Abstract
Context:
Side-alternating vibration (SAV) may help reduce the risk of falling by improving body balance control. Such training has been promoted as a strength-training intervention because it can increase muscle activation through an augmented excitatory input from the muscle spindles.
Objective:
To determine the effect of SAV training on static balance during 3 postural tasks of increasing difficulty and lower limb strength.
Design:
Randomized controlled clinical trial.
Setting:
Laboratory.
Patients or Other Participants:
A total of 21 healthy women were divided into training (n = 11; age = 43.35 ± 4.12 years, height = 169 ± 6.60 cm, mass = 68.33 ± 11.90 kg) and control (n = 10; age = 42.31 ± 3.73 years, height = 167 ± 4.32 cm, mass = 66.29 ± 10.74 kg) groups.
Intervention(s):
The training group completed a 9-week program during which participants performed 3 sessions per week of ten 15-second isometric contractions with a 30-second active rest of 3 exercises (half-squat, wide-stance squat, 1-legged half-squat) on an SAV plate (acceleration = 0.91–16.3g). The control group did not participate in any form of exercise over the 9-week period.
Main Outcome Measure(s):
We evaluated isokinetic and isometric strength of the knee extensors and flexors and ankle plantar flexors, dorsiflexors, and evertors. Static balance was assessed using 3 tasks of increasing difficulty (quiet bipedal stance, tandem stance, 1-legged stance). The electromyographic activity of the vastus lateralis, semitendinosus, medial gastrocnemius, tibialis anterior, and peroneus longus was recorded during postural task performance, baseline and pretraining, immediately posttraining, and 15 days posttraining.
Results:
After training in the training group, ankle muscle strength improved (P = .03), whereas knee muscle strength remained unaltered (P = .13). Improved ankle-evertor strength was observed at all angular velocities (P = .001). Postural sway decreased in both directions but was greater in the mediolateral (P < .001) than anteroposterior (P = .02) direction. The electromyographic activity of the peroneus longus increased during the sharpened tandem (P = .001) and 1-legged tasks (P = .007). No changes were seen in the control group for any measures.
Conclusions:
The SAV training could enhance ankle muscle strength and reduce postural sway during static balance performance. The reduction in mediolateral sway could be associated with the greater use of ankle evertors due to their strength improvement.
Key Words: whole-body vibration, posture, peroneus longus, ankle evertors
Key Points.
A 9-week, side-alternating vibration training program improved static balance control and postural stability, especially in the mediolateral direction.
Side-alternating vibration improved ankle strength but not knee strength.
Vibration exercise generates sinusoidal oscillations, and the load depends on frequency, amplitude, acceleration, and duration. During side-alternating vibration (SAV), the transfer of vibration is not synchronous; instead, it operates in a side-alternating way so the right foot is lowest when the left foot is highest.1 Researchers1 have suggested that SAV produces a reciprocal vertical displacement on the left and right sides of a fulcrum and continuous rotational movements around the hip and lumbosacral joints. These rotational movements evoke an elongation of the muscle-tendon complex at one time (stretch phase), followed by a period of shortening (shortening phase).2 Cochrane et al3 recently demonstrated that the muscle-tendon complex is elongated by 1% of its total length during 6-Hz vibration cycles. However, we believe the rotational movement that SAV evokes is controlled predominantly by the ankle evertors, such as the peronei, and to a lesser extent by the plantar flexors or dorsiflexors. If this is true, SAV training may lead to strength improvements in these muscles. Investigators4 know that the peronei evert the sole of the foot and are important determinants of lateral ankle stability. They stabilize the lower extremity on the foot, especially during standing on 1 limb, when the tendency of the superincumbent weight is to displace the limb medially. In addition, researchers5 have noted that postural control during difficult standing tasks is correlated highly with peroneal muscle activity.
Investigators5–8 have studied SAV in humans to determine its effects on strength and balance. Torvinen et al9 reported that a single bout of SAV comprising 4 minutes at a frequency of 15 to 30 Hz, amplitude of 0.72 to 10 mm, and acceleration of 3.5 to 14g rapidly improved muscle performance of the lower extremities and body balance in young healthy adults. A 12-week training program of 3 sessions per week at a frequency of 15 to 25 Hz, amplitude of 2 to 12.8 mm, and acceleration of 0.91 to 16.3g increased the isokinetic strength of the knee muscles in middle-aged women (P < .05).7 A concurrent vertical vibration session with a frequency of 30 Hz, amplitude of 4 mm, and acceleration of 7.33g also enhanced the short latency response of the hamstrings, improving knee-joint stability.10 Torvinen et al6 noted an 8-month training program with a frequency of 25 to 35 Hz, amplitude of 2 mm, and acceleration of 2.54 to 4.99g did not improve the isometric knee-extensor strength of healthy young adults. Similarly, in their 1-year study on older men, Bogaerts et al11 found that synchronous vibration training was as efficient as a fitness program for increasing the isometric and explosive knee-extension strength and muscle mass of the upper body.
Investigators5,6 have noted that whole-body vibration improves balance and mobility. For example, 1-year vibration training in elderly people has been associated with reduced frequency of falls.5–7 Moreover, Rittweger et al8 showed that vibration could minimize risk factors for falling by improving muscle strength and body balance. However, information on the effects of vibration training on static balance improvement is limited. Torvinen et al6 showed no effect of an 8-month vibration intervention on dynamic and static body balance in young adult volunteers.
Classically, the control of posture is accomplished by stereotyped muscle-activation patterns because these are reflected in the hip and ankle strategies.12 The ankle strategy mainly is used to describe the activation of the triceps surae and tibialis anterior muscles around the ankle.12 Given their anatomic function, these muscles principally control anteroposterior (A/P) sway.13 By contrast, descriptions of the role of the ankle muscles in controlling mediolateral (M/L) sway are limited. According to Winter et al,14 equilibrium in the M/L direction is achieved by activating the hip abductors and adductors, whereas the ankle invertors and evertors play a negligible role due to their limited moment-of-force capability. However, the assumption that strengthening the ankle evertors could decrease sway and improve postural control in the M/L direction seems reasonable.
