Abstract
Previously, we showed that inhibition of the protein kinase C β (PKCβ)/AKT pathway augments engagement of the histone deacetylase inhibitor (HDI)-induced apoptosis in lymphoma cells. In the present study, we investigated the cytotoxicity and mechanisms of cell death induced by the delta isoform-specific phosphatidylinositide 3-kinase (PI3K) inhibitor, GS-1101, in combination with the HDI, panobinostat (LBH589) and suberoylanilide hydroxamic acid (SAHA). Lymphoma cell lines and primary Non-Hodgkin Lymphoma (NHL) and chronic lymphocytic leukaemia (CLL) cells were simultaneously treated with the HDI, LBH589 and GS-1101. An interaction of the LBH589/GS-1101 combination was formally examined by using various concentrations of LBH589 and GS-1101. Combined treatment resulted in a synergistic inhibition of proliferation and showed synergistic effect on apoptotic induction in all tested cell lines and primary NHL and CLL cells. This study indicates that interference with PI3K signalling dramatically increases HDI-mediated apoptosis in malignant haematopoietic cells, possibly through both AKT-dependent or AKT- independent mechanisms. Moreover, the increase in HDI-related apoptosis observed in PI3K inhibitor-treated cells appears to be related to the disruption of the extracellular signal-regulated kinase (ERK) signalling pathway. This study provides a strong rational for testing the combination of PI3K inhibitors and HDI in the clinic.
Keywords: CLL, Apoptosis, Non-Hodgkin Lymphoma, PI3K, HDAC, Synergy
Introduction
The incidence of non-Hodgkin lymphoma (NHL) has nearly doubled in the last four decades with an annual increase of 4% (Jemal et al, 2008). B-cell lymphomas account for 85% of NHLs in Western countries. While advances in therapy have been realized, such as development of rituximab, low grade lymphomas remain largely incurable and aggressive B-cell lymphomas, such as high risk diffuse large B-cell lymphoma (DLBCL) have 5-year survivals of less than 50%. Thus, better therapies are needed.
Phosphatidylinositol 3-kinases (PI3K) are enzymes that transduce signals from cell surface receptors to effector molecules containing pleckstrin homology domains, such as BTK, or AKT (So & Fruman, 2012). Four isoforms (PI3K7agr;, PI3Kβ, PI3Kγ, and PI3Kδ) (Vanhaesebroeck et al, 2010) have been identified that regulate a variety of cellular functions through the production of phosphatidylinositol (3,4,5)-triphosphate (PIP3). Recently, clinical studies using the specific PI3Kδ inhibitor, GS-1101, as a single agent have shown durable remissions in a significant percentage of patients with chronic lymphocytic leukaemia (CLL), indolent NHL, or mantle cell lymphoma (MCL) (Fruman & Rommel, 2011; So & Fruman, 2012).
Previously, we showed that by disrupting multiple compensatory cytoprotective pathways, protein kinase C (PKC) inhibitors in combination with histone deacetylase (HDAC) inhibitors (HDI) might have potential therapeutic value in lymphoma treatment (Bodo et al, 2011). HDI represent an emerging class of therapeutic agents that induce tumour cell cycle arrest and apoptosis in various haematological malignancies (Chen et al, 2009; Glaser, 2007). The lack of selectivity of the currently available HDI (panobinostat [LBH589] and suberoylanilide hydroxamic acid [SAHA] are pan-HDI) results in modulating the acetylation status of a wide range of protein targets that leads to a therapeutic response, but also to undesired toxic effects, including haematological, gastrointestinal and cardiac toxicity (Haberland et al, 2009). SAHA monotherapy is approved by the Food and Drug Administration (FDA) for the treatment of cutaneous T-cell lymphoma, however it has not been demonstrated to have meaningful single agent activity in B-cell NHL patients (Crump et al, 2008). Therefore, future HDI-based therapies will probably be designed based on combination therapies with other agents with synergistic effects. Moreover, such an approach may overcome emerging resistance to targeted anti-cancer agents.
In this study we expand our previous work by investigating the antiproliferative and proapoptotic activity of the combination of PI3K inhibitors (PI3Ki) with HDI in a panel of B-cell lymphoma lines and primary lymphoma and leukaemia cells.
Methods
Materials, cell lines and treatment
All antibodies, except anti-PI3Kδ (Santa Cruz Biotechnology, Santa Cruz, CA) were obtained from Cell Signaling Technology (Danvers, MA). SU-DHL-6, SU-DHL-16, OCI-LY-19 cell lines were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany). OCI-LY-3 and OCI-LY-10 cell lines were kindly provided by Dr. Lossos (University of Miami, Miami, FL). Ramos and Raji cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). GS-1101 was obtained from Gilead Sciences (Foster City, CA). BKM120 and LBH589 were provided by Novartis Pharmaceuticals Inc. (Cambridge, MA). SAHA was purchased from Cayman chemicals (Ann Arbor, MI).
Primary cells were obtained from peripheral blood of patients. Informed consent was obtained according to protocols approved by the Institutional Review Board of the Cleveland Clinic. Isolated primary cells were resuspended to 2.0 × 106/ml in RPMI 1640 medium containing 20% fetal bovine serum. Isolated primary CLL cells consisted of 85–97% malignant B-CLL cells.
