Abstract
Background
Although adjuvant radiotherapy (RT) following surgery for breast cancer improves overall survival, controversy exists about its long-term adverse impact on cardiovascular health in older survivors.
Aim
To determine whether incident cardiovascular disease (CVD) is associated with RT and whether tumor laterality modifies this association.
Methods
Women aged 65+ years diagnosed with stage I and II breast cancer between 1990 and 1994 were identified from three health plans. Women were followed through CVD outcomes, health plan disenrollment, death, or study end (December 31, 2004). The main independent variable was RT use. Adjusted HRs and 95% CIs were estimated using Cox proportional hazards models with time-dependent tamoxifen and RT use status. We adjusted for age, race, stage, estrogen receptor/progesterone receptor, hypertension, and diabetes.
Results
In the full cohort (N = 806), RT was not associated with greater risk of CVD (maximum follow-up was 14 years). However, within the RT-exposed group (N = 340), women treated for left-side breast cancer had a significant increased risk of CVD outcomes (HR = 1.53, 95% CI: 1.06–2.21) compared with women with right-sided tumors.
Conclusion
Laterality is critical to understanding the effect of RT on CVD. Studies of more contemporary cohorts of women treated with RT should incorporate this variable to determine whether the risk persists with refinements in the dosing and delivery of RT.
Impact
As some irradiation to the heart is unavoidable even with refined modern RT techniques, continued effort is required to minimize such exposures, especially in older women with left-sided tumors.
Introduction
In the last quarter century, survival after breast cancer has markedly increased because of advances in earlier detection, hormonal treatments, and chemotherapy and improvements in radiation therapy (RT). Almost 75% of breast cancer patients worldwide are treated with adjuvant therapy, primarily RT. Despite the reduction in local breast cancer recurrence and related mortality reductions due to RT (1,2), vascular damage can also occur (3). Although data from clinical trials (1) and observational studies (4) have highlighted how this vascular damage may lead to symptomatic disease, the extent and nature of these late effects remain unclear due to discrepancies in prior reports. For example, conflicting results exist about the risk of cardiovascular disease (CVD) by laterality of breast cancer. Some reports suggest that women with left-sided breast cancer who received adjuvant RT are at greater risk of CVD including stroke due to increased radiation exposure to the heart (5–11) or to the carotid regions (12–15), whereas others found no differences in these outcomes due to laterality, possibly due to shorter study observation time (16, 17). Although current RT techniques have reduced, but not eliminated, incidental cardiac irradiation, heart and vascular diseases are typically found many years after RT (18). Hence, the long-term effects of RT on the cardiovascular system are not well understood (5). The rationale for evaluating stroke is that the carotid arteries may be included in the radiation fields among women with node-positive breast cancer (12, 14).
Furthermore, changes in RT technique over time and methodologic issues seem to account for the mixed research results (2, 6). Recent studies of RT (conducted in the late 1980s forward; refs. 5, 18) have not been able to include patients with adequate follow-up time and therefore are not reassuring about the long-term safety of RT (5, 6). In addition, older studies that show cardiovascular effects often include patients treated with earlier and perhaps outdated techniques. Beyond this, the 2 most common limitations in observational studies include difficulty in ascertaining accurate treatment information and separating RT effects from chemotherapy agents that also affect vascular disease. Single-institution studies can carefully characterize treatment but have limited generalizability due to patient selection and a lack of variability in RT technique. Surveillance, Epidemiology, and End Results (SEER)-Medicare population-based studies possess less detailed treatment and medical history data and, because of frequent reliance on death certificates to identify late effects, most have commonly examined mortality rather than incidence of CVD (4). In addition, although clinical trials are invaluable for their primary goal of assessing treatment efficacy, such studies tend to include younger, healthier women; however, older women probably are at greater risk given age-related vascular changes and associated comorbidities.
In contrast, our Breast Cancer Treatment in Older Women (BOW) I study represents a novel opportunity to overcome many of these limitations. Specifically, BOW I is a population-based study of women whose treatments were comparable over time and detailed treatment and comorbidity data were collected through medical record reviews, which reduced the impact of potentially strong confounders. Thus, our goal was to determine whether cardiovascular morbidity is associated with RT among early-stage breast cancer survivors 65 years or older and determine whether laterality of the breast cancer treatment modifies this risk.