Therefore, the purpose of our study was to investigate the effect of a 9-week SAV training protocol on (1) the A/P and M/L displacement of the center of pressure (CoP) and electromyographic (EMG) activity of lower limb muscles during 3 postural tasks of increasing difficulty (quiet bipedal stance, tandem stance, 1-legged stance) and (2) the moment/angular velocity (M/AV) relationship of the knee extensors, knee flexors, ankle plantar flexors, ankle dorsiflexors, and particularly ankle evertors. Thus, we hypothesized that SAV training would improve ankle-muscle strength and postural control mainly in the M/L direction. Furthermore, to evaluate the durability of the training effect, we performed all balance and strength tests immediately after and 2 weeks after the end of the training period. We proposed that vibration training could have delayed effects due to the high intensity of the training, which induces acute microdamage and, subsequently, the system requires a longer time to recover.15
METHODS
Participants
A total of 21 sedentary women were assigned randomly to 1 of 2 groups: training (n = 11; age = 43.35 ± 4.12 years, height = 169 ± 6.60 cm, mass = 68.33 ± 11.90 kg) or control (n = 10; age = 42.31 ± 3.73 years, height = 167 ± 4.32 cm, mass = 66.29 ± 10.74 kg). All participants were recruited through various announcements and local meetings organized by the Department of Physical Education and Sport Sciences, Aristotle University of Thessaloniki at Serres, promoting women's quality-of-life improvement based on exercise. They were healthy volunteers who did not have musculoskeletal or neurologic disease or impairment (eg, lower extremity injury, recent fracture, Parkinson disease, diabetes, multiple sclerosis, metabolic disease, osteoporosis, osteoarthrosis, epilepsy, or orthopaedic injuries) or any possible contraindication for whole-body vibration and did not participate in a systematic program of physical activity and exercise 24 months before the study. They were instructed not to take any medication or other supplements (eg, dietary) and not to perform any sports activities that could affect strength adaptations. Participants provided written informed consent, and the study was approved by the University Ethics Committee on Human Research of Aristotle University.
Procedures
The training group performed 27 training sessions comprising 3 sessions per week for 9 weeks with a 1-day rest after each session with the Galileo Fitness SAV platform (Novotec, Pforzheim, Germany). The training protocol was based on a protocol previously described by Spiliopoulou et al.7 The SAV induces rotational movements around the hip and lumbosacral joints1 so that when the right foot is at the lowest height, the left foot is at the highest point. Each session was divided into 3 phases: (1) warm-up comprising 5 minutes of stretching and walking, (2) main phase comprising 25 minutes of isometric exercises during SAV application with or without hand support, and (3) recovery phase comprising 10 minutes of stretching. An interval exercise protocol was applied by alternating muscular contraction and rest time (work-to-rest ratio = 1:2). In each training session during SAV application, the participants performed 10 isometric contractions, lasting 15 seconds each, of the following exercises: half-squat (knee angles of 120° and 90°), wide-stance squat (knee angle of 90°), and 1-legged half squat (knee angle of 120°) with a 30-second active rest. The intermalleolar distances during the half-squat, which were controlled strictly by the plate-specific signs on the left and right sides of the fulcrum, were 12, 23, 34, and 46 cm for weeks 1 to 2, 3 to 4, 5 to 6, and 7 to 9, respectively. No dynamic contractions and no additional weight were used during any exercises. The characteristics of vibration varied during the 9-week period (weeks 1–2: amplitude = 2–4 cm, frequency = 15–20 Hz, acceleration = 0.91–3.26g; weeks 3–4: amplitude = 4–8 cm, frequency = 18–22 Hz, acceleration = 3.36–7.89g; and weeks 5–9: amplitude = 8–12.8 cm, frequency = 22–25 Hz, acceleration = 7.89–16.3g) according to the principle of progressively increasing training. During isometric actions, participants were encouraged orally to strongly contract their muscles. They trained with socks, and no loss of contact of the foot with the vibration plate was observed during training. The “skidding” effect was controlled according to Rittweger1 and Rauch et al.16
The participants of the control group were women who lived sedentary lifestyles. We instructed them not to change their lifestyles during the study or to engage in any type of physical activity.
Measures
Strength and balance testing were performed before training (baseline/pretraining), 48 hours after the end of the last training session to avoid any fatigue effect (posttraining), and 15 days after the end of the training. All strength tests were performed on a KinCom dynamometer (model 500-11; Chattanooga Inc, Chattanooga, TN). We analyzed the repetition demonstrating the maximal moment. All moment values were gravity corrected; we took the dynamometer reading at selected static joint positions.17
During knee-extension and -flexion tests, participants were seated with a hip angle of 75° and the trunk, waist, and thigh of the right lower extremity stabilized with hook-and-loop straps. Three maximal concentric isometric (5 seconds at 120°), and eccentric efforts of the knee extensors and flexors (30°/s, 60°/s, 120°/s, 180°/s) were performed in a randomized order. The range of motion was 90° (full extension = 180°). During ankle plantar flexion, dorsiflexion, and eversion, participants were seated with the right thigh strapped down and the foot securely fastened with hook-and-loop straps to the footplate, so the ankle was in a neutral position (0° = 90° of ankle angle). For plantar flexion and dorsiflexion, the motion ranged from 20° of dorsiflexion to 30° of plantar flexion. For ankle eversion, the motion ranged from 15° of eversion to 25° of inversion (knee angle = 120°). Three maximal concentric isometric (5 seconds at 0°) and eccentric efforts of plantar flexors and dorsiflexors (30°/s, 60°/s, 120°/s) and evertors (30°/s, 60°/s, 90°/s) were performed. Kaminski et al18 reported high reliability of eversion measurements (intraclass correlation coefficients = 0.69–0.91).
All balance tests were performed on a Comex platform (Footchecker 3.2; Loran Engineering Ltd, Bologna, Italy). This pressure platform uses 2304 quartz piezoelectric sensing resistors in an active area of 70 × 50 cm to record pressure distribution at 50 Hz. To assess static balance, participants performed 3 tasks of increasing difficulty that we purposefully selected to evaluate balance dysfunction and were sufficient to distinguish postural responses19:
Quiet bipedal stance (QBS). Participants were instructed to stand as still as possible with a stance width of approximately 10 to 15 cm.
Tandem stance (TS). The heel of the nondominant foot was positioned in front of the toe of the dominant foot, leaving no space between the feet (upper extremities on the hips and elbows flexed to 100°). We measured the right foot of all participants and defined it as the dominant foot.
One-legged stance (OLS). Participants were instructed to stand on the dominant limb while the nonsupport limb was flexed at the knee with the plantar surface of the foot stabilized on the supporting limb.
Time was provided for familiarization with each test. Data recording started when the participant was stable in the required posture and lasted for 10 seconds. Two trials were performed, and the data were averaged. Tests were performed at the same time of day to avoid any chronobiological effect.
The A/P and M/L displacements in centimeters of the CoP were calculated from the instantaneous CoP (ay, ax) position based on the following equations:
|
|
|
|
where Rd indicates displacement in millimeters, y indicates A/P, and x indicates M/L.20
Postural sway was quantified by peak-to-peak amplitude (CoPmax) and the standard deviation (CoPsd) of CoP oscillations along the A/P and M/L axes. To avoid the dynamic phase of the task reflecting the postural adjustments caused by the weight transfer21 and to avoid any fatigue effect during the task, we discarded 2 seconds from the beginning and 2 seconds from the end of the analysis. The static phase of 6 seconds in the middle of the task was analyzed.
