Abstract
Recent paradigm in the field of cancer defines its origin from a small population of fast growing cells known as cancer stem cells (CSCs), and they are mainly responsible for disease aggressiveness, drug resistance and tumor relapse. The existence of CSCs has been proven in different types of cancer and possesses characteristic expression of a wide array of cell surface markers specific to the type of cancer. CSCs have been isolated and enriched using several surface markers in different cancer types. Self-renewal, drug resistance and the ability to transition from epithelial to mesenchymal phenotype are the major features attributed to this fraction of mutated stem cells. The CSC hypothesis proposes that these CSCs mimic stem cells by sharing similar pathways, such as Wnt, SHH, Notch and others. Further, the niche, which in this case is the tumor microenvironment, plays a very important role in the maintenance of CSCs. Altogether, this emerging field of research on CSCs is expected to unveil answers to the most difficult issues of one of the most dreadful diseases called cancer.
Keywords: Cancer stem cells, Drug Resistance, CSC markers and Therapy targets
Introduction
Cancer has been prevailing in this world for several years devouring the lives of millions of people. It has been estimated that in the current year, 1,638,910 new cases and 577,190 deaths would result due to cancer solely in the US (Siegel et al., 2012). There are approximately 26, 50000 articles in the NCBI database addressing different aspects of cancer. Despite extensive research going on in this malignant disease, no definitive treatment has yet been identified till date. This is due to the fact that cancer targets are difficult to study since it is controlled by an array of signaling events. Recent insights into cancer biology have uncovered several potential players responsible for this disease. The recent focus of many laboratories is cancer stem cells (CSCs) due to its prevalence in most of the cancer prone tissues such as breast, pancreas, prostate, leukemia, ovary and brain. Exploring the nature and behavior of these potent cells would provide a better understanding of the disease.
The history of cancer stem cells began in the year 1950, when Roy Stevens observed an elevated rate of testicular teratoma in a mice strain 129 (Stevens, 1981). In addition to the testicular teratomas being derived from the three primary germ layers, there were some embryonal carcinoma [EC] cells (Donovan and de Miguel, 2003). Later in 1964, Kleinsmith and Pierce formed the basis of CSC theory by demonstrating that single cell clones of EC cells also have the property of multipotency and pluripotency. (KLEINSMITH and PIERCE, Jr., 1964). The CSC hypothesis was later rediscovered by Bonnet and Dick when they identified that in acute myeloid leukemia, fewer number of (100-fold less) CD34+CD38− cells were required to cause leukemic proliferation as opposed to CD34+CD38+ cells (Bonnet and Dick, 1997). This led to the concept of CSC markers, wherein a set of markers are found to be characteristic of each CSC type. A population of 100 ESA+ CD44+CD24−/low cells were able to form breast tumors rapidly when compared to no tumor formation from as many as 20,000 CD44+CD24+ cells thereby reinforcing the concept of increased tumorigenic potency of a population of cells decorated by specific cell surface markers (Al-Hajj et al., 2003) (Figure 1).
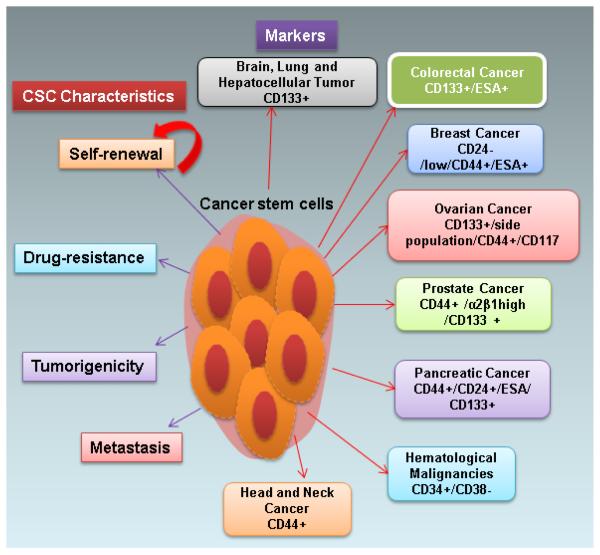
Figure 1.
A schematic representation of cancer stem cell (CSC) surface markers and its phenomenal characteristics. CSC surface markers are described for several cancers, such as brain, lung, hepatocellular, colorectal, breast, ovarian, prostate, pancreatic, hematological, and head and neck malignancies. The characteristics of CSCs are self-renewal, drug resistance, tumorigenicity and metastasis.