Participants and Methods
Design and study population
We conducted a cohort study of women 65 years or older who were diagnosed with early-stage breast cancer (American Joint Committee on Cancer TNM stage I, IIA, or IIB) from January 1, 1990, through December 31, 1994, and followed through December 31, 2004. These women were participants of the BOW I study. The study group was identified through the population-based cancer registries of the following 3 health plans: Kaiser Permanente, southern California; Group Health, western Washington; and Henry Ford Health System, Detroit, MI. The California, Washington, and Michigan registries are affiliated with the National Cancer Institute’s SEER program. The number of women identified from these health plans who met the study eligibility was 1,457 [did not have concurrent bilateral breast cancer, no personal history of cancer (except nonmelanoma skin cancer)]. For this analysis, we further excluded women with a history of CVD prior to their breast cancer diagnosis (n = 192), those who developed subsequent breast cancer (n = 267), received chemotherapy (n = 83) as such women would be at greater risk for CVD, or those who underwent BCS without radiation because they were systematically different from the analytic group at baseline (n = 93; such women were much older, had greater comorbidity, and were more likely to be from minority groups). Finally, we also excluded women who did not complete their adjuvant RT sessions (n = 16). This left 806 women for the analysis. The study protocol was reviewed and approved by the Institutional Review Boards at each participating site. A detailed description of the sampling and data collection protocols has been published elsewhere (BOW I study; ref. 19). The distribution of characteristics in this study sample was similar to that of the whole BOW I cohort in terms of age, demographics, and breast cancer treatment (19).
Identification of cardiovascular outcomes
The study outcomes included those CVD events that were serious enough to require hospitalization (ischemic heart disease, including acute myocardial infarction and angina, CVD, or stroke anytime following their initial breast cancer diagnosis). Electronic inpatient encounter codes (hospital discharge diagnoses) were used to identify these potential events. The Appendix includes the list of International Classification of Diseases (ICD) Version 9 and 10 codes for the study-specific outcomes. Outcome definitions were based on studies that assessed the validity of these ICD-9 and ICD-10 codes (20–23) and those used for Healthcare Effectiveness Data and Information Set (HEDIS) measures (24). Cases were identified by the first occurrence of a primary diagnosis of a study outcome. Women with multiple primary diagnoses on the same day were assigned an outcome based on a priority scale. This algorithm was developed in conjunction with a study cardiologist to assign an outcome to cases with multiple outcomes on the same day: 1, acute myocardial infarction; 2, stroke; 3, congestive heart failure; 4, cardiomyopathy; 5, dysrhythmia; 6, valvular dysfunction; 7, angina; and 8, pericarditis. Women were categorized as having the outcome (presence/absence) in the analysis if they developed any of these conditions during follow-up. Noncases (CVD free) included women in the cohort who had no evidence of these cardiovascular conditions.
Covariates and breast cancer treatment information
We gathered breast cancer treatment, tumor characteristics, demographic, and CVD risk factor (diabetes and hypertension) information from the medical charts. Age and race/ethnicity were collected at baseline. Data on risk factors for CVD (hypertension and diabetes mellitus) were collected 1 year prior to breast cancer diagnosis. Other covariates included stage of diagnosis, year of diagnosis, estrogen receptor (ER) status, progesterone receptor (PR) status, laterality (side of invasive tumor), and health plan site. Primary and adjuvant breast cancer treatment included surgery, tamoxifen use, and completion of the first course of RT (i.e., the patient completed all the RT sessions prescribed).
Statistical analyses
Follow-up commenced on the date women completed their first course of RT (1990–1994) and ended on the date of the CVD event, death, termination of health plan membership, or study end (December 31, 2004), whichever occurred first. Thus, the maximum follow-up time was 14 years. The definition of continuous health plan enrollment allowed for gaps of up to 60 days in enrollment during the study period, as these were likely to be administrative gaps. Completed RT was the exposure variable (yes/no). Differences in demographic and tumor characteristics by RT status were first examined by comparing χ2 or Fisher exact tests. We also estimated crude rates of CVD by RT use and laterality. HRs and 95% CIs were estimated using Cox models with time-dependent tamoxifen and RT (i.e., 0 up to start date; 1 after start date) and adjusted for the variables described earlier. In this way, the person-time accrued before RT contributed to the “no” RT group; after starting RT, the person-time contributed to the “yes” RT group.
