Abstract
Background
Based on preclinical studies, the vascular endothelial pathway is an important mechanism for estrogen receptor resistance. We conducted a phase II study of fulvestrant and bevacizumab in patients with aromatase inhibitor pretreated metastatic breast cancer.
Patients and methods
A single-stage phase II study was conducted with these objectives: 6-month progression-free survival (PFS), tumor response, toxic effect, and overall survival. Regimen: 250 mg fulvestrant days 1 and 15 (cycle 1) then day 1 (cycle 2 and beyond) and 10 mg/kg bevacizumab days 1 and 15 of each 4-week cycle.
Results
At interim analysis, 20 eligible patients initiated treatment, 11 were progression free and on treatment at 3 months, not meeting the protocol-specified efficacy requirements (at least 12 of 20). Accrual remained open during interim analysis with 36 patients enrolling before final study closure. Among the 33 eligible patients, the median PFS was 6.2 months [95% confidence interval (CI) 3.6–10.1 months]. Of the 18 with measurable disease, 4 (22%) patients (95% CI 6% to 48%) had a confirmed tumor response (1 complete, 3 partial). The most common grade 3/4 adverse events were hypertension 3 (9%) and headache 3 (9%).
Conclusions
The fulvestrant/bevacizumab combination is safe and tolerable; however, it did not meet its statistical end point.
Keywords: antiangiogenesis agent, aromatase inhibitor resistance, bevacizumab, estrogen receptor antagonist, fulvestrant, metastatic breast cancer
introduction
The use of monoclonal antibodies targeting the vascular endothelial growth factor (VEGF) pathway has been a significant addition to cancer therapy. Several phase III studies have shown an improvement in progression-free survival (PFS) when bevacizumab is combined with chemotherapy in breast cancer [1, 2]. Moreover, various studies have demonstrated that estrogen interacts with the VEGF pathway and is an important mechanism for resistance leading to the question of whether combination with antiangiogenesis and antiestrogen therapies could be an appropriate therapeutic modality [3]. Therefore, we embarked on a phase II study of fulvestrant, a complete ER suppressor, and bevacizumab, a VEGF monoclonal antibody, in aromatase refractory metastatic breast cancer (MBC) patients.
patients and methods
eligibility
Patients were eligible to enter on to the study if they had histologically or cytologically confirmed breast cancer with clinical evidence of metastatic disease. They must have had estrogen (ER) and/or progesterone (PgR) receptor-positive disease previously treated in the adjuvant or metastatic setting with an aromatase inhibitor. Patients with HER2-positive disease must have received at least one prior trastuzumab-containing regimen (unless there was a contraindication for trastuzumab). Patients were allowed, at most, one prior chemotherapy treatment of metastatic disease and up to two prior endocrine therapies in the neoadjuvant, adjuvant, or metastatic setting.
The following laboratory values were required within 2 weeks of registration: hemoglobin >8 g/dl, absolute neutrophil count ≥1000/mm3, platelets ≥100 000/mm3, white blood cells ≥3000/mm3, total bilirubin ≤1.5 × ULN, alkaline phosphatase ≤2.5 × ULN, AST ≤ 2.5 × ULN, ALT ≤ 2.5 × ULN, creatinine ≤1.5 × ULN, urinalysis <1+ protein. Women participants must have been postmenopausal (≥60 years old, ≥45 years old without a menstrual period in the previous year, estradiol and follicle-stimulating hormone levels in postmenopausal range, or history of bilateral oophorectomy).
