Abstract
Purpose
Bone acquisition in childhood impacts adult bone mass, and can be influenced by childhood socioeconomic conditions. Socioeconomic status is also associated with body weight which affects the load that bone is exposed to in a fall. We hypothesized that socioeconomic advantage in childhood is associated with greater bone strength relative to load in adulthood.
Methods
Hip dual x-ray absorptiometry scans from 722 participants in the Midlife in the United States Study were used to measure femoral neck size and bone mineral density, and combined with body weight and height to create composite indices of femoral neck strength relative to load in different failure modes: compression, bending, and impact. A childhood socioeconomic advantage score was created for the same participants from parental education, self-rated financial status relative to others, and not being on welfare. Multiple linear regression was used to determine the association of childhood socioeconomic advantage score with femoral neck composite strength indices, stratified by gender and race (white/non-white), and adjusted for study site, age, menopause status in women, education, and current financial advantage.
Results
Childhood socioeconomic advantage was independently associated with higher indices of all three composite strength indices in white men (adjusted standardized effect sizes, 0.19 to 0.27, all p values <0.01), but not in the other three race/gender groups. Additional adjustment for adult obesity, physical activity in different life stages, smoking, and heavy drinking over the life-course significantly attenuated the associations in white men.
Conclusions
Socioeconomic disadvantage in childhood is associated with lower hip strength relative to load in white men, and these influences are dampened by healthy lifestyle choices.
Keywords: bone strength, femoral neck, childhood socioeconomic advantage, health behaviors
1. Introduction
Low socioeconomic status (SES), which has strong, well-documented associations with a variety of adverse health outcomes, is also associated with increased risk for hip fracture in older ages [1,2,3,4,5,6]. Hip fractures are a major cause of morbidity, physical disability, and even early mortality [6], and its economic impact is projected to increase worldwide in the coming decades [7,8]. It is therefore becoming increasingly important to delineate the mechanisms underlying the association between low SES and greater hip fracture risk, in order to allow the design and targeting of preventive interventions.
The major predictor of greater hip fracture risk is low bone mineral density (BMD) in the femoral neck; yet, studies have not found strong associations between low SES and low femoral neck BMD [9,10,11,12,13]. Of the various indicators of adult SES, education level more than income, occupation, or wealth, has shown consistent associations with BMD [14,15,16,17,18]. Because adult bone mass is a function of both acquisition in younger ages and decline in later life [19,20], and because educational attainment is generally completed by young adulthood, this suggests that social circumstances in the early years may be more relevant to bone health than circumstances in later life. In fact two recent studies have documented strong positive associations between childhood social advantage and adult BMD [18,21].
However, both childhood socioeconomic advantage and adult education are associated only with BMD in the lumbar spine and not with BMD in the femoral neck [14,16,18], the more important determinant of hip fracture risk [22,23,24]. Femoral neck BMD, though important, is not the only driver of femoral neck bone strength, and thus of hip fracture risk. The size of the femoral neck size also contributes to its structural strength [24,25,26] (just as the strength of engineering structures depend on both material density and structure size), while body size determines the fracture forces that the hip is exposed to in a fall [27]. It is not enough for BMD to be high to reduce fracture risk; the composite of BMD and bone size must be adequately high relative to fracture forces to keep the risk of fracture low. Thus, composite indices [28] that integrate femoral neck BMD, femoral neck size, and body size, to quantify the strength of the femoral neck relative to load [29,30,31,32,33], are inversely associated with incident hip fracture risk [28,34,35]. Unlike BMD, which fails to correctly stratify fracture risk across ethnic groups [36,37,38,39], femoral neck composite strength indices do correctly stratify risk across ethnic groups [40], and predict fracture risk in a middle-aged woman without requiring knowledge of her race/ethnicity [41]. Furthermore, unlike BMD which is higher in diabetes [42] and thus inconsistent with the increased hip fracture risk in diabetes [43], femoral neck composite strength indices are indeed lower in diabetics than in non-diabetics [44]. Thus, the femoral neck composite strength indices might be better measures of the individual’s ability to resist hip fracture than is femoral neck BMD.
We postulate that the increased hip fracture risk in low SES groups is, at least partly, the result of inadequate bone acquisition in the femoral neck in childhood, and hypothesize that even if socioeconomic disadvantage in childhood is not associated with lower femoral neck BMD in adulthood, it will be associated with smaller indices of adult femoral neck strength relative to load. We used data from a national study to test this hypothesis.
2. Methods
The Study of Midlife in the United States (MIDUS), initiated in 1995, was designed to determine how social, psychological, and behavioral factors over the life course interact to influence health. The first wave of MIDUS collected demographic and psychosocial data on a national sample of English-speaking, non-institutionalized adults between 25 and 75 years of age residing in the coterminous United States whose household included at least one telephone (recruited by random digit dialing), and oversampled twin pairs and siblings [45]. In the second wave of data collection, 9–10 years later (MIDUS II), the sample was refreshed with African American residents recruited from Milwaukee, WI, specifically to increase the representation of urban African Americans. Details of sampling and recruitment are available online at http://www.icpsr.umich.edu/icpsrweb/NACDA/.
Of the 3191 MIDUS II participants deemed medically able to travel, 1255 participated between July 2004 and May 2009 in the MIDUS II Biomarker project, which required a 2-day commitment, including travel to one of the three clinical research centers (University of California at Los Angeles, Georgetown University, and University of Wisconsin). Reasons given for nonparticipation were travel, family, and work obligations. MIDUS II Biomarker Project participants were similar to the MIDUS II sample with respect to key characteristics (e.g., subjective health, chronic conditions, physical activity, alcohol use) [46], and the complete MIDUS II sample was similar to the MIDUS I sample [47]. As part of the Biomarker Project, BMD was measured in the lumbar spine and the left femoral neck using dual-energy x-ray absorptiometry (DXA). Funding for DXA scanning at two of the three sites (UCLA and Georgetown) was obtained after the Biomarker Project had commenced; thus, DXA scans were not available for every participant at these sites. Informed consent was provided by each participant, and each MIDUS center obtained institutional review board approval [46].