CSCs are also known as cancer initiating cells, side population (SP) or tumor initiating cells (TICs). There are several methods employed to isolate these altered stem cells from a heterogeneous population of cancer cells. Two methods using FACS are 1.DNA binding Hoechst dye33342 based exclusion method in which the dye bound cells will be sorted out using flow cytometry. This gives rise to two types of cells - SP and non-side population (NSP). Due to the presence of increased multi-drug transporter proteins (ABCG transporters) in the SP cells, they expel the dye and thus emit low fluorescence when compared to the NSP cells (Ponnusamy and Batra, 2008;Szotek et al., 2006)(Figure 2) and 2. Marker based isolation (Figure 2). For example; pancreatic CSCs can be isolated using antibody against CD44+CD24+ESA+. Other methods include magnetic cell sorting (MACS) (Cammareri et al., 2008), ALDEFLUR assay (Charafe-Jauffret et al., 2009) and in situ detection (Charafe-Jauffret et al., 2009).
Figure 2.
A schematic representation of cancer stem cell isolation and characteristics analysis. Most of the tumors are made up of heterozygous population of cells. For CSCs isolation, researchers use surface markers respective to the cancer type followed by flow cytometry method. The Hoechst 33342 dye is also used to isolate SP cells which are drug resistant population. The isolated CSC population is analyzed by in vitro (tumorsphere) and in vivo (xenograft) tumorigenesis assays. SP cells form larger tumorspheres in vitro and bigger tumors in vivo as opposed to NSP cells. Different serial dilutions of these assays are performed to analyze the stemness and tumorigenic nature of CSCs. CSC-Cancer Stem Cells, SP-Side Populations, and NSP-Non-Side Population.
After isolation, these CSCs are enriched in vitro by tumorsphere assays which use low attachment plates or soft agar assay to select for the anchorage independent cells- cells which can survive the harsh environment (mammosphere, pancreatosphere assay for breast and pancreatic CSCs, respectively). Further, these cells are tested for their tumorigenic potential in vivo by serial transplantation assays in which cells are injected into NOD-SCID mice either subcutaneously, orthotopically or intraperitoneally and are allowed to grow for few weeks. These CSCs leads to the formation of larger tumors compared to that of the small/no tumors in the non-CSCs injected region. Therefore, minimal number of CSCs is responsible to recreate the “tumor phenomenon.”
Finally, the combination of oncology and stem cell biology has led us to explore this dreadful disease further i.e. studying cancer with a different perspective which includes novel approaches and it has opened a new challenging concept left partially explored.
Specific markers of CSCs and therapeutic implications
CSCs share the characteristics of normal adult stem cells such as self-renewal, stemness and drug resistance. One of the hypotheses for CSCs describes that if the adult stem cells gets mutated and is involved in uncontrolled proliferation, it would become the CSC population. The differences between maintenance markers for adult and CSCs are not well understood. However, numerous surface markers were identified which were specifically attributed to CSCs. CSCs were isolated from several cancers such as brain, lung, hepatocellular, breast, ovarian, prostate, head and neck, hematological and pancreatic cancers (Figure 1). The identified cell surface markers are an important target for CSC therapy.