An MP100 acquisition unit (Biopac Systems, Inc, Goleta, CA) was used for EMG data collection. The EMG system was interfaced to a portable amplifier/transmitter (TEL100D; Biopac Systems, Inc) with a common-mode rejection ratio greater than 110 dB at 50/60 Hz and a bandwidth of 10 to 500 Hz. The EMG signals were fed through BNC connectors to a 12-bit analog-to-digital converter sampling at 1000 Hz per channel. Bipolar silver chloride surface electrodes (Motion control; IOMED, Inc, Montreal, Quebec, Canada) with a 2-cm center-to-center interface distance were placed over the bellies of 3 ankle muscles (peroneus longus [PL], tibialis anterior [TA], medial gastrocnemius [MGAS]) and 2 knee muscles (vastus lateralis [VL], semitendinosus [ST]). Electrode placement was accomplished according to the European recommendations for surface electromyography.22 The electrodes for the PL were placed at one-third of the line between the tip of the head of the fibula and the tip of the lateral malleolus; for the TA, at one-fourth of the line between the tip of fibula and the tip of the medial malleolus; for the MGAS, on the most prominent bulge of the muscle; for the VL, at two-thirds of the line from the anterior-superior iliac spine to the lateral side of the patella; and for the ST, at one-half of the line between the ischial tuberosity and the medial epicondyle of the tibia. The reference electrode was placed on the ankle. We ensured placement of the electrodes in the same skin position before and after training by marking the periphery of the skin electrodes with indelible ink. We prepared the skin by shaving, sanding, and cleaning it with alcohol. The fixation of the electrodes was accomplished carefully because we expected the susceptibility of electromyography to cross-talk to be higher in untrained individuals. However, if the location of the electrodes is identified accurately, the signal of a muscle is unlikely to be affected by cross-talk.23 After collection, the EMG data were full-wave rectified and averaged over 10-millisecond intervals, yielding an averaged electromyography. Subsequently, EMG signals in TS and OLS were expressed as a normalized value of the electromyography during QBS.
Statistical Analysis
For each dependent variable, we performed a 2 × 3 mixed-methods analysis of variance. The between-groups factor was group (training, control), and the within-group factor with repeated measures was time (baseline/pretraining, posttraining, 15 days posttraining). If we found a group × time interaction, we applied Tukey post hoc tests to identify specific differences across time points within each group. In addition, we performed post hoc between-groups comparisons with a t test for independent samples to assess differences at any of the 3 time points. We used SPSS (version 13; SPSS Inc, Chicago, IL) to perform all analyses, and the α level was set at .05 for all tests.
RESULTS
We found a group × time effect on eccentric moment of the knee extensors (60°/s: F1,19 = 5.79, P = .03; 30°/s: F1,19 = 5.00, P = .04) but not on concentric moment (F1,19 = 0.93, P = .76). Post hoc analysis confirmed that the 2 groups were no different at the baseline/pretraining testing (P = .77). However, the training group showed an increase in eccentric moment at 15 days posttraining (Table). We did not observe group or time effects in knee-flexor strength (Table).
Table. .
Mean ± SD of Eccentric, Isometric, and Concentric Moments
| Muscles |
Angular Velocity, N·m |
|||||||||
| −120 |
−90 |
−60 |
−30 |
0 |
30 |
60 |
90 |
120 |
180 |
|
| Knee extensors | ||||||||||
| Training | ||||||||||
| Baseline/pretraining | 59.21 ± 9.03 | NA | 64.72 ± 11.40 | 69.75 ± 13.34 | 64.00 ± 8.09 | 57.63 ± 7.43 | 45.87 ± 7.16 | NA | 35.09 ± 6.05 | 32.17 ± 6.44 |
| Posttraining | 60.45 ± 9.53 | NA | 67.64 ± 11.63 | 71.03 ± 14.14 | 65.28 ± 8.25 | 57.70 ± 9.09 | 46.78 ± 9.73 | NA | 38.67 ± 8.82 | 34.23 ± 7.98 |
| 15 d posttraining | 67.34 ± 9.81 | NA | 74.96 ± 11.75a,b | 77.72 ± 14.51a,b | 64.07 ± 8.10 | 56.64 ± 10.52 | 49.18 ± 17.19 | NA | 37.96 ± 12.39 | 33.97 ± 10.17 |
| Control | ||||||||||
| Baseline/ pretraining | 60.20 ± 6.16 | NA | 63.21 ± 7.82 | 70.01 ± 7.84 | 63.78 ± 6.25 | 56.57 ± 7.32 | 46.64 ± 4.45 | NA | 36.60 ± 6.72 | 32.99 ± 9.25 |
| Posttraining | 62.08 ± 8.00 | NA | 64.34 ± 8.23 | 70.14 ± 7.84 | 62.12 ± 6.08 | 55.60 ± 8.26 | 47.98 ± 5.51 | NA | 36.76 ± 6.72 | 33.14 ± 9.25 |
| 15 d posttraining | 61.04 ± 4.88 | NA | 64.77 ± 3.75 | 69.35 ± 7.23 | 63.33 ± 6.20 | 55.98 ± 7.77 | 47.32 ± 16.43 | NA | 34.02 ± 15.79 | 31.93 ± 9.24 |
| Knee flexors | ||||||||||
| Training | ||||||||||
| Baseline/ pretraining | NA | 59.62 ± 10.82 | 59.80 ± 12.06 | 62.69 ± 11.29 | 57.64 ± 8.52 | 55.7 ± 9.61 | 44.96 ± 10.59 | NA | 36.91 ± 12.19 | 31.30 ± 7.03 |