Of the 1255 participants in the MIDUS II Biomarker Project, we excluded data from 348 participants who did not have measureable DXA scans (and thus, femoral neck strength measurement), an additional 94 participants who reported the use of medications known to influence BMD (i.e., oral corticosteroids, alendronate, anastrozole, calcitonin, ibandronate, leuprolide, letrozole, raloxifene, risedronate, tamoxifen, zoledronic acid, testosterone, finasteride, dutasteride), another 88 women whose menopause transition stage could not be determined, and 3 participants for whom we lacked complete childhood SES information. Thus, the analytic sample for this study was comprised of 722 participants, 349 men and 373 women. An additional 10 participants were missing adult SES information and were excluded in analyses that included controls for adult SES.
2.1 Femoral Neck Strength Measurement
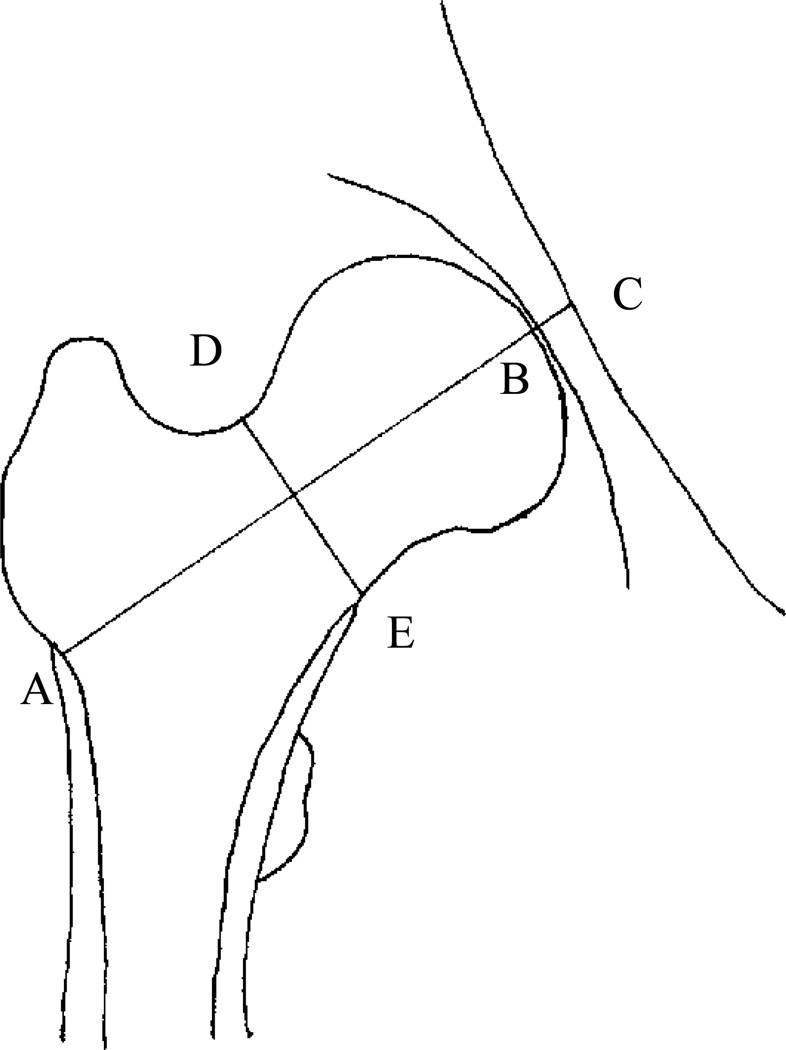
As part of the MIDUS Biomarker Project, DXA scans were performed with standardized protocols, using GE Healthcare Lunar Prodigy (U. Wisconsin - Madison) or Hologic 4500 (UCLA and Georgetown U.) machines, by technologists certified by the International Society for Clinical Densitometry. Three times per week, and on all days on which scans were obtained, instruments were calibrated. No densitometer shift or drift occurred during the course of this study. Adjudication of all DXA scans occurred centrally at the University of Wisconsin DXA center, using software provided by the manufacturers (Lunar, Inc., and Hologic, Inc.), and included measurement of the 2D projected areal BMD in the femoral neck, the femoral neck axis length (FNAL) - the distance on the 2D projected plane along the femoral neck axis from the lateral margin of the base of the greater trochanter to the apex of the femoral head, and the femoral neck width (FNW) - the smallest thickness of the femoral neck on the 2D projected plane along a line perpendicular to the femoral neck axis (Fig.1). Composite indices of femoral neck strength, that index bone strength relative to the load during a fall, were calculated from these DXA-based measurements and body height and weight, using the following formulas [28], which have been validated against 3D methods based on quantitative computed tomography [48].
CSI reflects the ability of the femoral neck to withstand axial compressive loading, BSI reflects its ability to withstand bending, and ISI its ability to absorb the energy of impact in a fall from standing height.
Figure 1.
Geometry of the Femoral Neck: AB is the femoral neck axis length and DE is the femoral neck width.
2.2 Childhood socioeconomic advantage assessment
In the first MIDUS data collection wave, participants were asked to recall three aspects of their socioeconomic environment during childhood: 1) educational level attained by father (or other male head of household who raised you for most of the time before you turned 17) and mother (or other female head of household who raised you for most of the time before you turned 17), 2) whether they had ever been on welfare for a period of six months or more during childhood and adolescence (yes or no), and 3) subjective assessment of financial status relative to others when you were growing up (response choices: worse off, same as, or better) We created a childhood socioeconomic advantage score (possible range, 0–6) by summing the three components: being on welfare (0: yes, 2: no), financial status relative to others (0: worse off, 1: same, 2: better), and highest parental education (0: < high school, 1: high school/general educational development [GED] certificate, 2: some college or more). Scores were only calculated for participants who supplied data for least 2 of the 3 components; the missing component was imputed as the rounded mean of the other two for 49 participants. Among twins and siblings in the MIDUS cohort, the intra-class correlation coefficient for childhood advantage score was 0.84, indicating a high degree of reliability in the recalled information about childhood socioeconomic status. This childhood advantage score has also been demonstrated to have inverse associations with physiological dysregulation in adulthood [49].
2.3 Other Measurements
Participants provided information on demographics and income during both MIDUS I and II. Race/ethnicity was self-identified as white, African American, other, or multiracial. For this analyses, we classified race as white vs. non-white; the latter group was mostly African American, but included a small number (n = 33) that were multi-racial or reported another race. During their visit to the clinical research centers (when the DXA scans were obtained), participants provided information on health behaviors, medication use (including sex steroid hormones), and menstrual bleeding patterns. Medication information was verified by examination of medication bottles brought to the clinical research center. We classified each woman participant’s menopausal transition stage as premenopausal if there had been no change in regularity of menses, early perimenopausal if there had been a change in regularity but at least one menses in the previous 3 months, late perimenopausal if the last menses had been more than 3 months prior but less than a year prior, or postmenopausal if there had been no menses for at least a year. We further classified the post-menopausal women by use of sex steroid hormones. Because very few (n=6) women were late perimenopausal, we combined them with the post-menopausal not using hormones group.