Cytotoxicity assay
Effect of drugs on cell survival was assessed with the water-soluble tetrazolium salt (WST)-1 assay. Cells were treated simultaneously with five serial dilutions of drugs for 48 H. Thereafter, the cells were incubated with WST-1 according to manufacturer’s instructions (Catalog No. 05015944001, Roche, Indianapolis, IN). Each dose was tested in triplicate; dose-effect and combination index curves were constructed using CalcuSyn software (Biosoft, Cambridge, UK).
Cell cycle analysis and apoptosis
Cell cycle phases and the percentage of subdiploid population were determined by propidium iodide flow cytometry staining. The cells were permeabilized with a solution of 0.05% Triton X-100 (Sigma-Aldrich, St. Louis, MO)/PBS with RNAase A (100 μg/ml) (Fisher-Scientific, Pittsburgh, PA) and stained with propidium iodide (50 μg/ml) (Sigma-Aldrich). Additionally, the percentage of subdiploid cells was correlated with Annexin V-phycoerythrin (PE)/7-AAD staining. The cells were stained with Annexin V-PE/7-aminoactinomycin D (7-AAD) according to the manufacturer’s instructions (BD Biosciences, San Jose, California).
Western blot analysis
Cells were harvested after treatment for 24 h and whole-cell protein extracts were prepared by incubating the cells in lysis buffer (R&D Systems, Minneapolis, MN) on ice for 30 min. Each lane of a 4–15% Ready Tris-HCl gel (Bio-Rad, Hercules, CA) was loaded with 20–30 μg of protein. After electrotransfer, blots were blocked with 5% milk/Tris-buffered saline-Tween 20 (TBS-T), incubated with the recommended dilutions of indicated antibodies overnight at 4 °C and then incubated with horseradish peroxidase-conjugated anti-rabbit- or anti-mouse-secondary antibody for 1 h at room temperature.
siRNA transfection
SUDHL-6 and SUDHL-16 cells were transfected with control-GFP or AKT1/2 siRNA (Santa Cruz Biotechnologies, Santa Cruz, CA, USA)’ using the Amaxa Nucleofector Kit V (Lonza, Walkersville, MD) according to the manufacturer’s protocol.
Statistical Analysis
For all results, values represent the mean from at least three independent experiments, with the exception of the experiments with primary cells where values derive from one analysis only. The interaction between drugs was examined according to the Chou and Talalay method (Chou & Talalay, 1984) by using the Calcusyn software (Biosoft). Combination index (CI) values served to determine the combined effect as synergistic (<1), additive (=1), or antagonistic (>1).
Results
Characterization of sensitivity of B-cell lymphoma cell lines to GS-1101
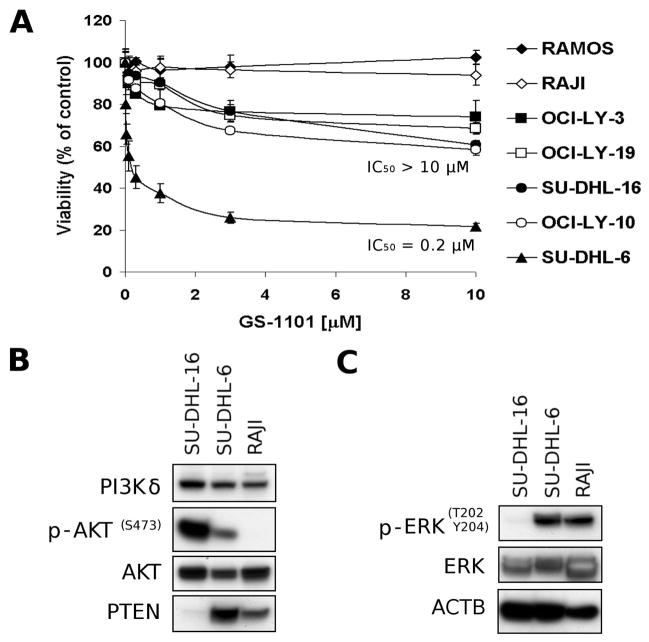
DLBCL and Burkitt lymphoma cell lines were treated with different concentrations of GS-1101 for 48 h. Dose-response curves are shown in Figure 1A. A wide diversity of cell responses to the GS-1101 treatment was observed. For example, the DLBCL cell line, SU-DHL-6, exhibited a 50% inhibitory concentration (IC50) of 0.2 μM, whereas the other tested DLBCL cell lines needed a GS-1101 concentration higher than 10 μM to induce 50% growth inhibition. The most resistant were Burkitt lymphoma cell lines, which did not respond to the GS-1101 treatment in tested concentration range at all.
Figure 1. Characterization of GS-1101 response in human B-cell lymphoma cell lines.
(A)Antiproliferative effect of GS-1101 in B-cell lymphoma cell lines. Cells were incubated with increasing concentrations of GS-1101 for 48 h before cell viability was determined by the WST-1 assay. Assessment of activity of (B) AKT pathway and (C) ERK pathway in untreated B-cell lymphoma cell lines using Western Blotting. IC50, 50 % inhibitory concentration.