| Posttraining | NA | 59.99 ± 12.16 | 62.53 ± 9.34 | 65.38 ± 12.25 | 58.28 ± 12.06 | 55.27 ± 7.44 | 47.68 ± 9.01 | NA | 37.85 ± 11.55 | 31.51 ± 8.87 |
| 15 d posttraining | NA | 60.70 ± 4.92 | 62.38 ± 5.76 | 68.10 ± 5.51 | 61.10 ± 9.03 | 53.41 ± 13.73 | 49.88 ± 20.68 | NA | 38.76 ± 16.58 | 32.36 ± 13.37 |
| Control | ||||||||||
| Baseline/ pretraining | NA | 58.14 ± 4.54 | 60.45 ± 4.54 | 64.77 ± 4.54 | 57.69 ± 8.55 | 56.31 ± 2.41 | 43.28 ± 4.93 | NA | 36.86 ± 6.79 | 30.11 ± 4.56 |
| Posttraining | NA | 57.80 ± 4.54 | 60.11 ± 4.54 | 64.43 ± 4.54 | 56.71 ± 8.40 | 57.42 ± 2.82 | 43.34 ± 4.84 | NA | 36.78 ± 6.83 | 30.07 ± 4.50 |
| 15 d posttraining | NA | 58.10 ± 3.57 | 62.20 ± 4.76 | 65.20 ± 1.98 | 57.05 ± 8.45 | 55.37 ± 16.14 | 45.54 ± 13.62 | NA | 36.26 ± 9.35 | 30.88 ± 3.76 |
| Ankle plantar flexors | ||||||||||
| Training | ||||||||||
| Baseline/ pretraining | NA | 39.04 ± 3.65 | 42.00 ± 6.41 | 43.15 ± 5.16 | 43.90 ± 6.43 | 36.66 ± 3.81 | 30.19 ± 5.06 | 26.24 ± 3.69 | NA | NA |
| Posttraining | NA | 42.85 ± 5.73 | 53.76 ± 8.21a | 49.84 ± 5.96a | 50.49 ± 7.40a | 42.16 ± 4.38a | 32.23 ± 7.07 | 29.47 ± 4.87 | NA | NA |
| 15 d posttraining | NA | 44.86 ± 3.54 | 54.60 ± 8.33a | 49.8 ± 5.96a | 51.10 ± 9.15a | 44.56 ± 3.71a | 29.80 ± 4.09 | 28.18 ± 3.75 | NA | NA |
| Control | ||||||||||
| Baseline/ pretraining | NA | 40.72 ± 5.34 | 43.82 ± 6.43 | 43.84 ± 6.15 | 43.99 ± 6.06 | 36.14 ± 7.93 | 30.22 ± 14.83 | 26.48 ± 5.50 | NA | NA |
| Posttraining | NA | 41.29 ± 6.43 | 43.02 ± 3.28 | 43.95 ± 6.15 | 44.66 ± 2.78 | 33.74 ± 9.40 | 29.89 ± 14.83 | 26.69 ± 5.50 | NA | NA |
| 15 d posttraining | NA | 40.74 ± 4.67 | 43.78 ± 5.89 | 43.56 ± 6.15 | 43.94 ± 4.16 | 33.84 ± 4.94 | 32.72 ± 10.90 | 29.29 ± 3.34 | NA | NA |
| Ankle dorsiflexors | ||||||||||
| Training | ||||||||||
| Baseline/ pretraining | NA | 19.11 ± 4.22 | 20.83 ± 4.16 | 20.94 ± 4.68 | 22.12 ± 2.86 | 10.15 ± 2.40 | 8.88 ± 1.24 | 7.91 ± 2.40 | NA | NA |
| Posttraining | NA | 20.69 ± 4.26 | 23.52 ± 1.98 | 26.46 ± 3.25a | 26.54 ± 3.44a | 13.09 ± 1.19a | 9.91 ± 3.07 | 8.53 ± 2.78 | NA | NA |
| 15 d posttraining | NA | 20.57 ± 4.03 | 23.56 ± 1.72 | 26.64 ± 3.14a | 26.87 ± 3.54a | 13.80 ± 2.03a | 9.91 ± 3.07 | 9.55 ± 1.48 | NA | NA |
| Control | ||||||||||
| Baseline/ pretraining | NA | 19.68 ± 2.17 | 21.77 ± 1.90 | 21.84 ± 2.89 | 21.24 ± 1.78 | 11.14 ± 1.88 | 9.93 ± 1.58 | 7.99 ± 1.27 | NA | NA |
| Posttraining | NA | 21.70 ± 3.79 | 22.92 ± 1.12 | 23.88 ± 2.96 | 21.40 ± 2.17 | 12.13 ± 1.52 | 11.27 ± 1.10 | 8.28 ± 1.15 | NA | NA |
| 15 d posttraining | NA | 19.62 ± 2.17 | 22.40 ± 1.68 | 22.37 ± 3.71 | 19.85 ± 1.73 | 11.35 ± 1.89 | 9.99 ± 1.37 | 7.97 ± 1.11 | NA | NA |
| Ankle evertors | ||||||||||
| Training | ||||||||||
| Baseline/ pretraining | NA | 27.26 ± 5.65 | 30.48 ± 6.17 | 34.92 ± 6.38 | 32.98 ± 8.15 | 28.47 ± 4.96 | 23.95 ± 5.38 | 21.64 ± 6.26 | NA | NA |
| Posttraining | NA | 37.90 ± 7.02a | 42.68 ± 9.42a | 49.44 ± 9.70a | 46.18 ± 11.41a | 39.86 ± 6.94a | 33.54 ± 7.54a | 30.29 ± 8.76a | NA | NA |
| 15 d posttraining | NA | 45.18 ± 8.16a,b | 48.78 ± 11.34a,b | 54.42 ± 10.02a,b | 52.78 ± 13.04a,b | 45.55 ± 5.98a,b | 38.33 ± 6.18a,b | 34.62 ± 6.55a,b | NA | NA |
| Control | ||||||||||
| Baseline/ pretraining | NA | 27.49 ± 7.60 | 30.85 ± 9.91 | 33.99 ± 11.67 | 33.70 ± 11.18 | 28.17 ± 8.91 | 22.41 ± 10.08 | 20.60 ± 10.28 | NA | NA |
| Posttraining | NA | 29.11 ± 6.77 | 28.38 ± 9.12 | 31.27 ± 10.74 | 31.34 ± 10.40 | 27.66 ± 8.75 | 21.07 ± 9.48 | 19.57 ± 9.76 | NA | NA |
| 15 d posttraining | NA | 27.10 ± 8.64 | 29.00 ± 9.32 | 30.93 ± 10.62 | 32.02 ± 10.62 | 27.95 ± 8.84 | 21.51 ± 9.68 | 19.15 ± 9.56 | NA | NA |
Abbreviation: NA indicates not applicable.
Indicates greater than baseline/pretraining at the same angular velocity (P < .05).
Indicates greater than posttraining at the same angular velocity (P < .05).
For the ankle dorsiflexors, the analysis revealed a group × time effect on concentric (30°/s: F2,38 = 19.21, P = .047), eccentric (30°/s: F2,38 = 12.19, P = .03), and isometric (0°/s: F2,38 = 9.14, P = .001) moments. Between-groups comparisons at baseline/pretraining testing revealed no differences between the groups for any of the measured moments (P > .05). Additional post hoc analysis showed an increase in isometric, concentric, and eccentric moments at 30°/s for the training group posttraining and at 15 days posttraining but not for the control group (Table). Similarly, for the plantar flexors, we found an interaction effect for eccentric (60°/s: F2,38 = 6.74, P = .03; 30°/s: F2,38 = 11.5, P = .01), concentric (30°/s: F2,38 = 9.82, P = .001), and isometric (F2,38 = 11.05, P = .001) moments. Whereas the 2 groups were not different at baseline/pretraining testing, post hoc analysis showed that the eccentric (30°/s, 60°/s), concentric (30°/s), and isometric moments increased from baseline/pretraining to posttraining and 15 days posttraining for the training group (Table).
