Abstract
Context:
Little is known about exercise intolerance or the utility of an exercise evaluation in patients with postconcussion syndrome (PCS).
Objective:
To assess exercise intolerance in male and female patients with PCS.
Design:
Cross-sectional study.
Setting:
Laboratory setting.
Patients or Other Participants:
Participants included a convenience sample of 34 patients with PCS (17 males, 17 females; age = 25.9 ± 10.9 years) and 22 uninjured individuals on whom we gathered historical deidentified laboratory data (control group; 11 males, 11 females; age = 23.3 ± 6.2 years).
Main Outcome Measure(s):
Self-reported symptoms, heart rate, systolic and diastolic blood pressures (BPs), and the Borg rating of perceived exertion were measured before, during each minute of, and immediately after a graded treadmill exercise test (Balke protocol). Exercise was stopped when participants could no longer maintain the effort or reported the onset of or increase in PCS symptoms.
Results:
Exercise test duration (8.5 ± 4.4 minutes versus 17.9 ± 3.6 minutes; t51 = 1.8, P < .001), heart rate (142.8 ± 24.1 versus 175.2 ± 17.4; t54 = −5.5, P < .001), and systolic BP (142.1 ± 18.3 mm Hg versus 155.5 ± 24.5 mm Hg; t53 = 2.3, P = .02) were lower, and diastolic BP (78.4 ± 10.2 mm Hg versus 73.5 ± 11.7 mm Hg; t53 = 2.2, P = .03) was higher at test cessation in the PCS than control group. Cox regression showed the odds of a shorter exercise duration were nearly 8 times greater in the PCS than control group (hazard ratio = 7.93; 95% confidence interval = 3.39, 18.56). In the general linear models that adjusted for differences in test duration, rating of perceived exertion was the only physiologic measure to show an overall difference between groups, with the control group reporting higher ratings than the PCS group (t53 = −6.0, P < .001). Within the PCS group, systolic BP was the only measure to show a sex effect, with males showing higher pressure readings than females throughout the exercise tests (t31 = 2.8, P = .009).
Conclusions:
Patients with PCS had a symptom-limited response to exercise, and the treadmill test was a potentially useful tool to monitor the recovery from PCS.
Key Words: brain injuries, concussions, treadmill test
Key Points.
All patients with postconcussion syndrome (PCS) reported increased symptoms or the appearance of additional symptoms from baseline that led to exercise cessation.
Patients with symptoms of PCS had limited ability to exercise when compared with uninjured control participants.
Whereas physical exertion is contraindicated in symptomatic patients after head injury, patients with PCS may be able to safely perform low-level exercise without risk of symptom exacerbation.
A lack of symptom exacerbation during exercise testing in patients after concussion could indicate a diagnosis other than PCS to account for their reports of prolonged PCS-like symptoms.
The treadmill test may provide another tool to help distinguish PCS from potential differential diagnoses and to monitor the clinical recovery of PCS, especially in athletes.
Brain injury and the resulting disability is a major public health concern,1 with 1.7 million traumatic brain injuries (TBIs) reported each year in the United States.2 Researchers3 have estimated that mild TBI represents between 70% and 90% of reported cases of TBI and that the rate of occurrence of mild TBI is more than 600 in 100 000 people per year. Symptoms from concussion typically resolve within 7 to 10 days4; however, up to 33% of individuals with concussions5,6 have postconcussion syndrome (PCS), with up to 30% continuing to meet criteria for PCS 6 months postinjury.7 Age and sex may play roles in the diagnosis of PCS because women but not girls report higher symptom scores than males on a commonly used PCS diagnostic, the Rivermead Post Concussion Symptoms Questionnaire.8
Postconcussion syndrome is the nonresolution of symptoms after a concussion. Definitions of PCS given by the American Psychiatric Association9 and the World Health Organization10 rely heavily on self-reported symptoms as the basis for diagnosis. The definition of PCS given by the World Health Organization10 includes a history of traumatic brain injury and 3 or more symptoms (headache; dizziness; fatigue; irritability; insomnia; difficulty in concentration or memory; intolerance of stress, emotion, or alcohol).10 No cognitive testing, exclusion of other disorders, or symptom threshold exists for the diagnosis of PCS.
Current recommendations11 state that, after a concussion, athletes may return to sport only after being asymptomatic at rest and during a graduated exercise program of increasing intensity and contact. Symptoms and neurocognitive performance, however, may be affected negatively by increased physical activity levels after concussion.12,13 Patients who develop the onset of or worsening concussive symptoms are directed to adopt a period of complete rest until they are asymptomatic again.
Symptom exacerbation during exercise has been proposed to be related to autonomic nervous system (ANS) dysfunction.14 An uncoupling between the ANS and cardiovascular systems has been demonstrated in acute brain injury.15,16 An exercise test may be useful in provoking physiologic markers of autonomic dysfunction that may not be observable during resting conditions. The stress of an exercise test may provoke an autonomic uncoupling similar to that demonstrated with mental stress in patients with PCS.17 An exercise evaluation also may be useful because lack of symptom exacerbation during exercise testing could indicate a diagnosis other than PCS to account for the presence of resting symptoms (eg, cervicogenic headache and dizziness,14 posttraumatic stress,18 migraine).