To investigate signalling pathways that may have an impact on GS-1101 treatment, three cell models (SU-DHL-6 as the most sensitive cell line, SU-DHL-16 with moderate sensitivity, and Raji, the most resistant cell line) were selected for Western blot analysis (Figure 1B, C). The analysis revealed high levels of phosphorylated AKT in untreated SU-DHL-16 cells, significantly lower levels in SU-DHL-6 cells and finally no detectable expression in Raji cells. In addition to the high level of phosphorylated (p-)AKT protein in SU-DHL-16 cells, a reduced level of PTEN protein was observed. This is in concordance with recent work showing that AKT is constitutively phosphorylated in SU-DHL-16 cells probably due to an aberrant overexpression of MIR155 that activates PI3K-AKT signalling by down-regulation of its suppressors (e.g. PTEN) (Huang et al, 2012). Furthermore, SU-DHL-16 cells were almost negative for a phosphorylated-ERK in comparison to relative high levels in Raji and SU-DHL-6 cells. These results showed that neither p-AKT or p-ERK can be used as a predictive marker for GS-1101 treatment. The absence of negative regulation of the AKT activity in SU-DHL-16 cells and low level of ERK protein might contribute to the higher resistance to GS-1101 treatment. In contrast, a relative loss of AKT activity in Raji cells may explain its lack of sensitivity to the GS-1101 treatment.
GS-1101 synergistically potentiates LBH589 induced proliferation inhibition in DLBCL cell lines and primary cells
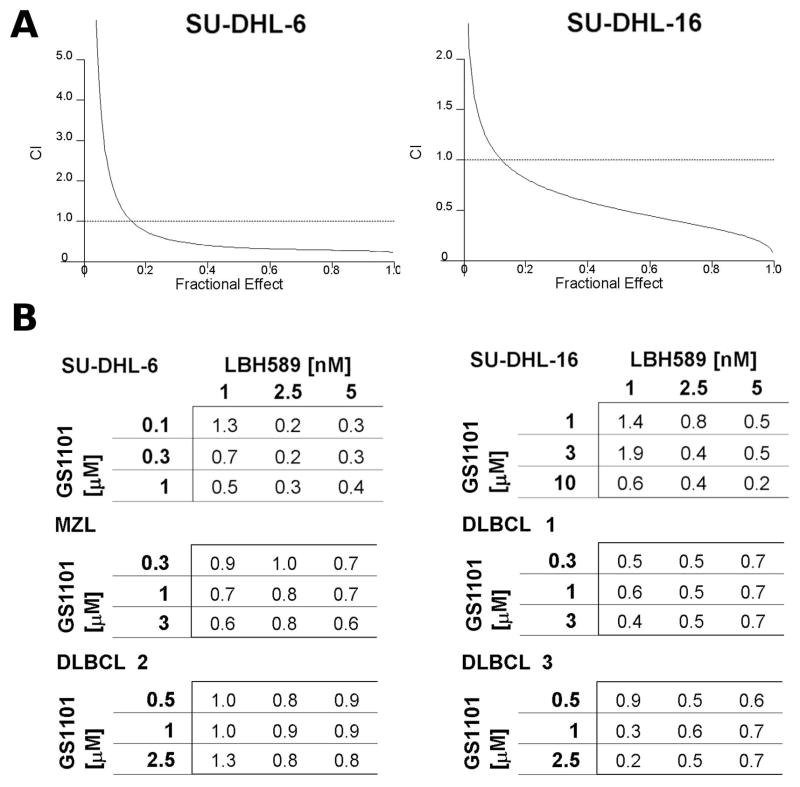
It is known that HDI can inhibit cytoprotective signalling pathways, including those related to AKT (Rahmani et al, 2003a; Rahmani et al, 2003b). To enhance the effect of GS-1101 in SU-DHL-16 cells, cells were simultaneously treated with the HDI, LBH589. The IC50 for LBH589 single treatment was 5.8 nM in SU-DHL-6 and 2.7 nM in SU-DHL-16 cells. To formally examine an interaction of the LBH589/GS-1101 combination, the cells were treated with varying concentrations of LBH589 and GS-1101 at fixed ratio. SU-DHL-6 cells were treated with 0.25–6.25 nM of LBH589 and 0.01–0.25 μM of GS-1101. SU-DHL-16 cells were treated with similar concentrations (0.25–5 nM) of LBH589, but with significantly higher concentrations (0.5–10 μM) of GS-1101. Eventually, fractional effect-CI curves were constructed (Figure 2A). These curves were created according to the Chou-Talalay method. The analysis of combined effects revealed highly synergistic interactions in both cell lines.
Figure 2. Interaction between GS-1101 and LBH589 is dose-dependent and synergistic.
(A) Fractional-effect/combination index (CI) curves were generated with the Chou-Talalay method of statistical analysis. Cells were treated for 48 h with indicated doses at fixed ratios. (B) Combination indexes for SU-DHL-6/16 cell lines and primary mantle zone lymphoma (MZL) and diffuse large B cell lymphoma (DLBCL) cells treated with different combinations of GS-1101 and LBH589 concentrations.
Further experiments with variable concentration ratio showed that this combination provides very strong synergistic effect with CIs ≤ 0.5 in tested cell lines and moderate synergistic effect with CIs ≤ 0.9 in all tested B-NHL primary samples (Figure 2B).
Additionally, a cytotoxicity test, which analysed the difference between sequential and simultaneous treatment, showed no significant difference if the cells were pretreated with GS-1101 or LBH589 or simultaneously exposed to both agents (data not shown).