Finally, patients must have been able to complete questionnaires, had a life expectancy of at least 3 months, had an ECOG PS of ≤2, and been disease free for at least 3 years of other invasive nonbreast malignancies. The following reasons were cause for exclusion: pregnant or nursing women; women of childbearing potential who are unwilling to employ adequate contraception; patients with microscopic residual disease only; major surgery, open biopsy, significant traumatic injury, chemotherapy, immunologic therapy, or radiotherapy (except if to a nontarget lesion only) within a month of registration; evidence of active brain metastasis; history of bleeding diathesis, uncontrolled coagulopathy, hypertensive crises, hypertensive encephalopathy, cerebrovascular accident, hemorrhage, or stroke; anticoagulants or thrombolytic agents within 2 weeks of registration; significant cardiac disease; arterial or venous thrombosis in the previous year; hemoptysis or gastrointestinal hemorrhage in the previous 6 months; nephritic syndrome or baseline proteinuria >1 g/24 h; history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within the past month; history of allergy or hypersensitivity to drug product excipients, murine antibodies, or agents chemically similar to fulvestrant or bevacizumab; active, unresolved infection; any serious concomitant medical condition that would make it undesirable for patients to participate in the trial or would jeopardize compliance with protocol treatment; and currently receiving treatment in a different clinical study in which investigational procedures are carried out or investigational therapies are administered.
treatment schedule
Treatment was administered in 28-day cycles. On days when both agents were administered, fulvestrant was given before bevacizumab. On day 1 of each cycle, fulvestrant was given intramuscularly at a dose of 250 mg (except in cycle 1 where patients received an additional loading dose of 250 mg) followed by 10 mg/kg of intravenous bevacizumab. Bevacizumab was also given on day 15 of each cycle. This study was conducted before the results of the COmparison of Faslodex In Recurrent or Metastatic breast cancer (CONFIRM) study were available, demonstrating that the lower dose used here appears to be inferior in terms of PFS in patients with ER-positive MBC [4].
Patients were allowed to discontinue one of the agents and still remain on study. Fulvestrant was discontinued for National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v3.0) grade 2+ allergic reaction, grade 3+ injection site reaction, and grade 4+ non-hematologic adverse events (at physician discretion). Bevacizumab was discontinued for cycle 1 grade 1+ allergic reaction; cardiac ischemia/infarction; grade 2+ hypertension (at physician discretion); continued grade 3 left ventricular systolic dysfunction; grade 4 left ventricular systolic dysfunction, new gastrointestinal (GI) fistula, leak, or perforation; grade 2+ hemorrhage (central nervous system [CNS], GI, or pulmonary); CNS cerebrovascular ischemia; grade 2+ thrombosis, thrombus, or embolism; grade 4 nonhematologic adverse events (including CNS bleed). In addition to discontinuation, fulvestrant was held due to grade 3+ vomiting; grade 3+ injection site reaction; or grade 3 nonhematologic adverse events (at physician's discretion). Bevacizumab was held for grade 2 or 3 hypertension; grade 3+ mucositis or stomatitis; grade 2+ bilirubin; or grade 3 nonhematologic adverse events.
response and adverse event criteria
Evaluation of response was carried out every 8 weeks. Criteria for response and progression were based on Response Evaluation Criteria in Solid Tumors (RECIST v1.0). Evaluation for adverse events was based on the NCI CTCAE v3.0 and was assessed after each cycle.
statistical design and analysis
The primary end point of this trial was the 6-month PFS rate. This was defined as a patient who was progression free and on study treatment at least 6 months from registration. A two-stage Simon optimal design was chosen to test the null hypothesis that the true 6-month PFS rate was at most 35% versus the alternative that it was at least 55%. This design had a significance level of 0.09% and 90% power. In order to complete the interim analysis in a shorter time frame, the decision at the time of the interim analysis was based on 3-month PFS and required that 12 of 20 assessable patients were progression free and on study treatment at 3 months. If the interim criterion was met, 27 additional patients would be accrued for a final planned sample size of 47 assessable patients.
Secondary end points included overall survival (OS), time-to-treatment failure (TTF), time-to-first cytotoxic agent, description of the adverse event profile, as well as the quality of life (QOL) of patients based on the 6-item Linear Analogue Self-Assessment (LASA) [5–7]. The LASA consists of six single-item numeric analog scales measuring overall QOL; mental, physical, emotional, and spiritual well-being; and level of activity each on a scale of 0 (‘As bad as it can be’) to 10 (‘As good as it can be’) during the past week. Paper booklets were administered to patients during clinic visits before treatment at baseline and then every other cycle. Items were transformed to a 0 (worst QOL or well-being) to 100 (best QOL or well-being) scale for statistical analysis. Additionally, for those patients with measurable disease, confirmed response rate and duration of response (DOR) was to be examined.