Whereas successful completion of a staged exercise evaluation is recommended before return to activity after concussion, little is known about its utility in patients with prolonged symptoms. Current return-to-play protocols are directed at recovery patterns from a concussion that typically resolves within 7 to 10 days. Unlike most patients after a concussion, patients with PCS are rarely asymptomatic at rest, which precludes them from attempting the exercise testing proposed by McCrory et al.11 In addition, the general descriptions used to date within the graduated return-to-play protocol (eg, light aerobic exercise to <70% maximal predicted heart rate [HR]) lack scientific evidence for their utility.11
Leddy et al19,20 showed that a graduated treadmill exercise test was safe and reliable in patients with PCS. Therefore, the main purpose of our study was to assess exercise intolerance as indicated by the appearance of or increases in symptoms in patients with PCS. We wanted to assess biometric differences during a graded exercise test between a group with PCS and a historically uninjured control group, assess exercise-related changes over time and differences between groups in the physiologic measures (HR, Borg Scale for rating of perceived exertion [RPE],21 systolic blood pressure [BP], and diastolic BP), assess the rates at which participants reached volitional test cessation in the PCS and control groups, and evaluate sex-related differences in both biometrics and rates of volitional test cessation within the PCS group.
METHODS
Participants
PCS Group
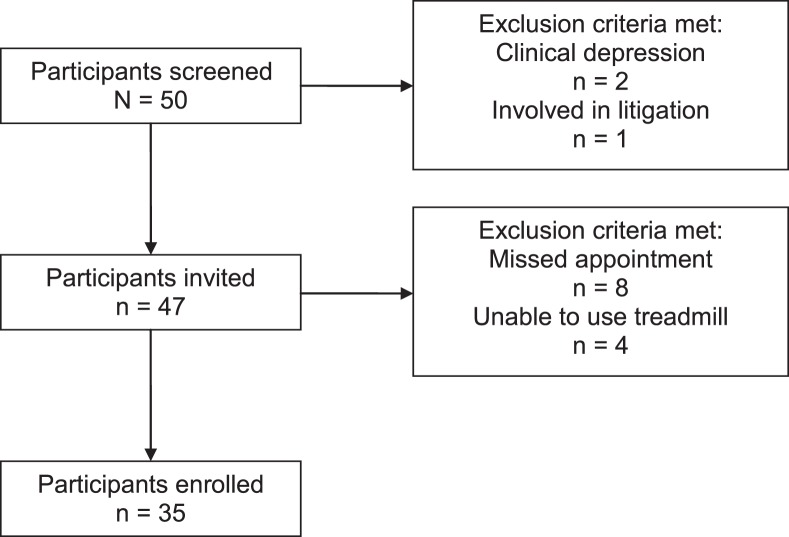
We considered all eligible individuals (17 males, 17 females; age = 25.9 ± 10.9 years) reporting consecutively to a local university sports medicine clinic with concerns of PCS for inclusion with no discrimination based on sex, race, or ethnic background (Figure 1). On average, participants were 226 days postinjury, and all self-reported that they were sedentary during the period preceding enrollment. For this experiment, sedentary was defined as less than 30 minutes of purposeful physical activity per week for the 3 months before the study. Participants were injured during sport- or nonsport-related incidents (eg, car accidents, falls, nonassault-related head contact). The diagnosis was confirmed in all patients through evaluation by a Board-certified internist (J.J.L.) and fulfillment of the World Health Organization10 criteria for symptoms of PCS. Eligibility was defined as healthy (aside from PCS diagnosis) with low-risk cardiovascular status (≤1 risk factor) as defined by the American College of Sports Medicine22 or with written authorization from the primary care physician and no associated psychological or cognitive abnormalities (eg, no pre-injury depression, anxiety, or bipolar disorder). Exclusion criteria included the use of medications that may alter symptom experience or exercise performance, including beta blockers, tricyclic antidepressants, selective serotonin reuptake inhibitors, and anticonvulsants; the inability to walk on a treadmill at grade; a history of orthopaedic injuries that would inhibit aerobic exercise activity; and pending litigation.
Figure 1. .
Participant recruitment process.
Those meeting eligibility requirements during a prescreen interview were invited to participate. The 90-minute laboratory visit included resting measures of HR and BP, symptom assessment, and a graded exercise test.
Control Group
We gathered historical deidentified laboratory data on 22 participants (11 males, 11 females; age = 23.3 ± 6.2 years), and they composed the control group. They were matched to the experimental group for age, sex, and sport participation history. At the time of testing, these participants completed a medical history questionnaire indicating they were sedentary but otherwise healthy individuals with no history of head injury. Participants then performed an exercise test identical to that performed by the PCS group.
All participants provided written informed consent or assent (parents or guardians provided informed consent in the latter case), and the study was approved by the Health Sciences Institutional Review Board of the University of Buffalo.