For the ankle evertors, the analysis revealed a group × time effect on eccentric (90°/s: F2,38 = 34.42, P = .03; 60°/s: F2,38 = 25.91, P = .001; 30°/s: F2,38 = 37.22, P = .001), concentric (30°/s: F2,38 = 19.11, P = .001; 60°/s: F2,38 = 11.93, P = .001; 90°/s: F2,38 = 4.69, P = .01), and isometric (F2,38 = 14.79, P = .001) moments. Post hoc analysis showed no between-groups differences at baseline/pretraining but revealed an increase in moment (eccentric 90°/s, 60°/s, and 30°/s; concentric 30°/s and 60°/s; and isometric) from baseline/pretraining to posttraining and 15 days posttraining for the training group but not for the control group (Table).
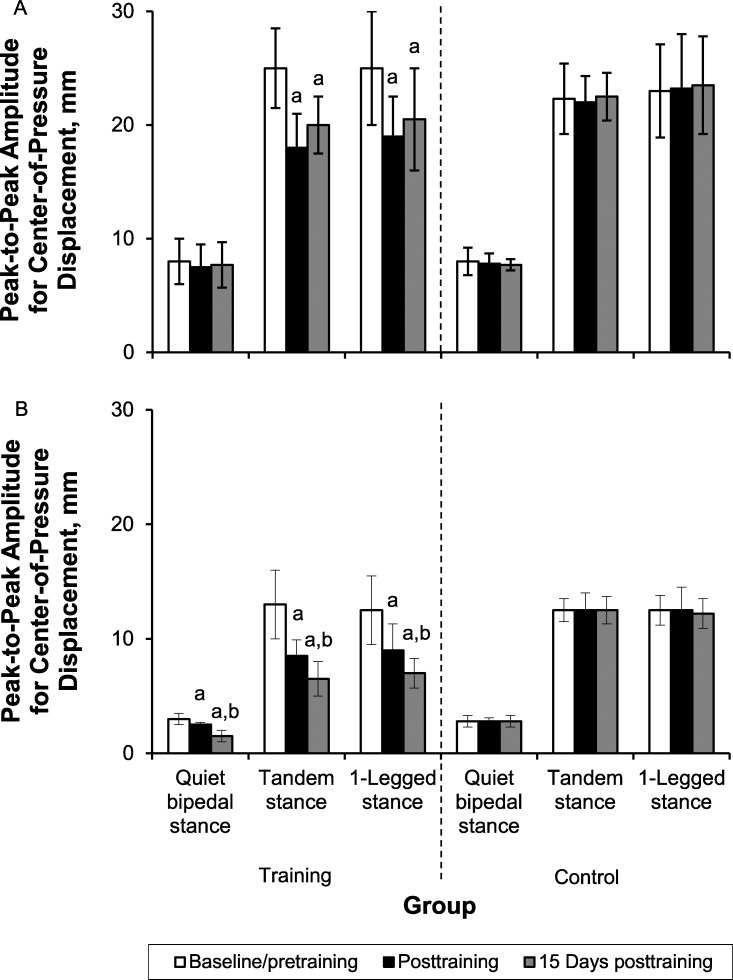
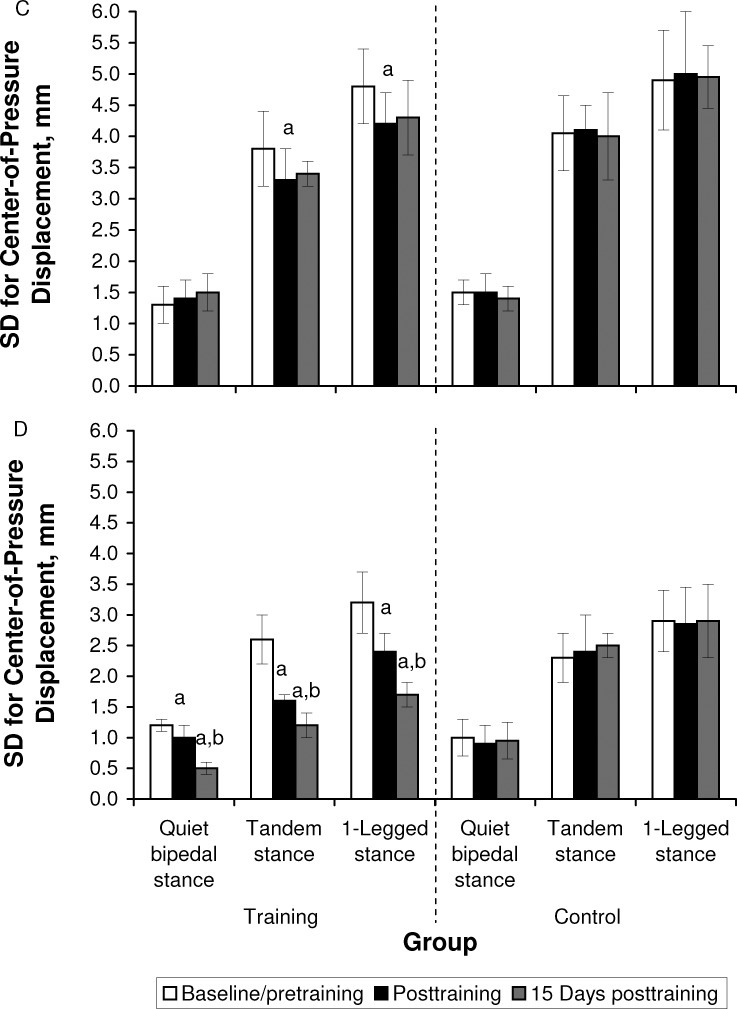
The CoP traces for a representative participant before and after training are shown in Figure 1. Group mean and SD values for the CoP measures are summarized in Figure 2. During the QBS task, the analysis revealed no group × time interaction of training on CoPmax (F1,19 = 1.91, P = .19) or CoPsd (F1,19 = 0.10, P = .75) in the A/P direction. However, a group × time interaction on CoPmax (F2,38 = 29.12, P = .004) and CoPsd (F2,38 = 22.25, P = .001) was shown in the M/L direction. We noted no differences between groups at baseline/pretraining testing in either measure (A/P: P = .67, M/L: P = .97). Post hoc Tukey tests across testing times revealed a decrease of CoPmax and CoPsd in the M/L direction only for the training group (P < .05 for posttraining and 15 days posttraining).
