We made several observations that have implications both for our patient population and also for the approach to future studies of childhood ILD. First, we found a spectrum of ILD cases that was similar to other published reports,4,7,8,20 suggesting that some of these disorders may be more prevalent than currently suspected, as they are likely to be found at all children’s hospitals, not only at large referral centers. Second, we achieved definitive diagnosis for previously unclassified ILD cases, highlighting the value of retrospective review. In particular, we identified 5 additional cases of NEHI, 1 case of PIG, 1 case with a lung growth abnormality, and 1 with suspected surfactant dysfunction. These diagnoses are associated with widely differing prognoses and some have genetic implications for families, underscoring the importance of defining specific ILD diagnoses.
NEHI is a form of childhood ILD that is now known to have well-defined clinical, radiographic, and histologic features.4,19,21,22 Of the 8 NEHI cases, 5 were recognized via the retrospective review process. Three of these 5 cases came to clinical attention before the original description of NEHI in the medical literature in 2005.21 We suggest that cases that may be high-yield for review include children with unexplained lung disease presenting with chronic tachypnea and hypoxemia, and those whose lung biopsies were previously described as chronic bronchiolitis, follicular bronchiolitis, or “normal.” NEHI comprised almost 10% of the total cases in our study. The incidence and prevalence of NEHI has not been established, although efforts are underway to develop a national registry for childhood diffuse lung diseases including NEHI. Based on our data, we propose that very broad participation from children’s hospitals, not simply those designated as “ILD centers,” will greatly enhance case ascertainment.
It has been well recognized that there are types of ILD distinct to infants and young children. Almost one-quarter of our cases were classified as “disorders more prevalent in infancy.” Furthermore, 52% of our population younger than 2 years of age at the time of diagnosis was classified in the infant disorders category, a proportion similar to the 60% reported in the original multicenter lung biopsy classification study by Deutsch et al.4 Of the 21 cases in our cohort classified as “disorders more prevalent in infancy,” only 6 were diagnosed in children presenting after 2 years of age. These data underscore the differing etiologic considerations for ILD in young children and the utility of this aspect of the classification structure.
The current childhood ILD classification system was developed based on clinical-pathologic criteria. This classification scheme does not provide guidance in the organization of cases in the absence of lung biopsy. We found that almost one-third of cases were classifiable based on clinical, genetic, and/or radiographic criteria without lung biopsy. The category most impacted by inclusion of non-biopsy cases was “disorders related to systemic disease processes,” in which most (65%) were diagnosed based on clinical features, serology, and/or biopsy of organs other than the lung, such as the kidney. Surfactant dysfunction disorders are another subgroup in which there have been significant advances facilitating diagnosis without lung biopsy. In our cohort, 2 cases originally evaluated by lung biopsy were later found to have genetic etiologies (1 SFTPC mutation and 1 with compound heterozygous ABCA3 mutations). We also identified 2 additional cases with radiologic and histopathologic findings consistent with surfactant dysfunction in which genetic testing was not previously performed. Therefore, in the current era of clinically available genetic testing, we anticipate reductions in the numbers of surgical lung biopsies in this patient population. Lastly, in retrospect, 5 of the 8 NEHI cases met criteria for diagnosis based on clinical and radiographic findings,19,22 and therefore lung biopsy would be unlikely to be performed in the current clinical practice pattern at our center and others.23
We recognize the limitations inherent to this type of retrospective study. First, it is likely that the occurrence of childhood ILD at our institution has been greater than the number of cases we identified by surgical and billing queries for case ascertainment. We anticipate that new International Classification of Diseases-9 codes will help to identify cases in future studies.24 Furthermore, our cohort size and highly variable follow-up duration did not permit analysis of therapeutic efficacy, outcomes, and risk factors associated with morbidity and mortality. We believe that multicenter prospective studies will be required to address these questions.
Conclusions
While childhood ILDs are individually rare diseases, we have demonstrated that these disorders are likely to be found at most children’s hospitals and that targeted retrospective review of previously undiagnosed cases, especially infants, is useful. Our findings also suggest that broad multicenter participation in future studies could facilitate case ascertainment and thus accelerate much needed progress in understanding the incidence, risk factors, and outcomes of various childhood ILDs. Lastly, with clinical availability of genetic testing and improved pattern recognition on chest CT imaging, clinicians should increasingly consider less invasive diagnostic strategies. Modification to the current childhood ILD classification system is needed to better accommodate the growing numbers of cases diagnosed without lung biopsy.