Resting Profiles
We manually took resting BP using standard sphygmomanometry (OMRON, Palatine, IL) of the left upper extremity and measured HR using a chest strap and wristwatch (model 810i T61; Polar, Kempele, Finland). To identify the presence of PCS symptoms at the time of and in the 24 hours before evaluation, we assessed all participants in the PCS group and 12 participants in the control group using a 27-item symptom checklist and the Head Injury Scale (Table 1).23,24
Table 1. .
Symptoms of Postconcussion Syndrome Assessed
| Symptom Checklista |
Head Injury Scaleb |
| Blurred vision | Balance difficulty/dizziness |
| Dizziness | Difficulty concentrating |
| Drowsiness | Drowsiness |
| Excess sleep | Fatigue |
| Easily distracted | Feeling slowed down |
| Fatigue | Feeling like “in a fog” |
| Feel “in a fog” | Headache |
| Feel “slowed down” | Nausea |
| Headache | Trouble falling asleep |
| Inappropriate emotions | |
| Irritability | |
| Loss of consciousness | |
| Loss of orientation | |
| Memory problems | |
| Nausea | |
| Nervousness | |
| Personality change | |
| Poor balance/coordination | |
| Poor concentration | |
| Ringing in ears | |
| Sadness | |
| Seeing stars | |
| Sensitivity to light | |
| Sensitivity to noise | |
| Sleep disturbance | |
| Vacant stare/glassy eyed | |
| Vomiting |
Presence was noted by checking all that applied.
Presence of symptoms was rated on a 7-point Likert scale, with 0 indicating never and 6 indicating always.
Graded Exercise Test
During their initial visits, participants walked on a treadmill following a standard Balke protocol.25 The treadmill speed was set at 3.3 miles per hour at a 0.0% incline (3.5 metabolic equivalents of task). Heart rate was recorded by chest strap as previously described. After 1 minute, we increased the treadmill grade to 2.0% and instructed the participant to maintain the same walking speed (4.4 metabolic equivalents of task). At each 2-minute interval, BP was taken. At the start of the third minute and each minute thereafter, we increased the grade by 1.0%, measured HR and RPE,21 and assessed the presence of symptoms. The participants in both groups were instructed repeatedly to notify the administrator (K.F.K.) of any increase in PCS symptom intensity or presence of new PCS symptoms so the test could be terminated immediately. This process continued until individuals could no longer maintain the appropriate speed, had onset or exacerbation of symptoms of PCS, or reached maximal allowable test capability (21 minutes). Immediately upon test termination, the final measurements were taken. Data from 1 participant were removed from statistical analysis because the values were highly variable and were greater than 2 times the standard deviation.
Data Analysis
Descriptive summaries of all variables were first calculated and stratified by group (PCS, control). Independent t tests were used to assess differences in group means. We used the Cohen d to determine the effect size of test duration, maximal HR, RPE, maximal systolic BP, and maximal diastolic BP. An effect size greater than 0.80 was considered high; greater than 0.050 to 0.80, moderate to high; and equal to or less than 0.050, low. To assess differences in rates of test cessation, including volitional and researcher-halted cessation, unadjusted differences in short-term survival rates (exercise test duration) were compared using Kaplan-Meier (Mantel-Cox log rank) analyses for PCS versus control and for males versus females within the PCS group only. Cox regression models were used to evaluate differences in time to exercise cessation while controlling for demographic factors (age, sex) that might have influenced exercise performance. The first model compared the PCS with the control group and included both sex and age. In a post hoc analysis, the second model with the PCS group only compared males and females and included age. To better characterize the risk of exercise cessation over time, adjusted cumulative survival curves were generated from both multivariable Cox regression models: PCS versus control group and males versus females within the PCS group only. We used SPSS (version 18.0; IBM Corporation, Armonk, NY) for all analyses. The α level was set to ≤ .05.
RESULTS
Physiologic Measures
Group-specific descriptive summaries and results of the t tests comparing group means are shown in Table 2. By definition, the PCS group reported experiencing symptoms within the 24 hours before testing, as indicated by symptom checklist score (9.9 ± 6.3) and Head Injury Scale severity scores (20.8 ± 13.7). The 12 control participants assessed for symptoms (not included in Table 2) indicated much lower average scores on the symptom checklist (1.9 ± 2.1) and Head Injury Scale (2.2 ± 4.0). We found no differences between the PCS and control groups in basic demographic information or mean resting physiologic measures (P > .05). In contrast, exercise performance (P < .05) and maximal physiologic responses to exercise (P < .05) differed between groups. Mean exercise test duration was 9.4 minutes less for the PCS than control group. Accordingly, mean percentage changes in HR and systolic BP from rest to test cessation were 12% and 10% less, respectively, in the PCS than the control group. Despite the PCS group having a 47% reduction in test duration, mean maximal diastolic BP increased by 1.3 mm Hg compared with a 0.4-mm Hg increase in the control group. The Cohen d effect size criteria for clinical differences demonstrated a high effect size (>0.80) for test duration (2.3), maximal HR (1.5), and RPE (1.6) and a moderate to high effect size (>0.50) for maximal systolic BP (0.61) and diastolic BP (0.58).
Table 2. .