GS-1101/LBH589 combination induces caspase-dependent apoptosis and represents class interaction between HDI and PI3Ki
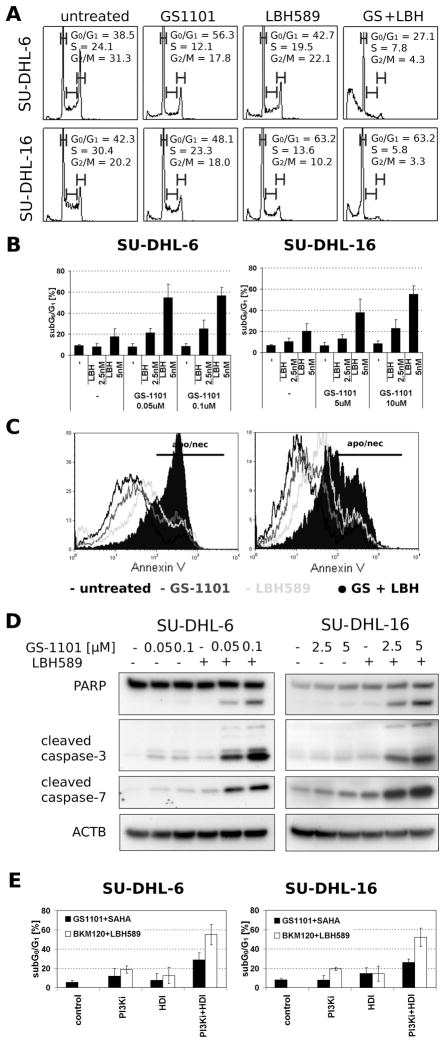
Cell cycle analysis (Figure 3A) showed that both GS-1101 and LBH589 induce G0/G1 arrest and a significant increase (p<0.05) in percentage of sub-G0/G1 cells, corresponding to subdiploid DNA content in both SU-DHL-6/16 cell lines (Figure 3B). An experiment with synchronized SU-DHL-6 cells treated simultaneously with GS-1101 and LBH589 further confirmed that the cells undergo apoptosis after they enter G1 phase (data not shown). The co-treatment of SU-DHL- 6 cells with 0.1 μM GS-1101 and 5 nM LBH589 induced a 47% increase in subdiploid population compared to the untreated sample, while GS-1101 alone showed little effect and LBH589 induced only a 9% increase in the sub-G0/G1 cell population. Likewise, in SU-DHL-16 cells, 10 μM GS-1101 and 5 nM LBH589 co-treatment induced a 48% increase in the subdiploid population compared to the untreated sample. GS-1101 single treatment induced a 2% increase and LBH589 induced a 14% increase in the sub-G0/G1 cell population. Similar results were obtained using an Annexin V/7-AAD assay (Figure 3C), confirming that sub-G0/G1 increase is due to the induction of apoptosis. Moreover, when the cells were pretreated with Caspase inhibitor I, a significant decrease in the sub-G0/G1 cell population was observed (data not shown).
Figure 3. Induction of apoptosis in SU-DHL-6/16 cells after exposure to GS-1101 and LBH589.
(A) Cells were incubated with GS-1101 and/or LBH589 (LBH) for 48 h, and the cell cycle was analysed by flow cytometry. (B) sub-G0/G1 DNA content analysis of SU-DHL-6/16 cell lines after 48 h treatment. (C) Histogram of annexin V/7-aminoactinomycin D (7-AAD) positive cells, where annexin V/7-AAD positivity reflects the number of apoptotic (apo) and necrotic (nec) cells. Annexin V/7-AAD staining was employed to confirm the extent of apoptosis after the combined treatment. (D) Combination of GS-1101 and LBH589 significantly induce PAPR cleavage and activation of caspase-3, and -7 in SU-DHL-6/16 cells. Cells were treated for 24 h and protein extracts were analysed by Western Blot. (E) sub-G0/G1 DNA content analysis of SU-DHL-6/16 cells after 48 h combined treatment where SAHA substitutes LBH589 and GS-1101 was replaced by BKM120.
Moreover, Western blot analysis showed that GS-1101 in combination with LBH589 induced cleavage of PARP, caspase-3 and -7, whereas single treatment had only a minimal effect on the cleavage of these proteins (Figure 3D).
To verify whether the synergism was due to a class interaction between HDI and PI3Ki or a drug-specific interaction, additional agents were tested (Figure 3E). Substituting the GS-1101 with the pan-PI3Ki, BKM120, similarly enhanced induction of apoptosis in combination with LBH589. For example, in SU-DHL-6 cells, co-treatment of 1 μM BKM120 with 5 nM LBH589 induced 50% increase of a subdiploid population in comparison to the untreated sample, while BKM120 alone induced a 13% increase in the sub-G0/G1 cell population. Similarly, GS-1101 enhanced SAHA-induced apoptosis. Together, these results suggest that the effect of GS-1101/LBH589 combination represents a class effect between HDI and PI3Ki.
Synergistic effect of GS-1101/HDI treatment on induction of apoptosis in primary CLL cells
Clinical studies with GS-1101 as a single agent have shown promising efficacy in a significant percentage of patients with CLL. Therefore, we also evaluated the apoptotic induction of combined GS-1101/HDI treatment in CLL primary cells.
