Abstract
Objective
Sphingomyelin Sphingolipid de novo biosynthesis is related with nonalcoholic fatty liver disease (NAFLD) or hepatic steatosis. However, the mechanism is still unclear. Sphingomyelin synthase (SMS), utilizing ceramide as one of the substrates to produce sphingomyelin, sits at the crossroads of sphingolipid biosynthesis. SMS has two isoforms: SMS1 and SMS2. SMS2 is the major isoform in the liver.
Approach and Results
To investigate the relationship between liver SMS2 activity-mediated sphingolipid changes and hepatic steatosis, we utilized two mouse models, SMS2 liver-specific transgenic (LTg) and SMS2 knockout (KO) mice. We found that SMS2LTg livers have lower ceramide and higher sphingomyelin, while SMS2 KO livers have higher ceramide and lower sphingomyelin. We also found that liver SMS2 overexpression promoted fatty acid uptaking and liver steatosis, while SMS2 deficiency had an opposite effect, in comparison with their respective controls. Importantly, the exogenous ceramide supplementation to Huh7 cells, a human hepatoma cell line, reduced the expression of PPAR γ2 and its target genes, CD36 and FSP27. PPAR γ reporter analysis confirmed this phenomenon. Moreover, PPARγ antagonist treatment significantly decreased triglyceride accumulation in SMS2LTg liver.
Conclusions
We attributed these effects to ceramide which can suppress of PPARγ2, thus reducing expression of CD36 and FSP27, and reducing liver steatosis. After all, SMS2 inhibition in the liver could diminish liver steatosis.
Keywords: SMS2 knockout mice, SMS2 liver-specific transgenic mice, Sphingolipids, Ceramide, Sphingomyelin, Liver lipids, Liver steatosis, proliferator-activated receptor γ (PPARγ) 2, CD36
There is a relationship between sphingolipid de novo biosynthesis and nonalcoholic fatty liver disease (NAFLD) or hepatic steatosis 1. However, the mechanism is still unclear, since many sphingolipids may be involved in the fatty liver formation. Sphingomyelin synthase (SMS), which utilizes ceramide as one of the substrates to produce sphingomyelin, sits at the crossroads of sphingolipid biosynthesis. Overexpression or blockage of SMS activity should influence not only sphingomyelin but also ceramide levels 2. SMS has two isoforms: SMS1 and SMS2. The major isoform in the liver is SMS2 2-3.
Ceramide is composed of sphingosine and a fatty acid. Ceramide can be generated through the de novo pathway 4. It also can be generated through the sphingomyelinase pathway, which breaks down sphingomyelin in the cell membranes and releases ceramide 5. Roles have been proposed for ceramide in heart disease, and it has been shown to induce apoptosis 6. Ceramide mediates an inflammatory response initiated by cytokines or oxidized LDL, a response that upregulates adhesion molecule expression and induces adhesion and migration of monocytes. These events are crucial in the initiation and progression of atherogenesis 7-8. Plasma ceramide may contribute to maladaptive inflammation in patients with coronary heart disease 9. It has been reported that plasma ceramide levels in apoE KO mice are higher than in controls 10. Plasma ceramides may also correlate with oxidized LDL, becoming a risk factor for atherosclerosis 10.
CD36 is a member of the class B scavenger receptor family, located on cell surface lipid rafts 11. CD36 expression was increased concomitantly with hepatic TG content in different animal models of liver steatosis 12-13. CD36 is regulated by PPARγ 14. PPARγ and CD36 mRNA expression was specifically up-regulated in high fat diet-induced liver steatosis in mice 15.
PPARγ is a member of a nuclear hormone superfamily that heterodimerize with the retinoid X receptor (RXR). These proteins are transcriptional regulators of genes encoding proteins involved in adipogenesis and lipid metabolism 16. PPARγ exists in three protein isoforms, PPARγ1, PPARγ2 and PPAR γ3, which are created by alternative promoter usage and alternative splicing at the 5′ end of the gene. PPARγ3 expression was restricted to colon and adipose tissue in man. PPARγ2 contains 30 additional amino acids at the N terminus compared with PPARγ1 17. PPARγ1 is expressed in many tissues, whereas significant PPARγ2 expression is limited in certain tissues, such as adipose tissues and the liver. Increased expression of either or both isoforms has been observed in livers of obese and insulin-resistant rodents 18-19. In fact, aberrant hepatic expression of PPARγ2 stimulates hepatic lipogenesis in a mouse model dealing with obesity, insulin resistance, dyslipidemia, and hepatic steatosis 20.
In this study, we specifically investigated diet-induced liver steatosis in both liver-specific SMS2 transgenic and SMS2 KO mice. We found that liver SMS2 overexpression promotes mouse liver steatosis, while SMS2 deficiency has opposite effect, in comparison with controls. We explored the potential mechanisms in this study.
Materials and Methods
Materials and Methods are available in online-only Supplement.
Results
Lipid analysis in SMS2LTg and SMS2 KO mice on chow diet
We utilized LC/MS/MS to measure liver sphingolipids and utilized enzymatic assay to measure liver total cholesterol, total phospholipid, triglyceride, and free fatty acid. As indicated in Table 1, livers from SMS2LTg mice contained significantly more sphingomyelin and less ceramide than controls (38% and 20%, P<0.01 and P<0.05), respectively, and livers from SMS2 KO mice contained significantly less sphingomyelin and more ceramide levels than controls (33% and 14%, P<0.01 and P<0.05), respectively. We also observed that liver free fatty acid levels were significantly increased in SMS2LTg (20%, P<0.05) and significantly decreased in SMS2 KO mice (18%, P<0.05). Triglyceride levels were significantly increased in SMS2LTg mice (50%, P<0.01), compared with controls. There was a decrease of triglyceride in SMS2 KO liver, but it was not statistically significant. There were no significant changes in total phospholipids and total cholesterol. All other liver sphingolipids have no noticeable difference (Supplemental Table I).