Exact confidence intervals are constructed for the primary end point and confirmed response rate based on the binomial distribution. The distributions of OS (time from study entry until death), PFS (time from study entry until disease progression or death regardless of whether receiving study treatment), and TTF (time from study entry until study termination due to progression, death, adverse events, or refusal) are estimated using the Kaplan–Meier method. Simple descriptive statistics are used to summarize the adverse event profile, baseline characteristics, and QOL end points.
results
Accrual remained open during the interim analysis but was subsequently closed (to allow for interim data to mature) because accrual was quicker than expected. At the time of the interim analysis only 11 of the first 20 assessable patients were progression free and on study treatment at 3 months. Thus, this study did not meet the efficacy requirements (i.e. at least 12 of 20 progression free and on study treatment at 3 months) specified in the protocol for reopening following the interim analysis. Therefore, this study was permanently closed to patient accrual effective December 2008.
patient population
Overall, 36 patients were accrued to the study from September 2007 to December 2008. Two patients cancelled participation before receiving any study drug, and another patient was deemed ineligible after beginning treatment due to being ER and PgR negative and excluded from all statistical analyses. Baseline patient characteristics for the 33 remaining patients can be found in Table 1. Thirty-two (97%) study participants were female, and the median age was 65 years (range 34–90 years). All 33 were ER positive and 23 (70%) were PgR positive. Two-thirds of the patients received prior hormonal therapy in the metastatic setting, 20 (60%) had prior chemotherapy in the adjuvant or neoadjuvant setting, and 1 (22%) had prior chemotherapy in the metastatic setting.
Table 1.
Baseline characteristics (N = 33)
| Dominant disease status | |
| Measurable | 18 (54.5%) |
| Other | 15 (45.5%) |
| ECOG performance status | |
| 0 | 19 (57.6%) |
| 1 | 13 (39.4%) |
| 2 | 1 (3%) |
| Most recent ER | |
| Positive | 33 (100%) |
| Most recent PgR | |
| Missing | 1 |
| Positive | 23 (71.9%) |
| Negative | 9 (28.1%) |
| HER2 status | |
| Positive | 3 (9.1%) |
| Negative | 29 (87.9%) |
| Not reported | 1 (3%) |
| Prior adjuvant or neoadjuvant chemotherapy | |
| Yes | 20 (60.6%) |
| No | 13 (39.4%) |
| Prior hormonal therapy in the metastatic setting | |
| Yes | 22 (66.7%) |
| No | 11 (33.3%) |
| Prior chemotherapy in the metastatic setting | |
| Missing | 1 |
| Yes | 7 (21.9%) |
| No | 25 (78.1%) |
follow-up
The median number of cycles administered was 6 (range 1–28). All 33 patients have discontinued treatment due to disease progression (24, 73%), refusal (4, 12%), adverse events (3, 9%), death (1, 3%: patient fell and developed a hematoma and subarachnoid hemorrhage), and alternate treatment (1, 3%: radiation therapy). At last follow-up, 10 (30%) patients remained alive with a median follow-up time of 26.4 months (range 1.7–42.5 months).