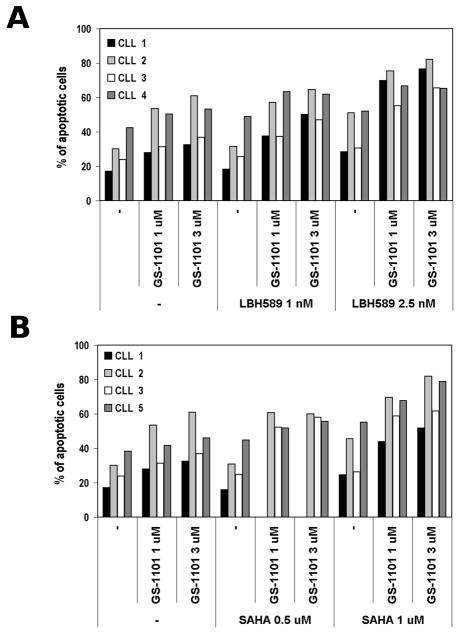
Leukaemia cells from 5 CLL cases were treated with GS-1101, either alone or in combination with LBH589 or SAHA. After 48 h, CD5/CD19 dual positive populations were analysed using the Annexin V/7-AAD assay (Figure 4, Figure S1). All tested drugs were effective as a single agent. However, the combination treatment further potentiated the induction of apoptosis. As summarized in Table I, CLL primary cells treated with the combination of GS-1101 and HDI resulted in a synergistic induction of apoptosis with CI values mostly lower than 0.8. Thus, the CI analysis showed a synergistic effect and provided proof of principle for testing the PI3Ki/HDI combination also in CLL patients.
Figure 4. Synergistic induction of apoptosis of GS-1101/HDI combined treatment in primary CLL cells.
(A). Isolated CLL peripheral blood mononuclear cells were treated with GS-1101 and/or LBH589 for 48 h followed by annexin V/7-aminoactinomycin D flow cytometry assay. (B) Dose-dependent increase of percentage of apoptotic cell population in primary CLL cases after treatment with GS-1101 and/or SAHA.
Table I.
Combination Index values calculated for the combination treatment of chronic lymphocytic leukaemia (CLL) cells
| CLL patient | LBH589 | SAHA | ||||
|---|---|---|---|---|---|---|
| 1 nM | 2.5 nM | 5 nM | 0.5 μM | 1 μM | 2 μM | |
| 1 | 0.38 | 0.26 | 0.61 | N/D | 0.62 | 0.74 |
| 2 | 1.09 | 0.66 | 0.74 | 0.98 | 0.61 | 0.72 |
| 3 | 0.29 | 0.19 | 0.27 | 0.12 | 0.18 | 0.28 |
| 4 | 0.44 | 0.51 | 0.38 | N/D | N/D | N/D |
| 5 | N/D | N/D | N/D | 0.39 | 0.10 | 0.19 |
CLL, chronic lymphocytic leukaemia; LBH589, panobinostat; SAHA, suberoylanilide hydroxamic acid.
GS-1101/LBH589 combination modulates multiple signalling pathways
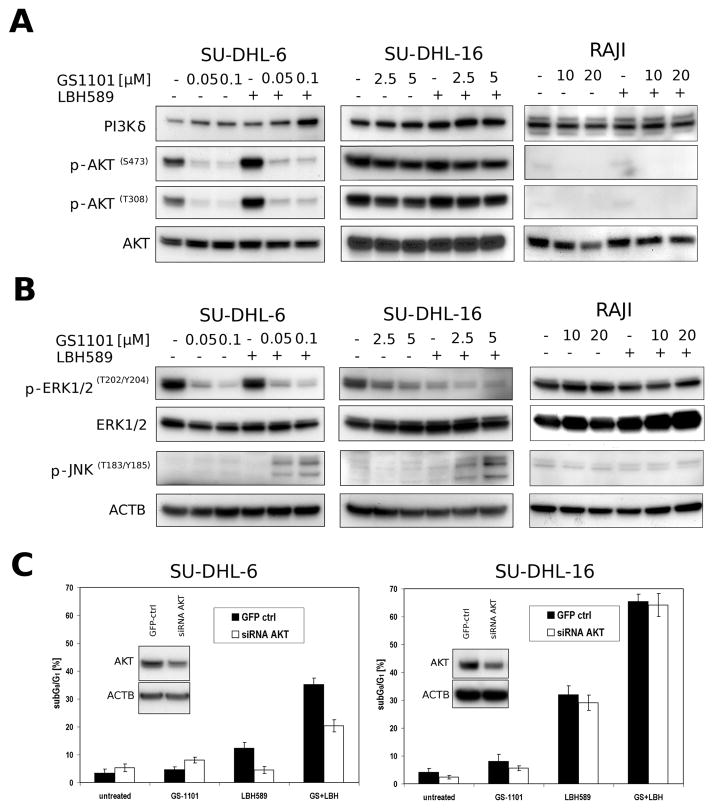
To gain an insight into the molecular mechanisms involved in combination treatment, several signalling pathways, such as ERK, AKT, JNK were investigated (Figure 5A, B). LBH589 single treatment slightly increased levels of p-AKT in SU-DHL-6 cells, but not in SU-DHL-16 or Raji cells. As we expected, the combined treatment of SU-DHL-6 cells mostly recapitulated the decrease of the p-AKT level seen in the cells treated with GS-1101 alone. Interestingly, in SU-DHL-16 cells no potentiation of the mild GS-1101-induced p-AKT downregulation was observed with the combined treatment.
Figure 5. GS-1101 and LBH589 modulate levels of several signalling proteins.