Sample Characteristics and Outcome Measures by Experimental Group
| Characteristic |
Experimental Group |
t |
df |
P |
|||
| Control (n = 22) |
Postconcussion Syndrome (n = 34) |
||||||
| Mean ± SD |
Range |
Mean ± SD |
Range |
||||
| Age, y | 23.3 ± 6.2 | 18–45 | 27.5 ± 12.9 | 15–53 | 1.8 | 51 | .08 |
| Test duration, min | 17.9 ± 3.6 | 11–21 | 8.5 ± 4.4a | 2–7 | –8.4 | 54 | <.001 |
| Resting heart rate, beats/ min | 69.7 ± 10.0 | 58–93 | 74.5 ± 11.3 | 48–96 | 1.5 | 46 | .15 |
| Maximal heart rate, beat/ min | 175.2 ± 17.4b | 123–200 | 142.8 ± 24.1a,b | 105–190 | –5.5 | 54 | <.001 |
| Age-predicted maximal heart rate, % | 88.8 ± 9.6 | 61.8–104.7 | 74.4 ± 12.1a | 58.0–106.3 | –4.8 | 54 | <.001 |
| Maximal rating of perceived exertion21 | 17.1 ± 2.1 | 13–20 | 13.0 ± 2.7a | 7–19 | –6.0 | 53 | <.001 |
| Resting systolic blood pressure, mm Hg | 115.0 ± 12.1 | 88–140 | 118.7 ± 9.3 | 98–136 | 1.3 | 53 | .21 |
| Maximal systolic blood pressure, mm Hg | 155.5 ± 24.5b | 112–220 | 142.1 ± 18.3b,c | 110–182 | –2.3 | 53 | .02 |
| Resting diastolic blood pressure, mm Hg | 73.1 ± 10.6 | 58–90 | 78.4 ± 10.2 | 42–94 | 1.9 | 53 | .07 |
| Maximal diastolic blood pressure, mm Hg | 73.5 ± 11.7 | 54–105 | 79.7 ± 9.2c | 62–100 | 2.2 | 53 | .03 |
| Time since injury, d | NA | NA | 226.2 ± 219.3 | 34–949 | |||
| Symptom checklist for previous 24-h period, sum | NA | NA | 9.9 ± 6.3 | 0–24 | |||
| Head Injury Scale24 for previous 24-h period, sum | NA | NA | 20.8 ± 13.7 | 0–47 | |||
Abbreviation: NA indicates not applicable.
Indicates different from control (P < .001).
Indicates different from resting (P < .001).
Indicates different from control (P < .05).
Rate of Test Cessation
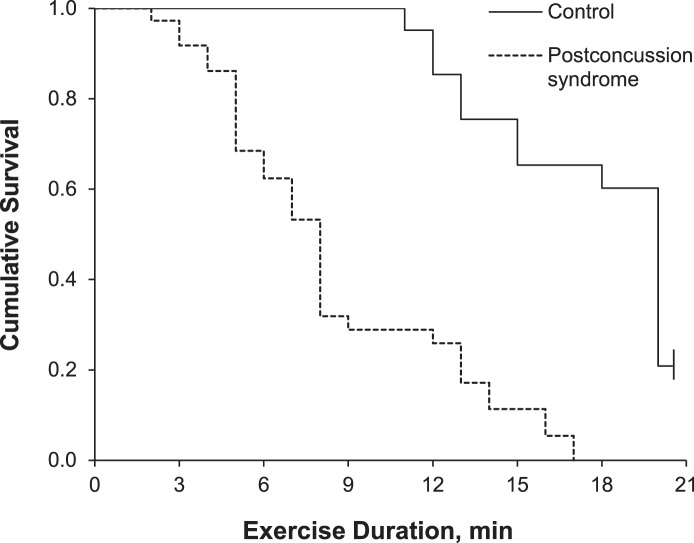
The graded exercise tests were terminated (censored) at 21 minutes. All tests on the PCS participants were terminated before the final stage due to exacerbation of PCS symptoms. No control group participants terminated their testing due to symptom exacerbation. The PCS group participants indicated the appearance of symptoms not present before testing (dizziness, blurred vision, poor balance) or the worsening of symptoms that were present before testing (headache, balance difficulty, dizziness). Five (23%) of the control participants completed the final stage and were censored. Unadjusted Kaplan-Meier analysis provided statistical evidence for the shorter exercise durations in the PCS versus control group (Mantel-Cox log rank = 35.51, P < .001). Median exercise durations for the PCS and control groups were 8 and 20 minutes, respectively. Multivariable Cox regression analysis showed the odds of a shorter exercise duration were nearly 8 times greater among PCS than control participants (hazard ratio = 7.93; 95% confidence interval [CI] = 3.39, 18.56). The model controlled for sex and age, but neither sex (male hazard ratio = 0.54; 95% CI = 0.29, 1.01) nor age (hazard ratio = 1.02; 95% CI = 0.99, 1.05) were different. Cumulative survival curves for PCS and control groups by exercise duration are provided in Figure 2; the values are adjusted for sex and age.
Figure 2. .