Men participants were categorized into three age groups: younger than 50 years, 50–59 years, and 60 years or older. The choice of age categories in men was guided by previous observations that notable age-related bone loss in men does not start until age 50 [50], and to age-match the oldest group to the post-menopausal women, because by age 60, more than 99% of women have completed the menopause transition [51]. In addition to these categories, age was also included as a continuous variable (in whole years) in analyses to control for bone strength declines with aging in men 60 years or older and in late-perimenopausal and postmenopausal women not using hormones.
The participant’s educational level was collapsed to a 3-category variable: no college vs. some college or Associate’s degree vs. Bachelor’s degree or more. We calculated a current adult financial advantage score (possible range 0–8) by summing 4 ordinal components: income (scored 0 if family income <3 times the poverty threshold specific to family size and composition, scored 1 if family income to poverty threshold ratio ≥3 but <6, scored 2 if the income-poverty ratio ≥6, reflecting tertiles of the income-poverty ratio distribution), self-rated current financial situation (0: worst, 1: average, 2: best), whether they had enough money to meet current needs (0: not enough, 1: just enough, 2: more than enough), and degree of difficulty paying bills (0: very, 1: not very, 2: not at all). Scores were only calculated for participants who supplied data regarding at least 3 of the 4 components; the missing component was imputed as the rounded mean of the other three components for 20 participants. Details of the construction of this financial advantage variable have been presented elsewhere [18,49].
At the time of BMD measurement, body weight and height were measured using standardized protocols, and questionnaires assessed current smoking status, total pack years of cigarette smoking, heavy alcohol consumption (>7 drinks per week or >3 drinks per occasion regularly for women, >14 drinks per week or >4 drinks per occasion regularly for men [52]) either in the past month or in the period in their lives when they consumed the most alcohol, and levels of physical activity in three different life stages: high school years (number of years of participation between ages 14 and 18 in competitive sports and in recreational sports), young adulthood between ages 20 and 35 years (number of years of regular physical activity for at least 20 minutes at a time, at least 3 times per week, in each of 3 self-categorized intensity levels: light, moderate, and vigorous), and current (average number of minutes per week currently spent doing light, moderate, and vigorous exercise). For both young adulthood and current physical activity, we created summary scores as weighted sums of reported durations of light (weight of 1), moderate (weight of 2), and vigorous (weight of 3) activity. Weights were chosen to reflect relative expenditure of energy in each category [53]. Self-reported physical activity life histories have been previously validated [54,55].
2.4 Statistical Analysis
We used linear mixed effects regression to examine the association of each of the femoral neck composite strength indices with the childhood socioeconomic advantage score (as a continuous predictor) adjusted for covariates, and included a random intercept at the family level to account for within-family correlations between siblings and twins in the sample. Analyses were stratified by race (white vs. non-white) and gender, because of the well-documented differences in the health implications of socioeconomic stressors by both gender and race [56,57,58,59].
Covariates were introduced in a stepwise fashion, based on biological plausibility and the extant literature on determinants of BMD. The base models controlled only for study site and age in men, and study site, age, and menopause transition stage (pre-menopause, early perimenopause, late perimenopause or post menopause not taking menopausal hormones, post menopause taking hormones) in women. Age was operationalized as a continuous variable in the oldest groups (men 60 years and older, and late perimenopausal and post menopausal women not taking hormones). To capture age/cohort differences in younger men, a 3-category age variable (<50 years, 50–59 years, and >= 60 years) was also included. In younger women, age was highly collinear with menopause transition stage, and was therefore not included separately.
To examine if the associations with childhood socioeconomic advantage were independent of adult socioeconomic status, we next added controls for adult education and current financial advantage. Because obesity is associated with both BMD and fracture risk, we next added body mass index (BMI) as a continuous covariate to the models. Finally we introduced health behaviors, specifically current smoking status, total pack years of smoking, heavy alcohol consumption (yes/no), physical activity between the ages of 14 and 18 years (number of years in competitive sports and number of years in recreational sports), a summary score for regular physical activity in young adulthood, and a current physical activity summary score, to examine whether health behaviors over the life course are at least partly responsible for the associations of socioeconomic advantage with femoral neck strength.
All statistical tests were 2-sided. SAS version 9.2 (SAS Institute Inc., Cary, North Carolina, U.S.A.) was used for all analyses.
3. Results
The study sample was similar to the complete MIDUS II Biomarker Project sample with respect to age, BMI, childhood socioeconomic advantage, educational attainment, current financial advantage, pack years of smoking, and physical activity in high school years and young adulthood (Table 1). The most common reasons for exclusion of Biomarker Project participants from the study sample were missing hip DXA scans (from two study sites where funding for the scans was delayed till after the Biomarker Project had started) and unclassifiable menopause transition stage in women. Therefore, compared to the Biomarker sample, the study sample had a smaller proportion of women and a larger proportion of African Americans (since the new urban African American participants from Milwaukee were seen in Madison, WI, the one site which did not have to wait to obtain funds to collect DXA scans).
Table 1.