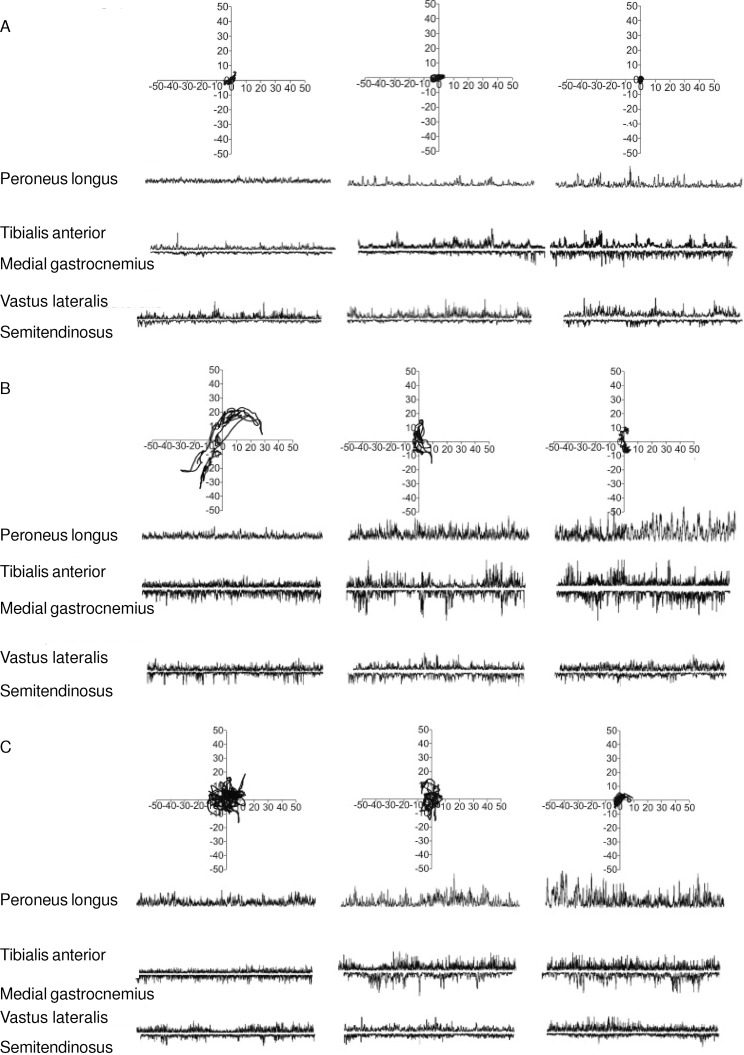
Figure 1. .
Representative trace of center-of-pressure displacement (mm) in the anteroposterior and mediolateral directions and electromyographic activity during the balance tasks of A, quiet bipedal stance, B, tandem stance, and C, 1-legged stance, for the peroneus longus, tibialis anterior, medial gastrocnemius, vastus lateralis, and semitendinosus at baseline/pretraining, posttraining, and 15 days posttraining.
Figure 2. .
The peak-to-peak amplitude of center-of-pressure displacement in the A, anteroposterior axis, and B, mediolateral axis; and the SD of center-of-pressure displacement in the C, anteroposterior axis, and D, mediolateral axis, across the 3 postural tasks of quiet bipedal stance, tandem stance, and 1-legged stance at baseline/pretraining, immediately posttraining, and at 15 days posttraining for the training and control groups. a Indicates lower than baseline/pretraining (P < .05). b Indicates lower than posttraining (P < .05). Figure continued on next page.
For the ST task, the analysis showed a group × time interaction on CoPmax and CoPsd in the A/P (CoPmax: F2,38 = 6.16, P = .002; CoPsd: F2,38 = 4.49, P = .02) and M/L (CoPmax: F2,38 = 27.1, P = .001; CoPsd: F2,38 = 10.01, P = .001) directions. We did not find between-groups differences at baseline/pretraining testing for any of these measures (P > .05). Furthermore, post hoc Tukey tests across testing times showed a decrease of CoPmax and CoPsd only for the training group (P < .05 for posttraining and 15 days posttraining). However, the training-induced decrease was greater in the M/L than the A/P direction.
For the OLS task, we found group × time interactions in the A/P (CoPmax: F2,38 = 3.77, P = .01; CoPsd: F2,38 = 3.24, P = .02) and M/L (CoPmax: F2,38 = 25.52, P = .001; CoPsd: F2,38 = 372.05, P = .001) directions, which confirmed the effect of training on sway measures. The higher F values in the M/L direction confirmed the greater effect of training in the M/L than A/P direction. Post hoc comparisons at baseline/pretraining confirmed the absence of between-groups differences for any of the postural-sway measures. For both M/L and A/P axes, post hoc Tukey tests across testing times showed a decrease in CoPmax and CoPsd only for the training group (P < .05 for posttraining and 15 days posttraining).
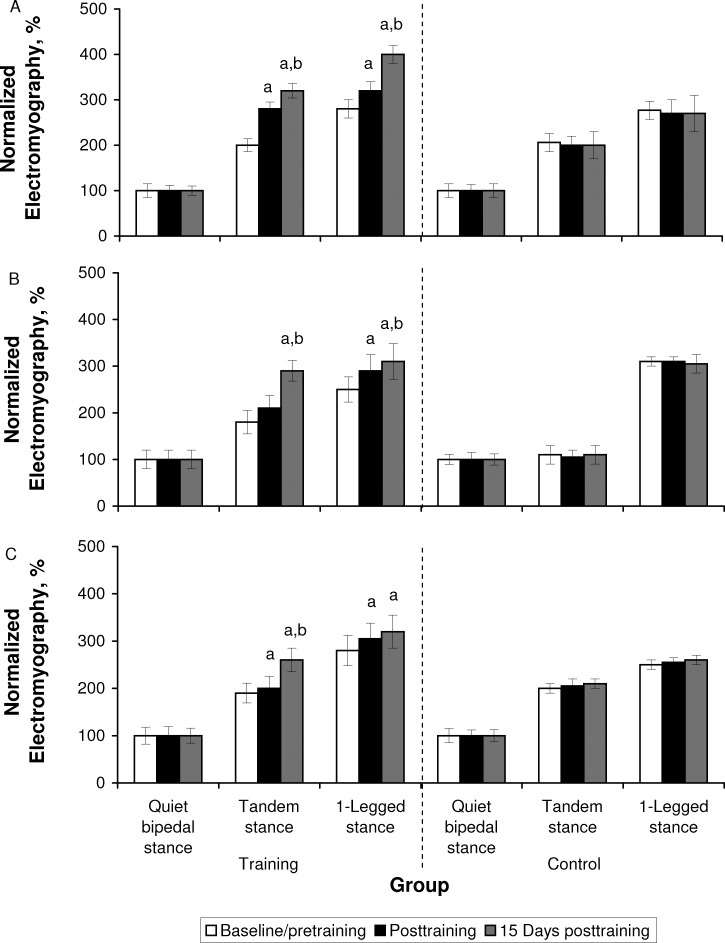
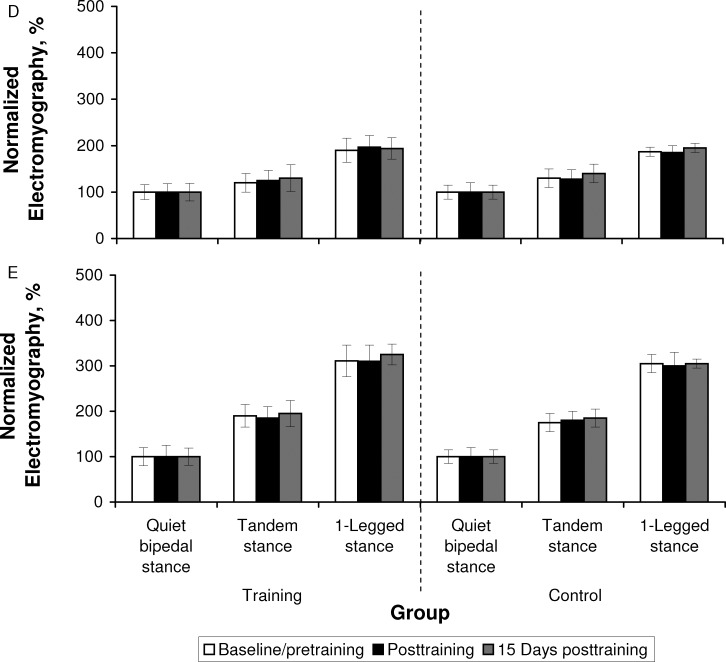
Analysis of the average EMG activity during postural task performance revealed group × time effects for PL (TS: F2,38 = 348.79, P = .001; OLS: F2,38 = 28.48, P = .001), TA (TS: F2,38 = 63.95, P = .001; OLS: F2,38 = 544.88, P = .001), and MGAS (TS: F2,38 = 23.20, P = .001; OLS: F2,38 = 217.84, P = .03) muscles during the TS and OLS balance tasks. Again, the higher F values for the PL muscle during the TS task suggested the greater effect of training on the PL relative to the TA and MGAS muscles. Baseline/pretraining between-groups comparisons confirmed the absence of differences between groups in the average EMG activity of the 3 muscles at baseline/pretraining. Further post hoc analysis indicated that the EMG activity of the 3 muscles increased posttraining and 15 days posttraining for the training group (Figure 3).