Cumulative survival curve by exercise duration for the postconcussion syndrome and control groups. Tests were censored at 21 minutes. Values are adjusted for mean of covariates (sex and age).
Sex
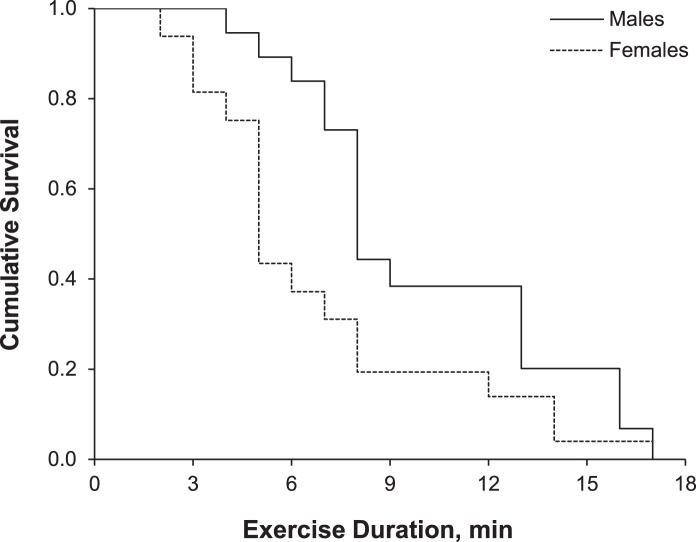
After our initial analysis, we wanted to identify any potential sex differences in our PCS group. A post hoc analysis within the PCS group showed no differences for HR (P = .63) or RPE (P = .28) between sexes. Resting BP measurements were lower in females than in males, and maximal SBP was also lower in females at test cessation. Characteristics of the PCS group by sex and the results of the t tests comparing group means are shown in Table 3. Whereas the overall test duration was less in females with PCS, the rate of exercise cessation was not different between sexes (Mantel-Cox log rank = 2.18, P = .14). Median exercise durations for males and females were 8 and 5 minutes, respectively. In the Cox regression model, neither sex (male hazard ratio = 0.50; 95% CI = 0.22, 1.15) nor age (hazard ratio = 1.02; 95% CI = 0.99, 1.05) was associated with the rate of exercise cessation. Cumulative survival curves for males and females by exercise duration among PCS participants are illustrated in Figure 3; the values are adjusted for age.
Table 3. .
Sample Characteristics and Outcome Measures in the Postconcussion Syndrome Group by Sex (Mean ± SD)
| Characteristic |
Postconcussion Syndrome Group |
t |
df |
P |
|
| Females (n = 17) |
Males (n = 17) |
||||
| Age, y | 25.3 ± 12.3 | 30.7 ± 13.2 | 1.2 | 32 | .22 |
| Test duration, min | 7.2 ± 4.4 | 9.8 ± 4.1a | 1.3 | 32 | .20 |
| Resting heart rate, beats/min | 77.5 ± 8.1 | 71.2 ± 13.6 | –1.6 | 28 | .13 |
| Maximal heart rate, beats/min | 144.8 ± 25.7 | 140.7 ± 22.8 | –0.5 | 32 | .63 |
| Age-predicted maximal heart rate, % | 74.3 ± 11.9 | 74.5 ± 12.6 | –0.3 | 32 | .80 |
| Maximal rating of perceived exertion21 | 12.5 ± 2.3 | 13.5 ± 3.1 | 1.1 | 31 | .28 |
| Resting systolic blood pressure, mm Hg | 115.5 ± 10.6 | 122.1 ± 6.7a | 2.1 | 31 | .04 |
| Maximal systolic blood pressure, mm Hg | 134.3 ± 13.4 | 150.4 ± 19.5b | 2.8 | 31 | .009 |
| Resting diastolic blood pressure, mm Hg | 73.8 ± 11.5 | 83.4 ± 5.7b | 3.0 | 31 | .005 |
| Maximal diastolic blood pressure, mm Hg | 78.9 ± 10.9 | 80.5 ± 7.2 | 0.5 | 31 | .63 |
| Time since injury, d | 223.1 ± 172.7 | 229.4 ± 263.4 | 0.1 | 32 | .94 |
| Symptom checklist for previous 24-h period, sum | 9.3 ± 6.0 | 10.6 ± 6.9 | 0.6 | 31 | .56 |
| Head Injury Scale24 for previous 24-h period, sum | 19.5 ± 13.8 | 22.3 ± 14.0 | 0.6 | 31 | .56 |
Indicates different from females (P < .05).
Indicates different from females (P < .01).
Figure 3. .
Cumulative survival curve by exercise duration for males and females within the postconcussion syndrome group. Values are adjusted for age.
DISCUSSION
Our results empirically demonstrate that patients who reported to our laboratory with symptomatic PCS had limited ability to exercise when compared with uninjured controls. The asymptomatic response to graded exercise is a primary criterion for return to sport.11 All participants with PCS reported an increase in symptom severity or the appearance of additional symptoms from baseline that led to exercise cessation. The treadmill test we used may provide another tool to help distinguish PCS from potential differential diagnoses and to monitor the clinical recovery of PCS, especially in athletes. Evidence from our study supports the theory that a lack of symptom exacerbation during exercise testing in patients after concussion could indicate a diagnosis other than PCS to account for their reports of prolonged PCS-like symptoms (eg, cervicogenic headache and dizziness,14 posttraumatic stress,18 migraine). In addition, monitoring increases in asymptomatic exercise tolerance during sequentially administered treadmill tests may indicate a clinically relevant recovery progression in patients with PCS.