We examined crude HRs and also evaluated additional multivariable models. One adjusted model included all covariates (fully adjusted), based on bivariate analyses and clinical relevance, whereas the other model included a subset of variables (parsimonious model). In addition, we examined the probability of CVD-free survival using Kaplan–Meier methods, with P values based on log-rank tests.
Results
The final study cohort consisted of 806 women (381 CVD event women and 425 CVD-free women) followed for a maximum of 14 years. Of the 425 CVD-free women, 75 died (18%) during the 14-year follow-up. Very few women were loss to follow-up [of 806 women, 52 (6.5%) disenrolled from the health plan]. The disenrollment was nondifferential by RT status, as roughly equal numbers of women left the health plans in each group. Table 1 shows the baseline characteristics of the BOW I women by RT status. The use of RT decreased substantially with age (P = 0.0011). In addition, RT use was greater among white non-Hispanic women (P = 0.0007), those diagnosed with stage I disease (P < 0.0001), and those with positive hormone receptors (P = 0.003 for ER+ and 0.0421 for PR+). Laterality of the tumor was not significantly related to RT use (P = 0.0599). Very few women who underwent mastectomy received ipsilateral RT (3%, 16 of 482). Those who received adjuvant tamoxifen treatment were less likely to undergo RT (P < 0.001). Roughly half (48%) of the women had hypertension and 10% had diabetes, although the prevalence of these did not differ by RT status.
Table 1.
Baseline characteristics of older breast cancer survivors by radiotherapy use
| RT |
No RT |
Total |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| N | 340 | 466 | 806 | 100 | ||
| Age, y | ||||||
| 65–69 | 132 | 38.82 | 144 | 30.91 | 276 | 34.24 |
| 70–74 | 107 | 31.47 | 139 | 29.83 | 246 | 30.52 |
| 75–79 | 57 | 16.76 | 72 | 15.45 | 129 | 16 |
| 80+ | 44 | 12.94 | 111 | 23.82 | 155 | 19.23 |
| P = 0.0011 | ||||||
| Year of diagnosis | ||||||
| 1990 | 57 | 16.76 | 92 | 19.74 | 149 | 18.49 |
| 1991 | 56 | 16.47 | 96 | 20.60 | 152 | 18.86 |
| 1992 | 67 | 19.71 | 99 | 21.24 | 166 | 20.60 |
| 1993 | 78 | 22.94 | 97 | 20.82 | 175 | 21.71 |
| 1994 | 82 | 24.12 | 82 | 17.60 | 164 | 20.35 |
| P = 0.113 | ||||||
| Race | ||||||
| White, non-Hispanic | 287 | 84.41 | 343 | 73.61 | 630 | 78.16 |
| Hispanic | 8 | 2.35 | 35 | 7.51 | 43 | 5.33 |
| African American | 35 | 10.29 | 61 | 13.09 | 96 | 11.91 |
| Asian/Pacific Islander | 10 | 2.94 | 26 | 5.58 | 36 | 4.47 |
| P = 0.0007 | ||||||
| Stage | ||||||
| I | 262 | 77.06 | 237 | 50.86 | 499 | 61.91 |
| II | 78 | 22.94 | 229 | 49.14 | 307 | 38.09 |
| P < 0.0001 | ||||||
| Laterality | ||||||
| Right | 140 | 41.18 | 223 | 47.85 | 363 | 45.04 |
| Left | 200 | 58.82 | 243 | 52.15 | 443 | 54.96 |
| P = 0.0599 | ||||||
| Estrogen status | ||||||
| Positive | 270 | 91.22 | 334 | 83.5 | 604 | 86.78 |
| Negative | 26 | 8.78 | 66 | 16.5 | 92 | 13.22 |
| P = 0.0030 | ||||||
| Progesterone status | ||||||
| Positive | 223 | 76.9 | 269 | 69.87 | 492 | 72.89 |
| Negative | 67 | 23.1 | 116 | 30.13 | 183 | 27.11 |
| P = 0.0421 | ||||||
| Primary therapy | ||||||
| BCS | 324 | 95.29 | 0 | 0.00 | 324 | 40.20 |
| Mastectomy | 16 | 4.71 | 466 | 100.00 | 482 | 59.80 |
| P < 0.0001 | ||||||
| Tamoxifen | ||||||
| Yes | 188 | 55.29 | 321 | 68.88 | 509 | 63.15 |
| No | 152 | 44.71 | 145 | 31.12 | 297 | 36.85 |
| P < 0.0001 | ||||||
| Comorbidities at baseline | ||||||
| Hypertension | ||||||
| Yes | 154 | 45.29 | 229 | 49.14 | 383 | 47.52 |
| No | 186 | 54.71 | 237 | 50.86 | 423 | 52.48 |
| P = 0.2800 | ||||||
| Diabetes | ||||||
| Yes | 26 | 7.65 | 53 | 11.37 | 79 | 9.80 |
| No | 314 | 92.35 | 413 | 88.63 | 727 | 90.20 |
| P = 0.0789 | ||||||
Table 2 displays follow-up by breast cancer laterality among the 340 women exposed to RT. Overall, the median follow-up time following RT was similar in women with left- and right-sided tumors (about 10 years), and the range was 0 to 14 years.