Descriptive Statistics for Study Sample and the Complete MIDUS II Biomarker Project Sample: Number (%) or Mean (standard deviation)
| Study Sample (n=722) |
Biomarker Sample1 (n=1255) |
|
|---|---|---|
| Age (years) | 56.8 (11.3) | 57.3 (11.5) |
| Gender: Men | 349 (48.3%) | 542 (43.2%) |
| Race: White | 518 (71.8%) | 967 (77.2%) |
| Body mass index (kg/m2) | 30.1 (6.8) | 29.8 (6.6) |
| Age in men | ||
| < 50 years | 112 (32.1%) | 156 (28.8%) |
| 50–59 years | 104 (29.8%) | 153 (28.2%) |
| ≥ 60 years | 133 (38.1%) | 233 (43.0%) |
| Menopause transition stage in women | ||
| Pre-menopausal | 63 (16.9%) | |
| Early perimenopausal | 53 (14.2%) | - |
| Late peri-menopausal or post-menopausal (not on hormone therapy) | 220 (59.0%) | - |
| Postmenopausal on hormone therapy | 37 (9.9%) | - |
| Currently smoking | 121 (16.8%) | 187 (14.9%) |
| Total smoking exposure (pack years) | 9.2 (17.4) | 8.8 (16.9) |
| Physical activity | ||
| Years in recreational sports, ages 14–18 | 1.7 (1.9) | 1.6 (1.8) |
| Years in competitive sports, ages 14–18 | 1.7 (1.8) | 1.6 (1.8) |
| Summary score for young adulthood2 | 34.3 (25.8) | 33.3 (26.0) |
| Current activity summary score3 | 692 (1219) | 638 (1090) |
| Heavy alcohol consumptiond | 288 (39.9%) | 450 (35.9%) |
| Childhood socioeconomic advantage scoree | 4.0 (1.5) | 4.0 (1.5) |
| Adult education attainment | ||
| High school or less | 222 (29.9%) | 357 (27.7%) |
| Some college or Associate’s degree | 206 (28.9%) | 371 (29.9%) |
| College degree or more | 294 (41.2%) | 527 (42.4%) |
| Adult financial advantage scoref | 3.9 (2.5) | 4.0 (2.5) |
| Adult femoral neck composite strength indices | ||
| Compression strength index (gram/kg-meter) | 3.6 (0.9) | - |
| Bending strength index (gram/kg-meter) | 1.2 (0.3) | - |
| Impact strength index (gram/kg-meter) | 0.2 (0.04) | - |
Most common reasons for exclusion of MIDUS II Biomarker Project participants from the Study Sample were missing bone scans (n=348), use of medications known to influence bone (n=94), and unclassifiable menopause transition stage (n=88).
Summary physical activity score for young adulthood = (number of years between ages 20 and 35 of regular light exercise) + 2*(number of years between ages 20 and 35 of regular moderate exercise) + 3*(number of years between ages 20 and 35 of regular vigorous exercise).
Summary score for current physical activity = (number of minutes per week of regular light exercise) + 2*(number of minutes per week of regular moderate exercise) + 3*(number of minutes per week of regular vigorous exercise)
Consumption of >7 drinks per week or >3 drinks per occasion regularly by women and >14 drinks per week or >4 drinks per occasion regularly by men, either in the past month or at the period in their lives when they felt they drank the most
Childhood socioeconomic advantage score = on welfare (0: yes, 2: no) + financial status relative to others (0: worse off, 1: same, 2: better) + parental education (0: < high school, 1: high school/general educational development [GED] certificate, 2: some college or more)
Current adult financial advantage score = family income (0 if income-poverty ratio <3, 1 if ratio ≥3 but <6, 2 if ratio ≥6) + self-rated financial situation (0: worst, 1: average, 2: best) + enough money to meet needs (0: not enough, 1: just enough, 2: more than enough) + degree of difficulty paying bills (0: very, 1: not very, 2: not at all)
MIDUS = Midlife in the United States Study
The average age of study participants was 56.8 years; 38% of men were 60 years or older, and 59% of women were either late peri-menopausal or post-menopausal and not taking sex steroid hormone therapy. Forty one percent of the participants were college graduates or better educated. The average childhood socioeconomic advantage score in the sample was 4.0; median score 4.0, standard deviation (SD) 1.5; inter-quartile range 3–5, 10th percentile 2, and 90th percentile 6. A substantial proportion of the sample experienced socioeconomic disadvantage in childhood: 11% had been on welfare, 26% reported being worse off financially relative to others, and 23% had parents with less than high school education. The higher end of the socioeconomic spectrum was also well represented: 32% reported being better off financially relative to others in childhood, and 40% had at least one parent with some college-level education. Childhood socioeconomic advantage was weakly correlated with the participant’s own adult education level (treated as a 3-level ordinal variable) and current financial advantage (Spearman r=0.35 and 0.19, respectively).
In stratified analyses, adjusted for age, study site, and menopause transition stage in women, childhood socioeconomic advantage was positively associated with all three composite indices of femoral neck strength in white men but not in the other three race/gender groups (Table 2). For every SD increment of childhood advantage score in white men, femoral neck strength indices increased by 0.18 SD to 0.26 SD (p values 0.0009 to 0.04). This translates to 0.48 SD higher compression strength index (95% confidence interval 0.17 SD, 0.79 SD) and 0.69 SD higher bending strength index (95% confidence interval 0.38 SD, 1.00 SD) at the 90th compared to the 10th percentile of childhood advantage score. These associations in white men were unchanged with adjustment for indicators of adult socioeconomic status: educational attainment and current financial advantage (Table 2). Additional adjustment for BMI attenuated the associations by 30–45%, but they remained statistically significant (Table 2). Further adjustment for health behaviors over the life course (smoking, heavy alcohol consumption, and physical activity) diminished the associations further, so that associations of childhood socioeconomic advantage with femoral neck compression strength and impact strength were no longer statistically significant, although childhood socioeconomic advantage continued to be associated positively with femoral neck bending strength in white men (Table 2): Each SD increment in childhood advantage score in white men was associated with 0.16 SD increment in the bending strength index (p=0.04).
Table 2.
Adjusted Associations1 of Childhood Socioeconomic Advantage with Femoral Neck Composite Strength Indices in Adulthood, Stratified by Race and Gender2
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Base Model | Model 1 + adult SES3 |
Model 2 + adult BMI4 |
Model 3 + health behaviors5 |
|
| Compression | ||||
| Strength Index | ||||
| White Men | 0.18 (0.06)** | 0.19 (0.05)** | 0.11 (0.04)* | 0.09 (0.04) |
| White Women | 0.02 (0.05) | 0.00 (0.06) | −0.02 (0.06) | −0.02 (0.06) |
| Non-White Men | −0.12 (0.10) | −0.05 (0.10) | −0.05 (0.07) | −0.04 (0.08) |
| Non-White Women | −0.06 (0.09) | −0.07 (0.10) | 0.01 (0.08) | 0.03 (0.08) |
| Bending Strength | ||||
| Index | ||||
| White Men | 0.26 (0.06)*** | 0.27 (0.06)*** | 0.19 (0.06)** | 0.16 (0.06)* |
| White Women | 0.04 (0.06) | −0.01 (0.07) | −0.02 (0.06) | −0.01 (0.06) |
| Non-White Men | −0.10 (0.11) | 0.00 (0.12) | 0.00 (0.09) | 0.04 (0.10) |
| Non-White Women | −0.08 (0.08) | −0.10 (0.08) | −0.02 (0.06) | −0.05 (0.06) |
| Impact Strength | ||||
| Index | ||||
| White Men | 0.20 (0.06)** | 0.20 (0.06)** | 0.11 (0.05)* | 0.09 (0.05) |
| White Women | 0.02 (0.05) | −0.01 (0.06) | −0.03 (0.05) | −0.01 (0.05) |
| Non-White Men | −0.22 (0.13) | −0.15 (0.13) | −0.15 (0.10) | −0.12 (0.11) |
| Non-White Women | −0.16 (0.08) | −0.18 (0.08)* | −0.10 (0.06) | −0.1 - (0.06) |
p<0.05
p <0.01
p<0.001
SES = Socioeconomic status
BMI = Body mass index
Associations presented in units of strength index standard deviations (SD) per SD increment in the socioeconomic advantage score. Standard errors (in same units) are in parentheses. Base model adjusted only for age, study site, and menopause transition stage.