Current recommendations10 state that after a concussion, athletes may return to sport only after being asymptomatic at rest and during a graduated exercise program of increasing intensity. Current protocols are directed at patterns of recovery from a concussion that typically resolves within 7 to 10 days, whereas our participants remained symptomatic an average of 226 days postinjury. Our participants progressed safely through gradual systematic increases in exercise intensity and were monitored closely for changes in the presence of symptoms. This controlled environment allowed us to safely assess the response to graded physiologic stress of individuals who had sustained concussions.
Controlled aerobic exercise rehabilitation after establishing symptom-free exercise capacity via treadmill testing has helped patients with PCS recover.20,26 For patients with symptoms lasting 6 weeks or longer, the treadmill test is safe and enables a faster recovery than a period of rest alone.20 This is consistent with animal data on recovery from concussion.27,28 Whereas patients with concussion must rest physically and cognitively within the acute phase (1–3 weeks),11 no evidence has shown that rest beyond this timeframe is beneficial to recovery. Baker et al26 reported that 77% of patients with PCS treated with controlled aerobic exercise returned to full daily functioning.
Test Duration
We evaluated the time to test cessation using Cox regression. Our results indicated that, when controlling for age and sex, patients with PCS whose exercise tests were discontinued due to the appearance or worsening of symptoms had a greater reduction in asymptomatic exercise capacity than healthy individuals. The median exercise duration among participants with PCS was 40% of the control group. The physiologic stress of a treadmill test, thus, can differentiate between patients with and without PCS.
Heart Rate
Similar to other investigators,16,17,20,29,30 we found that resting HR values did not differ between the PCS and control groups. The PCS group had lower unadjusted HRs at test termination, which reflected the shorter test time and, therefore, a lower achieved workload rather than injury status. Although their maximal HRs were lower than those of control participants, participants with PCS reached almost 75% of their age-predicted maximal HRs. This observation lends support to the recommendation by McCrory et al11 that the second stage of the graduated return-to-play protocol involve light aerobic exercise at less than 70% of maximal predicted HR.
To date, no one has evaluated the exercise response of patients who remain symptomatic after concussion. Investigators have found evidence of altered physiologic response to exercise in patients who recently have had concussions but are asymptomatic. Gall et al29 showed differences in HR during exercise between participants with and without concussions. We and Gall et al29 have evaluated exercise responses in patients after concussion; however, the participants in the latter study were asymptomatic. Differences in clinical presentation (symptomatic versus asymptomatic) after concussive injury lie along a continuum of recovery and, therefore, may exhibit progressively similar physiologic responses to exercise. Timing of the test after injury also may have played a role in the differences reported between studies. Our exercise evaluation was performed at a minimum of 34 days postinjury, which is well after the 7- to 10-day expected healing window.4 Gall et al evaluated participants with acute injuries who were 6.7 ± 1.8 days29 and 5.0 ± 1.4 days16 postinjury. Athletes with acute concussions demonstrated higher HRs during steady-state exercise and a greater rise in HR over time during a graded exercise test.29 Athletes with concussions also continue to exhibit differences in HR variability, a measure of ANS balance, during low to moderate steady-state exercise after they are asymptomatic.16 In addition, the higher intensity of the exercise intervals Gall et al29 used may have been responsible for the differences reported in HR between participants with and without concussions. Given the onset of postconcussion symptoms and, therefore, earlier test cessation, our participants with PCS did not reach an intensity comparable with that in the participants Gall et al29 tested.
Researchers30 have reported altered cardiovascular and pulmonary responses to exercise in patients with TBI. Mossberg et al30 found that HRs were lower in those with moderate and severe TBI than in nondisabled control participants. They also reported differences in oxygen consumption, oxygen saturation, and minute ventilation in the TBI group during a graded treadmill test. However, Mossberg et al30 did not report if patients were exercise limited due to the onset of symptoms or if they were forced to stop due to reaching physiologic capacity. All participants in our PCS group were symptom limited and were stopped before reaching their physiologic maximum.
Blood Pressure
In an exercise training study, Leddy et al20 concluded that PCS symptoms during exercise were triggered at a critical BP. We observed a higher diastolic BP in the PCS than control group only at peak exercise. This suggests the worsening or appearance of PCS symptoms during exercise may be related to the elevated end-test diastolic BP. In healthy participants, intact cerebral autoregulation is responsible for buffering BP oscillations and thereby protecting brain tissue against changes in perfusion.31 If a disruption in cerebral autoregulation causes or incorrectly detects changes in brain perfusion, an associated BP response may occur. Alterations in peripheral resistance and the resultant rise in diastolic BP observed in our study may be due to this ANS dysregulation. Others also have reported a similar loss of BP control with brain injury.15,32 Our results help substantiate the hypothesis that prolonged symptoms after a concussion may be associated with cerebral dysregulation.24 However, without direct measurement of ANS function or cerebral perfusion, we cannot identify the mechanisms responsible for possible dysregulation. Although our PCS and control groups were matched based on age, sex, and sport participation history, the differences we reported in diastolic BP might be artifacts of individual differences unaccounted for in our test groups. In addition, the lower systolic BP measures observed may reflect the shorter test time and, therefore, a lower achieved workload rather than injury status.