Table 1.
Mouse liver lipid measurement in SMS2 KO, SMS2LTg, and WT mice
| WT | SMS2LTg | WT | SMS2 KO | |
|---|---|---|---|---|
| Chow diet | ||||
| Cholesterol (μg/mg liver) | 1.0±0.1 | 1.2±0.6 | 0.8±0.2 | 0.7±0.1 |
| Phospholipids (μg/mg liver) | 2.7±0.3 | 3.2±0.4 | 2.9±0.3 | 2.7±0.2 |
| Triglyceride (μg/mg liver) | 10±2 | 15±3* | 11±3 | 9±2 |
| FFA (μmol/mg liver) | 7.1±0.6 | 8.5±0.4* | 7.9±1.1 | 6.5±0.3* |
| Sphingomyelin (μg/mg liver) | 0.8±0.2 | 1.1±0.1* | 0.9±0.1 | 0.6±0.1* |
| Ceramide (ng/mg liver) | 66±6 | 53±5* | 72±8 | 82±9* |
| High fat diet | ||||
| Cholesterol (μg/mg liver) | 3.0±0.7 | 3.3±0.4 | 3.0±0.6 | 2.7±0.3 |
| Phospholipids (μg/mg liver) | 5.1±0.3 | 5.3±0.6 | 5.4±0.5 | 5.1±0.2 |
| Triglyceride (μg/mg liver) | 25±5 | 38±8* | 21±4 | 11±3* |
| FFA (μmol/mg liver) | 11.4±1.8 | 14.1±2.0* | 12.0±2.5 | 9.2±2.1* |
| Sphingomyelin (μg/mg liver) | 1.5±0.3 | 2.0±0.2* | 1.6±0.3 | 1.2±0.2* |
| Ceramide (ng/mg liver) | 80±7 | 59±5* | 82±4 | 95±6* |
Value: mean±SD; n=5. FFA, free fatty acids.
P<0.05.
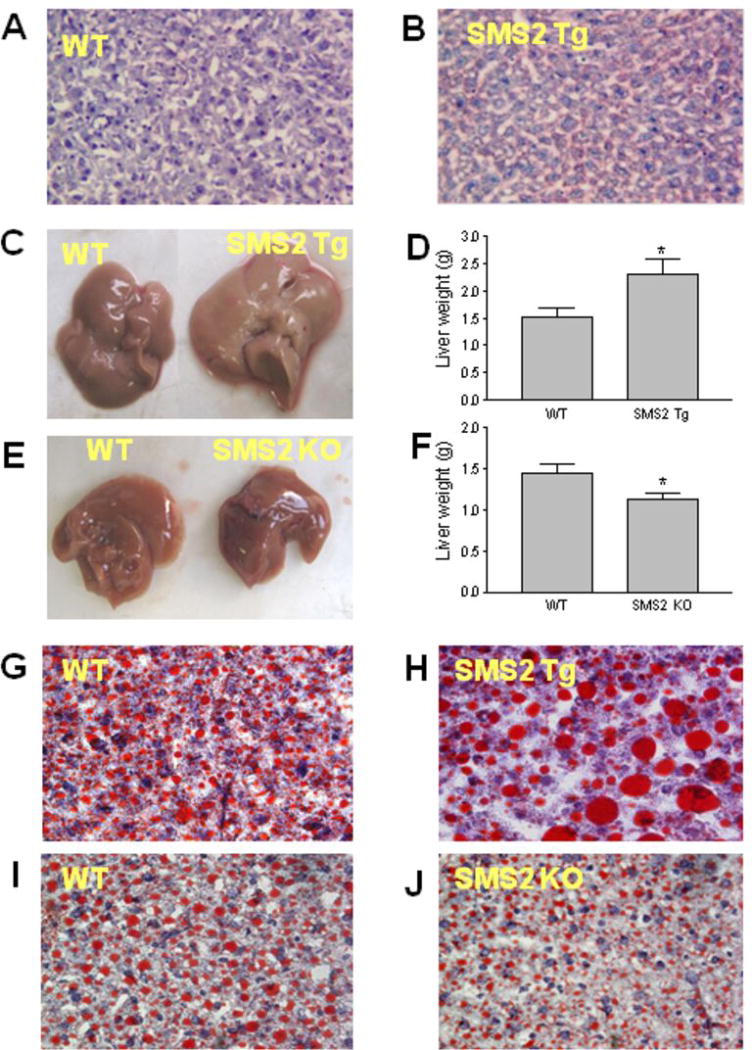
We next stained the liver section from SMS2LTg and control mice with Oil Red O and found that SMS2 overexpression in the liver promoted lipid accumulation (Figs. 1A and B, Supplemental Figs. IA-D). We performed insulin tolerance test on SMS2LTg and control mice and found that SMS2LTg mice, with lower liver ceramide levels, have lower insulin sensitivity (Supplemental Fig. II) than controls. Likewise, we reported previously that SMS2 deficient mice have higher insulin sensitivity 21.
Figure 1. The effect of SMS2 overexpression and deficiency on liver lipid accumulation.