(A) Western blot analysis of AKT phosphorylations after combined treatment with GS-1101/LBH589 for 24 h. (B) Western blot analysis of phospho-ERK and phospho-JNK after combined treatment with GS-1101/LBH589 for 24 h. (C) Sub-G0/G1 DNA content analysis of si RNA AKT transfected SU-DHL-6/16 cell lines after 48 h of GS-1101/LBH589 treatment. GFP ctrl, green fluorescent protein control; LBH, LBH589.
Moreover, in SU-DHL-6, low concentrations (0.5, 1 nM) of LBH589 increased p-ERK, while higher concentrations (5, 10 nM) reversed it (data not shown). In contrast, in SU-DHL-16 cells, the effect of LBH589 on p-ERK decrease was dose-dependent (data not shown). GS-1101 single treatment induced a decrease of p-ERK in both DLBCL cell lines but did not any impact on this protein in Raji cell line. In SU-DHL-6 cells, similarly as with p-AKT, the combination treatment did not change p-ERK levels in comparison to GS-1101 single treatment. However, a further diminution was detected in SU-DHL-16 cells. In Raji cells, the levels remained the same as with LBH589 treatment alone.
Interestingly, when each of the agents was used individually, it had no effect on p-JNK levels in SU-DHL-6/16 cells. However, the combined treatment markedly augmented JNK phosphorylation. This increase was not observed in Raji cells.
To better understand the mechanism of action of GS-1101/LBH589 combination in SU-DHL-6/16 cells, the cells were transfected with siRNA AKT, treated with both drugs and and the effect on cell cycle progression and apoptosis was further examined (Figure 5C). Western blot analysis showed a decreased AKT level in both cell lines transfected with siRNA AKT. However, a significant decrease (p<0.05) of the percentage of apoptotic cells after the combined treatment was detected only in SU-DHL-6 cells. The co-treatment of SU-DHL-6 cells transfected with the siRNA AKT, induced only a 15% increase in apoptotic population compared to 32% increase in control cells. Interestingly, siRNA AKT transfections increased the percentage of apoptotic cells in samples treated with GS-1101 alone and decreased in samples treated with LBH589 only.
Discussion
Recent evidence suggests that the proapoptotic effect of HDI is associated with modulation of protein kinase signalling pathways including MAPKs, PI3K and PKC pathways (Bodo et al, 2011;Espinos & Weber, 1998; Kim et al, 2006). The fact that the activity of the HDAC counterpart, histone acetyltransferase, is upregulated through phosphorylation by ERK, CDK2, or PKA (Kalkhoven, 2004) supports the importance of such pathways in HDI-induced lethality. Moreover, it has been shown that MAPK and PI3K pathways are increasingly associated with resistance to HDI (Kodani et al, 2005; Matsubara et al, 2009). Thus, combining HDI with inhibitors affecting these pathways is rational. In this study we demonstrated that PI3Ki/HDI combined treatment synergistically inhibits proliferation, primarily by promoting G1 cell cycle arrest, and induces apoptosis in B-cell lymphoma and leukaemia cells
The cytotoxic effects of PI3K modulation have frequently been attributed to AKT inactivation (Nesterov et al, 2001); however, previously it has been suggested that the interference with AKT signalling by PI3Ki plays little, if any, role in promoting HDI-mediated lethality (Rahmani et al, 2003b, Figure S2). In fact, our experiments showed that, after the GS-1101/LBH589 combined treatment, SU-DHL-16 cells underwent a synergistic inhibition of proliferation and apoptosis induction despite the failure of AKT inactivation. Similarly, the downregulation of AKT using siRNA AKT did not have any impact on the induction of apoptosis induced by combined treatment. It is obvious that in these cells, GS-1101 potentiation of GS-1101/LBH589-mediated proliferation, inhibition and apoptosis occurs through an alternative PI3K-dependent, but AKT independent pathway, such as, CDC42, RAC, or SGK among others (Murga et al, 2002; Park et al, 1999).
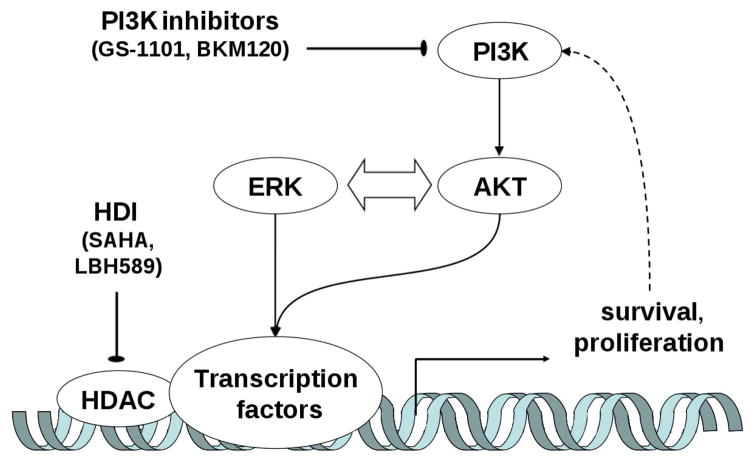
Although we do not completely understand the mechanisms that underlie the observed synergism, decreased levels of p-ERK may be an important indicator of efficacy. In our experiments, GS-1101 and LBH589 individually decreased ERK phosphorylation in SU-DHL-16 cells, and the combination was particularly effective in this regard. A similar pattern was seen in SUD-HL-6 cells. Although exceptions exist, ERK activation is generally associated with enhanced cell survival (Xia et al, 1995). ERK activation has also been shown to have an important function in protecting cells from the otherwise lethal effects of oxidative stress (Wang et al, 1998). Forced activation of MEK and ERK attenuated PI3Ki/HDI-mediated lethality. This might be explained by stabilization (Yang & Yu, 2003) of an antiapoptotic protein, BCL2, by ERK and eventually prevention of release of proapoptotic protein from the mitochondria (Dias & Bailly, 2005). We have found that, despite ERK inactivation when GS-1101 and LBH589 were administered individually, minimal toxicity indicates that this was insufficient, by itself, to potently induced apoptosis. Importantly, SU-DHL-6 cells were significantly more sensitive to PI3Ki/HDI-mediated apoptosis than SU-DHL-16 or Raji cells. The reason might be that both ERK and AKT pathways are active in SU-DHL-6 cells (Figure S3), whereas only one of these pathways is highly active in the other cell lines. The cross-talk between the ERK and AKT cascades has been previously described (Shelton et al, 2004), and combined downregulation of both AKT and ERK may be considerably more lethal than interruption of either pathway alone. Thus, a plausible explanation is that PI3K inhibitors (e.g. GS-1101, BKM120) disrupt cytoprotective signalling pathways that protect cells against the effect of HDI and it results in cell cycle arrest and potentiation of the apoptotic induction (Figure 6).