Ratings of Perceived Exertion
The RPE for the PCS group was lower at volitional cessation, possibly because the participants stopped exercise at a lower workload than control participants. Alternatively, given that the RPE was constructed to increase linearly with workload,21 the presence of brain injury may moderate this relationship. The literature contains discrepancies regarding the perceived exertion reported by participants with and without brain injury.33–35 Similar to our findings, Dawes et al34 reported that individuals with brain injury had shorter exercise tests; however, they found no differences in ratings of exertion. This inconsistency may be due to differences in brain injury between studies because Dawes et al34 evaluated participants with TBI and stroke. We also may have observed differences in RPE if participants with PCS interpreted the meaning of the written descriptors attached to the RPE scale (eg, very light, somewhat hard) differently than uninjured control participants.35 Dawes et al35 reported that participants with prolonged acquired brain injury (average = 14.2 ± 12.03 months postinjury) may have cognitive limitations in understanding the RPE scale. Merritta et al33 studied patients with mild to moderate brain injury and excluded those with cognitive impairments to limit the effect of cognitive function on physical performance and RPE. They showed increases in perceived exertion in the patients with brain injuries when compared with healthy control participants. Although the authors of these studies included participants with brain injury, the differences in severity of brain injury may have contributed to the disparities observed among studies.
Limitations
Oxygen consumption, ventilation, and cerebral blood flow measures may have enhanced our ability to detect differences in exercise responses in the PCS group. We did not take respiratory measurements because, in pilot studies, the collection mask or mouthpiece seemed to increase our participants' anxiety and thereby elicited an early symptom response unrelated to exercise tolerance. Researchers17 have shown evidence of increased symptom response to cognitive stressors unrelated to physical performance.
Another limitation is that we used data from a historical control group. Although the use of a standard exercise protocol, such as the Balke,25 allowed us to compare our PCS group with existing control group data, we could not directly assess the presence of PCS symptoms in all control participants before exercise.36 A final factor limiting the generalizability of our results was that the population with PCS we evaluated was a convenience sample of patients who reported consecutively to a local university sports medicine clinic.
Investigators should directly assess cardiorespiratory responses and cerebral blood flow during exercise in patients with and without PCS. Currently, subjective symptom reporting is the most commonly used clinical criterion indicating recovery from PCS. Information gathered with direct physiologic measures may allow an exercise tolerance threshold (which does not rely on subjective symptom report) to be identified in patients with PCS. In addition, researchers could follow the possibility of delayed symptom exacerbation in patients with PCS over time in the days after participation in a graded exercise test. Researchers also may use an exercise test protocol longitudinally to follow changes in exercise intolerance over time in patients with PCS and identify physiologic indicators of recovery.
Clinical Implications
The graded exercise test that we used allowed us to objectively measure intolerance and physiologic responses to exercise in patients with prolonged symptoms after concussion. This is clinically relevant because PCS is defined by the persistence of subjective symptoms at rest.9,10 We need to recognize that outcomes based on commonly used subjective measures of exercise intensity, including RPE, may be affected by the presence of PCS. Our work provides an additional tool for sports medicine professionals in diagnosing PCS from a physiologic standpoint. Given that all of our patients with PCS terminated exercise due to the presence of or increase in symptoms, if increasing exercise intensity fails to exacerbate symptoms, the possibility of a condition other than PCS (migraine, cervicogenic origin of symptoms, posttraumatic stress) should be investigated. In addition, the information gathered during the exercise test may provide a starting point for developing appropriate physical rehabilitation programs for patients with PCS.
CONCLUSIONS
We experimentally quantified a symptom-limited response to exercise in patients with PCS. Whereas physical exertion currently is contraindicated in symptomatic patients after head injury, patients with PCS may be able to safely perform low-level exercise (eg, <75% age-predicted maximal HR) without risk of symptom exacerbation.
ACKNOWLEDGMENT
Funding for this study was provided in part by the New York State Athletic Trainers' Association.