efficacy
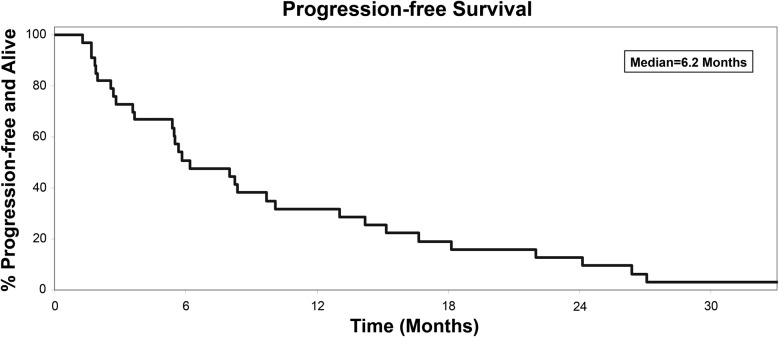
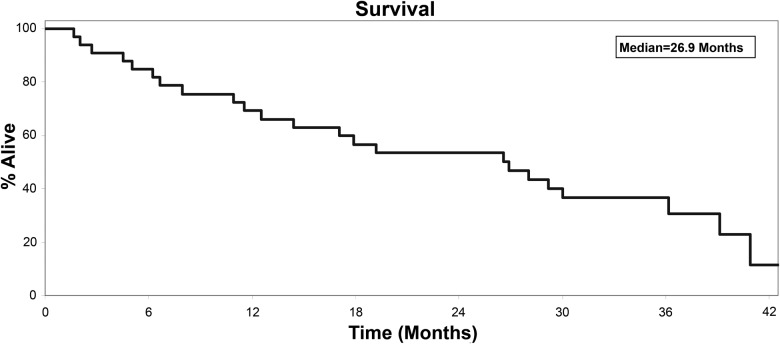
Overall, of the 33 assessable patients, there were 13 [39%, 95% confidence interval (CI) 23% to 58%) patients progression free and on study treatment at 6 months. For the time-to-event analyses, the median PFS was 6.2 months (95% CI 3.6–10.1; Figure 1), the median OS was 26.9 months (95% CI 12.5–36.2; Figure 2), the median TTF was 5.6 months (95% CI 2.7–8.2), and the median time to first dose of cytotoxic agent was 9.9 months (95% CI 6.2–19.5). There were 18 assessable patients on the trial with measurable disease. Of these, four (22%, 95% CI 6% to 22%) achieved a confirmed tumor response including one complete and three partial responses. These four patients maintained response for 3.7 months (partial response, progression), 3.7 (partial response, progression), 20.1 (partial response, progression), and 29.4 (complete response, nonprogression) months.
Figure 1.
Progression-free survival, events = 31, median 6.2 months (95% confidence interval 3.6–10.1).
Figure 2.
Overall survival, events = 23, median 26.9 months (95% confidence interval 12.5–36.2).
adverse events
All grade 4 and 5 adverse events and grade 3 adverse events occurring in at least 5% of patients appear in Table 2. The most common grade 3 or higher AEs were hypertension (3, 9%) and headache (3, 9%). There was one grade 5 CNS hemorrhage which occurred during cycle 3 (patient fell and struck her head resulting in a brain bleed followed by surgery, after which the patient continued to bleed postoperatively and subsequently died). No autopsy was done. Seventeen (52%) patients experienced a grade 3+ nonhematologic AE of which five (15%) experienced a grade 4+ nonhematologic AE.
Table 2.
Adverse events (N = 33)
| Adverse events | Grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| N | % | N | % | N | % | N | % | N | % | |
| Hypertension | 8 | 24.2 | 2 | 6.1 | 1 | 3.0 | ||||
| Proteinuria | 4 | 12.1 | 5 | 15.2 | 2 | 6.1 | ||||
| Headache | 4 | 12.1 | 3 | 9.1 | ||||||
| Confusion | 1 | 3.0 | 1 | 3.0 | 1 | 3.0 | ||||
| Catheter infection | 2 | 6.1 | ||||||||
| Creatinine increased | 2 | 6.1 | ||||||||
| Disseminated intravascular coagulation | 1 | 3.0 | ||||||||
| Intracranial hemorrhage | 1 | 3.0 | ||||||||
| Ischemia cerebrovascular | 1 | 3.0 | ||||||||
| Left ventricular fail | 1 | 3.0 | ||||||||
All grade 4 and 5 adverse events and grade 3 adverse events occurring in at least 5% of patients are shown. Maximum grade per patient is reported.