Table 2.
Follow-up among the 340 women exposed to RT by breast cancer laterality
| Left | Right | |
|---|---|---|
| Follow-up, y | ||
| Mean | 7.4 | 7.9 |
| Median | 9.4 | 10.0 |
| Range | 0–14.0 | 0–14.0 |
Crude rates and overall and adjusted HRs stratified by RT and laterality are given in Table 3. The fully adjusted model included all variables that were significantly associated with RT use in Table 1 or clinically relevant. Furthermore, this model included diabetes and hypertension, which were strongly associated with CVD (P < 0.0001 for both, data not shown). The parsimonious adjusted model excluded certain variables (year of diagnosis, health plan, and stage). Removal of these specific variables did not change the effect measures. Furthermore, we did not find an association when comparing CVD risk among women treated with and without RT [Table 3 (models 1 and 2)]. In contrast, within the RT-exposed subset (N = 340), women treated for cancer of the left breast were 53% more likely to develop CVD [adjusted HR = 1.53, 95% CI: 1.06–2.21; Table 3 (model 3, parsimonious model)] than women treated with RT for the right breast. As expected, we found no difference in CVD risk in left- versus right-sided breast cancer among women who did not receive RT [adjusted HR = 1.14, 95% CI: 0.88–1.41; Table 3 (model 4)]. In addition, we evaluated CVD-free probability in the subset of 340 women exposed to RT by breast cancer laterality. As shown in Figure 1, women with left-sided tumors had significantly lower CVD-free survival probability than women with right-sided tumors (P = 0.0296).
Table 3.
Overall and adjusted HRs for CVD according to RT at baseline and stratified by laterality
| RT and laterality | Total, n | With CVD, n | Woman-years | Crude rates (per 1,000 woman-years) |
Crude HR (95% CI) |
Adjusted HRa (95% CI) |
Adjusted HRb (95% CI) |
|---|---|---|---|---|---|---|---|
| Radiation yes, left side vs. |
443 | 88 | 1,441 | 61.07 | 0.86 (0.65–1.14) | 0.97 (0.72–1.31) | 1.00 (0.75–1.35) |
| Radiation no, left side |
125 | 1,564 | 79.92 | 1.00 (ref) | ref | ||
| Radiation yes, right side vs. |
363 | 48 | 1,094 | 43.88 | 0.63 (0.45–0.88) | 0.73 (0.52–1.05) | 0.75 (0.53–1.06) |
| Radiation no, right side |
120 | 1,542 | 77.82 | 1.00 (ref) | ref | ||
| Radiation yes, left side vs. |
340 | 88 | 1,441 | 61.07 | 1.42 (0.99–2.03) | 1.54 (1.07–2.23) | 1.53 (1.06–2.21) |
| Radiation yes, right side |
48 | 1,094 | 43.88 | 1.00 (ref) | ref | ||
| Radiation no, left side vs. |
466 | 125 | 1,564 | 79.92 | 1.03 (0.80–1.32) | 1.17 (0.91–1.51) | 1.14 (0.88–1.47) |
| Radiation no, right side |
120 | 1,542 | 77.82 | 1.00 (ref) | ref |
Fully adjusted model includes age, year, and stage of breast cancer diagnosis, race, ER/PR status, health plan, diabetes, and hypertension.
