Abstract
Background
This phase I/II study examined the safety and efficacy of Sepantronium Bromide (S), a small-molecule selective survivin suppressant, administered in combination with carboplatin (C) and paclitaxel (P).
Patients and methods
Forty-one patients were treated on study. Twenty-two patients received escalating doses of S (3.6–12 mg/m2) and 19 with untreated stage IV non-small-cell lung cancer (NSCLC) were treated with the maximum tolerated dose of 10 mg/m2 in combination with standard doses of C (AUC6) and P (200 mg/m2) for six cycles. S was administered as a continuous intravenous infusion (CIVI) over 72 h in 21-day treatment cycles. Study end points included safety and toxic effect, response rate, progression-free and overall survival (PFS and OS), as well as exploratory pharmacodynamic correlates.
Results
Treatment with S was well tolerated, and toxic effects were mostly hematological in the phase II study. Two (11%) partial responses were observed with a median PFS of 5.7 months and median OS 16.1 months. Pharmacodynamic analysis did not demonstrate an association with response.
Conclusion
The combination of S (10 mg/m2/day 72-h CIVI) administered with C and P every 3 weeks exhibited a favorable safety profile but failed to demonstrate an improvement in response rate in advanced NSCLC.
Clinical trial number
Keywords: survivin, non-small-cell lung cancer, apoptosis, sepantronium bromide
introduction
The success of anticancer therapies is often limited by the development of resistance, which may result from defects in the apoptotic pathways [1]. Inhibition of survivin in tumor cells has been proposed as a mechanism to promote both spontaneous apoptosis and to enhance tumor cell response to apoptosis induced by chemotherapy [2]. Survivin is a member of the family of inhibitors of apoptosis proteins (IAPs) [3, 4] of which eight members are known, including X-linked inhibitor of apoptosis (XIAP), cIAP1, cIAP2, NAIP (NLR family, apoptosis inhibitory protein), Livin, ILP2 (IAP-like protein 2), BRUCE and surviving [5, 6]. Survivin has been implicated in both cell survival and regulation of mitosis in cancer [7, 8] and has consistently been identified by molecular profiling analysis to be associated with higher grade more advanced disease, abbreviated survival, accelerated rates of recurrence and chemotherapy and radiation resistance [9–11]. Survivin is preferentially and highly expressed in cancer cells, with little expression in most normal nondividing adult tissues making it a therapeutic target of interest [7].
YM155 (sepantronium bromide), a small imidazolium-based compound (1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazolium bromide) was originally identified by high-throughput screening of chemical libraries for inhibitors of activity of the survivin gene promoter using a survivin promoter luciferase assay [12]. The mode of action of sepantronium bromide has been demonstrated to be mediated through a 2-Kb promoter region of the survivin gene. Treatment with sepantronium bromide causes the dissociation of the paraspeckle regulatory 54-kDa nuclear RNA-binding protein (p54nrb) from interleukin enhancer-binding factor 3 (ILF3), which results in different subcellular localizations of ILF3 and p54nrb, which ultimately results in survivin downregulation [13, 14].
Preclinical studies involving sepantronium bromide showed inhibition of survivin at both the mRNA and protein levels and exhibited impressive anticancer activity in mouse models [12]. Phase I studies carried out to date have involved administration of sepantronium bromide as a single agent via continuous intravenous infusion (CIVI) over 168 h every 3 weeks. The maximum tolerated dose (MTD) has been determined as 4.8 mg/m2/day in the US population and 8 mg/m2/day in Japanese patients [15, 16]. A phase II trial investigated sepantronium bromide 4.8 mg/m2/day × 168 h in 37 patients with advanced NSCLC who had progressive disease after platinum-based therapy. Single-agent sepantronium bromide was well tolerated and had a modest response rate of 5.4% [17]. It has been recently demonstrated that sepantronium bromide acts in a synergistic manner to promote the induction of apoptosis by platinum compounds both in vitro and in vivo in NSCLC cell lines and with taxanes in prostate cancer [12, 18]. This is the first completed trial whereby a small molecule survivin suppressant (sepantronium bromide) has been combined with carboplatin and paclitaxel and administered over a shorter time period of 72 h CIVI.