Figure 6. A schematic model of the mechanism of PI3K inhibitors and HDI synergy.
Disruption of the cytoprotective signalling pathways potentiates HDI effects and results in cell cycle arrest and apoptotic induction. HDAC, histone deacetylase HDI, HDAC inhibitor PI3K, phosphatidylinositide 3-kinase.
GS-1101/LBH589 treatment was also accompanied by JNK activation. It is know that HDI have the ability to induce DNA damage in tumour cells and to promote γH2A.X formation, which participates in DNA damage repair (Rosato et al, 2008). In this context, the JNK-related stress pathway might be activated by DNA damage. Hypothetically, LBH589, by inducing DNA damage, and GS-1101, by inhibiting DNA damage repair, may potentiate the DNA damage and contribute to the proliferation inhibition and cell death. However, treatment with the JNK inhibitor, SP600125, failed to attenuate PI3Ki/HDI-mediated lethality (Figure S4). This finding suggests that JNK activation represents a consequence of PI3Ki/HDI-mediated apoptosis rather than its cause.
While cell lines represent an excellent tool for initial work, they cannot always accurately predict effects in primary tumour cells. As GS-1101 showed promise in indolent B-cell lymphoproliferative disorders, such as CLL, a response of CLL cells to the LBH589/GS-1101 treatment was also studied. This treatment resulted in a synergistic apoptosis induction with CI values mostly < 0.8. Furthermore, the experiments using alternate PI3K and HDI suggested that the effect seen is not dependent on the individual specific compound, which strengthens the pre-clinical rationale for this combination.
In summary, we have demonstrated the synergistic anti-tumour activity achieved by combining PI3Ki with HDI in established B-cell lymphoma cell lines and primary NHL/CLL cells. Further, this study indicates that interference with PI3K signalling dramatically increases HDI-mediated apoptosis in malignant haematopoietic cells - this might be through either AKT-dependent or AKT-independent mechanisms. Moreover, the dramatic increase in HDI-related apoptosis observed in PI3Ki-treated cells appears to be related to the disruption of the ERK signalling pathway. Given that PI3Ki (e.g. GS-1101, BKM120) and HDI (panobinostat) are in clinical trials, and some HDI (e.g. SAHA, romidepsin) are already FDA-approved, this study provides a strong rational for testing the combination of PI3Ki and HDI in the clinic.
Supplementary Material
Fig S1. The effect of GS-1101/LBH589 treatment on the induction of the apoptosis in CLL primary cells cultured with and without HS-5 stroma cells.
Fig S2. The effect of GS-1101/LBH589 treatment on (A) p-AKT, p-ERK, and p-JNK and (B) induction of apoptosis in GS-1101 resistant SU-DHL-6 cell line.
Fig S3. sub-G0/G1 DNA content analysis of siRNA AKT and siRNA ERK transfected SUDHL-6 cell lines after 48 h of GS-1101/LBH589 treatment.
Fig S4. The effect of JNK inhibitor SP600125 pretreatment on the percentage of apoptotic cells after GS-1101/LBH589 treatment.
Acknowledgments
The authors would like to thank Lisa Durkin for her technical expertise in cell culturing. This work was supported by grant from National Institutes of Health (CA127264).
Footnotes
Authorship and Disclosures
JB was the principal investigator and takes primary responsibility for the paper. BTH and CAP provided the patient samples. JB, XZ, and AS performed the laboratory work for this study. JB, XZ, and EDH co-ordinated the research and wrote the paper. AS, AA, BTH, CAP, and BJL collaborated on writing the paper. JB, XZ, AS, BTH, CAP, AA, and EDH reported no potential conflicts of interest. BJL is an employee of Gilead Sciences Inc.