quality of life
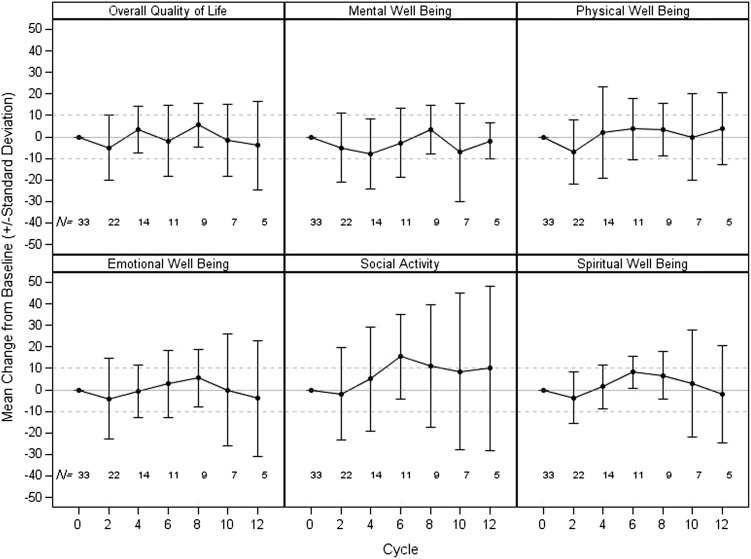
Mean changes from baseline by cycle for the six LASA items are depicted in Figure 3. Overall QOL and physical well-being appeared to have very little change from cycle to cycle. The largest mean decline in overall QOL and physical well-being occurred at cycle 2 (overall QOL: mean change −5.0, standard deviation [SD] 15.0; physical well-being: mean change −6.8, SD 14.9); however, the magnitude of these declines was not clinically meaningful. Similarly, none of the other domain items were shown to have substantial declines at any assessment time point for patients who remained on treatment.
Figure 3.
Quality of life as measured by the Linear Analogue Self-Assessment.
discussion
Fulvestrant, a ‘pure’ antiestrogen, has a steroid structure that allows it to compete with estrogen for the ER receptor [8–12]. Fulvestrant effectively blocks ER dimerization and DNA binding, increases ER turnover, and inhibits nuclear uptake of the receptor. Because it blocks ER function before co-activator binding, fulvestrant can theoretically overcome resistance that is driven by the agonist properties of tamoxifen [1].
Two pivotal phase III trials (and a combined analysis of both trials) suggested that fulvestrant (250 mg monthly) is at least as effective and as well tolerated as anastrozole (1 mg by mouth daily) in postmenopausal women with advanced tamoxifen-resistant breast cancer [2, 13, 14].
The dose of fulvestrant used in our study was the original 250 mg approved by regulatory agencies, before the results of the CONFIRM trial. However, data from the current trial are still relevant. In the phase III trial CONFIRM, 736 postmenopausal women with ER-positive advanced breast cancer recurring or progressing after prior endocrine therapy were randomly assigned to fulvestrant at the approved dose (250 mg monthly) or at 500 mg monthly (after the initial three doses at 500 mg every 14 days). The 500 mg fulvestrant dose was associated with a significant increase in time to progression [TTP; hazard ratio (HR) 0.80; 95% CI 0.68–0.94) without an increase in toxic effect [15]. The FACT study was an open-label randomized phase III study of fulvestrant and anastrazole in combination in comparison to anastrazole as a first-line therapy for postmenopausal breast cancer showed no benefit of combination treatment compared with single agent anastrazole. The primary end point was TTP (10.8 versus 10.2 months) and median OS was 37.8 versus 38.2 months [16].
Presented at the 2011 San Antonio Breast Cancer Symposium, a phase III trial comparing sequential anastrazole followed by fulvestrant at tumor progression to anastrazole in combination with fulvestrant showed that the combination treatment improved median OS (47.7 versus 41.3 months) and PFS (15 versus 13.5 months) although only 40% received prior tamoxifen and 12 women received prior aromatase inhibitors. The combination did not benefit those who received prior tamoxifen [17].
Both phase III studies used combined hormonal treatment, one supports combination treatment the other does not. This is still an area of active investigation. There is an ongoing Alliance trial (NCT 000601900) of tamoxifen or letrozole with or without bevacizumab as treatment of stage III or VI breast cancer that is open to accrual. Our study combined two potentially minimally toxic drugs together with the goal of improving PFS using the combination of fulvestrant and bevacizumab.