Pancreatic, liver, colorectal, breast, ovarian and prostate cancer stem cell characterization and its markers are well established in CSC research. (1) Pancreatic cancer is the fourth most common cause of cancer related deaths in the Unites States, showing worst prognosis of all human cancers and resistant to chemotherapeutic agents. CSCs in pancreatic cancer constitute less than 1% of the total tumor cell population and express specific surface markers such as CD44+/CD24+/ESA/CD133+ (Ponnusamy and Batra, 2008). Furthermore, the isolated pancreatic CSCs also show overexpression of sonic hedgehog (SHH) and polycomb group (PCG) gene family member Bmi-1 gene (Lee et al., 2008;Li et al., 2007a;Li et al., 2009) which are responsible for the maintenance of CSCs. Recent studies have also shown the increased expression of CXC chemokine receptor 4 (CXCR4) in CD133+ pancreatic cancer stem cells (Hermann et al., 2007). The liver is one of the highly regenerative potential organs which carry oval progenitor cells around the peripheral branches of the bile ducts (Roskams et al., 2004). The normal liver progenitor cells share molecular markers with hepatocytes (albumin, CK7, CK19), oval cell markers (OV6, A6, OV1), fetal hepatocyte marker (α fetoprotein) and are also positive for common stem cell markers such as CD34+, Thy-1+, c-Kit+ and Flt-3+ (Roskams, 2006;Burke et al., 2007). In human hepatocellular carcinoma, CD133+ cells possess the potential to self-renew by expressing Notch, BMI and Oct3/4 (Ma et al., 2007;Ma et al., 2008). Colorectal adult stem cells reside at the bottom of the crypt and differentiate into >1010 new cells daily and mutations even in a single stem cell located at the bottom of the crypt develops colorectal cancer (Ricci-Vitiani et al., 2007). Colorectal cancer is the third most frequent cancer worldwide and is the second most reason for cancer related deaths in western countries and it develops more drug resistance and tumor recurrence after chemotherapeutic treatment. CSCs in colon cancer express CD133+, CD44+, CD166, CD49f and ESA markers (Dalerba et al., 2007;Ricci-Vitiani et al., 2007) and these markers can be used to enrich colon CSCs (4). Breast cancer is one of the major causes of cancer related deaths in women worldwide. Breast CSCs originate from multi-potent stem cells present in the mammary fat pad and genetic mutations and alterations affect self-renewal and differentiation features. The mammary adult stem cells of murine express CD29f+, CD24+ and Lin− markers (Shackleton et al., 2006) and CSCs of mammary stem cells express CD24−/low/CD44+/ESA+ (Charafe-Jauffret et al., 2009;Dontu et al., 2004;Ginestier et al., 2007). The self-renewal factors for both normal and malignant mammary stem cells are Notch (Dontu et al., 2004), Hedgehog (SHH) (Hatsell and Frost, 2007), Wnt/β-catenin (Lindvall et al., 2007), epidermal growth factors and receptors (EGF/EGFR) (Dontu et al., 2003), Leukemia inhibitory factor (LIF) (Kritikou et al., 2003), Oct3/4, ALDH1 and SOX1 (Wright et al., 2008;Ginestier et al., 2007). The terminal differentiation of prostate epithelium is derived from three different epithelial layers such as luminal, basal and rare endocrine cells. The candidate prostate CSCs are identified by their preferential expression of CD44+/α2β1high/CD133+ surface markers (Collins et al., 2005). Ovarian cancer is also one of the lethal diseases worldwide and is the most challenging gynecological cancer. The enrichment of ovarian CSCs were developed using side population (hoechst33342 dye) and CD133+/CD44+/CD117 surface markers (Ferrandina et al., 2008;Ponnusamy and Batra, 2008;Szotek et al., 2006). Several other cancer stem cell markers such as CD133+ for brain and lung, CD34+/CD38−/CD33+ for hematological malignancies (Lapidot et al., 1994;ten et al., 2010) and CD44+ for head and neck cancer were also established (Prince et al., 2007).
Most of the cancers are resistant to chemotherapeutic drugs due to CSC population which ultimately results in tumor relapse and thus decrease the survival of patients (Dean et al., 2005;Ponnusamy and Batra, 2008). In several recent studies, the activation and upregulation of some specific signaling pathways such as PI3K/Akt/mTOR, Hedgehog, HMG-CoA reductase, and Notch were exclusively involved in the CSC population (Martelli et al., 2010;Martelli et al., 2009;Bleau et al., 2009;Von Hoff et al., 2009;Hofmann et al., 2009;Fang et al., 2009;Balyasnikova et al., 2010;Wei et al., 2009). CD34 in leukemia cells was also strongly correlated with ABC transporter, which plays a role in drug efflux, which can be used as a chemotherapeutic target. CD33 expression is detected in 80-90% of leukemia cells as well as CSC populations (ten et al., 2009;Leith et al., 1997). The antibody against CD33 (Gemtuzumab ozogamacin-GO) became an important target for both leukemia cells and CSC targeted therapy. Targeting these pathways has been focused on by many researchers. PI3K/Akt/mTOR pathway has been inhibited in renal cell carcinoma using Temsirolimus and Everolimus. Hedgehog inhibitors GDC-0449, PF04449913, BMS833923 and LDE225 were used for solid tumors, colorectal, hematological, basal cell and meduloblastoma tumors respectively (Clayton and Mousa, 2011). Notch inhibitors MK0752, RO4929097, PF-03084041 were used for breast, pancreatic, renal cell and leukemia cancers respectively (Clayton and Mousa, 2011). Recent studies explored that HMG-CoA reductase inhibitor plays a role in CSCs. The CSC population is believed to escape from oxidative stress which results in increased survival (Fang et al., 2009;Kotamraju et al., 2007). The inhibitors of HMG-CoA namely imatinib and simvastatin are showing a synergistic effect in Chronic myelogenous leukemia cells (Kotamraju et al., 2007). Mesenchymal stem cells (MSCs) are also most widely used adult stem cells for therapeutic applications of several diseases. Recent studies have shown that the effect of MSCs in reduced proliferation and decreased apoptosis in leukemia stem cells (Balyasnikova et al., 2010;Wei et al., 2009). The effective treatment for cancer lies in targeting CSCs with specific molecules or pharmacological inhibitors.