patients and methods
patient population
Eligible patients on the phase I part of the study had pathologically confirmed solid malignancies refractory to standard therapy or for whom no standard therapy existed. Patients were not allowed to have had more than one line of prior chemotherapy but there was no limit to the amount of prior targeted therapy received. The phase II part of the study enrolled only patients with stage IV NSCLC who had received no prior chemotherapy (for additional eligibility criteria, see supplementary text, available at Annals of Oncology online).
study drugs and trial design
In the phase I component, patients received escalating doses of sepantronium bromide from 3.6 to 12 mg/m2 administered by CIVI over 72 h in combination with standard doses of carboplatin (AUC6) and paclitaxel (200 mg/m2). As sepantronium bromide had not been previously been combined with carboplatin or paclitaxel before commencing this study, the starting dose of 3.6 mg/m2 was less than the MTD determined in previous phase I and phase II studies which had evaluated single-agent sepantronium bromide administered over 168 h. The 72-h infusion schedule was selected based on favorable pharmacokinetics observed in previous phase I trials, and that it provided a more feasible, patient-friendly schedule than a 168-h infusion. Preclinical data have demonstrated that the efficacy of sepantronium bromide can be maintained with a 3-day infusion when compared with a 168-h infusion (Astellas, not published) (see supplementary text, available at Annals of Oncology online).
drug administration
The starting dose of sepantronium bromide was 3.6 mg/mg2 per day, subsequent doses included 5.0, 6.0, 8.0, 10.0 and 12.0 mg/m2 per day. Sepantronium bromide was supplied in vials containing 30 mg of the drug in 3-ml lactic acid based buffer (pH3.6), and it was prepared for administration in a controlled light and temperature environment (for additional details, see supplementary text, available at Annals of Oncology online).
study assessments
The primary end point of the phase II study was objective tumor response. Tumor assessment was done by CT on days 18–21 every two cycles. Response evaluation was according to RECIST 1.1 [19]. Additional efficacy variables included progression-free and overall survival (PFS and OS).
pharmacokinetics
In the phase I study, plasma samples were collected on multiple time points during cycle 1, including immediately before starting sepantronium bromide, 1, 2, 4, 8, 24 and 48 h after starting sepantronium bromide and at the end of the infusion and after 30 min, 1, 2, 4, 8, 24 and 48 h. Plasma concentrations of sepantronium bromide were determined using liquid chromatography coupled with mass spectrometric detection (LC-MS). The pharmacokinetic parameters were evaluated by compartmental analysis using WinNonlin 5.3 (Pharsight Corporation, Mountain View, CA) [20]. Plasma sepantronium bromide concentration–time profiles for each patient during cycle 1 were analyzed individually. The primary pharmacokinetic descriptors estimated were area under concentration–time curve extrapolated to infinity (AUC0–∞), steady-state concentrations of sepantronium bromide (Css), terminal half-life (T1/2), volume of distribution at steady state (Vss) and clearance (CL).
pharmacodynamics
Blood samples were taken before commencing study and throughout all cycles on day 1 and day 4 (before and post 72-h sepantronium bromide infusion) to determine whether biomarkers could be associated with a clinical response. Serum values of M30 apoptosense (a caspase-3 cleavage product of cytokeratin 18) were measured in an effort to determine the rate of apoptosis. Preclinical data had shown that suppressed survivin expression correlates with a decrease in angiogenesis and hence IL8 and VEGF were measured. The KRAS status of tumor tissue was also evaluated at baseline as pathway mapping from global expression microarray studies had suggested that the MAPK pathway may be a resistance pathway that tumor cells use to escape the proposed combination treatment with sepantronium bromide (Astellas, unpublished). Mutation analyses were carried out on paraffin embedded tumors using pyrosequencing (PyroMark Q24, Qiagen, Valencia, CA) to detect the most common point mutations, and capillary gene electrophoresis to detect small deletion/insertions [21]. For KRAS, all potential mutations involving codons 12, 13 and 61 were assessed.