Sizes of race/gender strata in base model analyses: 272 White men, 246 White women, 77 non-White men, and 127 non-White women. Strata sizes are slightly reduced in models 2–4 due because of missing one or more covariates
With additional controls for educational attainment in adulthood and current financial advantage
With additional control for current body mass index (continuous)
With additional controls for smoking (current and total lifetime) and regular physical activity (in high school years, adulthood, and currently).
4. Discussion
As hypothesized, socioeconomic advantage in childhood was associated with higher values of femoral neck composite strength indices in white men, pointing to an influential role for childhood social circumstances in bone acquisition in this group. The association was attenuated and became statistically non-significant for two of the three strength indices when analyses were controlled for differences in health behaviors. Childhood socioeconomic advantage was not associated with femoral neck strength indices in non-whites and in women, even before controlling for health behaviors. Previous studies have found associations between childhood social circumstances and BMD in the lumbar spine [18], but to our knowledge, this is the first study to demonstrate such an association with femoral neck strength, which is more relevant to hip fracture risk. In older, community-dwelling women, each SD increment in femoral neck strength indices is associated with 57–66% relative reduction in hip fracture risk over 10 years [28]. If the strength indices had comparable fracture risk associations in older white men, we would expect that hip fracture risk would be 36% lower in white men at the 90th percentile of childhood advantage score compared to those at the 10th percentile of childhood advantage score.
Several mechanisms have been postulated to explain associations of childhood SES with adult health, including SES differences in nutrition, neighborhood environment, and parents’ lifestyle choices (such as smoking which would expose young children to second hand smoke) during the critical growing years [60]. Another major, potential mechanism by which socioeconomic disadvantage could affect bone strength in adulthood is via the effect of early life stresses on adult physiology, including the hypothalamic-pituitary-adrenal axis, the sympathetic nervous system, glucose regulation, and chronic inflammation [61,62,63], all of which have been linked to poor bone health [64,65,66]. In addition, low SES is also associated with increased body weight, which can positively influence bone mass by increasing loading [67]. These opposing influences on bone mass might explain why we and others have not found associations between low SES and low femoral neck BMD [14,16,18]. This study shows that when bone strength is assessed relative to load, socioeconomic disadvantage in childhood is indeed associated with less femoral neck strength in white men.
Our inability to observe an SES association with femoral neck strength in non-whites is consistent with the literature on race/ethnicity differences in SES effects on health. For instance, SES associations with cardiovascular health are generally larger in whites than in minority race/ethnicity groups in the US [68,69,70], partly because high SES does not necessarily translate to less life stresses in minority groups [71,72,73,74]. We were also hampered by the smaller sizes of the non-white strata. Our inability to observe childhood SES associations with adult femoral neck strength in women was more surprising, since low adult SES is strongly associated with increased risk of hip fractures in older women. However, in our study, childhood socioeconomic advantage was not strongly correlated with measures of adult SES, and only the latter may be associated with hip fracture risk in older women. It may also be that the childhood SES measure we used does not adequately reflect the social stressors experienced by impoverished girls and young women. A third possibility is that childhood SES effects on femoral neck strength in women are masked by much larger changes in bone strength during the menarche [75] and the menopause transition [76].
The association of childhood socioeconomic advantage with greater femoral neck strength in white men was significantly attenuated by controls for BMI. Increased body weight affects bone mass by stimulating osteoblasts [77], and it also affects the load on bone in a fall; thus differences in adult BMI are likely to represent a major pathway by which childhood SES affects adult bone strength relative to load. The childhood SES association with femoral neck strength relative to load was further attenuated by controls for health behaviors over the life course, suggesting that differences in health behaviors by childhood SES might be responsible for the majority of the childhood SES effects on adult bone strength, and that even if childhood socioeconomic disadvantage adversely affects bone health, its effects can be undone by healthy lifestyle choices. In fact, several studies have demonstrated the protective effects of physical activity over the life course on adult bone health [78], and the especially important role of physical activity in the growing years [79] and of competitive sports in early life in Swedish men [80]. There is similarly increasing evidence of the deleterious effects of smoking on bone health in general [81], and especially that of smoking in the teenage and young adult years on peak bone mass in European men [82,83]. Not all of the childhood SES association with adult bone strength in the femoral neck was explained by health behaviors in this study, suggesting that other mechanisms such as food choices and nutrition adequacy may also play a mechanistic role.
Our study has a few limitations, the most important one being its observational design, which cannot be used to establish a causal pathway between childhood SES and hip strength. We cannot for instance rule out the possibility that the associations seen here reflect only the benefits of healthy lifestyle choices in preventing declines in bone mass in adulthood, and not the effects of childhood social circumstances on bone acquisition in the growing years. Secondly, we did not adjust for multiple testing because this is the first examination of childhood SES associations with indices of femoral neck strength relative to load. Further confirmatory studies are needed before clinical recommendations for risk stratification can be made. Thirdly, childhood SES was ascertained from recalled self-report and is therefore susceptible to bias. However, among twins and siblings, there is excellent agreement in recalled childhood social class and parental education [84,85]. Among MIDUS twin and siblings participants, the intra-class correlation coefficient for the childhood socioeconomic advantage score was 0.84, indicating a high degree of reliability. The intra-class correlation coefficient changed little when we dropped non-twin siblings from the sample and when we examined parental education separately. Finally, the smaller number of non-whites (relative to whites) in the sample may have limited our power to find small effects in non-white men and women; and the small strata sizes meant we could not tease apart independent influences of the components of the childhood SES index.