CSCs in drug resistance
Although chemotherapy eradicates most tumor cells, small populations of tumor stem cells which have resistance to cancer therapy are left behind resulting in recurrence of tumor. This resistance is due to the protection imparted by an increased expression of the ATP-binding cassette (ABC) drug transporters (Dean et al., 2005;Ponnusamy and Batra, 2008). For instance; it was shown that the CD44+ CD24− breast cancer stem cells had increased ABCG2 mRNA expression when compared to the alternative phenotype (Stavrovskaya and Stromskaya, 2008). These multi drug transporters use their drug efflux mechanisms to toss out the drug thereby decreasing the intracellular drug levels and thus being indifferent to the cancer treatment. The ABC transporters constitute the largest trans-membrane protein family (Fletcher et al., 2010). This large family is further divided into 7 subfamilies which are designated A to G based on their structural and sequence homology (Fletcher et al., 2010). The most challenging research of reversing the multi-drug resistance in these CSCs by inhibiting specific ABC transporters has been carried out for more than two decades (Fletcher et al., 2010). There were 3 generations of ABCB1 inhibitors designed to be administered along with front line chemotherapy although all of them proved to be a failure and none of them had any additional effect on prognosis. First generation inhibitors like cyclosporine A and verapamil were approved in the 1980s for non-cancer conditions, unfortunately these inhibitors were found to be highly toxic (Fletcher et al., 2010). Though the second generation inhibitors such as valspodar were improved in terms of decreased toxicity and increased potency they remained a failure as well since the patient was indifferent to these drugs during a Phase III trial (Catherine Lhomme’ et al., 2008;Fletcher et al., 2010). Even the third generation drugs such as Zosuquidar failed during a Phase II trial (Ruff et al., 2009) . Therefore, understanding the underlying mechanisms of stem cell resistance due to these MDR transporters is critical to improve our anticancer strategies and thereby identify new therapeutic targets (Dean et al., 2005).
CSCs and Metastasis
The successful process of seeding and colonization of CSCs results in distant organ metastasis (Li et al., 2007b). From the heterogeneous population of primary tumor cells only a small fraction moves into circulation and 1/100th fraction of these cells develops into metastases. (Gupta et al., 2005). These cells are believed to undergo EMT during which their epithelial characteristics are lost thus acquiring invasiveness and stem cell traits (Polyak and Weinberg, 2009). Two major metastasis models have been framed in the breast cancer field. Firstly, the classical model in which metastasis is treated as the final stage of cancer evolution where, the tumors acquire several mutations that lead to invasiveness which further leads into dissemination (Cairns, 1975). Secondly, another model was proposed in which metastasis was considered to be an early event which behaves as an inherent property of the tumor itself (Hellman, 1994). Both models prevail but the classical model is considered conceptually flawed as (i) metastatic lesions do appear after resection of small tumors which were not metastatic at diagnosis (ii) unknown primary tumors are metastatic and account for 4-5% of total clinical metastases (Rhim et al., 2012). The second model however, has been recently proven in pancreatic cancer where it is shown that EMT and dissemination occur before tumor formation (Rhim et al., 2012). CSCs are found to involve in the process of metastasis, For example; in colorectal cancer when CD26+ CSCs were injected into mice it progressed into distant metastasis whereas CD26− cells did not show that phenotype (Pang et al., 2010). Similarly, CD133+CXCR4+ CSCs were found to be responsible for the metastatic phenotype in pancreatic cancer (Hermann et al., 2007). Identification and careful targeting of this subset of CSCs which have the metastatic capacity would help in fighting against cancer.