statistical methods
The primary objectives of this study were to conduct a phase I trial to determine the maximum tolerated dose (MTD) of sepantronium bromide when combined with standard dose levels of carboplatin and paclitaxel, and to determine in a phase II study if this drug combination was associated with an objective response rate which exceeds that of 15% historically seen with carboplatin and paclitaxel alone in patients with stage IV NSCLC [22]. The initial portion of the trial was a phase I dose escalation study using a standard 3 + 3 design to determine a MTD over six dose levels of sepantronium bromide (3.6–12 mg/m2). The phase II trial was conducted using a Simon two-stage optimal design in order to rule out an unacceptably low 15% response rate (PR+CR), in favor of a modestly high response rate of 35% (for additional statistical methods and for correlative studies method of analyses, see supplementary text, available at Annals of Oncology online).
results
patients
From February 2010 to July 2012, a total of 41 patients were enrolled in the study and all patients received at least one 72-h infusion of sepantronium bromide with chemotherapy. Patient demographics are summarized in Table 1. In the phase I study, the majority of patients had NSCLC (18 of 22, 82%), one had small cell lung cancer, one adenoid cystic carcinoma, one angiosarcoma and one carcinoma of unknown origin. All 19 patients on the phase II section had treatment naïve stage IV NSCLC. Nineteen patients (100%) completed at least two cycles and 10 patients (53%) completed all six cycles.
Table 1.
Patient demographics and molecular/clinical characteristics
| Characteristic | YM155 3.6–12 mg/m2 (N=22, phase I) | YM155 10 mg/m2 (N=19, phase II) |
|---|---|---|
| Sex | ||
| Male | 10 (45%) | 11 (58%) |
| Female | 12 (55%) | 8 (42%) |
| Histology | ||
| Lung cancer | ||
| Adenocarcinoma | 11 (50%) | 14 (74%) |
| Bronchioalveolar (BAC) | 3 (14%) | 1 (5%) |
| Squamous cell carcinoma | 3 (14%) | 2 (11%) |
| Large cell | 1 (4.5%) | 2 (11%) |
| Small cell | 1 (4.5%) | |
| Adenoid cystic | 1 (4.5%) | |
| Angiosarcoma | 1 (4.5%) | |
| Unknown origin | 1 (4.5%) | |
| Mutational status (18 NSCLC patients on phase I) | ||
| EGFR | ||
| Mutated | 2 (11%) | 1 (5%) |
| Wild-type | 15 (83%) | 16 (84%) |
| Not done | 1 (6%) | 2 (11%) |
| KRAS | ||
| Mutated | 8 (44%) | 3 (16%) |
| Wild-type | 8 (44%) | 14 (74%) |
| Not done | 2 (11%) | 2 (10%) |
| Ethnicity | ||
| White | 13 (59%) | 14 (74%) |
| African American | 1 (4%) | 3 (16%) |
| Asian | 5 (23%) | 1 (5%) |
| Hispanic/Latino | 3 (14%) | 1 (5%) |
| Age | ||
| Median age (years) | 62 | 59 |
| Range | 38–79 | 41–74 |
| No. of prior regimens | ||
| 0 | 9 (41%) | 0 (100%) |
| 1 | 7 (32%) | |
| >1 | 6 (27%) | |
EGFR, epidermal growth factor receptor; KRAS, Kirsten RNA-associated rat Sarcoma 2 virus gene.
dose-limiting toxic effect and safety/tolerability
No dose-limiting toxic effects (DLTs) were observed in three subjects treated at doses of 3.6, 5, 6 or 8 mg/m2. A sudden unexplained death was observed at the 10 mg/m2 dose (see supplementary text, available at Annals of Oncology online). As per protocol, this dose level enrolled an additional three patients, and no further DLTs were seen in the five other patients treated at the 10 mg/m2 dose. At the 12 mg/m2 dose, two DLTs were observed. A 77-year-old male patient with bronchioalveolar carcinoma (BAC) had a probable drug induced grade 5 pneumonitis (see supplementary text, available at Annals of Oncology online). A second DLT of a transient grade 3 amylase and grade 4 lipase were noted in the patient with an adenoid cystic carcinoma. The MTD was determined as 10 mg/m2 (supplementary Table S1, available at Annals of Oncology online for phase I toxic effect). Treatment with carboplatin, paclitaxel and sepantronium bromide (10 mg/m2) was generally well tolerated in the phase II section. Grade 3/4 adverse events are listed in Table 2. Hematological toxic effects were the most common adverse event with 47% of patients having grade 3/4 neutropenia or lymphopenia. No subjects discontinued treatment of an adverse event.