Liver samples were embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek), frozen, and sectioned (7 μm), then were stained Oil Red O and hematoxylin. Sections were photographed at ×400 magnification. Panel A, liver from control mice on chow; Panel B, liver from SMS2LTg mice on chow. Panel C-J, All the mice were on a high fat high cholesterol diet for 8 weeks. Panel C-D, liver, from control and SMS2LTg mice, size comparison. Panel E-F, liver, from control and SMS2 KO mice, size comparison. Panel G, Control liver. Panel H, SMS2LTg mouse liver. Panel I, Control liver. Panel J, SMS2 KO mouse liver. This set of result is the representatives of 6 WT and 6 SMS2LTg mice, and 6 WT and 6 SMS2 KO mice. *P<0.05.
Liver SMS2 overexpression promotes, while liver SMS2 deficiency diminishes diet-induced steatosis in mice
We found that SMS2LTg mice had significantly larger (Figs. 1C and D), while SMS2 KO mice had smaller livers (Figs. 1E and F), compared with their respective controls, after 8-weeks high fat high cholesterol diet feeding. We also noticed that SMS2LTg liver had a milk-like color (Fig. 1C).
We further stained the liver frozen sections with Oil Red O, finding that SMS2 overexpression caused substantial lipid accumulation in the liver (Figs. 1G and H, Supplemental Figs. IE-H), while SMS2 deficiency had the opposite results (Figs. 1I and J; Supplemental Figs. II-L).
As indicated in Table 1, after a high fat feeding, the liver from SMS2LTg mice contained significantly higher (33%, P<0.01), while the liver from SMS2 KO mice contained significantly less sphingomyelin levels (25%, P<0.01), compared with controls. Liver triglyceride levels were significantly increased in SMS2LTg mice (52%, P <0.001) and significantly decreased in SMS2 KO mice (48%, P<0.001). Liver free fatty acid levels were significantly increased in SMS2LTg (24%, P<0.05) and significantly decreased in SMS2 KO mice (23%, P<0.05). There were no significant changes in total phospholipids and total cholesterol (Table 1).
We also measured sphingolipid levels using LC/MS/MS. As indicated in Table 1, liver ceramide levels were significantly decreased in SMS2LTg mice (26%, P<0.01) and significantly increased in SMS2 KO mice (16%, P<0.05), compared with controls. There were no significant changes of sphingosine, sphingosine-1-phosphate, and dihydroxyl-sphingosin-1-phosphate (data not shown).
We next sought to isolate plasma membranes from mouse liver with relatively pure quality (Supplemental Fig. III), and measured SM and ceramide as well as other sphingolipid levels, using LC/MS/MS. We found that SMS2 overexpression significantly increased, while deficiency decreased SM levels, compared with that of controls (Table 2). Moreover, we did not observe any significant changes of other sphingolipids, such as ceramide and sphingosine-1-phosphate (Table 2). Similar results were obtained for mice on a chow diet (Table 2).
Table 2.
Mouse liver plasma membrane lipid analysis by LC/MS/MS
| WT | SMS2LTg | WT | SMS2 KO | |
|---|---|---|---|---|
| Chow diet | ||||
| PC(nmole/mg) | 68±8 | 65±15 | 62±10 | 60±13 |
| SM(nmole/mg) | 26±7 | 37±6* | 22±5 | 12±3* |
| Ceramide(ng/mg) | 755±90 | 702±81 | 820±108 | 783±120 |
| DHCer(ng/mg) | 116±29 | 129±39 | 121±31 | 133±23 |
| GlyCer(ng/mg) | 291±42 | 246±35 | 231±52 | 266±44 |
| GM3(ng/mg) | 99±16 | 110±23 | 120±36 | 102±13 |
| High fat diet | ||||
| PC(nmole/mg) | 88±11 | 77±21 | 82±9 | 79±14 |
| SM(nmole/mg) | 33±9 | 51±8* | 35±6 | 22±5* |
| Ceramide(ng/mg) | 937±100 | 902±91 | 990±79 | 883±81 |
| DHCer(ng/mg) | 144±19 | 121±59 | 129±26 | 124±33 |
| GlyCer(ng/mg) | 308±51 | 292±41 | 330±61 | 289±50 |
| GM3(ng/mg) | 120±30 | 99±29 | 116±22 | 105±21 |
Sph, sphingosine; SM, sphingomyelin; PC, phosphatidylcholine; DHCer, dihydroceramide; Glycer, Glucosylceramide. Value: mean±SD; n=4-5.
P<0.05.
We have already characterized HDL and apoA-I levels in SMS2LTg and SMS2 KO mice. We did not observe HDL-C and apoA-I changes 2. We also measured ABCA1 levels on mouse liver homogenants using Western blot, and we did not find significant changes (Supplemental Fig. IV).
Ceramide, but not sphingomyelin or phosphatidylcholine can suppress PPARγ expression
To gain insight into the mechanisms of how SMS2 overexpression promotes and deficiency prevents the formation of fatty livers, we examined the expression levels of the responsible genes using real time quantitative PCR in the liver. We found that PPARγ2 was significantly elevated in high fat-fed SMS2 transgenic mice (2.1-fold, P<0.001), but suppressed in SMS2 KO mice (69%, P<0.001) (Table 3). Consequently, CD36 and FSP27, two downstream effecters of PPARγ 2 were dramatically increased in SMS2 transgenic mice (3.7-fold, P<0.0001, and 5.8-fold, P<0.0001) and decreased in SMS2 KO mice (48%, P<0.001, and 61%, P<0.001) (Table 3). We also found that diacylglycerol acyltransferase (DAGT) 1 and DAGT2 were significantly increased in SMS2 transgenic liver (Table 3). All other genes related to lipid metabolism had no statistically significant changes under SMS2 overexpression and deficiency (Table 3). Our observation suggested that PPARγ 2 is at least one of the key factors mediating SMS2 activity-related liver steatosis.