CSCs and Tumor Microenvironment
CSCs that originally underwent mutation in various ways are the chief reason for the development of tumor and its progression. Yet, these CSCs solely cannot lead to malignancy. They require the assistance of an environment which is corrupted as well and is thereby able to support the growth of these altered stem cells or CSCs (Hanahan and Coussens, 2012). Similar to stem cells which requires specific signals to maintain its stem cell nature the CSCs also rely on the microenvironment to maintain its properties (Hanahan and Coussens, 2012;Ward and Dirks, 2007). The tumor microenvironment (TME) is made up of several constituents such as i) cancer cells that undergo dysregulated proliferation, ii) cancer stem cells, which constitutes a minor fraction (<5%) of the total population of cells, iii) stromal cells consisting of cancer associated fibroblastic cells, infiltrating immune cells and angiogenic vascular cells (Hanahan and Coussens, 2012), iv) Extracellular matrix (ECM) components such as laminin, collagen, fibronectin and elastin and v) non cancer cells (Friedl and Alexander, 2011;Hanahan and Coussens, 2012). The strong interactions between all these diverse cells, ECM and various stromal factors in addition to the geometric constraints that a cell undergoes defines a tumor microenvironment (TME) (LaBarge, 2010). TME contributes to tumor escape by activating the intricate molecular mechanisms thereby inhibiting the immune cells to function (Whiteside, 2008). In addition to the specialized niche, both the genetic or environmental variations imposed on the cells lead to aberrant signaling, thereby facilitating the production of altered stem cells (Vera-Ramirez et al., 2010). Moreover, these aberrant stem cells can lead to EMT promoting tumor metastasis (Jing et al., 2011). By understanding the role of different components of the TME, novel therapeutic targets could be used against these potential cells to impede or reduce the tumor growth.
CSCs and Pathways
Several pathways that regulate the controlled proliferation of stem cells are found to be disrupted in CSCs. The three most widely studied signaling pathways are Notch, Wnt/ β-catenin and Sonic Hedgehog.
Wnt signaling/Notch/SHH
Wnt/β-catenin signaling is found to play a chief role in the maintenance and homeostasis of stem cells originating from prostate, intestine, breast, pancreatic, ovarian and brain tumors (Lindvall et al., 2007;Pinto and Clevers, 2005;Polakis, 2000;Ponnusamy and Batra, 2008). Wnt signaling facilitates the entry of β-catenin into the nucleus and interacts with DNA binding proteins and results in repression or/and activation of target gene transcription (Lindvall et al., 2007;Pinto and Clevers, 2005). In the intestine, Wnt signaling is essential for the maintenance and homeostasis of stem cells (Pinto and Clevers, 2005;Scoville et al., 2008). During a cancerous condition, Wnt signaling functions abruptly due to mutations in its key components of the pathway such as a well-known tumor suppressor namely adenomatous polyposis coli (APC) gene that targets β-catenin for degradation (Lindvall et al., 2007;Pinto and Clevers, 2005;Su et al., 2012). Recent studies showed that in the APC loss-of-function mutant (Apc1322T) mice there was an elevated expression of stem cell markers such as Lgr5, Bmi1, Musashi1 and the Wnt target CD44 (later identified as the colorectal CSC marker) (Dalerba et al., 2007;Lewis et al., 2010). Further, when CD44 was deleted in APCMin/+ it was found to attenuate intestinal tumorigenesis (Zeilstra et al., 2008). Overall, the above studies show that Wnt signaling is found to play an important role in the contribution of tumorigenesis and thus supports the CSC model.
Notch-signaling creates paracrine communication between two cells. Notch family members are identified as oncogenes in several malignancies (Allenspach et al., 2002;Roy et al., 2007). They are expressed during mammalian development in numerous tissues and are also involved in proliferation, differentiation and survival of cells (Allenspach et al., 2002;Pajcini et al., 2011). Notch directly plays a role in the formation of definitive hematopoietic stem cells (Pajcini et al., 2011). Notch is one of the signaling pathways which is involved in the maintenance of stem cells and plays an important role in cancer (Pajcini et al., 2011;Reya et al., 2001). Recent studies have also demonstrated that Notch inhibitors (MK0752, RO4929097, PF-03084041) target cancer cells and CSCs (Clayton and Mousa, 2011).