Our study also has several strengths, including the national study sample with good representation of low-income urban residents, comprehensive assessment of childhood socioeconomic standing based on both subjective and objective information, the collection of health behavior data over the life course, and the examination of hip strength relative to load, which is more relevant to hip fractures than BMD on its own.
In conclusion, this study found that, independent of adult SES, socioeconomic advantage in childhood is associated with higher femoral neck strength relative to load in white men. Previous studies have found that childhood socioeconomic advantage is associated positively with bone density in the lumbar spine in both men and women. Taken together this suggests that socioeconomic factors in childhood influence the acquisition of bone mass during the growing years, and that healthy lifestyle choices should be strongly encouraged from a young age. Targeting this counseling to children in disadvantaged socioeconomic environments may be a potentially untapped way to decrease the hip fracture burden worldwide.
Highlights.
White men in the US who report greater socioeconomic advantage in childhood have greater hip bone strength.
Socioeconomic factors in childhood appear to influence bone acquisition in boys from the majority race/ethnicity group
Healthy lifestyle choices over the lifecourse appear to reduce the hip strength deficit associated with childhood socioeconomic disadvantage.
Childhood socioeconomic position was not associated with hip strength in non-whites and in women.
Acknowledgements
This research was supported by National Institutes of Health grant numbers 1R01AG033067, R01-AG-032271, and P01-AG-020166. The UCLA GCRC helped support this study (UCLA GCRC Grant # M01-RR000865).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacon WE, Hadden WC. Occurrence of hip fractures and socioeconomic position. J Aging Health. 2000;12:193–203. doi: 10.1177/089826430001200203. [DOI] [PubMed] [Google Scholar]
- 2.Farahmand BY, Persson PG, Michaelsson K, Baron JA, Parker MG, Ljunghall S for the Swedish Hip Fracture Study Group. Socioeconomic status, marital status, and hip fracture risk: A population-based case-control study. Osteoporos Int. 2000;11:803–808. doi: 10.1007/s001980070060. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RT, Chase GA, Chrischilles EA, Wallace RB. Hip fracture risk among community-dwelling elderly people in the United States: A prospective study of physical, cognitive, and socioeconomic indicators. Am J Pub Health. 2006;96:1210–1218. doi: 10.2105/AJPH.2005.077479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zingmond DS, Soohoo NF, Silverman SL. The role of socioeconomic status on hip fracture. Osteoporos Int. 2006;17:1562–1568. doi: 10.1007/s00198-006-0161-7. [DOI] [PubMed] [Google Scholar]
- 5.Brennan SI, Henry MJ, Kotowicz MA, et al. Incident hip fracture and social disadvantage in an Australian population aged 50 years or greater. Bone. 2011;48:607–610. doi: 10.1016/j.bone.2010.10.175. [DOI] [PubMed] [Google Scholar]
- 6.Quah C, Boulton C, Moran C. The influence of socioeconomic status on the incidence, outcome and mortality of fractures of the hip. J Bone Joint Surg Br. 2011;93:801–805. doi: 10.1302/0301-620X.93B6.24936. [DOI] [PubMed] [Google Scholar]
- 7.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SY, Levy AR, Lefaivre KA, Guy P, Kuramoto L. Sobolev B Geographic trends in incidence of hip fractures: a comprehensive literature review. Osteoporos Int. 2011:12. doi: 10.1007/s00198-011-1596-z. [DOI] [PubMed] [Google Scholar]
- 9.Elliot JR, Gilchrist NL, Wells JE. The effect of socioeconomic status on bone density in a male Caucasian population. Bone. 1996;18:371–373. doi: 10.1016/8756-3282(96)00006-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang MC, Dixon LB. Socioeconomic influences on bone health in postmenopausal women: findings from NHANES III, 1988–1994. Osteoporos Int. 2006;17:91–98. doi: 10.1007/s00198-005-1917-1. [DOI] [PubMed] [Google Scholar]
- 11.Brennan SL, Henry MJ, Wluka AE, Nicholson GC, Kotowicz MA, Pasco JA. Socioeconomic status and bone mineral density in a population-based sample of men. Bone. 2010;46:993–999. doi: 10.1016/j.bone.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal N, Raveendran A, Khandelwal N, et al. Prevalence and related risk factors of osteoporosis in peri- and postmenopausal Indian women. J Midlife Health. 2011;2:81–85. doi: 10.4103/0976-7800.92537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabipour I, Cumming R, Handelsman DJ, et al. Socioeconomic status and bone health in community-dwelling older men: the CHAMP Study. Osteoporos Int. 2011;22:1343–1353. doi: 10.1007/s00198-010-1332-0. [DOI] [PubMed] [Google Scholar]
- 14.Varenna M, et al. Prevalence of osteoporosis by educational level in a cohort of postmenopausal women. Osteoporos Int. 1999;9:236–241. doi: 10.1007/s001980050143. [DOI] [PubMed] [Google Scholar]
- 15.Gur A, Jale Sarac A, Nas K, Cevik R. The relationship between education level and bone mineral density in postmenopausal women. BMC Family Practice. 2004;5:18. doi: 10.1186/1471-2296-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Mittal S, Orito S, et al. Impact of dietary intake, education, and physical activity on bone mineral density among North Indian women. J Bone Miner Metab. 2010;28:192–201. doi: 10.1007/s00774-009-0118-y. [DOI] [PubMed] [Google Scholar]
- 17.Brennan SL, Pasco JA, Urquhart DM, Oldenburg B, Wang Y, Wluka AE. Association between socioeconomic status and bone mineral density in adults: a systematic review. Osteoporos Int. 2011;22:517–527. doi: 10.1007/s00198-010-1261-y. [DOI] [PubMed] [Google Scholar]
- 18.Crandall CJ, Merkin SS, Seeman TE, Greendale GA, Binkley N, Karlamangla AS. Socioeconomic status over the life-course and adult bone mineral density: The Midlife in the U.S. Study. Bone. 2012;51:107–113. doi: 10.1016/j.bone.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ. 1991;303:961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riis BJ, Hansen MA, Jensen AM, Overgaard K, Christiansen C. Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a 15-year follow-up study. Bone. 1996;19:9–12. doi: 10.1016/8756-3282(96)00102-0. [DOI] [PubMed] [Google Scholar]