Table 3.
Hepatic gene expression in mice after high fat feeding.
| WT | SMS2LTg | WT | SMS2 KO | |
|---|---|---|---|---|
| TG and DAG hydrolysis | ||||
| HSL | 100±41 | 95±36 | 100±37 | 199±59 |
| ATGL | 100±31 | 136±27 | 100±43 | 109±37 |
| FFA oxidation | ||||
| AOX | 100±28 | 145±31 | 100±44 | 113±53 |
| CPT1 | 100±37 | 141±66 | 100±18 | 94±19 |
| PPARα | 100±37 | 149±42 | 100±28 | 188±54 |
| FFA uptake and transport | ||||
| PPARγ1 | 100±11 | 114±20 | 100±23 | 81±22 |
| PPARγ2 | 100±40 | 209±31* | 100±33 | 31±9* |
| CD36 | 100±21 | 370±56* | 100±29 | 52±15* |
| FSP27 | 100±27 | 582±102* | 100±20 | 39±14* |
| Lipid synthesis and storage | ||||
| FAS | 100±16 | 126±36 | 100±73 | 76±32 |
| SREBP1c | 100±11 | 74±10 | 100±43 | 135±60 |
| DGAT1 | 100±31 | 173±36* | 100±54 | 143±52 |
| DGAT2 | 100±45 | 183±41* | 100±58 | 159±37 |
| Mitochondrial bioenergetic/lipid metabolism | ||||
| UCP2 | 100±21 | 78±48 | 100±51 | 75±9 |
HSL, hormone-sensitive lipase; ATGL, adipose triglyceride lipase; AOX, acyl-CoA oxidase; CPT1, Carnitine palmitoyltransferase; PPAR, peroxisome proliferator-activated receptor; FAS27, Fat-specific protein of 27 kDa; FAS, fatty acid synthase; SREBP, sterol-responsive element-binding protein; DGAT, diacylglycerol acyltransferase; UCP1, uncoupling protein 1, or UCP1. Value: mean±SD; n=4. FFA, free fatty acids.
P<0.01.
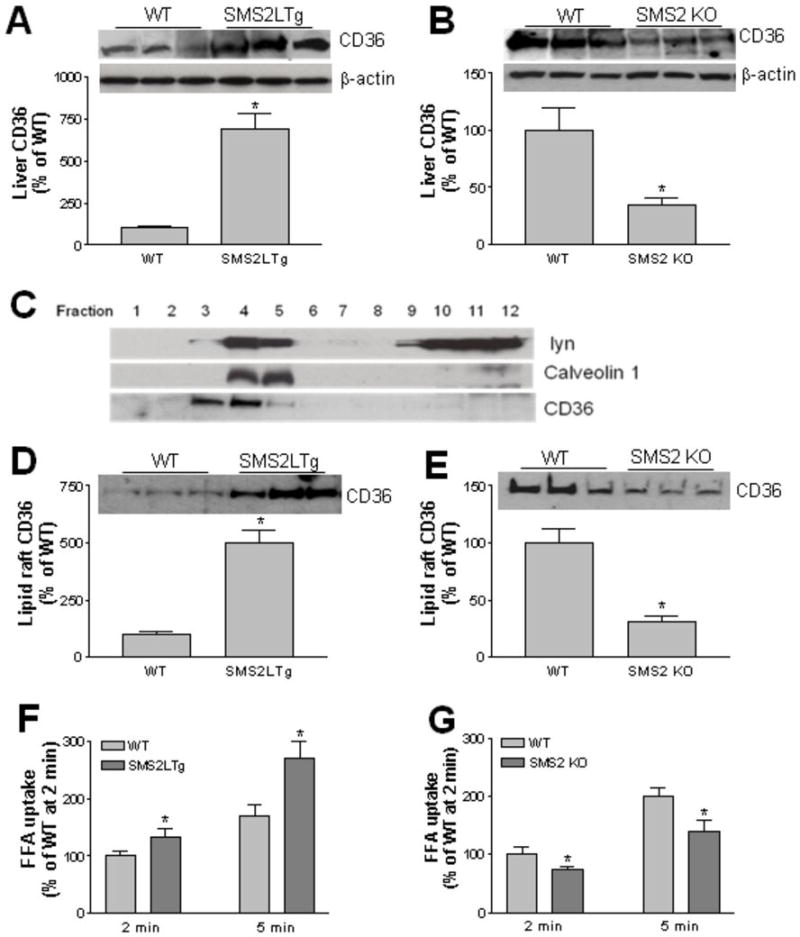
We next sought to examine protein levels of CD36 in SMS2LTg and SMS2 KO livers. We found that SMS2LTg liver had significantly higher CD36 protein levels (7-fold, P<0.001) (Fig. 2A), while SMS2 KO liver had significantly lower CD36 protein levels (70%, P<0.01) (Fig. 2B), compared with their controls, respectively.
Figure 2. SMS2 overexpression increases and deficiency decreases CD36 levels and FFA uptake.