In mammalian cells, SHH ligands mediate SHH signaling pathways (Pasca di and Hebrok, 2003). The pathway is activated when the ligand binds to Patched (PTCH1), a 12-pass transmembrane-spanning receptor (Merchant and Matsui, 2010). The absence of SHH leads to the constitutive repression of a 7-pass transmembrane protein namely Smoothened (SMO) (Merchant and Matsui, 2010) . When the ligand binds to PTCH, SMO is derepressed thus activating the Gli zinc-finger transcription factors such as Gli1, Gli2 and Gli3 (Merchant and Matsui, 2010;Pasca di and Hebrok, 2003). In cancer conditions, the prime reason for the contribution of basal cell carcinoma is due to mutations in PTCH1 and SMO leading to the constitutive activation of SHH signaling (Su et al., 2012). Other mutations such as Gli1 and Gli3 mutations are also observed in pancreatic adenocarcinoma and in glioblastoma, Gli1 gene amplification was seen (Merchant and Matsui, 2010). In Glioblastoma CSCs, the Hh signaling pathway was found to be active which was inhibited using siRNA based method or using cyclopamine against the components of the pathway thereby reversing the tumorigenesis (Bar et al., 2007;Clement et al., 2007). In breast cancer, Hh ligands were used to activate the CSC pathway which resulted in GLI1 or GLI2 expression (Godlewski et al., 2008;Liu et al., 2006). On inhibiting this pathway using siRNA directed against GLI1 and GLI2 or by using cyclopamine the expression of BMI-1 (a self-renewal factor/oncogene) was altered (Godlewski et al., 2008;Liu et al., 2006).
Other Pathways
Other signaling pathways such as the Polycomb group transcriptional repressor, Bmi-1 was found to be essential for the self-renewal of stem cells in the hematopoietic system, peripheral and central nervous system (Molofsky et al., 2003;Park et al., 2003). It was observed that Bmi-1 deficient neural stem cells had a reduced capacity of self-renewal which led to their postnatal depletion (Molofsky et al., 2003). Depletion of Bmi-1 led to increased β-catenin signaling which led to blockage of the self-renewal activity of p63+ stem cells (Lukacs et al., 2010). Another study showed that on targeting the Bmi-1 in glioma using microRNA-128 both self-renewal and proliferation was inhibited (Godlewski et al., 2008).
To summarize, in CSCs simple pathways turn complex due to certain alterations in their underlying molecular mechanisms. Thus Wnt, Notch and SHH signaling controls the CSC fate decisions with the assistance of a tumor microenvironment. Therefore, a thorough understanding of these complex pathways is required in order to target and inactivate CSCs.
Future Directions
Understanding the critical details in the field of CSCs holds great promise towards the cure of cancer. To eradicate these CSCs, safe targeted therapies should be developed against this small population of transformed stem cells. Greater focus should be imparted towards several areas in CSC research, which has attained partial success such as in areas designing new inhibitors/drugs to inhibit ABC transporters and in areas identifying highly specific markers (both on the surface and within cells) for each cancer type (Fletcher et al., 2010;Mimeault and Batra, 2012). Although the technical advancements in the CSC field has provided various methods for the isolation, characterization and have provided the properties of CSCs, their functional properties have not been well evaluated (Mimeault and Batra, 2012;Mimeault and Batra, 2010). Further investigations of the behavior of CSCs should be extended even in progressive cancer models. Identification of novel molecules, which plays an important role in the maintenance of CSCs, may serve as therapeutic targets. In the future, effective treatment for cancer requires targeting bio-molecules which can eliminate both differentiated cancer cells and the potential CSC populations specific to different cancers.
Acknowledgements
The authors on this article were supported by grants from the National Institutes of Health (RO1 CA138791, EDRN UO1CA111294, SPORE P50CA127297, and TMEN U54CA163120). The authors acknowledge the invaluable support from Mrs. Kavita Mallya.
Footnotes
Author Disclosure Statement The authors declare no conflict of interest.