Table 2.
National Cancer Institute Common Toxicity Criteria for Adverse Events Grade 3 and 4 treatment-related adverse events in the phase ii study population (n=19)
| Number of patients |
No. of events by grade |
|||
|---|---|---|---|---|
| Adverse event | No. | % | Grade 3 | Grade 4 |
| Neutropenia | 9 | 47 | 7 | 2 |
| Anemia | 2 | 11 | 2 | 0 |
| Lymphopenia | 9 | 47 | 8 | 1 |
| Leucopenia | 2 | 11 | 1 | 1 |
| Hyperuricemia | 1 | 5 | 0 | 1 |
| Hypokalemia | 1 | 5 | 1 | 0 |
| Vomiting | 1 | 5 | 1 | 0 |
| Upper respiratory infection | 1 | 5 | 1 | 0 |
| Vascular access complication | 2 | 10 | 2 | 0 |
pharmacokinetics
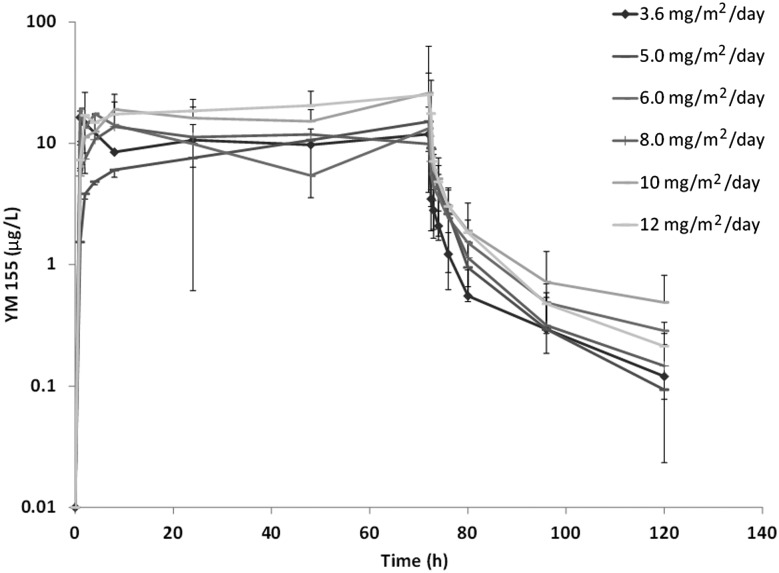
Pharmacokinetic profiles were available in 21 of 22 patients on the phase I part of the study. Plasma sepantronium bromide concentrations versus time profiles are shown in Figure 1. Dose proportionality of sepantronium bromide and pharmacokinetic parameters are shown in supplementary Table S2 and Figure S1 available at Annals of Oncology online. After the end of infusion, the plasma concentrations of sepantronium bromide declined rapidly in a biphasic manner, and the two-compartment model best described the PK profiles of sepantronium bromide. At the MTD of 10 mg/m2, the geometric mean terminal half-life and CL was ∼33 h, and 74 L/h, respectively.
Figure 1.