Several bevacizumab/chemotherapy combination trials including ECOG trial 2100 [18], AVADO trial [19], and RIBBON-1 trial [20] have shown improvement of PFS with bevacizumab but no improvement in OS. Presently, bevacizumab is not an approved option for patients with MBC. Although cross-comparison of trials can be problematic, it could be hypothesis generating (Table 3). We compared our study with the results of Ingle et al. [21] in the second-line setting in a similar population, the response rate was 14.3% and median PFS was 3 months, and to a phase III study in the same population, Chia et al. [22] showed a 7.4% partial response rate with fulvestrant and a median PFS of 3.7 months. In the first-line trials with fulvestrant, the median PFS was 5.5 months [13] and 5.4 months [14]. The CONFIRM trial comparing fulvestrant 250 mg with fulvestrant 500 mg in the first-line and second-line metastatic setting showed median PFS of 5.5 months with low dose and 6.5 months with high dose [15]. Our study showed at least a doubling of the median PFS and confirmed tumor response rate, the exact clinical relevance of this is not known.
Table 3.
Results of similar studies involving fulvestrant
| Author | Type of study | Drug used | Publ | Patient population | Response | Median PFS (months) |
|---|---|---|---|---|---|---|
| Ingle | Phase II | Fulvestrant | JCO | 79 prior AI or Tam | PR 14% | 3 |
| Chia | Phase III | Fulvestrant | JCO | 351 prior AI | PR 7.4% | 3.7 |
| Exemestane | 342 prior AI | PR 6.7% | 3.7 | |||
| Tan | Phase II | Fulvestrant/bevacizumab | 33 AI refractory (18 with measurable disease) | CR + PR 22% (4 of 18 with measurable disease) | 6.2 |
Yardley et al. conducted a trial similar to ours involving a phase II study of 79 patients (38 treated with anastrazole and bevacizumab and 41 treated with fulvestrant and bevacizumab). Among the patients treated with fulvestrant and bevacizumab, the response rate was 27%; CR 2 of 41 (5%), PR 9 of 41 (22%) and 14 of 41 (34%) had stable disease for more than 6 months. The median TTP was 9 months. Grade 3 AEs included hypertension (15%), proteinuria (5%), thrombocytopenia (2%), anemia (2%), fatigue (2%), headache (2%), neuropathy (2%), and hypersensitivity reaction (2%); with a single grade 4 AE occurring (wound dehiscence, 2%) [23]. Compared with our study, the tolerability and efficacy was relatable.
Recently presented at the 2012 San Antonio Breast Cancer Symposium, the LEA trial compared patients treated with endocrine therapy (letrozole or fulvestrant) with or without bevacizumab. The median TTP of patients treated with endocrine therapy alone was 13.8 months compared with 18.4 months for patients treated with endocrine therapy plus bevacizumab (HR 0.83; 95% CI 0.65–1.06, P = 0.14). The study did not meet its primary end point of decreasing risk of progression by 31%. Additionally, the addition of bevacizumab did not improve median OS; median OS for patients on endocrine therapy alone was 42 months compared with 41 months for patients treated with endocrine therapy plus bevacizumab (HR 1.18; 95% CI 0.77–1.81, P = 0.469) [24].
The BOLERO-2 trial was a phase III study comparing exemestane to exemestane plus everolimus. The addition of everolimus to exemestane improved the median PFS to 6.9 months compared with 2.8 months (HR 0.43; P < 0.001). The everolimus/exemestane combination, however, had greater incidence of grade 3/4 AEs. [25]. Presently, everolimus and exemestane are approved as a treatment of postmenopausal women with MBC by the Food and Drug Administration. Among the different AI combinations, only the everolimus and exemestane trial has shown a significant improvement in median PFS.
conclusion
Our study has shown that the combination of fulvestrant and bevacizumab is safe and tolerable; however, it did not meet its statistical end point of improving PFS. With the availability of newer combinations such as everolimus and exemestane, its clinical utility is attenuated.