Supplementary Material
Acknowledgments
We thank all of the providers involved in the care of these patients. We thank Dr Lawrence M. Nogee, of the Division of Neonatology at Johns Hopkins University, Baltimore, Maryland, for helpful discussion and review of this manuscript, and for inclusion of genetic testing results in 1 case from his research studies.25
Glossary
- ABCA3
gene encoding member A3 of the ATP Binding Cassette Family
- ACD-MPV
alveolar capillary dysplasia with misalignment of the pulmonary veins
- ChILD
childhood interstitial lung disease
- CT
computed tomography
- ILD
interstitial lung disease
- IQR
interquartile range
- NEHI
neuroendocrine cell hyperplasia of infancy
- NKX2.1
gene encoding for thyroid transcription factor-1
- PIG
pulmonary interstitial glycogenosis
- SFTPB
gene encoding surfactant protein B
- SFTPC
gene encoding surfactant protein C
- TTF-1
thyroid transcription factor-1
Footnotes
Dr Soares conceptualized and designed the study, designed the data collection instrument, performed data acquisition and analysis, and drafted the initial manuscript; Dr Deutsch contributed to the study design, performed the pathology review, and contributed to data analysis and drafting of the manuscript; Dr Moore contributed to the study design, data acquisition, and drafting of the manuscript; Dr Fazili contributed to the data acquisition and drafting of the manuscript; Drs Austin, Brown, and Hilmes contributed to the study design, data acquisition and analysis, and drafting of the manuscript; Dr Sokolow contributed to data acquisition and drafting of the manuscript; Dr Young conceptualized and designed the study, contributed to data acquisition and analysis and writing of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the American Thoracic Society/ChILD Foundation/chILD (Lung) Foundation-UK (Dr Young); NIH K08HL082757 (Dr Young). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Fan LL, Deterding RR, Langston C. Pediatric interstitial lung disease revisited. Pediatr Pulmonol. 2004;38(5):369–378 [DOI] [PubMed] [Google Scholar]
- 2.Fan LL, Kozinetz CA. Factors influencing survival in children with chronic interstitial lung disease. Am J Respir Crit Care Med. 1997;156(3 pt 1):939–942 [DOI] [PubMed] [Google Scholar]
- 3.Deterding R. Evaluating infants and children with interstitial lung disease. Semin Respir Crit Care Med. 2007;28(3):333–341 [DOI] [PubMed] [Google Scholar]
- 4.Deutsch GH, Young LR, Deterding RR, et al. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176(11):1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan LL, Mullen AL, Brugman SM, Inscore SC, Parks DP, White CW. Clinical spectrum of chronic interstitial lung disease in children. J Pediatr. 1992;121(6):867–872 [DOI] [PubMed] [Google Scholar]
- 6.Fan LL, Kozinetz CA, Deterding RR, Brugman SM. Evaluation of a diagnostic approach to pediatric interstitial lung disease. Pediatrics. 1998;101(1 pt 1):82–85 [DOI] [PubMed] [Google Scholar]
- 7.Clement A. Task force on chronic interstitial lung disease in immunocompetent children. Eur Respir J. 2004;24(4):686–697 [DOI] [PubMed] [Google Scholar]
- 8.Griese M, Haug M, Brasch F, et al. Incidence and classification of pediatric diffuse parenchymal lung diseases in Germany. Orphanet J Rare Dis. 2009;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan N, Taam RA, Epaud R, et al. A national internet-linked based database for pediatric interstitial lung diseases: the French network. Orphanet J Rare Dis. 2012;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinwiddie R, Sharief N, Crawford O. Idiopathic interstitial pneumonitis in children: a national survey in the United Kingdom and Ireland. Pediatr Pulmonol. 2002;34(1):23–29 [DOI] [PubMed] [Google Scholar]
- 11.Thomas AQ, Lane K, Phillips J, III, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322–1328 [DOI] [PubMed] [Google Scholar]
- 12.Hamvas A, Deterding RR, Wert SE, et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2-1. [published online ahead of print February 23, 2013] Chest. 10.1378/chest.12-2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350(13):1296–1303 [DOI] [PubMed] [Google Scholar]
- 14.Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328(6):406–410 [DOI] [PubMed] [Google Scholar]
- 15.Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol. 2009;12(4):253–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344(8):573–579 [DOI] [PubMed] [Google Scholar]
- 17.Guillerman RP. Imaging of childhood interstitial lung disease. Pediatr Allergy Immunol Pulmonol. 2010;23(1):43–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch DA, Hay T, Newell JD, Jr, Divgi VD, Fan LL. Pediatric diffuse lung disease: diagnosis and classification using high-resolution CT. AJR Am J Roentgenol. 1999;173(3):713–718 [DOI] [PubMed] [Google Scholar]
- 19.Brody AS, Guillerman RP, Hay TC, et al. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol. 2010;194(1):238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langston C, Dishop MK. Diffuse lung disease in infancy: a proposed classification applied to 259 diagnostic biopsies. Pediatr Dev Pathol. 2009;12(6):421–437 [DOI] [PubMed] [Google Scholar]
- 21.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40(2):157–165 [DOI] [PubMed] [Google Scholar]
- 22.Young LR, Brody AS, Inge TH, et al. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest. 2011;139(5):1060–1071 [DOI] [PubMed] [Google Scholar]
- 23.Kerby GS, Wagner BD, Popler J, et al. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy [published online ahead of print November 20, 2012]. Pediatr Pulmonol. doi: 10.1002/ppul.22718 [DOI] [PubMed] [Google Scholar]
- 24.Popler J, Lesnick B, Dishop MK, Deterding RR. New coding in the International Classification of Diseases, Ninth Revision, for children's interstitial lung disease. Chest. 2012;142(3):774–780 [DOI] [PubMed] [Google Scholar]
- 25.Bullard JE, Wert SE, Whitsett JA, Dean M, Nogee LM. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172(8):1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.