Panel A-B, liver CD36 in homogenates was detected by Western blotting. Panel C – E, Lipid raft was isolated according to the method described in the text. Fractions (1 to 12) were collected from the top to the bottom after gradient centrifugation. Each fraction was used for the detection of Lyn kinase, caveolin-1, and CD36. Fractions 3 to 5 (lipid rafts) were pooled, and CD36 levels were detected by Western blotting. Panel F – G, free fatty acid uptake. Hepatocytes from SMS2LTg or SMS2 KO or WT mice were isolated and incubated with [14C]oleate, then the lipids were extracted using Hexanes/Isopropanol (3/2, v/v), radioactivity was analyzed by scintillation counting. Values are Mean ± SD., n=5, *P<0.01.
CD36 is located on plasma membrane lipid rafts 22. We then investigated how SMS2 overexpression or deficiency affected CD36 in lipid rafts. We isolated these rafts from the whole liver, using reported protocols 23-24. CD36 was found in light fractions enriched with the raft markers, Src kinase lyn and caveolin-1 (Fig. 2C). We pooled raft fractions (3-5) and nonraft fractions (10-12) to perform a CD36 Western blot. SMS2LTg liver raft regions contained significantly more CD36 (5-fold, P<0.001) (Fig. 2D), while SMS2 KO liver raft regions contained significantly less (72%, P<0.001) (Fig. 2E).
To further evaluate the effect of SMS2 liver overexpression or deficiency on CD36, we examined the free fatty acid uptake. Hepatocytes from SMS2LTg or SMS2 KO mice were isolated and incubated with [14C]Oleic acid. We found that SMS2LTg hepatocytes took up more free fatty acid (Fig. 2F), while SMS2 KO hepatocytes took up less (Fig. 2G), compared with their controls, respectively.
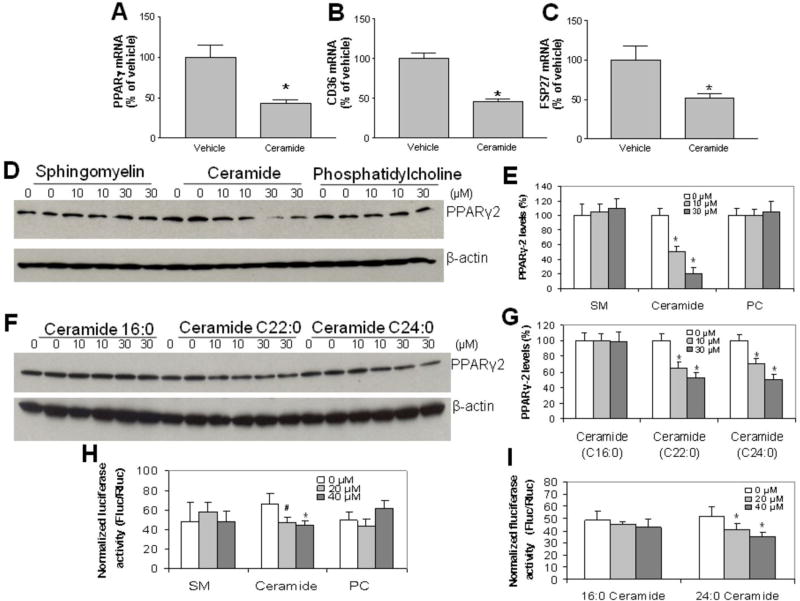
We noticed that SMS2 overexpression decreases ceramide and increases sphingomyelin in the liver homogenates, while SMS2 deficiency has opposite effects (Table 1). To directly examine the effect of lipid changes on PPARγ2, we examined whether exogenous ceramide supplementation to Huh7 cells, a hepatoma cell line, could stimulate PPARγ 2 suppression. For this, we treated the cells with ceramide (10 μM). As shown in Figure 3A, B, and C, the exogenous supplementation of ceramide in culture significantly reduced PPARγ2, CD36, and FSP27 mRNA levels. We also did PPARγ2 Western blot and found that the protein levels of PPARγ2 were suppressed in a dose dependant manner (Fig. 3D and E). We used the same concentration of sphingomyelin and phosphatidylcholine to treat Huh7 cells, but no significant changes were found, in terms of PPARγ2 protein levels (Fig. 3D and E). Moreover, we found that ceramide 22:0 and 24:0 but not 16:0 are suppressors of PPARγ2 (Fig. 3F and G).
Figure 3. The exogenous supplementation of ceramide in culture significantly reducesPPARγ2 protein levels.
Huh7 cells were incubated with exogenous 0, 10, 30 μM of sphingomyelin, ceramide or phosphatidylcholine for 24 h. Cellular PPARγ2, CD36 and FSP27 mRNA levels were measured by real-time PCR. Panel A, PPARγ2 mRNA levels. Panel B, CD36 mRNA levels. Panel C, FSP27 mRNA levels. Cellular PPARγ2 mass were measured by Western blotting. Panel D and E, PPARγ2 Western blot after treatment with exogenous 0, 10, 30 μM sphingomyelin, ceramide or phosphatidylcholine for 24 h. Panel F and G, PPARγ2 Western blot after treatment with exogenous 0, 10, 30 μM of ceramides C16:0, C22:0, and C24:0 for 24 h. Panel H and I, PPAR reporter assay. Huh7 cells were transfected with PPAR reporter, negative control or positive control, respectively. After 24 h of transfection, the cells were treated with exogenous sphingomyelin, ceramide or phosphatidylcholine for 24 h, and then dual-luciferase reporter assay was performed. Panel H, dual-luciferase reporter assay after treatment with exogenous 0, 20, 40 μM of sphingomyelin, ceramide or phosphatidylcholine for 24 h. Panel I, dual-luciferase reporter assay after treatment with exogenous 0, 20, 40 μM of ceramides C16:0 and C24:0 for 24 h. Values are Mean ± SD., n=5, *P<0.05, **P<0.01.