Mean (±SD) Plasma concentrations of sepantronium bromide (YM155) in NSCLC patients after 72-h continuous infusion.
efficacy
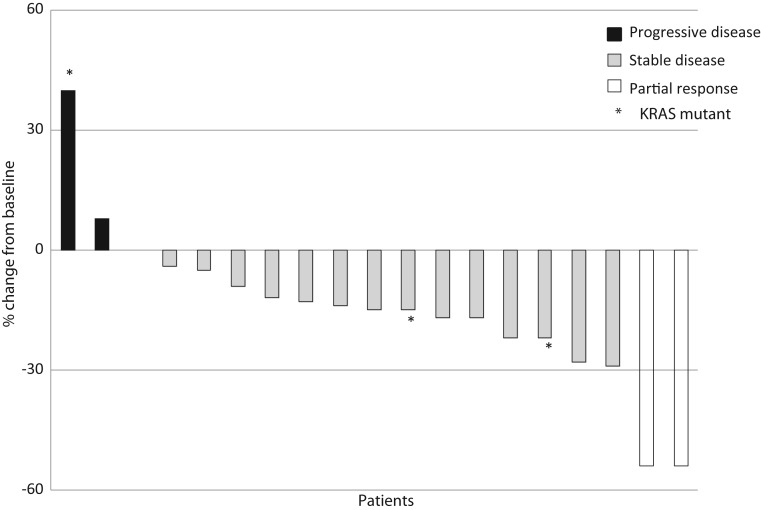
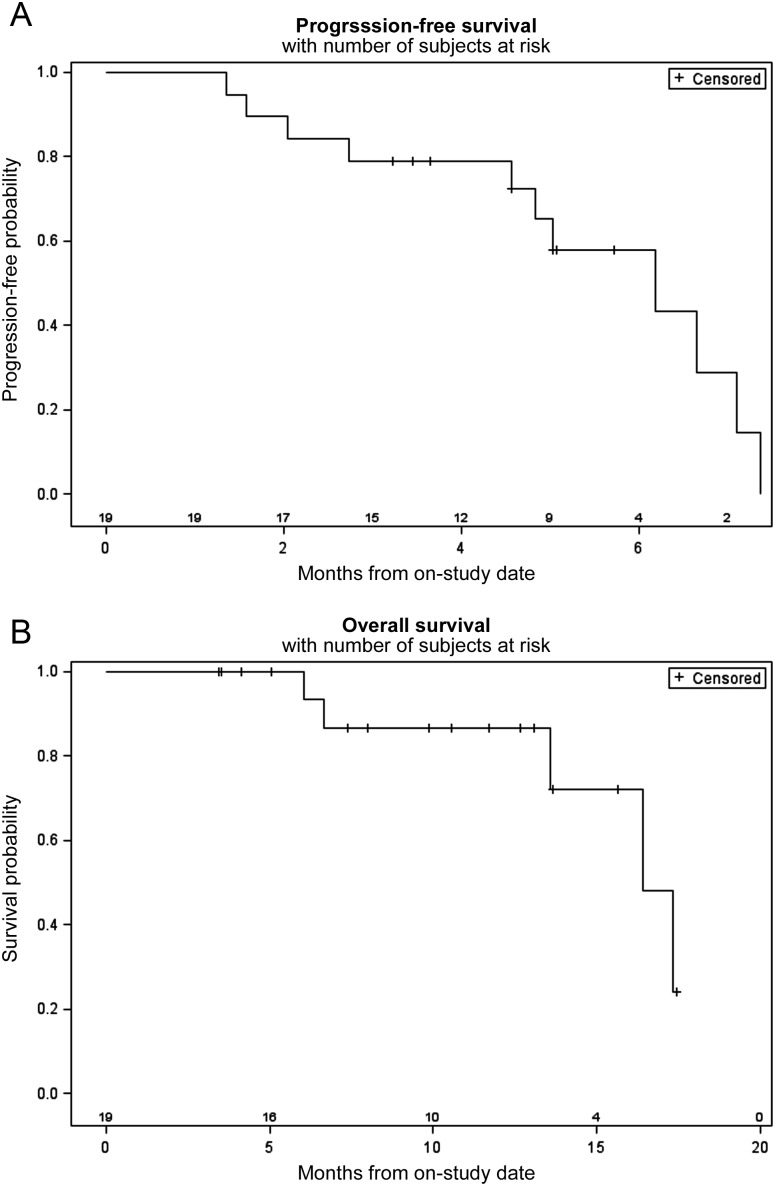
Of the 22 subjects treated on the phase I study, 16 were assessable for response as they had completed at least two cycles of therapy and had a restaging CT post cycle 2. Of the six patients who were not considered assessable, one patient had a TIA that was not considered drug related, two died after cycle 1 secondary to disease progression and an unexplained death (as discussed in DLT section), one did not have measurable disease and two withdrew due to DLTs on dose level 6. No complete and four confirmed partial responses (two NSCLC, one small-cell lung cancer and one angiosarcoma) and two unconfirmed responses were noted. Ten subjects had stable disease as best response. On the phase II study, all 19 patients were assessable for response (Figure 2). Only two partial responses [11%, 95% confidence interval (CI) 1.3% to 33.1%], one in adenocarcinoma and one in squamous cell carcinoma were seen, and 15 (75%) patients had stable disease ranging from 6 weeks to 9 months. Median survival was 16.2 months; 12-month OS is 87% (95% CI 62.1% to 96.3%). Median PFS was 5.7 months (Figure 3); 3-month PFS probability is 79.0%. Median follow-up for surviving patients is 10.2 months. Median potential follow-up (from on-study until date of analysis) is 15.3 months. KRAS and EGFR mutations were found in 16% and 5% of patients, respectively, but the mutational status was not associated with response.