Parsimonious model is adjusted for the same variables excluding year of diagnosis, health plan, and breast cancer stage at diagnosis.
Figure 1.
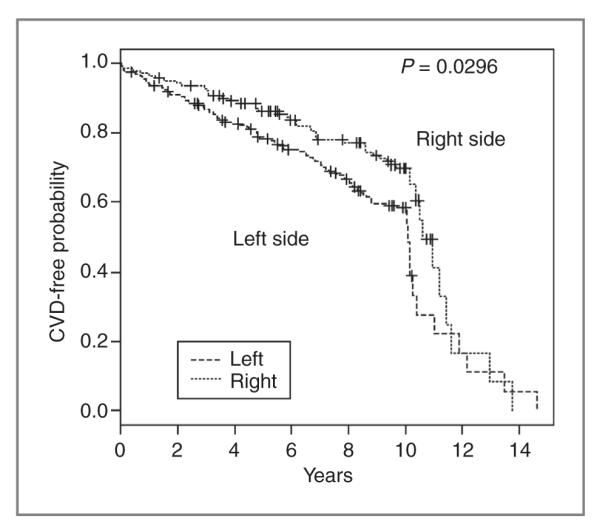
Probability of CVD-free survival among those exposed to radiation treatment by breast cancer laterality (n = 340)
Discussion
In this study of more than 800 women treated in 3 health care delivery systems nationwide, we found that risk of CVD differed by laterality of breast cancer. Among older women exposed to RT, those with left-sided tumors were 53% more likely to develop CVD up to 14 years posttreatment (adjusted HR = 1.53, 95% CI: 1.06–2.21). Our results show that it is important to stratify by laterality of breast cancer to understand the effect of RT on these outcomes. Radiation-induced heart damage is primarily caused by lesions to the microvasculature or by conduction abnormalities and arrhythmias related to autonomic dysfunction (3). The biological mechanism for these damages is not clear but involves a series of inflammatory reactions and formation of fibrous tissue in the heart and vascular system (6).
Our study has a number of strengths. Data for this investigation were derived from a medical record review–based study and not just from electronic clinical data. Our study could overcome a number of limitations of previous studies. To minimize confounding, we excluded women who underwent chemotherapy and therefore would be at increased risk of cardiac outcomes. We also excluded women who received breast-conserving surgery only who were sicker and more likely to have subclinical CVD. Participants for this study were drawn from 3 community-based integrated health care delivery organizations and their care should reflect the general cancer treatment patients receive in other delivery systems in the United States. In addition, we included information on hypertension and diabetes, two of the most common and strongest risk factors for CVD. Furthermore, retention was excellent in the health plans as few women disenrolled; only 52 of 806 women (6.5%) were lost to follow-up (disenrollment was nondifferential by RT status).
Certain limitations of the study must also be considered. Although we did not examine radiation dose, the exposure assessment period was short (RT between 1990 and 1994), thus reflecting a period when radiation techniques were similar over the years. Capturing radiation dose information from the medical charts was incomplete in this study. Although these exposures occurred nearly 20 years ago when RT regimens may have been different from those used in the current practice, examination of these CVD health effects require long observation periods. Despite the small sample sizes in the RT-exposed group (n = 340), we found a statistically significant increased risk of CVD when examining the HRs by laterality [Table 3 (model 3)]; these results were supported by our evaluation of disease-free probability using Kaplan–Meier methods (Fig. 1). In addition, although we did not examine other known risk factors for CVD such as body mass index and smoking, these behavioral factors likely operate through hypertension and diabetes mellitus; thus, our adjustment for these comorbidities accounts for the majority of the contribution of these variables. In our new study (BOW II), which was designed to examine the late effects of cancer treatments, we will extend follow-up (up to a maximum of 20 years post–breast cancer diagnosis) and collect a wider set of CVD risk factors.