In order to confirm the suppression effect of ceramide on PPARγ, we performed PPARγ-reporter assay and found that ceramide, but not sphingomyelin and phosphatidylcholine, decreased luciferase activity (Fig. 3H). Moreover, we found that ceramide 24:0 but not 16:0 reduced luciferase activity in a dose dependant manner (Fig. 3I). These results demonstrated that ceramide can suppress PPARγ activity. Furthermore, we treated SMS2LTg mice with PPARγ antagonist (GW9662, 4 mg/kg by intraperitoneal injection) for four weeks, we found that triglyceride accumulation was significantly reduced, compared with vehicle treatment (30%, P<0.05, Supplemental Fig. V), suggesting that PPAR-γ is one of the factors for liver steatosis.
Discussion
SMS2 is the major SMS isoform in the liver. It contributes more than 70% of the liver total SMS activity 2. In this study, we demonstrated that 1) SMS2 overexpression decreases hepatic ceramide and increase sphingomyelin levels, while SMS2 deficiency has an opposite effect; 2) SMS2 overexpression increases while SMS2 deficiency decreases liver plasma membrane sphingomyelin levels; 3) liver SMS2 overexpression promotes while SMS2 deficiency prevents high fat diet-induced triglyceride and free fatty acid accumulation in the liver; 4) SMS2 overexpression induces hepatic PPARγ2 and its downstream genes, CD36 and FSP27 levels, while SMS2 deficiency reduces all these levels; 5) exogenous ceramide but not sphingomyelin or phosphatidylcholine suppresses PPARγ2, CD36, and FSP27 expression levels, and 6) PPARγ antagonist reduces triglyceride accumulation in mouse liver.
There is a relationship between sphingolipid de novo biosynthesis and hepatic steatosis 1. Pharmacological inhibition of serine palmitoyltransferase 25 or glucosylceramide synthase 26 or the genetic depletion of acid sphingomyelinase 27 reduces hepatic triglyceride levels in mice susceptible to the development of a fatty liver. However, the mechanism is still unknown and sometimes the results are incongruous. Previously, Mitsutake et al. have reported that SMS2 deficiency in mice diminishes the development of obesity and fatty liver, and they attributed this phenomenon to the reduction of sphingomyelin levels in cell membrane lipid rafts 28. We also reported that SMS2-deficiency-mediated reduction of plasma membrane sphingomyelin increases insulin sensitivity and decreases high fat-induced obesity 21. It is known that sphingomyelin levels in plasma membrane lipid rafts are important in mediating many important cell functions, such as insulin signaling 21, cholesterol efflux 29, inflammatory responses 3, 30, and lipid uptake and transportation. Plasma membrane sphingomyelin-mediated effect is still observed in this study. We showed in this study that SMS2LTg liver plasma membrane contained significant more sphingomyelin (Table 2) and significantly more amount of CD36 (Fig. 2D), while SMS2 KO liver plasma membrane contained significantly less sphingomyelin (Table 2) and CD36 (Fig. 2E).
However, there is one thing which could not be fully explained by lipid raft sphingomyelin changes: why do SMS2LTg livers have significantly higher and SMS2 deficient livers have significantly lower CD36 and FSP27, a well known protein involved in hepatocyte lipid droplet formation and liver steatosis 31, mRNA levels (Table 3). There must be an upstream mechanism which can upregulate both CD36 and FSP27 expression. Although Mitsutake et al. 28 had indicated that PPARγ, which is upstream of CD36 and FSP27, was decreased in SMS2 deficient mouse livers, they did not show why and how PPARγ was regulated under such conditions.
Does ceramide play an import role in mediating liver steatosis? Previous researchers have reported that high ceremide levels contribute to the development of NAFLD via multiple ways involved in insulin resistance, oxidative stress, inflammation, and apoptosis32. Yetukuri et al even demonstrated that in ob/ob mice, hepatic ceramide levels were strongly correlated with the degree of steatosis 33. However, what we found in this study was different from the study reported by Yetukuri et al 33. First of all, we showed that although SMS2 overexpression or deficiency does not change ceramide levels on liver plasma membrane (Table 2), SMS2LTg mice had significantly lower, while SMS2 KO mice have higher ceramide levels in liver homogenates (Table 1). The former had severe liver steatosis with (Fig. 1G-H, Supplemental Fig. IE-H) or without high fat diet feeding (Fig. 1A-B, Supplemental Fig. IA -D), while the latter had much less liver steatosis (Fig. 1 I-J, Supplemental Fig. I I-L). It is plausible that ceramide depletion in the liver causes steatosis, while ceramide accumulation has an opposite effect. Secondly, exogenous ceramide suppressed PPARγ 2 in a dose dependent fashion (Figs. 3D and E). We further confirmed this by using PPARγ reporter analysis (Fig. 3H). Thirdly, it has been reported that, in primary cultured adipocytes, ceramide treatment reduced PPARγ expression in a time and concentration dependent manner 34. It has also been reported that, in murine mesenchymal stem cells, ceramide treatment reduced PPARγ expression and reduced levels of both triglyceride and specific fatty acids 35. Recently, we reported that ceramide does not cause cardiac toxicity 36 and insulin resistance 21. As one of the potential mechanisms, we believe that ceramide can reduce liver steatosis through the direct suppression of PPARγ2 expression in the liver. Although the role of PPARγ in liver steatosis is still uncertain37, our PPARγ antagonist study (Supplemental Fig. 5), suggests PPAR-γ is one of the factors for liver steatosis.
