Abstract
Context:
Clinicians perform therapeutic interventions, such as stretching, manual therapy, electrotherapy, ultrasound, and exercises, to increase ankle dorsiflexion. However, authors of previous studies have not determined which intervention or combination of interventions is most effective.
Objective:
To determine the magnitude of therapeutic intervention effects on and the most effective therapeutic interventions for restoring normal ankle dorsiflexion after ankle sprain.
Data Sources:
We performed a comprehensive literature search in Web of Science and EBSCO HOST from 1965 to May 29, 2011, with 19 search terms related to ankle sprain, dorsiflexion, and intervention and by cross-referencing pertinent articles.
Study Selection:
Eligible studies had to be written in English and include the means and standard deviations of both pretreatment and posttreatment in patients with acute, subacute, or chronic ankle sprains. Outcomes of interest included various joint mobilizations, stretching, local vibration, hyperbaric oxygen therapy, electrical stimulation, and mental-relaxation interventions.
Data Extraction:
We extracted data on dorsiflexion improvements among various therapeutic applications by calculating Cohen d effect sizes with associated 95% confidence intervals (CIs) and evaluated the methodologic quality using the Physiotherapy Evidence Database (PEDro) scale.
Data Synthesis:
In total, 9 studies (PEDro score = 5.22 ± 1.92) met the inclusion criteria. Static-stretching interventions with a home exercise program had the strongest effects on increasing dorsiflexion in patients 2 weeks after acute ankle sprains (Cohen d = 1.06; 95% CI = 0.12, 2.42). The range of effect sizes for movement with mobilization on ankle dorsiflexion among individuals with recurrent ankle sprains was small (Cohen d range = 0.14 to 0.39).
Conclusions:
Static-stretching intervention as a part of standardized care yielded the strongest effects on dorsiflexion after acute ankle sprains. The existing evidence suggests that clinicians need to consider what may be the limiting factor of ankle dorsiflexion to select the most appropriate treatments and interventions. Investigators should examine the relationship between improvements in dorsiflexion and patient progress using measures of patient self-reported functional outcome after therapeutic interventions to determine the most appropriate forms of therapeutic interventions to address ankle-dorsiflexion limitation.
Key Words: chronic ankle instability, range of motion, stretching, joint mobilization
Key Points.
A static-stretching intervention as part of a standardized home exercise program had the strongest effects on ankle-dorsiflexion improvement after acute ankle sprains.
Clinicians need to consider what may be the limiting factor of ankle dorsiflexion to select the most appropriate treatments and interventions.
Investigators should examine the long-term effects of treatments on ankle dorsiflexion and a relationship between an improvement in ankle dorsiflexion and measures of patient self-reported and physical function to determine the most appropriate forms of therapeutic interventions to address limited dorsiflexion.
Lateral ankle sprain has been documented to be the most common lower extremity injury sustained during sport participation.1–4 Approximately 85% of all ankle sprains result from an inversion mechanism and damage to the lateral ligamentous complex of the ankle.5 Injury to the lateral ligamentous complex at the ankle joint results in pain, swelling, and limited osteokinematics.6 A loss of normal ankle dorsiflexion usually is observed at the talocrural joint after lateral ankle sprain.7–12
The amount of available ankle dorsiflexion plays a key role in the cause of lower extremity injuries.7,13–22 Limitation of dorsiflexion may be a predisposition to reinjury of the ankle11,16 and several future lower limb injuries, including plantar fasciopathy,13,20,21 lateral ankle sprains,13,15,17,19 iliotibial band syndrome,14 patellofemoral pain syndrome,18 patellar tendinopathy,22 and medial tibial stress syndrome.14
The importance of restoring ankle dorsiflexion after an acute ankle sprain often is emphasized in rehabilitation guidelines,9 and proper recovery of ankle dorsiflexion is a vital component of ankle rehabilitation. Inadequate restoration of ankle dorsiflexion may increase the risk of developing recurrent ankle sprain11,16 and limit functional activities, such as walking, with long-term pain and disability.23 Limited ankle-dorsiflexion range of motion (ROM) after lateral ankle sprain has been considered a predisposing factor for recurrent ankle sprain because diminished dorsiflexion prevents the ankle from reaching its closed-pack position by holding the ankle in a hypersupinated position. Therefore, ensuring appropriate restoration of ankle dorsiflexion after ankle sprain has important clinical implications for restoring full functional abilities, ultimately leading to reduced risk of recurrent ankle sprain.
Clinicians perform several therapeutic interventions, such as stretching, manual therapy, electrotherapy, ultrasound, and exercises, to increase ankle dorsiflexion. However, the intervention or combination of interventions that most effectively improves ankle dorsiflexion has not been established. In previous systematic reviews,24–26 researchers have examined the effects of specific intervention techniques of manipulative therapy on various outcome variables. In addition, Bleakley et al27 conducted a systematic review with a comprehensive search of various therapeutic interventions to provide evidence for the management of ankle sprains and the prevention of long-term complications; however, the authors focused only on patients with an acute ankle sprain. Therefore, the purpose of this systematic review was to determine the magnitude of therapeutic intervention effects on and the most effective therapeutic interventions for restoring normal ankle dorsiflexion after ankle sprain. In contrast to previous reviews,24–26 we comprehensively searched the existing literature to determine the effectiveness of various therapeutic intervention techniques in restoring ankle dorsiflexion in patients with acute, subacute, or recurrent ankle sprains. By providing a quantitative estimate of the magnitude of the effect of therapeutic interventions, our review provides a new perspective on the evidence of interventions to restore ankle dorsiflexion in various stages of ankle-sprain conditions.
METHODS
Search Strategy
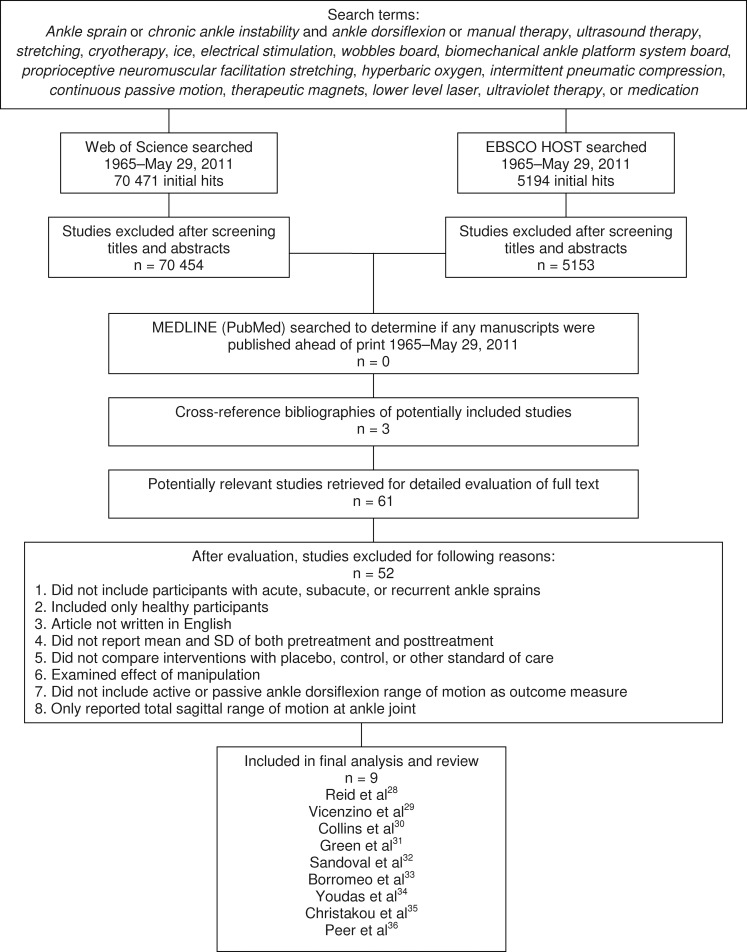
The primary author performed a comprehensive search for articles in the electronic databases Web of Science and EBSCO HOST based on combinations of the key words shown in Figure 1. In addition, articles that met inclusion criteria and were published electronically on MEDLINE (PubMed) ahead of print were included. The articles had to be written in English and published from 1965 to May 29, 2011. The references in the citation lists of potentially included articles were screened to identify additional articles that may have met the inclusion criteria and were not identified during the original database searches.