Our results are consistent with findings from several earlier studies (4, 16, 25). Although other studies have not found an association between laterality of RT and CVD risk, this may be due to inclusion of younger women in their studies (12, 22) and shorter follow-up times (15) or use of differing definitions of outcomes. In our study, our definitions for the CVD outcomes relied on previously validated ICD-9 and ICD-10 codes (20–23) and on the health care system codes used for HEDIS measures (24). Indeed, recent articles warn clinicians that some irradiation to the anterior part of the heart is unavoidable even with refined modern RT techniques and that continued effort is required to minimize such exposures (5, 6, 26–30). For older women with cancer of the left breast, RT may increase CVD risk. Very few studies provide rates of CVD by left versus right breast cancer by age; therefore, we cannot make direct comparisons. However, in Table 3 (model 3), the HRs are consistent with published findings. That is, among women exposed to RT, the risk of CVD is about 40% to 50% greater in women with radiation to the left side than those with radiation to the right side. For example, a SEER-based study found that the heart disease mortality ratio in 60- to 69-year-old women was 1.40 (95% CI: 1.15–1.70; ref. 26). Although this cited article evaluated mortality, whereas we examined disease incidence, our results are consistent with results of the SEER-based study. In addition, in this managed care population, we also determined that women who were exposed to RT had a lower risk of CVD. Our hypothesis for this finding is that women who received RT were systematically different (healthier) from women who do not receive RT in these health plans. This is supported by our results in Table 1; in terms of proportions, women with hypertension were less likely to receive RT than those without hypertension (45% vs. 55%). Likewise, women with diabetes were less likely to receive RT than those without diabetes (7% vs. 93%). Other studies based on the SEER population have also encountered selection bias issues and have therefore focused their analyses on subsets of women exposed to RT (and excluded those exposed to chemotherapy; refs. 15, refs. 16). To be consistent with those studies, we similarly focused our main analyses (Table 3 and Fig. 1) on the subset of women exposed to RT. Future outcome studies of more contemporary cohorts of women treated with RT should incorporate laterality to determine whether the risk persists with refinements in the dosing and delivery of RT.
Acknowledgments
We thank the research support teams in each of the participating sites. This study was conducted under the auspices of the HMO Cancer Research Network (CRN), a consortium of 14 integrated heath care delivery systems with more than 11 million enrollees nationwide: (i) Fallon Community Health Plan, Meyers Primary Care Institute; (ii) Geisinger Health System; (iii) Group Health; (iv) Harvard Pilgrim Health Care Institute and Harvard Medical School; (v) HealthPartners; (vi) Henry Ford Hospital and Health System; (vii) Kaiser Permanente Colorado; (viii) Kaiser Permanente Georgia; (ix) Kaiser Permanente Hawaii; (x) Kaiser Permanente Northern California; (xi) Kaiser Permanente Northwest; (xii) Kaiser Permanente Southern California; (xiii) Lovelace Health System; and (xiv) Marshfield Clinic. The overall goal of the CRN, and the NCI initiative under which it was funded, is to use this consortium to conduct research on cancer prevention, early detection, treatment, long-term care, surveillance, and cancer communication and dissemination and implementation research (U19 CA079689; principal investigator: Ed Wagner). The authors also thank Ms. Nora Kasparian for assistance with the manuscript.
Grant Support This work was supported by NCI grants R01CA093772, R01CA093772-05A2, and U19CA79689 and Kaiser Permanente Southern California.
Appendix.
| CVD/stroke endpoints |
ICD-9 codes | ICD-10 codes |
|---|---|---|
| Ischemic heart disease | ||
| Acute myocardial infarction | 410.X1 (Inpatient) | I21.X–I22.X |
| 410.X (NDI) | ||
| Angina pectoris | 411.XX, 413.XX, 414.XX | I20.X, I24.X–I25.X |
| Other heart disease | ||
| Pericarditis | 420.XX, 423.XX | I30.X–I32.X, I23.X |
| Valvular dysfunction | 424.XX | I34.X–I39.X |
| Cardiomyopathy | 425.0X, 425.1X, 425.3X, 425.4X, 425.8X, 425.9X | I42.X–I43.X |
| Dysrhythmia | 427.XX | I46.X–I49.X, R001 |
| Congestive heart failure | 428.XX | I50.X |
| Stroke | 430.XX, 431.XX, 432.XX, 434.XX, 436.XX | I60.X–I62.X, I64.X, I66.X |
Priority assignment for cases with multiple diagnoses on same day:
Acute myocardial infarction
Stroke
CHF
Cardiomyopathy
Dysrhythmia
Valvular dysfunction
Angina
Pericarditis
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Early Breast Cancer Trialists Collaborative Group Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and15-year survival: an overview of randomized trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, et al. American Society of Clinical Oncology Clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 3.Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174:865–9. doi: 10.1667/RR1862.1. [DOI] [PubMed] [Google Scholar]