funding
The work was supported by Genentech, the Breast Cancer Research Foundation, and the National Institute of Health (CA25224).
disclosure
PJF is a member of the speakers' bureau for Roche/Genentech. Authors receive funding from Genentech. the Breast Cancer Research Foundation, and the National Institutes of Health.
references
- 1.Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 2.Howell A, Pippen J, Elledge RM, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: a prospectively planned combined survival analysis of two multicenter trials. Cancer. 2005;104:236–239. doi: 10.1002/cncr.21163. [DOI] [PubMed] [Google Scholar]
- 3.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13:905–919. doi: 10.1677/erc.1.01221. [DOI] [PubMed] [Google Scholar]
- 4.Di Leo A, Jerusalem G, Petruzelka L, et al. Final analysis of overall survival for the Phase III CONFIRM trial: fulvestrant 500 mg versus 250 mg. Cancer Res. 2012;72:S1–S4. [Google Scholar]
- 5.Bretscher M, Rummans T, Sloan J, et al. Quality of life in hospice patients. A pilot study. Psychosomatics. 1999;40:309–313. doi: 10.1016/S0033-3182(99)71224-7. [DOI] [PubMed] [Google Scholar]
- 6.Grunberg SM, Groshen S, Steingass S, et al. Comparison of conditional quality of life terminology and visual analogue scale measurements. Qual Life Res. 1996;5:65–72. doi: 10.1007/BF00435970. [DOI] [PubMed] [Google Scholar]
- 7.Gudex C, Dolan P, Kind P, et al. Health state valuations from the general public using the visual analogue scale. Qual Life Res. 1996;5:521–531. doi: 10.1007/BF00439226. [DOI] [PubMed] [Google Scholar]
- 8.Kansra S, Yamagata S, Sneade L, et al. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol. 2005;239:27–36. doi: 10.1016/j.mce.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Obermiller AM, Copur MS. The longstanding quest for a better endocrine therapy continues high-dose fulvestrant: have we found its effective dose, combination, setting, or sequence? Contemporary Oncol. 2011;3:1–5. [Google Scholar]
- 10.Croxtall JD, McKeage K. Fulvestrant: a review of its use in the management of hormone receptor-positive metastatic breast cancer in postmenopausal women. Drugs. 2011;71:363–380. doi: 10.2165/11204810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Valachis A, Mauri D, Polyzos NP, et al. Fulvestrant in the treatment of advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol. 2010;73:220–227. doi: 10.1016/j.critrevonc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Flemming J, Madarnas Y, Franek JA. Fulvestrant for systemic therapy of locally advanced or metastatic breast cancer in postmenopausal women: a systematic review. Breast Cancer Res Treat. 2009;115:255–268. doi: 10.1007/s10549-008-0137-8. [DOI] [PubMed] [Google Scholar]
- 13.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 14.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 15.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 16.Bergh J, Jonsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30:1919–1925. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RS, Barlow WE, Albain KS, et al. A phase III randomized trial of anastrozole versus anastrozole and fulvestrant as first-line therapy for postmenopausal women with metastatic breast cancer: SWOG S0226. Cancer Res. 2011;71:S1-1. [Google Scholar]
- 18.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 19.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 20.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 21.Ingle JN, Suman VJ, Rowland KM, et al. Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J Clin Oncol. 2006;24:1052–1056. doi: 10.1200/JCO.2005.04.1053. [DOI] [PubMed] [Google Scholar]
- 22.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 23.Yardley DA, Burris HA, 3rd, Clark BL, et al. Hormonal therapy plus bevacizumab in postmenopausal patients who have hormone receptor-positive metastatic breast cancer: a phase II Trial of the Sarah Cannon Oncology Research Consortium. Clin Breast Cancer. 2011;11:146–152. doi: 10.1016/j.clbc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Loibl S, von Minckwitz G, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer—first efficacy results from the LEA study. Cancer Res. 2012;72:91s. doi: 10.1200/JCO.2014.57.2388. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]