Figure 1.
Flow chart of included and excluded studies.
Selection Criteria
We identified research articles in which authors evaluated various therapeutic intervention techniques. We examined the full text of studies identified through the electronic searches and the cross-referenced bibliographies of these studies to determine whether they met the following inclusion criteria (Figure 1):
The authors included participants only with acute, subacute, or recurrent ankle sprains. We defined acute ankle sprain as occurring within 96 hours before study participation and resulting in swelling, pain, and limited function. Subacute ankle sprain was defined as occurring within 1 to 8 weeks before study participation. Finally, recurrent ankle sprain was defined as a history of at least 1 lateral ankle sprain and a recurrent episode of the ankle giving way (instability).
The authors examined the efficacy of at least 1 of the following interventions: manual therapy, therapeutic modalities, therapeutic exercises, or medication.
The authors did not examine the effect of manipulation with high velocity as an intervention because it is outside of the scope of practice for some clinicians, and we desired to report outcomes that would have broad clinical application.
The authors compared interventions with placebo, control, or other standard-of-care conditions.
The authors assessed active, passive, or functional ankle dorsiflexion ROM as an outcome measure.
The authors reported ankle-dorsiflexion means and standard deviations of both pretreatment and posttreatment or baseline and follow-up measurements; these data are necessary to calculate a standardized effect size for inclusion of these studies in the final data analysis. If the required statistical and numerical data were not reported in the published studies, we contacted authors through e-mail to request these data before deciding whether to exclude the published article from this review.
Data Extraction
The primary author extracted the relevant information in the selected studies. The data of interest were assessments of ankle dorsiflexion. After the inclusion criteria were applied to the title and abstracts, 70 454 of 70 471 articles and 5153 of 5194 articles were excluded from Web of Science and EBSCO HOST, respectively (Figure 1). Our electronic search using MEDLINE and cross-referenced bibliographies of potentially included studies produced an additional 3 articles. We read the remaining 61 articles in their entirety and excluded 52 because they did not meet the inclusion criteria. Nine studies met the inclusion criteria and were included for final data analysis.28–36 Discrepancies in data extraction were solved by discussion.
Methodologic Quality
After the research articles were identified for inclusion in the review, we applied the Physiotherapy Evidence Database (PEDro) scale37,38 to rate their quality. Each author used the PEDro scale to independently rate the studies that met the specified criteria. If agreement in a study's score was not achieved, the authors discussed and came to a consensus on a score. In addition, levels of evidence and grades of recommendation presented in the Oxford Centre for Evidence-based Medicine39 were applied to all included articles.
Data Synthesis
To be combined for data analysis, we categorized the included studies into 2 primary groupings of evidence based on onset of injury. The 2 groups were termed as (1) acute/subacute ankle sprain and (2) recurrent ankle sprain. We further subdivided the acute/subacute ankle sprain group into 4 subcategories of interventions: (1.1) manual therapy, (1.2) therapeutic modalities, (1.3) therapeutic exercises, and (1.4) psychological intervention. Only 2 articles included participants with recurrent ankle sprain, so this group was not subdivided.
To assess the magnitude of treatment effects, we calculated Cohen d effect sizes using means and standard deviations of pretreatment and posttreatment data or baseline and follow-up data for each study.40,41 If effect sizes were provided in the original publication, we recalculated the effect size for consistency in comparison across all the studies included in this review. The strength of an effect size was interpreted as weak (Cohen d ≤ 0.4), moderate (Cohen d range = >0.40–≤ 0.8), or strong (Cohen d > 0.8).40,41 We also calculated 95% confidence intervals (CIs) around the effect size. When interpreting an effect size, the researcher should note the magnitude of the point measure and the width of the 95% CI and should consider whether the range of CIs crosses zero. One cannot determine if a truly beneficial or harmful effect would be present in 95% of the samples collected when the 95% CI crosses zero. Therefore, studies yielding large effect sizes and small CIs that do not cross zero have the strongest clinical importance. We used Excel 2010 (Microsoft Corporation, Redmond, WA) for statistical analysis.
RESULTS
A full overview of interventions performed in the 9 studies included in this systematic review is provided in Table 1.28–36 The authors of these 9 studies conducted randomized control, randomized, or outcome measures trials. A total of 196 patients with acute, subacute, or recurrent ankle sprains participated in the included trials.
Table 1.