- 4.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–6. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 5.Demerici S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation induced cardiac toxicity after therapy for breast cancer: Interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys. 2009;73:980–7. doi: 10.1016/j.ijrobp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Senkus-Konefka E, Jassem J. Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. 2007;33:578–93. doi: 10.1016/j.ctrv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Stewart H, Rutquist L, Houghton J, Edwards R, Redmond C, et al. Cause specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–53. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 8.Jones JM, Ribeiro GG. Mortality patterns over 34 years of breast cancer patients in a clinical trial of post-operative radiotherapy. Clin Radiol. 1989;40:204–8. doi: 10.1016/s0009-9260(89)80099-6. [DOI] [PubMed] [Google Scholar]
- 9.Paszat LF, Mackillop WJ, Groome PA, Boyd C, Schulze K, Holowaty E. Mortality from myocardial infarction after adjuvant therapy for breast cancer in the surveillance, epidemiology, and end results cancer registries. J Clin Oncol. 1998;16:2625–31. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 10.Roychoudri R, Robinson D, Putcha V, Cuzick J, Darby S, Moller H. Increased cardiovascular mortality more than 15 years after radiotherapy for breast cancer: a population based study. BMC Cancer. 2007;7:9. doi: 10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early stage breast cancer. J Clin Oncol. 2007;25:3031–7. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 12.Jagsi R, Griffith KA, Koelling T, Roberts R, Pierce LJ. Stroke rates and risk factors in patients treated with radiation therapy for early-stage breast cancer. J Clin Oncol. 2006;24:2779–85. doi: 10.1200/JCO.2005.04.0014. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson G, Holmberg L, Garmo H, Terent A, Blomqvist C. Radiation to supraclavicular and internal mammary lymph nodes in breast cancer increases the risk of stroke. Brit J Cancer. 2009;100:811–6. doi: 10.1038/sj.bjc.6604902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson G, Holmberg L, Garmo H, Terent A, Blomqvist C. Increased incidence of stroke in women with breast cancer. Eur J Cancer. 2005;41:423–9. doi: 10.1016/j.ejca.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, Buchholz TA, et al. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23:7475–82. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- 16.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–24. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle JT, Neugut AI, Jacobson JS, Wang J, McBride R, Grann A, et al. Radiation therapy, cardiac risk factors and cardiac toxicity in early stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68:82–93. doi: 10.1016/j.ijrobp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Lee LJ, Harris JR. Innovations in radiation therapy for breast cancer. Breast. 2009;18:S103–11. doi: 10.1016/S0960-9776(09)70284-X. [DOI] [PubMed] [Google Scholar]
- 19.Enger SM, Thwin SS, Buist DS, Field T, Frost F, Geiger AM, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–83. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004;160:1152–8. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 21.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 22.Hooning MJ, Dorresteijn LD, Aleman BM, Kappelle AC, Klijn JG, Boogerd W, et al. Decreased risk of stroke among 10-year survivors of breast cancer. J Clin Oncol. 2006;24:5388–94. doi: 10.1200/JCO.2006.06.5516. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–6. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 24.National College for Quality Assurance [cited 2011 Feb 1];HEDIS Quality Measurements, Technical Resources. Available from: http://www.ncqa.org/tabid/1223/Default.aspx.
- 25.Jagsi R, Griffith KA, Koelling T, Roberts R, Pierce LJ. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer. 2007;109:650–7. doi: 10.1002/cncr.22452. [DOI] [PubMed] [Google Scholar]
- 26.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 27.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–65. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correa CR, Darby SC. Cardiac disease and second lung cancer after radiotherapy for breast cancer. Eur J Cancer. 2009;45S:420–1. doi: 10.1016/S0959-8049(09)70073-5. [DOI] [PubMed] [Google Scholar]
- 29.Taylor CW, McGale P, Darby SC. Cardiac risks of breast-cancer radiotherapy: a contemporary view. Clin Oncol. 2006;18:236–46. doi: 10.1016/j.clon.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Taylor CW, Brønnum D, Darby SC, Gagliardi G, Hall P, Jensen MB, et al. Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol. 2011 Mar 4; doi: 10.1016/j.radonc.2011.01.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