References
- Bodo J, Sedlak J, Maciejewski JP, Almasan A, Hsi ED. HDAC inhibitors potentiate the apoptotic effect of enzastaurin in lymphoma cells. Apoptosis. 2011;16:914–923. doi: 10.1007/s10495-011-0617-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Fiskus W, Eaton K, Fernandez P, Wang Y, Rao R, Lee P, Joshi R, Yang Y, Kolhe R, Balusu R, Chappa P, Natarajan K, Jillella A, Atadja P, Bhalla KN. Cotreatment with BCL-2 antagonist sensitizes cutaneous T-cell lymphoma to lethal action of HDAC7-Nur77-based mechanism. Blood. 2009;113:4038–4048. doi: 10.1182/blood-2008-08-176024. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Crump M, Coiffier B, Jacobsen ED, Sun L, Ricker JL, Xie H, Frankel SR, Randolph SS, Cheson BD. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann Oncol. 2008;19:964–969. doi: 10.1093/annonc/mdn031. [DOI] [PubMed] [Google Scholar]
- Dias N, Bailly C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem Pharmacol. 2005;70:1–12. doi: 10.1016/j.bcp.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Espinos E, Weber MJ. Activation of the MAP kinase cascade by histone deacetylase inhibitors is required for the stimulation of choline acetyltransferase gene promoter. Brain Res Mol Brain Res. 1998;56:118–124. doi: 10.1016/s0169-328x(98)00036-9. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Rommel C. PI3Kdelta Inhibitors in Cancer: Rationale and Serendipity Merge in the Clinic. Cancer Discov. 2011;1:562–572. doi: 10.1158/2159-8290.CD-11-0249. [DOI] [PubMed] [Google Scholar]
- Glaser KB. HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Shen Y, Liu M, Bi C, Jiang C, Iqbal J, McKeithan TW, Chan WC, Ding SJ, Fu K. Quantitative Proteomics Reveals miR-155 Regulates the PI3K-AKT Pathway in Diffuse Large B-Cell Lymphoma. Am JPathol. 2012;181:26–33. doi: 10.1016/j.ajpath.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E. CBP and p300: HATs for different occasions. Biochem Pharmacol. 2004;68:1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Kim YK, Seo DW, Kang DW, Lee HY, Han JW, Kim SN. Involvement of HDAC1 and the PI3K/PKC signaling pathways in NF-kappaB activation by the HDAC inhibitor apicidin. Biochem Biophys Res Commun. 2006;347:1088–1093. doi: 10.1016/j.bbrc.2006.06.196. [DOI] [PubMed] [Google Scholar]
- Kodani M, Igishi T, Matsumoto S, Chikumi H, Shigeoka Y, Nakanishi H, Morita M, Yasuda K, Hitsuda Y, Shimizu E. Suppression of phosphatidylinositol 3-kinase/Akt signaling pathway is a determinant of the sensitivity to a novel histone deacetylase inhibitor, FK228, in lung adenocarcinoma cells. Oncol Rep. 2005;13:477–483. [PubMed] [Google Scholar]
- Matsubara H, Watanabe M, Imai T, Yui Y, Mizushima Y, Hiraumi Y, Kamitsuji Y, Watanabe K, Nishijo K, Toguchida J, Nakahata T, Adachi S. Involvement of extracellular signal-regulated kinase activation in human osteosarcoma cell resistance to the histone deacetylase inhibitor FK228 [(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene- 3,6,9,19,22-pentone] JPharmacol Exp Ther. 2009;328:839–848. doi: 10.1124/jpet.108.147462. [DOI] [PubMed] [Google Scholar]
- Murga C, Zohar M, Teramoto H, Gutkind JS. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene. 2002;21:207–216. doi: 10.1038/sj.onc.1205036. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Yu C, Dai Y, Reese E, Ahmed W, Dent P, Grant S. Coadministration of the heat shock protein 90 antagonist 17-allylamino- 17-demethoxygeldanamycin with suberoylanilide hydroxamic acid or sodium butyrate synergistically induces apoptosis in human leukemia cells. Cancer Res. 2003a;63:8420–8427. [PubMed] [Google Scholar]
- Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, Grant S. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene. 2003b;22:6231–6242. doi: 10.1038/sj.onc.1206646. [DOI] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Maggio SC, Coe S, Atadja P, Dent P, Grant S. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther. 2008;7:3285–3297. doi: 10.1158/1535-7163.MCT-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JG, Blalock WL, White ER, Steelman LS, McCubrey JA. Ability of the activated PI3K/Akt oncoproteins to synergize with MEK1 and induce cell cycle progression and abrogate the cytokine-dependence of hematopoietic cells. Cell Cycle. 2004;3:503–512. [PubMed] [Google Scholar]
- So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J. 2012;442:465–481. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333 ( Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yu X. Regulation of apoptosis: the ubiquitous way. FASEB J. 2003;17:790–799. doi: 10.1096/fj.02-0654rev. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. The effect of GS-1101/LBH589 treatment on the induction of the apoptosis in CLL primary cells cultured with and without HS-5 stroma cells.
Fig S2. The effect of GS-1101/LBH589 treatment on (A) p-AKT, p-ERK, and p-JNK and (B) induction of apoptosis in GS-1101 resistant SU-DHL-6 cell line.
Fig S3. sub-G0/G1 DNA content analysis of siRNA AKT and siRNA ERK transfected SUDHL-6 cell lines after 48 h of GS-1101/LBH589 treatment.
Fig S4. The effect of JNK inhibitor SP600125 pretreatment on the percentage of apoptotic cells after GS-1101/LBH589 treatment.