Article Content Summary
| Study |
Participants |
Length of Intervention |
Experimental Intervention |
Control Intervention |
Form of Measurement |
Result |
|||
| Condition |
N |
Description |
n |
Description |
n |
||||
| Manual therapy | |||||||||
| Reid et al28 | Recurrent ankle sprain | 23 | 1 session | Weight-bearing movement with mobilization: 2 sets × 10 repetitions | 12 (Session 1: movement with mobilization; session 2: sham) | Sham mobilization: 2 sets × 10 repetitions | 11 (Session 1: sham; session 2: movement with mobilization) | Weight-bearing ankle dorsiflexion | Increased weight-bearing dorsiflexion after movement with mobilization |
| Vicenzino et al29 | Recurrent ankle sprain | 16 | Nonweight-bearing movement with mobilization: 1 session; weight-bearing movement with mobilization: 1 session | 1. Nonweight-bearing movement with mobilization: 4 sets × 4 glides; 2. weight-bearing movement with mobilization: 4 sets × 4 glides | 16a | No movement with mobilization | 16a | Weight-bearing ankle dorsiflexion | Increased weight-bearing dorsiflexion after nonweight-bearing and weight-bearing movement with mobilization |
| Collins et al30 | Grade 2 subacute lateral ankle sprain | 16 | 1 session | Weight-bearing movement with mobilization: 3 sets × 10 repetitions | 16a | 1. Placebo: sham mobilization; 2. control: no movement with mobilization | 16a | Weight-bearing ankle dorsiflexion | Increased weight-bearing dorsiflexion after movement with mobilization |
| Green et al31 | Acute lateral ankle sprain | 38 | Until full range of motion restored within 6 treatment sessions over the 14-d treatment period | 1. Rest, ice, compression, and elevation for 20 min; 2. passive anteroposterior small-amplitude oscillatory joint mobilization of the talus: 2 sets of 60 s | 19 | Rest, ice, compression, and elevation only | 19 | Pain-free ankle dorsiflexion with the Lidcombe template (100 N) | Increased dorsiflexion after joint mobilization. The joint mobilization group achieved full, pain-free dorsiflexion with fewer sessions. |
| Therapeutic modalities | |||||||||
| Sandoval et al32 | Grade 1 or 2 acute lateral ankle sprain | 27 | Until the participant reached the end of the treatment or until he or she completed the 8-wk treatment. Treatments were provided once a day with 5 sessions per wk. | 1. High-voltage pulsed-current electrical stimulation (+) for 30 min and conventional treatmentsb; 2. high-voltage pulsed-current electrical stimulation (–) for 30 min and conventional treatmentsb | High-voltage pulsed-current electrical stimulation (+) = 8; high-voltage pulsed-current electrical stimulation (–) = 9 | Conventional treatmentb | 10 | Ankle dorsiflexion with a goniometer | High-voltage pulsed-current electrical stimulation (+) = high-voltage pulsed-current electrical stimulation (–) = conventional treatmentsb |
| Borromeo et al33 | Acute lateral ankle sprain | 32 | 3 treatment sessions within a 7-d period | Hyperbaric oxygen therapy (100% oxygen at 2 atm absolute pressure: 90 min for session 1 and 60 min for sessions 2 and 3) and standardized treatmentc | 16 | Air at 1.1 atm absolute pressure and standardized treatment | 16 | Active and passive dorsiflexion with a goniometer | Increased dorsiflexion over the course of treatment sessions in both groups |
| Peer et al36 | Grade 1 or 2 acute lateral ankle sprain | 5 | Biomechanical muscle stimulation: 6 min; control treatment: 20 min | Biomechanical muscle stimulation (swisswing): 2 min each at 20 Hz on bottom of foot and gastrocnemius belly | 5a | 20 min of rest, ice, compression, and elevation | 5a | Active dorsiflexion with 20° to 30° of knee flexion using a goniometer | Increased dorsiflexion after the biomechanical muscle stimulation treatment |
| Therapeutic exercises | |||||||||
| Youdas et al34 | Grade 1 or 2 acute lateral ankle sprain | 21 | 6 wk | A standing static stretch to the calf muscle-tendon unit of the injured ankle Group 1: 30 s × 3 sets per day; group 2: 1 min × 3 sets per day; group 3: 2 min × 3 sets per day with a home exercise programd | 30 s = 7 1 min = 8 2 min = 7 | No control group | 0 | Active ankle dorsiflexion with the knee extended using a goniometer | Increased dorsiflexion from baseline to 2-, 4-, and 6-wk measurements regardless of the duration of stretching |
| Psychological intervention | |||||||||
| Christakou et al35 | Grade 2 acute lateral ankle sprain | 18 | 4 sessions of the intervention phase and 12 sessions of imagery for 45 min | Relaxation and imagery program: 12 45-min sessions and physiotherapy programe (34.66 ± 4.33 d) | 9 | Physiotherapy program only (33.77± 4.57 d) | 9 | Passive dorsiflexion with a goniometer | Experimental = control |
The authors used the crossover experimental study design, and all participants received both experimental and control interventions.
Conventional treatment included crushed ice bag for 20 min with elevation and isometric and resisted active exercises in all degrees of freedom of the ankle joint until the limits of pain without weight bearing.
Standardized treatment programs included naproxen; rest, ice, compression, and elevation; range-of-motion and strengthening exercises; and proprioceptive training.
The home exercise program included active ankle range of motion; active resistive exercise to the affected ankle in all planes; proprioceptive training; and rest, ice, compression, and elevation.
Physiotherapy program included hydromassage, ultrasound, a laser device, range-of-motion exercises, strengthening exercises, proprioceptive training, cycling on a stationary bicycle, forward lunges against a wall, step-ups and step-downs, diagonal hops, and stretching exercises.
The methodologic quality scores of included studies can be found in Table 2. Scores on the PEDro scale ranged from 1 to 8 out of 10 points (mean = 5.22 ± 1.92). The level of evidence of each study included in this review was 2b or 2c. Level 2b is a relatively weak recommendation with moderate-quality evidence, indicating that alternative interventions may be better for some patients with an ankle sprain under some circumstances. Level 2c is a weak recommendation with low-quality evidence, meaning that the effect of an intervention is uncertain. Although some of the 8 studies categorized as level of evidence 2b had moderate to large standard deviations, the method of randomized control trials was considered acceptable.
Table 2.
Methodologic Quality of the Included Studies
| Study |
Study Design |
Oxford Centre for Evidence-based Medicine Level39 |
Physiotherapy Evidence Database (PEDro) Scale Score37 |
| Manual therapy | |||
| Reid et al28 | Randomized control crossover trial | 2b− | 6 |
| Vicenzino et al29 | Randomized control crossover trial | 2b− | 7 |
| Collins et al30 | Randomized control crossover trial | 2b− | 5 |
| Green et al31 | Randomized control trial | 2b | 6 |
| Therapeutic modalities | |||
| Sandoval et al32 | Randomized control trial | 2b | 8 |
| Borromeo et al33 | Randomized control trial | 2b− | 6 |
| Peer et al36 | Outcome studies (crossover trial) | 2c | 2 |
| Therapeutic exercises | |||
| Youdas et al34 | Randomized trial | 2c | 4 |
| Psychological intervention | |||
| Christakou et al35 | Randomized control trial | 2b− | 3 |
Acute/Subacute Ankle Sprain
Seven of 9 studies that fit the inclusion criteria incorporated the use of movement with mobilization (MWM),30 passive oscillatory joint mobilization,31 local vibration therapy,36 high-voltage pulsed stimulation (HVPS),32 static stretching,34 hyperbaric oxygen,33 and psychological interventions35 for the improvement in ankle dorsiflexion. The effect sizes for treatment groups in these 7 studies ranged from −0.02 to 1.99, and the CIs across effect sizes for 5 studies crossed zero. Effect sizes and associated 95% CIs are shown in Table 3.
Table 3.