Figure 2.
Response rate. Maximal change in size of indicator lesions of phase II patients. RECIST 1.1, Response Evaluation Criteria in Solid Tumors.
Figure 3.
Progression-free and overall survival. Kaplan–Meier curves of (A) progression-free survival and (B) overall survival of phase II patients. Ticks represent censored patients.
pharmacodynamics
Biomarker analysis included all phase II patients. Baseline values of M30 apoptosense, IL8 and VEGF, values of these markers from cycle 2, day 4 (C2D4) measurements and changes between baseline and C2D4 were compared for their association with response. There was no association between any of these biomarkers, or the changes in their levels and response rate (supplementary Table S3, available at Annals of Oncology online).
discussion
Survivin has often been implicated in conferring tumor resistance to both chemotherapy and radiotherapy by antagonizing apoptosis independently of caspase activation by binding to other adaptor or cofactor molecules [3]. This phase I/II trial is the first completed study to our knowledge that has combined a small molecule survivin suppressant, sepantronium bromide (administered over 72 h), with chemotherapy in an effort to maximize apoptosis. Previously published studies have suggested that sepantronium bromide should be given with cytotoxic chemotherapy, and preclinical data have indicated significant synergy between sepantronium bromide and both platinums and taxanes [18]. The results of this study are disappointing given the observed response rate of 11% in patients treated at the MTD of 10 mg/m2 dose of YM155 with standard doses of carboplatin and paclitaxel is below historical response rates of 15% seen with carboplatin/paclitaxel alone in patients with metastatic non-small-cell lung cancer [22]). The confidence intervals are broad, and they do not cross our goal of a 35% response rate; however, response rates are considered a poor surrogate for survival, and we did see a prolonged median survival of 16.2 months. The 1-year OS probability of 87% is in fact very high but may be explained by small patient numbers and the relatively short follow-up time. In addition, following the six cycles of treatment on this trial, many patients with nonprogressive disease were enrolled in an International multicenter, randomized, double-blinded, placebo-controlled study involving a vaccine maintenance therapy. We do not feel that the lack of response was due to the shorter infusion time or as a result of drug scheduling. The 7-day infusion that has previously been evaluated [15–17] has proved extremely arduous on patients in the clinical setting. Preclinical data have demonstrated that the efficacy of sepantronium bromide can be maintained with a 3-day infusion when compared with a 168-h infusion (Astellas, not published). Furthermore, preclinical modeling employing Calu-6 lung cancer xenografts has demonstrated that the concomitant administration demonstrated a slightly enhanced efficacy when compared with other drug schedules (Astellas, not published).
The combined treatment was well tolerated, and we did not see toxic effects similar to those observed in the previous phase I studies involving sepantronium bromide administered over 168h CIVI . A drug-induced death secondary to pneumonitis was observed in one patient but pneumonitis of any grade did not occur in the other 40 patients treated on study. The plasma exposure of sepantronium bromide appeared linear across the dose range tested (supplementary Figure S1, available at Annals of Oncology online). There was however an apparent increase in PK parameters (CL and Vss) at higher doses. This potential change in PK behavior may have contributed to reaching the maximum tolerable dose at 10 mg/m2. The Css of sepantronium bromide when administered in combination with carboplatin and paclitaxel are similar to previously published data [15, 16].