- 21.Pearce MS, Birrell FN, Francis RM, Rawlings DJ, Tuck SP, Parker L. Lifecourse study of bone health at age 49–51 years: the Newcastle thousand families cohort study. J Epidemiol Community Health. 2005;59:475–480. doi: 10.1136/jech.2004.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D. Vogt TM for The SOF Research Group. Risk factors for hip fractures in white women. New Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 23.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 24.Rivadeneira F, Zillikens MC, De Laet CEDH, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: The Rotterdam Study. J Bone Miner Res. 2007;22:1781–1790. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 25.Allolio B. Risk factors for hip fracture not related to bone mass and their therapeutic implications. Osteoporos Int. 1999;10:S9–S16. doi: 10.1007/pl00004166. [DOI] [PubMed] [Google Scholar]
- 26.Alonso CG, Curiel MD, Carranza FH, Cano RP, Perez AD. Femoral bone mineral density, neck-shaft angle and mean femoral neck width as predictors of hip fracture in men and women. Multicenter Project for Research in Osteoporosis. Osteoporos Int. 2000;11:714–720. [PubMed] [Google Scholar]
- 27.Robinovitch SN, Hayes WC, McMahon TA. Prediction of femoral impact forces in falls on the hip. Trans ASME. 1991;113:366–374. doi: 10.1115/1.2895414. [DOI] [PubMed] [Google Scholar]
- 28.Karlamangla AS, Barrett-Connor E, Young J, Greendale GA. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int. 2004;15:62–70. doi: 10.1007/s00198-003-1513-1. [DOI] [PubMed] [Google Scholar]
- 29.Baptista F, Varela A. Sardinha LB Bone mineral mass in males and females with and without Down syndrome. Osteoporos Int. 2005;16:380–388. doi: 10.1007/s00198-004-1687-1. [DOI] [PubMed] [Google Scholar]
- 30.Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18:963–972. doi: 10.1007/s00198-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sardinha LB, Baptista F, Ekelund U. Objectively measured physical activity and bone strength in 9-year old boys and girls. Pediatrics. 2008;122:e728. doi: 10.1542/peds.2007-2573. [DOI] [PubMed] [Google Scholar]
- 32.Xu XH, Xiong DH, Liu XG, Guo Y, Chen Y, Zhao J, Recker RR, Deng HW. Association analyses of vitamin D-binding protein gene with compression strength index variation in Caucasian nuclear families. Osteoporos Int. 2009;21:99–107. doi: 10.1007/s00198-009-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong SS, Liu XG, Chen Y, et al. Association analyses of RANKL/RANK/OPG gene polymorphisms with femoral neck compression strength index variation in Caucasians. Calcif Tissue Int. 2009;85:104–112. doi: 10.1007/s00223-009-9255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu N, Liu YJ, Pei Y, Zhang L, et al. Evaluation of compressive strength index of the femoral neck in Caucasians and Chinese. Calcif Tissue Int. 2010;87:324–332. doi: 10.1007/s00223-010-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G-W, Chang S-X, Xu Z, et al. Prediction of hip osteoporotic fractures from composite indices of femoral neck strength. Skeletal Radiol. doi: 10.1007/s00256-012-1473-7. (Early Epub June 2012) [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Turner CH, Yoshikawa T, et al. Do variations in hip geometry explain differences in hip fracture risk between Japanese and white Americans? J Bone Miner Res. 1994;9:1071–1076. doi: 10.1002/jbmr.5650090715. [DOI] [PubMed] [Google Scholar]
- 37.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 38.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 39.Robbins J, Aragaki AK, Kooperberg C, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298:2389–2398. doi: 10.1001/jama.298.20.2389. [DOI] [PubMed] [Google Scholar]
- 40.Ishii S, Cauley JA, Greendale GA, Danielson ME, Safaei Nili N, Karlamangla A. Ethnic differences in composite indices of femoral neck strength. Osteoporos Intl. 2012;23:1381–1390. doi: 10.1007/s00198-011-1723-x. Erratum 23 (2012) 1391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishii S, Greendale G, Cauley J, Crandall C, Huang MH, Danielson M, Karlamangla A. Fracture risk assessment without race/ethnicity information. J Clin Endocrin Metab. doi: 10.1210/jc.2012-1997. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–2192. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishii S, Cauley J, Crandall C, Srikanthan P, Greendale G, Huang MH, Danielson M, Karlamangla A. Diabetes and femoral neck strength: Findings from the Hip Strength Across the Menopausal Transition Study. J Clin Endocrinol Metab. 2012;97:190–197. doi: 10.1210/jc.2011-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brim OG, Ryff CD, Kessler RC. How Healthy are We? A National Study of Well-being at Midlife. Chicago: University of Chicago Press; 2004. [Google Scholar]
- 46.Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: Protocol, Measures, Sample, and Comparative Context. J Aging Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radler BT, Ryff CD. Who Participates? Accounting for Longitudinal Retention in the MIDUS National Study of Health and Well-Being. J Aging Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danielson ME, Beck TJ, Karlamangla AS, et al. A comparison of DXA and CT based methods for estimating the strength of the femoral neck in post-menopausal women. Osteoporos Intl. doi: 10.1007/s00198-012-2066-y. (Epub ahead of print July 19, 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruenewald T, Karlamangla AS, Hu P, et al. History of socioeconomic disadvantage and multi-system physiological health in later life. Soc Sci Med. 2012;74:75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton LJ., 3rd Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981;67:328–335. doi: 10.1172/JCI110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treloar AE. Menstrual cyclicity and the pre-menopause. Maturitas. 1981;3:249–264. doi: 10.1016/0378-5122(81)90032-3. [DOI] [PubMed] [Google Scholar]
- 52.National Institute on Alcohol Abuse and Alcoholism. National Institute on Alcohol Abuse and Alcoholism; 2005. Helping patient who drink too much: A clinician's guide updated 2005 edition. U.S. Department of Health and Human Services, National Institutes of Health. [Google Scholar]
- 53.Chasan-Taber L, Erickson JB, McBride JW, et al. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155:282–289. doi: 10.1093/aje/155.3.282. [DOI] [PubMed] [Google Scholar]