In line with the association between low ceramide levels and NAFLD observed in our SMS2LTg mice, Deevska et al. reported that sphingomyelinase (which hydrolyzes sphingomyelin and produce ceramide) deficiency reduced hepatic steatosis and improved insulin sensitivity27. Bijl et al. reported that glucosylceramide synthase (which utilizes ceramide to produce glucosylceramide) inhibition markedly reduced liver steatosis in mice26. The linkage may be that ceramide can suppress PPARγ2 expression in the liver (Fig. 3).
Although PPARγ was once considered an orphan receptor, native and modified polyunsaturated fatty acids have emerged as strong candidates for endogenous activators of this receptor 38-40. However, so far no endogenous suppressors of PPARγ have been reported. We hypothesized that certain species of ceramide could be native lipid suppressors for PPARγ. Our results indicated that ceramide 22:0 and 24:0 but not 16:0 can suppress PPARγ2 in a Huh7 cell culture system (Fig. 3F, G and I). The detailed mechanism which causes this deserves further investigation.
Another possible mechanism linking between SMS2 activity and NAFLD might be related with cellular sphingomyelin levels. We have reported previously that SMS2 deficiency-mediated reduction of sphingomyelin in the plasma membranes leads to an improvement in tissue and whole body insulin sensitivity 21 and this might be associated with less liver steatosis in these mice. However, exogenous sphingomyelin had no effect on PPARγ2 expression (Fig. 3).
The importance of this study is the finding that increase of ceramide levels can prevent liver steatosis, through suppression of PPARγ2. Increasing ceramide levels in the liver can be achieved by SMS2 inhibition.
Supplementary Material
Significance.
Imbalances in input, oxidation, synthesis, and output of fatty acids could contribute to nonalcoholic fatty liver disease (NAFLD). There is a relationship between sphingolipid de novo biosynthesis and NAFLD. Sphingomyelin synthase (SMS) overexpression reduces liver ceramide level, thus inducing liver steatosis, while SMS2 deficiency has opposite effect. Current study is the first one indicates that ceramide can suppress of PPARγ2 and its target genes, CD36 and FSP27, thus diminishing liver steatosis. Regulation ceramide levels through manipulation of SMS activity could have a clinical impact on the treatment of NAFLD.
Acknowledgments
We thank Drs. David Peake, Hai H. Bui, and Youyan Zhang (Eli Lilly) for their valued support and technical guidance.
Sources of Funding
This work was supported by grants National Institute of Health HL69817 and VA Merit 000900-01.
Footnotes
Disclosures
No author reports any conflict of interest.
References
- 1.Bikman BT, Summers SA. Sphingolipids and hepatic steatosis. Adv Exp Med Biol. 2011;721:87–97. doi: 10.1007/978-1-4614-0650-1_6. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Zhang H, Li Z, Hailemariam TK, Chakraborty M, Jiang K, Qiu D, Bui HH, Peake DA, Kuo MS, Wadgaonkar R, Cao G, Jiang XC. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler Thromb Vasc Biol. 2009;29:850–856. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Fan Y, Liu J, Li Y, Quan C, Bui HH, Kuo MS, Park TS, Cao G, Jiang XC. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:1577–1584. doi: 10.1161/ATVBAHA.112.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merrill AH., Jr Characterization of serine palmitoyltransferase activity in chinese hamster overy cells. Biochimica et biophysica acta. 1983;754:284–291. doi: 10.1016/0005-2760(83)90144-3. [DOI] [PubMed] [Google Scholar]
- 5.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 6.Mallat Z, Tedgui A. Current perspective on the role of apoptosis in atherothrombotic disease. Circulation research. 2001;88:998–1003. doi: 10.1161/hh1001.090571. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee S. Sphingolipids in atherosclerosis and vascular biology. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:1523–1533. doi: 10.1161/01.atv.18.10.1523. [DOI] [PubMed] [Google Scholar]
- 8.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 9.de Mello VD, Lankinen M, Schwab U, Kolehmainen M, Lehto S, Seppanen-Laakso T, Oresic M, Pulkkinen L, Uusitupa M, Erkkila AT. Link between plasma ceramides, inflammation and insulin resistance: Association with serum il-6 concentration in patients with coronary heart disease. Diabetologia. 2009;52:2612–2615. doi: 10.1007/s00125-009-1482-9. [DOI] [PubMed] [Google Scholar]
- 10.Ichi I, Takashima Y, Adachi N, Nakahara K, Kamikawa C, Harada-Shiba M, Kojo S. Effects of dietary cholesterol on tissue ceramides and oxidation products of apolipoprotein b-100 in apoe-deficient mice. Lipids. 2007;42:893–900. doi: 10.1007/s11745-007-3067-z. [DOI] [PubMed] [Google Scholar]