Figure 3. .
Normalized electromyography during quiet bipedal stance, tandem stance, and 1-legged stance recorded at baseline/immediately pretraining, immediately posttraining, and 15 days posttraining from the A, peroneus longus, B, tibialis anterior, C, medial gastrocnemius, D, vastus lateralis, and E, semitendinosus. a Indicates higher than baseline/pretraining (P < .05). b Indicates higher than posttraining (P < .05). Figure continued on next page.
DISCUSSION
Our results showed that 9 weeks of SAV training improved ankle-muscle strength and reduced postural sway in sedentary women. It is interesting that the improvement of ankle-evertor strength was accompanied by a greater decrease in M/L than A/P sway. Thus, improvements in postural-balance tasks after SAV training may be determined largely by an increase in ankle-muscle strength.
Vibration training did not improve knee-muscle isokinetic strength. The main reason could be the low intensity of the training stimulus during vibration training. This training position does not seem to be sufficient to induce maximal strength adaptations in the knee muscles. Another reason could be the incompatibility (specificity principle) between the muscle contraction modes used during training (isometric) and testing (concentric and eccentric). Thus, we still do not know if SAV affects maximal isokinetic performance of the knee muscles. Our results are in agreement with the findings of Rees et al,24,25 who did not report any improvement in knee-joint torque and power after 8 weeks of vibration in a healthy elderly population.
We found an enhancement of ankle muscle in contrast to knee-muscle strength after training (Table). The ankle muscles are the first to adapt to the vibration stimulus because they are the nearest to the site of vibration.24 Researchers have shown that whole-body vibration (frequency = 30–45 Hz, amplitude = 2–5 mm) increases the activation levels (0.3%–34.5%) of the lower limb muscles.26 Muscle activity increases linearly with the frequency and amplitude of vibration. High vibration amplitudes induce greater muscle activation. In any case, the vibration-induced muscle activation level could vary depending on the type of platform,2 the muscle contraction (squatting or relaxing) during vibration, and the different frequencies and amplitudes. This greater muscle activation could be due to an augmented excitatory input from the muscle spindle.8 However, researchers27,28 have demonstrated that reflex activity either remains unchanged or decreases after a bout of whole-body vibration. Although spinal reflex excitability decreased, the changes in homosynaptic depression (presynaptic mechanism that regulates the function of the Ia afferent–motoneuron synapse) point to a particular mechanism that may directly improve neuromuscular function after whole-body vibration.27 However, we did not measure reflex activity in response to vibration, and in future studies, researchers should investigate the exact mechanism underlying the vibration effect on the greater muscle activation.
In addition, our results are in line with those reported in other studies confirming that balance and physical performance could be improved after 3 months7 and 8 months6 of vibration training. The improvement in static balance often is accompanied by the assumption of increased proprioceptive input induced by the excessive excitation of the muscle spindles due to whole-body vibration.5 For example, Melnyk et al10 showed that a single vibration session led to an increase in the short latency response of the hamstrings, improving knee-joint stability and causing a postactivation potentiation mainly downstream from the neuromuscular junction.3 Moreover, mechanical stimuli causes the muscle fibers to stretch, evoking a stretch reflex and enhancing the neuromuscular function through neurogenic excitability and recruitment.1 However, researchers27 have shown that after a bout of vibration, a decrease in spinal excitability occurs independently of the muscle group. This decrease is highly variable and may be associated with individual differences other than sex, training specificity, or conditioning.28
Postural sway in the M/L direction is considered a better predictor of the risk of falling than A/P sway, even for individuals who have no recent history of falling.29 Our results showed a greater sway decrease in the M/L than A/P direction (approximately 50% versus 25%) after SAV training. This also was accompanied by an increase in PL EMG activity after training (Figure 3). During quiet standing, Gatev et al30 observed an overreliance on the ankle muscles and suggested the goal of “minimal neural effort,” focusing on greater ankle proprioceptive input. Amiridis et al19 suggested that trained elderly people, showing greater reliance on their stronger ankle muscles, may substantially limit the use of the more fall-prone hip strategy when responding to balance threats. Therefore, strengthening the ankle muscles for controlling M/L sway could explain the positive effects of vibration on postural performance. Furthermore, when narrowing the base of support in the TS in our study, the activation of the PL muscle was much greater than the activation of the other ankle or hip muscles (Figure 3). This confirms that postural control in the M/L plane is dominated by the ankle evertors and invertors, with mixed and minimal contribution by the hip load-and-unload mechanism. Finally, Tropp and Odenrick4 noted the position of the CoP during single-limb stance was correlated highly with ankle position and peroneal muscle activity. Both the PL and peroneus brevis stabilize the lower extremity on the foot, especially in standing on 1 limb, and are considered important determinants of lateral ankle stability.4
Figure 3. .
Continued.
Notably, in our study, the effects of vibration training were optimized (or maximized) at 15 days posttraining. We could postulate that the substantial training load and the acute fatigue effect led to slightly better performance immediately posttraining. However, by 15 days posttraining, the body seems to repair the microdamage produced by the exercise. Therefore, the well-described overcompensation phenomenon15 that frequently is observed in untrained populations seems to explain the evident optimization of the training outcomes.