- 54.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30:266–274. doi: 10.1097/00005768-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein L, Patel AV, Ursin G, Sullivan-Halley J, Press MF, Deapen D, Berlin JA, Daling JR, McDonald JA, Norman SA, Malone KE, Strom BL, Liff J, Folger SG, Simon MS, Burkman RT, Marchbanks PA, Weiss LK, Spirtas R. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst. 2005;97:1671–1679. doi: 10.1093/jnci/dji374. [DOI] [PubMed] [Google Scholar]
- 56.MacIntyre S, Hunt K. Socio-economic position, gender, and health: How do they interact? J Health Psychol. 1997;2:315–334. doi: 10.1177/135910539700200304. [DOI] [PubMed] [Google Scholar]
- 57.Williams DR. Race/ethnicity and socioeconomic status: Measurement and methodological issues. Intl J Health Serv. 1996;26:483–505. doi: 10.2190/U9QT-7B7Y-HQ15-JT14. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: The problem of residual confounding and the resiliency of race. Epidemiology. 1997;8:621–628. [PubMed] [Google Scholar]
- 59.LaVeist TA, Wallace JM., Jr Health risk and inequitable distribution of liquor stores in African American neighborhood. Soc Sci Med. 2000;51:613–617. doi: 10.1016/s0277-9536(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 60.Adler NE, Stewart J. Health disparities across the lifespan: Meaning, methods, and mechanisms. Ann NY Acad Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- 61.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blair C, Cybele Raver C, Granger D, Mills-Koonce R, Hibel L the Family Life Project Key Investigators. Allostatis and allostatic load in the context of poverty in early childhood. Development and Psychopathology. 2011;23(3):845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson PO, Goodyear IM. Childhood adversity and allostatic overload of the hypothalamic-pituitary-adrenal axis. A vulnerability model for depressive disorders. Development and Psychopathology. 2011;23(4):1017–1037. doi: 10.1017/S0954579411000472. [DOI] [PubMed] [Google Scholar]
- 64.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys. 2008;473:231–236. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dennison E, Hindmarsh P, Fall C, Kellingray S, Barker D, Phillips D, et al. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J Clin Endocrinol Metab. 1999;84:3058–3063. doi: 10.1210/jcem.84.9.5964. [DOI] [PubMed] [Google Scholar]
- 66.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7:134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 67.Nordstrom A, Hogstrom M, Nordstrom P. Effects of different types of weight-bearing loading on bone mass and size in young adults: A longitudinal study. Bone. 2008;42:565–571. doi: 10.1016/j.bone.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Watkins LO, Neaton JD, Kuller LH. Racial differences in high-density lipoprotein cholesterol and coronary heart disease incidence in the usual-care group of the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1986;57:538–545. doi: 10.1016/0002-9149(86)90831-3. [DOI] [PubMed] [Google Scholar]
- 69.Kraus JF, Borhani, Franti CE. Socioeconomic status, ethnicity, and risk of coronary heart disease. Am J Epidemiol. 1980;111:407–414. doi: 10.1093/oxfordjournals.aje.a112915. [DOI] [PubMed] [Google Scholar]
- 70.Karlamangla AS, Singer B, Williams D, Schwartz J, Matthews K, Kiefe C, Seeman TE. Impact of socioeconomic status on accumulation of cardiovascular risk factors in young adults: The CARDIA Study. Soc Sci Med. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 71.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. Am Psychol. 1999;54:805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- 72.Williams DR, Neighbors H. Racism, Discrimination & Hypertension: Evidence & needed Research. Ethnicity & Disease. 2001;11:800–816. [PubMed] [Google Scholar]
- 73.Meyer IH. Prejudice as stress: Conceptual and measurement problems. Am J Pub Health. 2003;93:262–265. doi: 10.2105/ajph.93.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kessler RC, Neighbors HW. A new perspective on the relationships among race, social class, and psychological distress. J Health and Soc Behav. 1986;27:107–115. [PubMed] [Google Scholar]
- 75.Seselj M, Nahhas RW, Sherwood RJ, et al. The influence of age at menarche on crosssectional geometry of bone in young adulthood. Bone. 2012:38–45. doi: 10.1016/j.bone.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greendale GA, Sowers MF, Han WJ, et al. Bone mineral density loss in relation to the final menstrual period in a multi-ethnic cohort: Results from the Study of Women’s Health Across the Nation (SWAN) J Bone Miner Res. doi: 10.1002/jbmr.534. (Epub ahead of print 2011 Oct 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greendale GA, Barrett Connor E, Edelstein S, et al. Lifetime leisure exercise and osteoporosis. Am J Epidemiol. 1995;141:951–959. doi: 10.1093/oxfordjournals.aje.a117362. [DOI] [PubMed] [Google Scholar]
- 79.Rideout CA, McKay HA, Barr SI. Self-reported lifetime physical activity and areal bone mineral density in healthy postmenopausal women: The importance of teenage activity. Calcif Tissue Int. 2006;79:214–222. doi: 10.1007/s00223-006-0058-7. [DOI] [PubMed] [Google Scholar]
- 80.Nilsson M, Ohlsson C, Eriksson AL, et al. Competitive physical activity early in life is associated with bone mineral density in elderly Swedish men. Osteoporos Int. 2008;19:1557–1566. doi: 10.1007/s00198-008-0600-8. [DOI] [PubMed] [Google Scholar]
- 81.Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68:259–270. doi: 10.1007/bf02390832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taes Y, Lapauw B, Vanbillemont G, et al. Early smoking is associated with peak bone mass and prevalent fractures in young, healthy men. J Bone Miner Res. 2010;25:379–387. doi: 10.1359/jbmr.090809. [DOI] [PubMed] [Google Scholar]
- 83.Rudang R, Darelid A, Nilsson M, et al. Smoking is associated with impaired bone mass development in young adult men: A 5-year longitudinal study. J Bone Miner Res. 2012;27:2189–2197. doi: 10.1002/jbmr.1674. [DOI] [PubMed] [Google Scholar]
- 84.Krieger N, Okamoto A, Selby JV. Adult female twins' recall of childhood social class and father's education: a validation study for public health research. Am J Epidemiol. 1998;147:704–708. doi: 10.1093/oxfordjournals.aje.a009512. [DOI] [PubMed] [Google Scholar]
- 85.Robins LN, Schoenberg SP, Holmes SJ, Ratcliff KS, Benham A, Works J. Early home environment and retrospective recall: a test for concordance between siblings with and without psychiatric disorders. Am J Orthopsychiatry. 1985;55:27–41. doi: 10.1111/j.1939-0025.1985.tb03419.x. [DOI] [PubMed] [Google Scholar]