- 11.Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. Fat/cd36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16:24–31. doi: 10.1091/mbc.E04-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buque X, Martinez MJ, Cano A, Miquilena-Colina ME, Garcia-Monzon C, Aspichueta P, Ochoa B. A subset of dysregulated metabolic and survival genes is associated with severity of hepatic steatosis in obese zucker rats. J Lipid Res. 2010;51:500–513. doi: 10.1194/jlr.M001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degrace P, Moindrot B, Mohamed I, Gresti J, Du ZY, Chardigny JM, Sebedio JL, Clouet P. Upregulation of liver vldl receptor and fat/cd36 expression in ldlr-/- apob100/100 mice fed trans-10, cis-12 conjugated linoleic acid. J Lipid Res. 2006;47:2647–2655. doi: 10.1194/jlr.M600140-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson AC, Hajjar DP. Cd36, oxidized ldl and ppar gamma: Pathological interactions in macrophages and atherosclerosis. Vascul Pharmacol. 2004;41:139–146. doi: 10.1016/j.vph.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, Suzuki Y, Saito H, Kohgo Y, Okumura T. Increased expression of ppargamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 16.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 17.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. Mppar gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 18.Memon RA, Tecott LH, Nonogaki K, Beigneux A, Moser AH, Grunfeld C, Feingold KR. Up-regulation of peroxisome proliferator-activated receptors (ppar-alpha) and ppar-gamma messenger ribonucleic acid expression in the liver in murine obesity: Troglitazone induces expression of ppar-gamma-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141:4021–4031. doi: 10.1210/endo.141.11.7771. [DOI] [PubMed] [Google Scholar]
- 19.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: A transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YL, Hernandez-Ono A, Siri P, Weisberg S, Conlon D, Graham MJ, Crooke RM, Huang LS, Ginsberg HN. Aberrant hepatic expression of ppargamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J Biol Chem. 2006;281:37603–37615. doi: 10.1074/jbc.M604709200. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Zhang H, Liu J, Liang CP, Li Y, Teitelman G, Beyer T, Bui HH, Peake DA, Zhang Y, Sanders PE, Kuo MS, Park TS, Cao G, Jiang XC. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol Cell Biol. 2011;31:4205–4218. doi: 10.1128/MCB.05893-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: Implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller G, Jung C, Wied S, Welte S, Jordan H, Frick W. Redistribution of glycolipid raft domain components induces insulin-mimetic signaling in rat adipocytes. Molecular and cellular biology. 2001;21:4553–4567. doi: 10.1128/MCB.21.14.4553-4567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Stralfors P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. Faseb J. 1999;13:1961–1971. [PubMed] [Google Scholar]
- 25.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bijl N, Sokolovic M, Vrins C, Langeveld M, Moerland PD, Ottenhoff R, van Roomen CP, Claessen N, Boot RG, Aten J, Groen AK, Aerts JM, van Eijk M. Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology. 2009;50:1431–1441. doi: 10.1002/hep.23175. [DOI] [PubMed] [Google Scholar]
- 27.Deevska GM, Rozenova KA, Giltiay NV, Chambers MA, White J, Boyanovsky BB, Wei J, Daugherty A, Smart EJ, Reid MB, Merrill AH, Jr, Nikolova-Karakashian M. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J Biol Chem. 2009;284:8359–8368. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsutake S, Zama K, Yokota H, Yoshida T, Tanaka M, Mitsui M, Ikawa M, Okabe M, Tanaka Y, Yamashita T, Takemoto H, Okazaki T, Watanabe K, Igarashi Y. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem. 2011;286:28544–28555. doi: 10.1074/jbc.M111.255646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Huan C, Chakraborty M, Zhang H, Lu D, Kuo MS, Cao G, Jiang XC. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ Res. 2009;105:295–303. doi: 10.1161/CIRCRESAHA.109.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hailemariam TK, Huan C, Liu J, Li Z, Roman C, Kalbfeisch M, Bui HH, Peake DA, Kuo MS, Cao G, Wadgaonkar R, Jiang XC. Sphingomyelin synthase 2 deficiency attenuates nfkappab activation. Arterioscler Thromb Vasc Biol. 2008;28:1519–1526. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]
- 31.Okumura T. Role of lipid droplet proteins in liver steatosis. J Physiol Biochem. 2011;67:629–636. doi: 10.1007/s13105-011-0110-6. [DOI] [PubMed] [Google Scholar]
- 32.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yetukuri L, Katajamaa M, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M. Bioinformatics strategies for lipidomics analysis: Characterization of obesity related hepatic steatosis. BMC Syst Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajita K, Mune T, Kanoh Y, Natsume Y, Ishizawa M, Kawai Y, Yasuda K, Sugiyama C, Ishizuka T. Tnfalpha reduces the expression of peroxisome proliferator-activated receptor gamma (ppargamma) via the production of ceramide and activation of atypical pkc. Diabetes Res Clin Pract. 2004;66(Suppl 1):S79–83. doi: 10.1016/j.diabres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Yang CC, Gomillion C, Burg KJ. Effect of ceramide on mesenchymal stem cell differentiation toward adipocytes. Appl Biochem Biotechnol. 2010;160:197–212. doi: 10.1007/s12010-008-8505-8. [DOI] [PubMed] [Google Scholar]
- 36.Lee SY, Kim JR, Hu Y, Khan R, Kim SJ, Bharadwaj KG, Davidson MM, Choi CS, Shin KO, Lee YM, Park WJ, Park IS, Jiang XC, Goldberg IJ, Park TS. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J Biol Chem. 2012;287:18429–18439. doi: 10.1074/jbc.M111.296947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ables GP. Update on ppargamma and nonalcoholic fatty liver disease. PPAR Res. 2012;2012:912351. doi: 10.1155/2012/912351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-delta 12, 14-prostaglandin j2 is a ligand for the adipocyte determination factor ppar gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 39.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin j2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 40.Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: At the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.