In an effort to determine the rate of apoptosis with sepantronium bromide, a previous phase II trial in NSCLC evaluated mean mRNA expression levels of survivin in peripheral blood mononuclear cells (PBMCs) [17], and in this trial, we evaluated serum levels of M30 (a caspase-3 cleavage product of cytokeratin 18). To date, no association has been established between survivin downregulation in PBMCs and changes in serum levels of M30 and response rate. Establishing predictive biomarkers for apoptotic pathway targeted therapy remains a significant challenge and discovery and validation will have to be tailored to the known mechanism of action of a certain agent in a certain disease. Successful targeting of survivin may be more difficult to measure than Bcl-2 or TRAIL as survivin acts as a nodal protein linking multiple pathways and in addition to regulating apoptosis, it is a regulator of cell division, a stress response factor and a promoter of tumor-associated angiogenesis [3]. Although this is not a randomized study, there did not appear to be a significant improvement in response rate when sepantronium bromide was administered in combination with standard doses of carboplatin and paclitaxel in unselected patients with metastatic NSCLC. Drug combinations targeting several components within apoptotic pathways may be more effective, and additional research to help unravel the complicated web of signaling interactions is required if we are to gain further insight into programmed cell death.
funding
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organization imply endorsement by the U.S. Government.
references
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3(1):46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 3.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8(1):61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396(6711):580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 5.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12(9):1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 6.Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64(20):7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 8.Blanc-Brude OP, Mesri M, Wall NR, et al. Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res. 2003;9(7):2683–2692. [PubMed] [Google Scholar]
- 9.Kawasaki H, Altieri DC, Lu CD, et al. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58(22):5071–5074. [PubMed] [Google Scholar]
- 10.Span PN, Sweep FC, Wiegerinck ET, et al. Survivin is an independent prognostic marker for risk stratification of breast cancer patients. Clin Chem. 2004;50(11):1986–1993. doi: 10.1373/clinchem.2004.039149. [DOI] [PubMed] [Google Scholar]
- 11.Vischioni B, van der Valk P, Span SW, et al. Nuclear localization of survivin is a positive prognostic factor for survival in advanced non-small-cell lung cancer. Ann Oncol. 2004;15(11):1654–1660. doi: 10.1093/annonc/mdh436. [DOI] [PubMed] [Google Scholar]
- 12.Nakahara T, Kita A, Yamanaka K, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67(17):8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura N, Yamauchi T, Hiramoto M, et al. Interleukin enhancer-binding factor 3/NF110 is a target of YM155, a suppressant of survivin. Mol Cell Proteomics. 2012;11(7):M111.013243. doi: 10.1074/mcp.M111.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamauchi T, Nakamura N, Hiramoto M, et al. Sepantronium bromide (YM155) induces disruption of the ILF3/p54(nrb) complex, which is required for survivin expression. Biochem Biophys Res Commun. 2012;425(4):711–716. doi: 10.1016/j.bbrc.2012.07.103. [DOI] [PubMed] [Google Scholar]
- 15.Tolcher AW, Mita A, Lewis LD, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26(32):5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh T, Okamoto I, Miyazaki M, et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15(11):3872–3880. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- 17.Giaccone G, Zatloukal P, Roubec J, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27(27):4481–4486. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 18.Iwasa T, Okamoto I, Takezawa K, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103(1):36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Gabrielsson J. Stockholm, Sweden: Swedish Pharmaceutical Press; 1994. pp. 107–114. Pharmacokinetic/pharmacodynamic data analysis: concepts and applications. pp. 9–40. [Google Scholar]
- 21.Ronaghi M, Uhlen M, Nyren P. A sequencing method based on real-time pyrophosphate. Science. 1998;281(5375):363. doi: 10.1126/science.281.5375.363. 365. [DOI] [PubMed] [Google Scholar]
- 22.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.