Effect Size With 95% Confidence Interval for Improved Ankle Dorsiflexion After 1 Dose or Multiple Doses of Therapeutic Interventions
| Study |
Intervention |
Effect of 1 Dose (95% Confidence Interval) |
Effect of Multiple Doses (95% Confidence Interval) |
| Acute ankle sprain | |||
| Green et al31,a | Passive joint mobilization | Session 1: 0.45 (−0.20, 1.08) | 2 Sessions: 1.28 (0.55, 1.96)b,c |
| Session 2: 0.19 (−0.47, 0.84) | 3 Sessions: 1.63 (0.81, 2.36)b,c | ||
| Session 3: 0.11 (−0.61, 0.83) | 4 Sessions: 1.49 (0.44, 2.44)b,c | ||
| Session 4: 0.37 (−0.80, 1.48) | 5 Sessions: 1.71 (0.47, 2.83)b,c | ||
| Session 5: 0.09 (−1.31, 1.46) | |||
| Control (rest, ice, compression, and elevation) | Session 1: 0.09 (−0.55, 0.73) | 2 Sessions: 0.76 (0.09, 1.40)c | |
| Session 2: 0.21 (−0.43, 0.84) | 3 Sessions: 0.95 (0.26, 1.60)b,c | ||
| Session 3: −0.13 (−0.76, 0.51) | 4 Sessions: 1.11 (0.37, 1.80)b,c | ||
| Session 4: −0.17 (−0.86, 0.53) | 5 Sessions: 1.65 (0.76, 2.45)b,c | ||
| Session 5: 0.27 (−0.58, 1.10) | |||
| Sandoval et al32 | High-voltage pulsed-current electrical stimulation (+) and conventional treatmentd | NA | 0.35 (−0.66, 1.31) |
| High-voltage pulsed-current electrical stimulation (−) and conventional treatmentd | NA | 0.94 (−0.02, 1.82) | |
| Conventional treatmentd only | NA | 0.81 (−0.13, 1.69) | |
| Borromeo et al33 | Hyperbaric oxygen therapy and standardized treatmentse | 0.46 (−0.26, 1.15) | 1.32 (0.53, 2.05)b |
| Air and standardized treatmentse | 0.50 (−0.21, 1.19) | 1.22 (0.44, 1.94)b | |
| Peer et al36 | Biomechanical muscle stimulation | 0.52 (−0.79, 1.72) | NA |
| Rest, ice, compression, and elevation | −0.05 (−1.28, 1.20) | NA | |
| Youdas et al34 | Static stretching for 30 s and home exercise programf | NA | 1.36 (0.12, 2.42)b |
| Static stretching for 1 min and home exercise programf | NA | 1.86 (0.60, 2.91)b | |
| Static stretching for 2 min and home exercise programf | NA | 1.99 (0.60, 3.10)b | |
| Christakou et al35 | Relaxation and imagery program and physiotherapy programg | NA | 1.22 (0.16, 2.16)b |
| Physiotherapy programg only | NA | 1.27 (0.20, 2.21)b | |
| Subacute ankle sprain | |||
| Collins et al30 | Weight-bearing movement with mobilization | 0.38 (−0.32, 1.07) | NA |
| Placebo | 0.05 (−0.65, 0.74) | NA | |
| Control | −0.06 (−0.75, 0.64) | NA | |
| Recurrent ankle sprain | |||
| Reid et al28 | Weight-bearing movement with mobilization | 0.15 (−0.44, 0.72) | NA |
| Sham mobilization | 0.05 (−0.53, 0.62) | NA | |
| Vicenzino et al29 | Weight-bearing movement with mobilization | 0.39 (−0.32, 1.08) | NA |
| Nonweight-bearing movement with mobilization | 0.29 (−0.41, 0.98) | NA | |
| Control | 0.13 (−0.57, 0.82) | NA | |
Abbreviation: NA indicates not applicable.
The authors performed 6 sessions of the interventions for each group; however, only 1 patient received passive oscillatory joint mobilization in the last session. Therefore, we could not calculate the effect size and 95% confidence interval because of the lack of mean and standard deviation.
Indicates large effect size with 95% confidence interval that did not cross zero.
Indicates effect size was calculated from baseline measurement before the first session to measurement immediately after each session.
Conventional treatment included crushed ice bag for 20 min with elevation and isometric and resisted active exercises in all degrees of freedom at the ankle joint until the limits of pain without weight bearing.
Standardized treatment programs included naproxen; rest, ice, compression, and elevation; range-of-motion and strength exercises; and proprioceptive training.
The home exercise program included active ankle range of motion; active resistive exercise to the affected ankle in all planes; proprioceptive training; and rest, ice, compression, and elevation.
Physiotherapy program included hydromassage, ultrasound, a laser device, range-of-motion exercises, strengthening exercises, proprioceptive training, cycling on a stationary bicycle, forward lunges against a wall, step-ups and step-downs, diagonal hops, and stretching exercises.
Manual Therapy
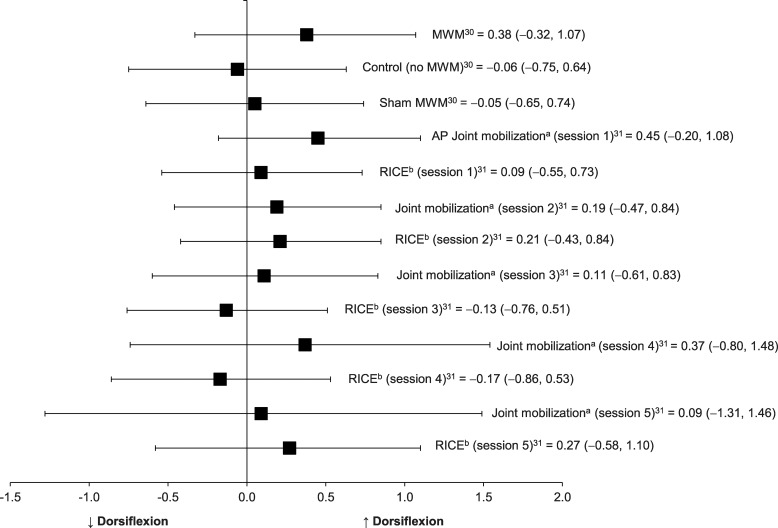
Two included studies30,31 in which the investigators evaluated MWM and Maitland passive oscillatory joint mobilization after acute or subacute ankle sprain were categorized into this group (Table 3, Figure 2). These authors examined the immediate pre-post changes in dorsiflexion from 1 dose of these interventions. Collins et al30 provided only single treatment sessions of MWM to 16 patients with subacute ankle sprain, whereas Green et al31 performed 6 sessions of passive oscillatory joint mobilization with standard-of-care treatments for 19 patients with acute lateral ankle sprains until full recovery of ankle-dorsiflexion ROM was achieved. We graded the strength of recommendation as 2b because the randomized control study design yielded improvements in dorsiflexion that were observed consistently after 1 passive joint mobilization or MWM. However, the effect sizes for pre-post improvement in ankle dorsiflexion were small (Cohen d = 0.09) to moderate (Cohen d = 0.45) after each single application of passive oscillatory joint mobilization, and 95% CIs crossed zero.27 The effects of MWM on weight-bearing dorsiflexion among patients with subacute ankle sprain were small (Cohen d = 0.38), and 95% CIs crossed zero. These small effect sizes with large 95% CIs indicate that the improvements in ankle dorsiflexion after 1 dose of joint mobilization or MWM were not clinically important. The control groups from both studies demonstrated harmful or small effects (range = −0.06–0.27) on ankle dorsiflexion with 95% CIs overlapping zero. The effect sizes for the immediate pre-post changes in dorsiflexion from 1 dose of these interventions are presented in Table 3 and Figure 2.26
Figure 2.