REFERENCES
- 1.Consensus conference. Rehabilitation of persons with traumatic brain injury: NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury. JAMA. 1999;282(10):974–983. [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald MM, Coronado V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths, 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control;; 2010. [Google Scholar]
- 3.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;((43 suppl)):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 4.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 5.Binder LM, Rohling ML, Larrabee GJ. A review of mild head trauma: part I. Meta-analytic review of neuropsychological studies. J Clin Exp Neuropsychol. 1997;19(3):421–431. doi: 10.1080/01688639708403870. [DOI] [PubMed] [Google Scholar]
- 6.Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA. Disability caused by minor head injury. Neurosurgery. 1981;9(3):221–228. [PubMed] [Google Scholar]
- 7.Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829–836. doi: 10.1076/jcen.23.6.829.1022. [DOI] [PubMed] [Google Scholar]
- 8.Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PMR. 2009;1(3):245–253. doi: 10.1016/j.pmrj.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington, VA: American Psychiatric Association;; 1994. pp. 704–706. [Google Scholar]
- 10.World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th revision. Geneva, Switzerland: World Health Organization;; 1993. pp. 63–64. [Google Scholar]
- 11.McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. J Athl Train. 2009;44(4):434–448. doi: 10.4085/1062-6050-44.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majerske CW, Mihalik JP, Ren D, et al. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J Athl Train. 2008;43(3):265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Clin J Sport Med. 2005;15(2):48–55. doi: 10.1097/01.jsm.0000159931.77191.29. [DOI] [PubMed] [Google Scholar]
- 14.Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol. 2006;8(5):415–426. doi: 10.1007/s11940-006-0031-9. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein B, Toweill D, Lai S, Sonnenthal K, Kimberly B. Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am J Physiol. 1998;275((4 pt 2)):R1287–R1292. doi: 10.1152/ajpregu.1998.275.4.R1287. [DOI] [PubMed] [Google Scholar]
- 16.Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc. 2004;36(8):1269–1274. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- 17.Hanna-Pladdy B, Berry ZM, Bennett T, Phillips HL, Gouvier WD. Stress as a diagnostic challenge for postconcussive symptoms: sequelae of mild traumatic brain injury or physiological stress response. Clin Neuropsychol. 2001;15(3):289–304. doi: 10.1076/clin.15.3.289.10272. [DOI] [PubMed] [Google Scholar]
- 18.Sallis RE, Jones K. Prevalence of headaches in football players. Med Sci Sports Exerc. 2000;32(11):1820–1824. doi: 10.1097/00005768-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Leddy JJ, Baker JG, Kozlowski K, Bisson L, Willer B. Reliability of a graded exercise test for assessing recovery from concussion. Clin J Sport Med. 2011;21(2):89–94. doi: 10.1097/JSM.0b013e3181fdc721. [DOI] [PubMed] [Google Scholar]
- 20.Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med. 2010;20(1):21–27. doi: 10.1097/JSM.0b013e3181c6c22c. [DOI] [PubMed] [Google Scholar]
- 21.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 22.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins;; 2006. pp. 18–39. [Google Scholar]
- 23.Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers' Association position statement: management of sport-related concussion. J Athl Train. 2004;39(3):280–297. [PMC free article] [PubMed] [Google Scholar]
- 24.Leddy JJ, Kozlowski K, Fung M, Pendergast DR, Willer B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: implications for treatment. NeuroRehabilitation. 2007;22(3):199–205. [PubMed] [Google Scholar]
- 25.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92(1):39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 26.Baker JG, Freitas MS, Leddy JJ, Kozlowski KF, Willer BS. Return to full functioning after graded exercise assessment and progressive exercise treatment of postconcussion syndrome. Rehabil Res Pract. 2012;2012:705309. doi: 10.1155/2012/705309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016(2):154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- 28.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Gall B, Parkhouse WS, Goodman D. Exercise following a sport induced concussion. Br J Sports Med. 2004;38(6):773–777. doi: 10.1136/bjsm.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mossberg KA, Ayala D, Baker T, Heard J, Masel B. Aerobic capacity after traumatic brain injury: comparison with a nondisabled cohort. Arch Phys Med Rehabil. 2007;88(3):315–320. doi: 10.1016/j.apmr.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Brys M, Brown CM, Marthol H, Franta R, Hilz MJ. Dynamic cerebral autoregulation remains stable during physical challenge in healthy persons. Am J Physiol Heart Circ Physiol. 2003;285(3):H1048–H1054. doi: 10.1152/ajpheart.00062.2003. [DOI] [PubMed] [Google Scholar]
- 32.DeWitt DS, Prough DS. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J Neurotrauma. 2003;20(9):795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- 33.Merritta C, Cherian B, Macaden AS, John JA. Measurement of physical performance and objective fatigability in people with mild-to-moderate traumatic brain injury. Int J Rehabil Res. 2010;33(2):109–114. doi: 10.1097/MRR.0b013e32832e6b37. [DOI] [PubMed] [Google Scholar]
- 34.Dawes H, Scott OM, Roach NK, Wade DT. Exertional symptoms and exercise capacity in individuals with brain injury. Disabil Rehabil. 2006;28(20):1243–1250. doi: 10.1080/09638280600554595. [DOI] [PubMed] [Google Scholar]
- 35.Dawes HN, Barker KL, Cockburn J, Roach N, Scott O, Wade D. Borg's rating of perceived exertion scales: do the verbal anchors mean the same for different clinical groups? Arch Phys Med Rehabil. 2005;86(5):912–916. doi: 10.1016/j.apmr.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 36.Iverson GL, Lange RT. Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003;10(3):137–144. doi: 10.1207/S15324826AN1003_02. [DOI] [PubMed] [Google Scholar]