LIMITS AND PERSPECTIVES
The absence of an experimental group doing the same isometric exercises without vibration could be considered a limitation in our study. However, finding an exercise imitating the half-squat position and promoting ankle-evertor activation at the same time is difficult. A systematic investigation of the effects of SAV on various training protocols and the durability of the training effects would be valuable.
CONCLUSIONS
Nine weeks of SAV training decreased postural sway during standing in untrained women, especially in the M/L direction. This improvement was accompanied by increased strength of the ankle muscles, particularly the ankle evertors. Vibration training did not provoke improvements in knee isokinetic strength.
Figure 2. .
Continued.
REFERENCES
- 1.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 2.Abercromby AFJ, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski WH. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39(9):1642–1650. doi: 10.1249/mss.0b013e318093f551. [DOI] [PubMed] [Google Scholar]
- 3.Cochrane D, Stannard SR, Firth EC, Rittweger J. Acute whole-body vibration elicits post-activation potentiation. Eur J Appl Physiol. 2010;108(2):311–319. doi: 10.1007/s00421-009-1215-2. [DOI] [PubMed] [Google Scholar]
- 4.Tropp H, Odenrick P. Postural control in single-limb stance. J Orthop Res. 1988;6(6):833–839. doi: 10.1002/jor.1100060607. [DOI] [PubMed] [Google Scholar]
- 5.Bruyere O, Wuidart MA, Palma E, et al. Controlled whole body vibration to decrease fall risk and improve health-related quality of life of nursing home residents. Arch Phys Med Rehabil. 2005;86(2):303–307. doi: 10.1016/j.apmr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Torvinen S, Kannus P, Sievanen H, et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res. 2003;18(5):876–884. doi: 10.1359/jbmr.2003.18.5.876. [DOI] [PubMed] [Google Scholar]
- 7.Spiliopoulou SI, Amiridis IG, Tsiganos G, Economides D, Kellis E. Vibration effects on static balance and strength. Int J Sports Med. 2010;31(9):610–616. doi: 10.1055/s-0030-1249618. [DOI] [PubMed] [Google Scholar]
- 8.Rittweger J, Beller G, Felsenberg D. Acute physiological effects of exhaustive whole-body vibration exercise in man. Clin Physiol Funct Imaging. 2000;20(2):134–142. doi: 10.1046/j.1365-2281.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 9.Torvinen S, Kannus P, Sievanen H, et al. Effect of a vibration exposure on muscular performance and body balance: randomized cross-over study. Clin Physiol Funct Imaging. 2002;22(2):145–152. doi: 10.1046/j.1365-2281.2002.00410.x. [DOI] [PubMed] [Google Scholar]
- 10.Melnyk M, Kofler B, Faist M, Hodapp M, Gollhofer A. Effect of a whole-body vibration session on knee stability. Int J Sports Med. 2008;29(10):839–844. doi: 10.1055/s-2008-1038405. [DOI] [PubMed] [Google Scholar]
- 11.Bogaerts A, Delecluse C, Claessens AL, et al. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(6):630–635. doi: 10.1093/gerona/62.6.630. [DOI] [PubMed] [Google Scholar]
- 12.Nashner LM, McCollum G. The organization of human postural movements: a formal basis and experimental synthesis. Behav Brain Sci. 1985;8(1):135–152. [Google Scholar]
- 13.Amiridis IG, Hatzitaki V, Arabatzi F. Age-induced modifications of static postural control in humans. Neurosci Lett. 2003;350(3):137–140. doi: 10.1016/s0304-3940(03)00878-4. [DOI] [PubMed] [Google Scholar]
- 14.Winter D, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75(6):2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- 15.Werhosansky JW. L'Entraînement Efficace. Paris, France: Presses Universitaires de France;; 1992. [Google Scholar]
- 16.Rauch F, Sievanen S, Boonen M, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- 17.Mayhew TP. Rothsteln, Finucane SDG, Lamb RL. Performance characteristics of the Kin-Com dynamometer. Phys Ther. 1994;74(11):1047–1054. doi: 10.1093/ptj/74.11.1047. [DOI] [PubMed] [Google Scholar]
- 18.Kaminski TW, Perrin DH, Szczerba JE, Bernier J. The reliability and validity of ankle inversion and eversion torque measurements from the Kin Com II isokinetic dynamometer. J Sport Rehabil. 1995;4(3):210–218. [Google Scholar]
- 19.Amiridis IG, Arabatzi F, Violaris P, Stavropoulos E, Hatzitaki V. Static balance improvement in elderly after dorsiflexors electrostimulation training. Eur J Appl Physiol. 2005;94(4):424–433. doi: 10.1007/s00421-005-1326-3. [DOI] [PubMed] [Google Scholar]
- 20.Rose J, Wolff DR, Jones VK, Bloch DA, Oehlert JW, Gamble JG. Postural balance in children with cerebral palsy. Develop Med Child Neurol. 2002;44(1):58–63. doi: 10.1017/s0012162201001669. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson E, Henriksson M, Hirschfeld H. Age-related differences in postural adjustments in connection with different tasks involving weight transfer while standing. Gait Posture. 2007;26(4):508–515. doi: 10.1016/j.gaitpost.2006.11.206. [DOI] [PubMed] [Google Scholar]
- 22.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(3):561–574. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 23.Winter DA, Fuglevand AJ, Archer SE. Crosstalk in surface electromyography: theoretical and practical estimates. J Electromyogr Kinesiol. 1994;4(1):15–26. doi: 10.1016/1050-6411(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 24.Rees SS, Murphy AJ, Watsford ML. Effects of whole body vibration on postural steadiness in an older population. J Sci Med Sport. 2009;12(4):440–444. doi: 10.1016/j.jsams.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Rees SS, Murphy AJ, Watsford ML. Effects of whole-body vibration exercise on lower-extremity muscle strength and power in an older population: a randomized clinical trial. Phys Ther. 2008;88(4):462–470. doi: 10.2522/ptj.20070027. [DOI] [PubMed] [Google Scholar]
- 26.Cardinale M, Lim J. The acute effects of two different whole body vibration frequencies on vertical jump performance. Med Sport (Roma) 2003;56(4):287–292. [Google Scholar]
- 27.Kipp K, Johnson ST, Doeringer JR, Hoffman MA. Spinal reflex excitability and homosynaptic depression after a bout of whole-body vibration. Muscle Nerve. 2011;43(2):259–262. doi: 10.1002/mus.21844. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong WJ, Nestle HN, Grinnell DC, et al. The acute effect of whole-body vibration on the Hoffmann reflex. J Strength Cond Res. 2008;22(2):471–476. doi: 10.1519/JSC.0b013e3181660605. [DOI] [PubMed] [Google Scholar]
- 29.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49(2):72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 30.Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514((pt 3)):915–928. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]