Effect sizes with 95% confidence intervals for improvements in ankle- dorsiflexion range of motion immediately after joint mobilization in individuals with acute or subacute ankle sprain. Abbreviations: AP, anteroposterior; MWM, movement with mobilization. a Indicates a passive small-amplitude oscillatory talocrual joint mobilization was applied in the AP direction. b The group receiving rest, ice, compression, and elevation was considered the control group in the study by Green et al.31
From baseline measurement before the first treatment session to follow-up measurement after each treatment session, improvements in ankle-dorsiflexion ROM were observed in both the joint mobilization and control groups with large effect sizes and small 95% CIs that did not cross zero.31 Patients in the control group received the standard-of-care treatment alone that included the rest, ice, compression, and elevation (RICE) protocol. Effect sizes for the improvements in dorsiflexion from multiple treatment sessions are presented in Table 3. The large effect sizes observed in both groups indicate the combination of joint mobilization and RICE protocol or the RICE protocol alone are both clinically beneficial for improving ankle dorsiflexion, but the true beneficial effect of multiple consecutive applications of a passive oscillatory joint mobilization alone for improving ankle dorsiflexion is inconclusive. In addition, patients were discharged from the trials when they restored full dorsiflexion ROM, so the number of treatment sessions was not the same for all patients.
Therapeutic Modalities
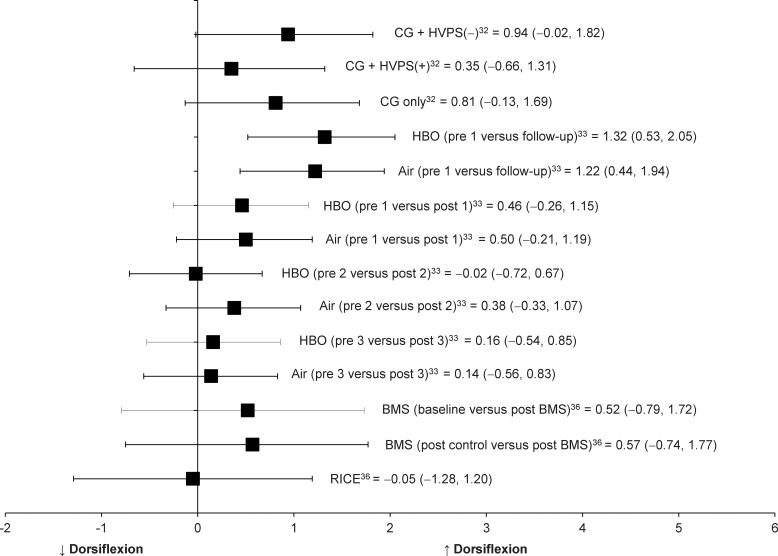
In 3 of the 7 studies, investigators examined the effectiveness of therapeutic modalities, specifically HVPS plus conventional treatment,32 hyperbaric oxygen,33 and biomechanical muscle stimulation (BMS)36 after an acute ankle sprain on dorsiflexion. Borromeo et al33 and Peer et al36 reported the immediate pre-post changes in dorsiflexion from 1 dose of hyperbaric oxygen and BMS, respectively. The effect sizes at immediate follow-up for the treatment groups in these 2 studies were small (hyperbaric: Cohen d = 0.46; BMS: Cohen d = 0.52), with 95% CIs around the effect sizes that crossed zero (Table 3, Figure 3).
Figure 3.
Effect sizes with 95% confidence intervals for improvements in ankle dorsiflexion range of motion immediate after therapeutic modalities in individuals with acute or subacute ankle sprain. Abbreviations: BMS, biomechanical muscle stimulation; CG, conventional treatment group; HVPS (–), negative-polarity high-voltage pulsed-current electrical stimulation; HVPS (+), positive-polarity high-voltage pulsed-current electrical stimulation; HBO, hyperbaric oxygen therapy; and RICE, rest, ice, compression, and elevation.
Borromeo et al33 and Sandoval et al32 examined changes in dorsiflexion between baseline and follow-up from multiple consecutive doses of hyperbaric oxygen and HVPS. The effect sizes for these pre-post intervention series changes in dorsiflexion for treatment groups in the included studies ranged from 0.35 to 1.32 (Table 3, Figure 3). The 95% CI around effect sizes did not cross zero for the hyperbaric oxygen treatment group but crossed zero for the HVPS treatment groups.
The control groups from all 3 studies produced a broad range of small to large effect sizes (Cohen d range = −0.05 to 0.81), but all associated 95% CIs crossed zero.
Therapeutic Exercises
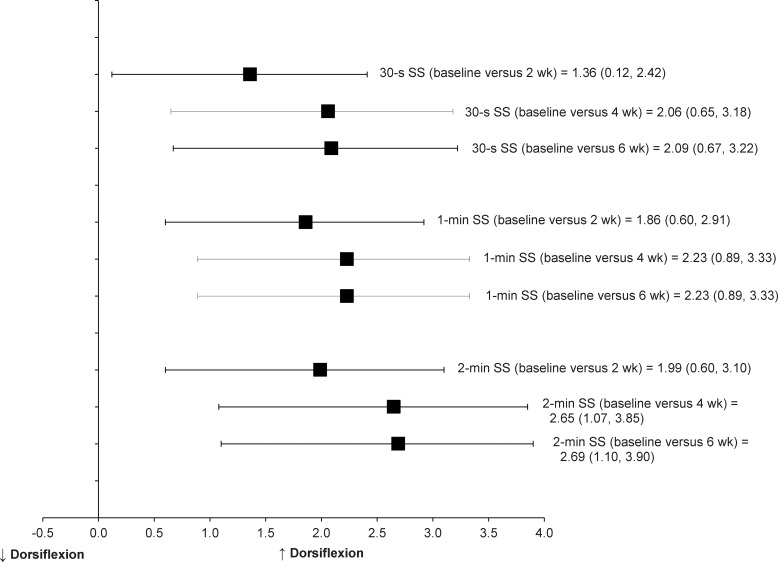
Only 1 study was categorized into this group. Youdas et al34 conducted a randomized trial to examine improvements in active ankle-dorsiflexion ROM after static stretching was added to standardized treatments consisting of cryotherapy, strengthening, and proprioceptive training for acute lateral ankle sprains. A total of 21 patients received 30 seconds, 1 minute, or 2 minutes of static calf stretching with standard-of-care treatments for 6 weeks after their acute ankle sprains. Active ankle-dorsiflexion ROM increased after 2, 4, and 6 weeks of static calf stretching with standard-of-care treatments. Strong effects for ankle-dorsiflexion improvements were found after a standardized home treatment program with static stretching, and 95% CIs did not cross zero (Table 3, Figure 4).