References
- Al-Hajj M, Wicha MS, ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allenspach EJ, Maillard I, Aster JC, Pear WS. Notch signaling in cancer. Cancer Biol Ther. 2002;1(5):466–476. doi: 10.4161/cbt.1.5.159. [DOI] [PubMed] [Google Scholar]
- Balyasnikova IV, Ferguson SD, Sengupta S, Han Y, Lesniak MS. Mesenchymal stem cells modified with a single-chain antibody against EGFRvIII successfully inhibit the growth of human xenograft malignant glioma. PLoS One. 2010;5(3):e9750. doi: 10.1371/journal.pone.0009750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Burke ZD, Thowfeequ S, Peran M, Tosh D. Stem cells in the adult pancreas and liver. Biochem J. 2007;404(2):169–178. doi: 10.1042/BJ20070167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Cammareri P, Lombardo Y, Francipane MG, Bonventre S, Todaro M, Stassi G. Isolation and culture of colon cancer stem cells. Methods Cell Biol. 2008;86:311–324. doi: 10.1016/S0091-679X(08)00014-9. [DOI] [PubMed] [Google Scholar]
- Lhomme’ Catherine, Joly Florence, Walker Joan L., Lissoni Andrea A., Nicoletto Maria O., Manikhas Gregory M., Baekelandt Mark M.O., Gordon Alan N., Fracasso Paula M., Mietlowski William L., Jones Gary J., Dugan Margaret H. Phase III Study of Valspodar (PSC 833) Combined With Paclitaxel and Carboplatin Compared With Paclitaxel and Carboplatin Alone in Patients With Stage IV or Suboptimally Debulked Stage III Epithelial Ovarian Cancer or Primary Peritoneal Cancer. J Clin Oncol. 2008;26(16):2674–2681. doi: 10.1200/JCO.2007.14.9807. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Birnbaum D. Breast cancer stem cells: tools and models to rely on. BMC Cancer. 2009;9:202. doi: 10.1186/1471-2407-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton S, Mousa SA. Therapeutics formulated to target cancer stem cells: Is it in our future? Cancer Cell Int. 2011;11:7. doi: 10.1186/1475-2867-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de TN, Radovanovic I, Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Donovan PJ, de Miguel MP. Turning germ cells into stem cells. Curr Opin Genet Dev. 2003;13(5):463–471. doi: 10.1016/j.gde.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61(4):290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, Zannoni G, Mancuso S, Scambia G. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18(3):506–514. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10(2):147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68(22):9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Mani S, Yang J, Hartwell K, Weinberg RA. The evolving portrait of cancer metastasis. Cold Spring Harb Symp Quant Biol. 2005;70:291–297. doi: 10.1101/sqb.2005.70.033. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hatsell S, Frost AR. Hedgehog signaling in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2007;12(2-3):163–173. doi: 10.1007/s10911-007-9048-2. [DOI] [PubMed] [Google Scholar]
- Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. 1994;12(10):2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hofmann I, Stover EH, Cullen DE, Mao J, Morgan KJ, Lee BH, Kharas MG, Miller PG, Cornejo MG, Okabe R, Armstrong SA, Ghilardi N, Gould S, de Sauvage FJ, McMahon AP, Gilliland DG. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4(6):559–567. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci. 2011;1:29. doi: 10.1186/2045-3701-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith LJ, Pierce GB., jr. Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964;24:1544–1551. [PubMed] [Google Scholar]
- Kotamraju S, Williams CL, Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67(15):7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- Kritikou EA, Sharkey A, Abell K, Came PJ, Anderson E, Clarkson RW, Watson CJ. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development. 2003;130(15):3459–3468. doi: 10.1242/dev.00578. [DOI] [PubMed] [Google Scholar]
- LaBarge MA. The difficulty of targeting cancer stem cell niches. Clin Cancer Res. 2010;16(12):3121–3129. doi: 10.1158/1078-0432.CCR-09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26(17):2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, Head DR, Appelbaum FR, Willman CL. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89(9):3323–3329. [PubMed] [Google Scholar]
- Lewis A, Segditsas S, Deheragoda M, Pollard P, Jeffery R, Nye E, Lockstone H, Davis H, Clark S, Stamp G, Poulsom R, Wright N, Tomlinson I. Severe polyposis in Apc(1322T) mice is associated with submaximal Wnt signalling and increased expression of the stem cell marker Lgr5. Gut. 2010;59(12):1680–1686. doi: 10.1136/gut.2009.193680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007a;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009;568:161–173. doi: 10.1007/978-1-59745-280-9_10. [DOI] [PubMed] [Google Scholar]
- Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007b;17(1):3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- Lindvall C, Bu W, Williams BO, Li Y. Wnt signaling, stem cells, and the cellular origin of breast cancer. Stem Cell Rev. 2007;3(2):157–168. doi: 10.1007/s12015-007-0025-3. [DOI] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7(6):682–693. doi: 10.1016/j.stem.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Chiarini F, Evangelisti C, Grimaldi C, Ognibene A, Manzoli L, Billi AM, McCubrey JA. The phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin signaling network and the control of normal myelopoiesis. Histol Histopathol. 2010;25(5):669–680. doi: 10.14670/HH-25.669. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs. 2009;18(9):1333–1349. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16(12):3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent advancements on the identification of immature cancer cells with the stem cell-like properties in different aggressive and recurrent cancer types. Anticancer Agents Med Chem. 2010;10(2):103. doi: 10.2174/187152010790909335. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Great promise of tissue-resident adult stem/progenitor cells in transplantation and cancer therapies. Adv Exp Med Biol. 2012;741:171–186. doi: 10.1007/978-1-4614-2098-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajcini KV, Speck NA, Pear WS. Notch signaling in mammalian hematopoietic stem cells. Leukemia. 2011;25(10):1525–1532. doi: 10.1038/leu.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, Tan VP, Yau TC, Poon RT, Wong BC. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6(6):603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Pasca di MM, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3(12):903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- Pinto D, Clevers H. Wnt, stem cells and cancer in the intestine. Biol Cell. 2005;97(3):185–196. doi: 10.1042/BC20040094. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Ponnusamy MP, Batra SK. Ovarian cancer: emerging concept on cancer stem cells. J Ovarian Res. 2008;1(1):4. doi: 10.1186/1757-2215-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1-2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De MR. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25(27):3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39(6):1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17(1):52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Ruff P, Vorobiof DA, Jordaan JP, Demetriou GS, Moodley SD, Nosworthy AL, Werner ID, Raats J, Burgess LJ. A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer Chemother Pharmacol. 2009;64(4):763–768. doi: 10.1007/s00280-009-0925-9. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134(3):849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Stavrovskaya AA, Stromskaya TP. Transport proteins of the ABC family and multidrug resistance of tumor cells. Biochemistry (Mosc ) 2008;73(5):592–604. doi: 10.1134/s0006297908050118. [DOI] [PubMed] [Google Scholar]
- Stevens LC. Genetic influences on teratocarcinogenesis and parthenogenesis. Prog Clin Biol Res. 1981;45:93–104. [PubMed] [Google Scholar]
- Su W, Meng F, Huang L, Zheng M, Liu W, Sun H. Sonic hedgehog maintains survival and growth of chronic myeloid leukemia progenitor cells through beta-catenin signaling. Exp Hematol. 2012;40(5):418–427. doi: 10.1016/j.exphem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten CB, Bremer E, de BM, Bijma T, Samplonius D, Schwemmlein M, Huls G, Fey G, Helfrich W. A novel AML-selective TRAIL fusion protein that is superior to Gemtuzumab Ozogamicin in terms of in vitro selectivity, activity and stability. Leukemia. 2009;23(8):1389–1397. doi: 10.1038/leu.2009.34. [DOI] [PubMed] [Google Scholar]
- Ten CB, de BM, Wei Y, Bremer E, Helfrich W. Targeted elimination of leukemia stem cells; a new therapeutic approach in hemato-oncology. Curr Drug Targets. 2010;11(1):95–110. doi: 10.2174/138945010790031063. [DOI] [PubMed] [Google Scholar]
- Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa CL, Quiles JL, Ramirez-Tortosa MC, Alvarez JC, Fernandez-Navarro M, Lorente JA. Gene-expression profiles, tumor microenvironment, and cancer stem cells in breast cancer: latest advances towards an integrated approach. Cancer Treat Rev. 2010;36(6):477–484. doi: 10.1016/j.ctrv.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM, Lum BL, Darbonne WC, Marsters JC, Jr., de Sauvage FJ, Low JA. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Dirks PB. Cancer stem cells: at the headwaters of tumor development. Annu Rev Pathol. 2007;2:175–189. doi: 10.1146/annurev.pathol.2.010506.091847. [DOI] [PubMed] [Google Scholar]
- Wei Z, Chen N, Guo H, Wang X, Xu F, Ren Q, Lu S, Liu B, Zhang L, Zhao H. Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res. 2009;28:141. doi: 10.1186/1756-9966-28-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10(1):R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra J, Joosten SP, Dokter M, Verwiel E, Spaargaren M, Pals ST. Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res. 2008;68(10):3655–3661. doi: 10.1158/0008-5472.CAN-07-2940. [DOI] [PubMed] [Google Scholar]