Figure 4.
Effect sizes with 95% confidence intervals for an improvement in ankle dorsiflexion after static stretching with a home exercise program in individuals with acute ankle sprain (active ankle range of motion; active resistive exercise to the affected ankle in all planes; proprioceptive training; and rest, ice, compression, and elevation). Abbreviation: SS, static stretch.
Psychological Intervention
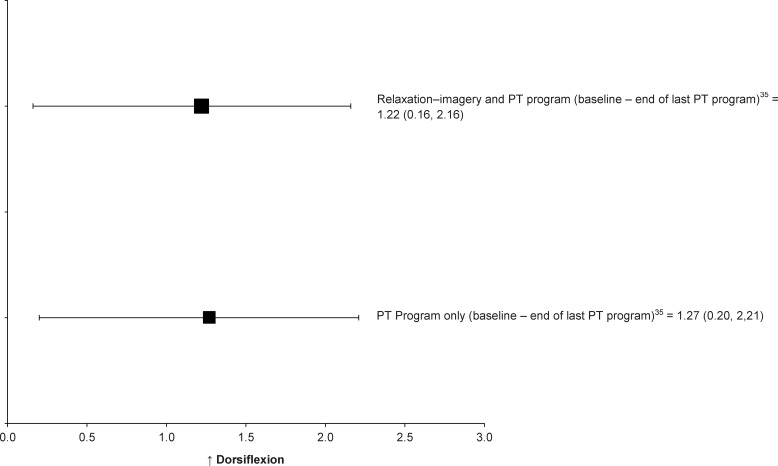
This category included only 1 study. Christakou et al35 investigated the efficacy of mental relaxation and imagery interventions combined with a physiotherapy program on ankle ROM, pain, and edema after acute ankle sprain. The effect sizes for an increase in ankle dorsiflexion from the beginning of the first treatment session to the end of the last treatment session were large in both the experimental (Cohen d = 1.22; 95% CI = 0.16, 2.16) and control groups (Cohen d = 1.27; 95% CI = 0.20, 2.21; Table 3, Figure 5). Both groups received the standard physiotherapy program and produced large effect sizes with small 95% CIs, suggesting that the addition of mental relaxation and imagery intervention to a standard physiotherapy program has a small or no clinical benefit for ankle dorsiflexion improvements.
Figure 5.
Effect sizes with 95% confidence intervals for improved ankle dorsiflexion after psychological intervention in individuals with acute ankle sprain. Abbreviation: PT (physiotherapy) program included hydromassage, ultrasound, laser, range-of-motion exercises, strengthening exercises, proprioceptive training, cycling on a stationary bicycle, forward lunges against a wall, step-ups and down, diagonal hops, and stretching exercises.
Recurrent Ankle Sprain
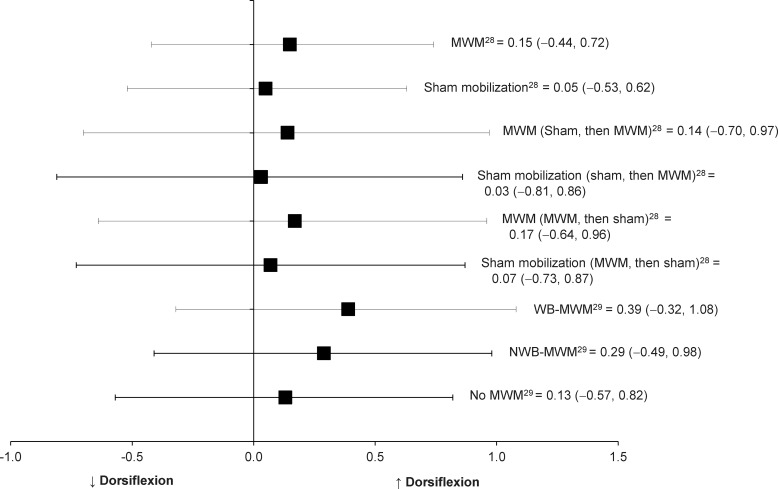
In 2 reports, researchers28,29 studied 39 patients with recurrent ankle sprains. They performed MWM of the talocrual joint and assessed weight-bearing dorsiflexion. In both studies, weight-bearing dorsiflexion improved after the single dose of MWM in patients with recurrent ankle sprain compared with the control treatment. We graded the level of evidence as 2b because the randomized control crossover study designs for the MWM interventions yielded improvements in closed kinetic chain dorsiflexion after 1 dose of MWM. However, the effects of MWM on weight-bearing dorsiflexion among patients with recurrent ankle sprains ranged from 0.15 to 0.39, and associated 95% CIs crossed zero (Table 3, Figure 6). The small effect sizes with 95% CIs crossing zero indicates the improvements in ankle dorsiflexion after 1 dose of MWM in patients with recurrent ankle sprains were not clinically important. Therefore, the effects of MWM on weight-bearing dorsiflexion among participants with recurrent ankle sprains are inconclusive in both studies.
Figure 6.
Effect sizes with 95% confidence intervals for improved ankle-dorsiflexion range of motion immediately after movement with mobilization (MWM) in individuals with recurrent ankle sprain. Abbreviations: NWB, nonweight bearing; and WB, weight bearing.
DISCUSSION
A static-stretching intervention as part of a standardized home exercise program had the strongest effects on ankle-dorsiflexion improvement after acute ankle sprains. This was not unexpected because limited ankle dorsiflexion often is attributed to inflexibility of the triceps surae muscles.9,11,19,42 Tightness in the gastrocnemius-soleus complex likely is not caused directly by acute lateral ankle sprain but may develop as an adaptive response to immobilization and result from an abnormal gait pattern. For example, limited dorsiflexion ROM has been observed after cast immobilization for ankle conditions.43,44 A stretching technique commonly is incorporated to restore full ROM by targeting flexibility of the gastrocnemius-soleus muscle complex. The stretching intervention may increase flexibility before pain perception and allow the viscoelastic properties of junctions between muscle and tendon to overcome the stretch reflex or increase the stretch tolerance.43,45 In our review of the literature, we found stretching techniques added to the standard-of-care treatments produced improvements in ankle dorsiflexion in patients with acute ankle sprains that were supported by strong effect sizes. However, this finding should be interpreted with caution because the experimental design of Youdas et al34 did not include a group that received only passive stretching or a control group that did not receive any treatment. All patients who participated in the study received the same interventions other than static stretching. Authors of several studies46–48 not included in this review have provided some evidence to support the use of stretching to improve dorsiflexion in healthy participants. However, determining the effectiveness of stretching is difficult in patients who have ankle conditions and may have had associated swelling, pain, adhesion, and altered arthrokinematics. We included this study in our systematic review because stretching has been accepted as part of the standard-of-care treatment to restore ankle-dorsiflexion ROM, and we considered that the standardized effect sizes allow us to compare the interventions performed by Youdas et al34 with other forms of interventions investigated in other studies included in this review. Therefore, the addition of stretching to a standard of care may be beneficial, but the clinical effects of stretching techniques alone on ankle dorsiflexion after acute ankle sprains are still unknown. Further investigation is necessary to determine if adaptive tightness in the gastrocnemius-soleus complex develops after acute ankle sprains and if the stretching intervention in isolation for acute ankle sprains is the source of the clinical benefit for dorsiflexion improvement.
When ankle dorsiflexion is limited, a joint-mobilization technique may be used to address a potential arthrokinematic restriction. Proper accessory motion is necessary to achieve full physiologic ROM. During normal ankle dorsiflexion, the convex talus glides posteriorly, rolls upward, and rotates externally on the concave surface of the mortise, and the fibula glides superiorly and moves laterally away from the tibia.10,11,31,49 However, after an acute ankle sprain, posterior gliding of the talus may be restricted during dorsiflexion because disruption of the anterior talofibular ligament may induce anterior subluxation and internal rotation of the talus on the mortise and anterior and inferior displacement of the distal fibula.8,10,11,49–51 Therefore, anterior talar translation often is theorized to limit accessory motions in the talocrural joint, which may impair normal ankle dorsiflexion. Passive oscillatory talocrual joint mobilization is purported to increase accessory joint motions by passively restoring posterior glide of the talus and correcting the positional fault of the talus and distal fibula.31,49,52 Mulligan49 introduced the MWM treatment techniques for correcting the positional fault of the bony segments, improving joint ROM, and restoring pain-free function by combining physiologic (osteokinematic) and accessory joint movements. In the MWM treatment techniques, a sustained manual gliding force is applied to a joint in a direction perpendicular to the plane of motion or impaired motion while the patient actively performs a painful and restricted motion of that joint.49,53 An application of MWM for improving dorsiflexion after ankle sprain consists of a passive glide of the tibia relative to the talus in a posteroanterior direction or a glide of the talus on the tibia in an anteroposterior direction that is sustained during active dorsiflexion to the end of pain-free ROM.49
Although increases in ankle dorsiflexion were demonstrated after 1 passive oscillatory joint mobilization in patients with acute ankle sprain31 or MWM in patients with subacute30 or recurrent ankle sprain28,29 in 4 studies included in this review,28 the clinical relevance of conclusions drawn from the current literature is limited because the associated effect sizes were small to moderate, with 95% CIs that crossed zero. However, the studies in which authors evaluated passive oscillatory joint mobilization or MWM were categorized as level 2b evidence and produced a mean PEDro score of 6.0, allowing for moderately supportive evidence and practical recommendation for considering the use of these interventions as a part of rehabilitation for individuals with ankle sprains. These patients already may have had restored ankle dorsiflexion, or the observed restriction to ankle dorsiflexion may have been multifactorial; therefore, 1 treatment may have limited benefit. We could not find studies in which authors have examined the effects of more than 1 bout of MWM on ankle dorsiflexion after an ankle sprain. Investigators should examine the effect of MWM with multiple consecutive treatments to determine the clinical relevance of dorsiflexion improvements. Green et al31 provided 6 treatment sessions of joint mobilization with RICE or RICE alone to patients with acute ankle sprains. The multiple consecutive treatments of joint mobilization with RICE produced large effect sizes for ankle-dorsiflexion improvement and 95% CIs did not cross zero. However, caution is needed when interpreting the effect sizes of multiple consecutive treatments of passive joint mobilization in the study. Normal ankle-dorsiflexion ROM was restored with fewer treatment sessions in patients who received passive joint-mobilization interventions with RICE than in patients who received only RICE. Patients were discharged from the trial when full ankle dorsiflexion was restored. Subsequently, not all patients in the experimental group received the same number of treatment sessions because the total number of patients declined progressively over the 6 intervention sessions upon achieving full ankle dorsiflexion. Furthermore, the control group, which received the RICE protocol alone, showed large effect sizes with small 95% CIs for multiple consecutive treatments for ankle-dorsiflexion improvements. Therefore, the evidence is inconclusive with respect to the beneficial effect of a passive oscillatory joint mobilization alone for ankle-dorsiflexion improvement. In addition, this study lasted 2 weeks with 6 maximal treatment sessions; however, no long-term follow-up was conducted to determine if these improvements in ankle dorsiflexion ROM were maintained. Investigators should examine the permanence of the improvements in ankle dorsiflexion.
Ankle dorsiflexion typically is restricted by pain, spasm, and swelling, and subsequently, therapeutic modalities controlling pain and swelling may effect moderate improvement in ankle dorsiflexion. Cryotherapy and electrotherapy often are incorporated to minimize pain, spasm, and neural inhibition, thereby allowing for earlier and more aggressive interventions to restore motion.54 Hyperbaric oxygen treatment, a less traditional therapeutic intervention, is purported to cause vasoconstriction, facilitate reabsorption of extravascular fluid into the circulation, and accelerate debridement by increasing oxygen delivery to macrophages, thus assisting in controlling the amount of swelling. Green et al31 reported a small associated effect size for acute increase in ankle dorsiflexion after a RICE protocol in the control group (Cohen d = 0.09), whereas Peer et al36 reported negative effects for immediate improvement in ankle dorsiflexion after a RICE protocol in patients with acute ankle sprain (Cohen d = −0.05). Sandoval et al32 noted strong effect sizes after negative-polarity HVPS with a standardized intervention program (Cohen d = 0.94) or the standardized intervention program alone (Cohen d = 0.81); however, the associated 95% CIs crossed zero. Borromeo et al33 reported small to moderate effect sizes for increases in ankle dorsiflexion after 1 dose of hyperbaric oxygen (Cohen d = 0.46), but again the 95% CIs crossed zero. Therefore, although associated with mostly favorable results, the improvement in ankle dorsiflexion after 1 dose of cryotherapy, electrotherapy, and hyperbaric oxygen may not be clinically relevant.
We did not find evidence to support the use of relaxation and imagery techniques to increase ankle dorsiflexion after ankle sprain. Christakou et al35 noted that mental relaxation and imagery intervention for patients sustaining lateral ankle sprains had no clinical benefit for ankle-dorsiflexion improvement. The effect sizes for the improvements in ankle dorsiflexion were also large in both the experimental (Cohen d = 1.22) and control groups (Cohen d = 1.27) with narrow 95% CIs, indicating that the combination of mental relaxation and imagery combined with standard-of-care treatments was not more beneficial than the standard-of-care treatment alone for improving ankle dorsiflexion. However, the design of the groups and treatment protocol, the use of a weak measure of internal and external imagery, and a small sample size limited the strength of clinical evidence. Whereas some investigators55,56 have suggested that imagery and relaxation may be able to reduce pain and improve neuromuscular control through central nervous system changes in spinal and cortical pathways, further research is needed to investigate the influence of psychological interventions on restoration of ankle dorsiflexion and the mechanisms behind it.
Some researchers have suggested that a combination of factors restricts ankle dorsiflexion after ankle sprain. Vicenzino et al29 demonstrated a relationship between an improvement in posterior talar glide and weight-bearing dorsiflexion after weight-bearing MWM in patients with recurrent ankle sprain. However, Denegar et al8 found no differences in ankle dorsiflexion between the injured and uninjured ankles in 12 patients who had histories of lateral ankle sprain within the 6 months before the study and who had returned to sport participation; yet the amount of posterior talar glide was reduced in the injured ankle (8° ± 5.8°) compared with the uninjured ankle (16.6° ± 3.4°) in the 6 months after ankle sprain. The difference was associated with a very strong effect size and a 95% CI that did not cross zero (Cohen d = 1.81; 95% CI = 0.81, 2.69). Denegar et al8 further explained that restored ankle dorsiflexion may be due to flexibility of the gastrocnemius-soleus complex and hypermobility of other joints. The selection of therapeutic interventions for improving ankle dorsiflexion after ankle sprains depends on multiple limiting factors to ankle dorsiflexion. Therefore, a clinician should consider an approach to improve ankle dorsiflexion that may necessitate recognizing which factors limit ankle dorsiflexion after an ankle sprain.
Although the authors of 8 of the studies we included in this review assessed ankle dorsiflexion along with the level of pain or the amount of swelling to determine treatment effectiveness, only Borromeo et al33 also provided patient self-reported functional scores, showing a strong effect for improved self-reported functional scores after 7 days of hyperbaric oxygen treatments (Cohen d = 18.66; 95% CI = 13.68, 22.70). However, none of the authors of the studies included in our review examined the relationship between improvements in dorsiflexion and patient progress using patient self-reported functional outcome measures. A patient-oriented approach using self-reported function may be necessary to determine maximal treatment efficiency and efficacy in addition to dorsiflexion measurements.
CONCLUSIONS
Restoring normal ROM of ankle dorsiflexion after ankle sprains is important to minimize the risk of reinjury and quickly restore full functional abilities. From our review, the existing evidence suggests that clinicians need to consider what may be the limiting factor of ankle dorsiflexion to select the most appropriate treatments and interventions. The small amount of patient-oriented evidence coupled with heterogeneous patient populations across studies may limit the clinical relevance of conclusions drawn from the literature. Investigators should examine the long-term effects of treatments on ankle dorsiflexion and a relationship between an improvement in ankle dorsiflexion and measures of patient self-reported and physical function to determine the most appropriate forms of therapeutic interventions to address ankle-dorsiflexion limitation.
Table 1.
Continued
ACKNOWLEDGMENTS
We thank Alfred A. Bove, MD, PhD, and Ann Christakou for granting permission to use their unpublished data.
REFERENCES
- 1.Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641–645. doi: 10.1197/j.aem.2007.03.1354. [DOI] [PubMed] [Google Scholar]
- 2.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 3.Cumps E, Verhagen E, Meeusen R. Prospective epidemiological study of basketball injuries during one competitive season: ankle sprains and overuse knee injuries. J Sports Sci Med. 2007;6(2):204–211. [PMC free article] [PubMed] [Google Scholar]
- 4.Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37(1):73–94. doi: 10.2165/00007256-200737010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Baumhauer JF, Alosa DM, Renstrom AF, Trevino S, Beynnon B. A prospective study of ankle injury risk factors. Am J Sports Med. 1995;23(5):564–570. doi: 10.1177/036354659502300508. [DOI] [PubMed] [Google Scholar]
- 6.Safran MR, Benedetti RS, Bartolozzi AR, III, Mandelbaum BR. Lateral ankle sprains: a comprehensive review. Part 1: etiology, pathoanatomy, histopathogenesis, and diagnosis. Med Sci Sports Exerc. 1999;31((7 suppl)):S429–S437. doi: 10.1097/00005768-199907001-00004. [DOI] [PubMed] [Google Scholar]
- 7.Tabrizi P, McIntyre WM, Quesnel MB, Howard AW. Limited dorsiflexion predisposes to injuries of the ankle in children. J Bone Joint Surg Br. 2000;82(8):1103–1106. doi: 10.1302/0301-620x.82b8.10134. [DOI] [PubMed] [Google Scholar]
- 8.Denegar CR, Hertel J, Fonseca J. The effect of lateral ankle sprain on dorsiflexion range of motion, posterior talar glide, and joint laxity. J Orthop Sports Phys Ther. 2002;32(4):166–173. doi: 10.2519/jospt.2002.32.4.166. [DOI] [PubMed] [Google Scholar]
- 9.Denegar CR, Miller SJ., III Can chronic ankle instability be prevented? Rethinking management of lateral ankle sprains. J Athl Train. 2002;37(4):430–435. [PMC free article] [PubMed] [Google Scholar]
- 10.Hubbard TJ, Hertel J. Mechanical contributions to chronic lateral ankle instability. Sports Med. 2006;36(3):263–277. doi: 10.2165/00007256-200636030-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 12.Hertel J. Functional instability following lateral ankle sprain. Sports Med. 2000;29(5):361–371. doi: 10.2165/00007256-200029050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Neely FG. Biomechanical risk factors for exercise-related lower limb injuries. Sports Med. 1998;26(6):395–413. doi: 10.2165/00007256-199826060-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman KR, Brodine SK, Shaffer RA, Johnson CW, Cullison TR. The effect of foot structure and range of motion on musculoskeletal overuse injuries. Am J Sports Med. 1999;27(5):585–593. doi: 10.1177/03635465990270050701. [DOI] [PubMed] [Google Scholar]
- 15.Pope R, Herbert R, Kirwan J. Effects of ankle dorsiflexion range and pre-exercise calf muscle stretching on injury risk in army recruits. Aust J Physiother. 1998;44(3):165–172. doi: 10.1016/s0004-9514(14)60376-7. [DOI] [PubMed] [Google Scholar]
- 16.Drewes LK, McKeon PO, Kerrigan DC, Hertel J. Dorsiflexion deficit during jogging with chronic ankle instability. J Sci Med Sport. 2009;12(6):685–687. doi: 10.1016/j.jsams.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Willems TM, Witvrouw E, Delbaere K, Mahieu N, De Bourdeaudhuij I, De Clercq D. Intrinsic risk factors for inversion ankle sprains in male subjects: a prospective study. Am J Sports Med. 2005;33(3):415–423. doi: 10.1177/0363546504268137. [DOI] [PubMed] [Google Scholar]
- 18.Lun V, Meeuwisse WH, Stergiou P, Stefanyshyn D. Relation between running injury and static lower limb alignment in recreational runners. Br J Sports Med. 2004;38(5):576–580. doi: 10.1136/bjsm.2003.005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright IC, Neptune RR, van den Bogert AJ, Nigg BM. The influence of foot positioning on ankle sprains. J Biomech. 2000;33(5):513–519. doi: 10.1016/s0021-9290(99)00218-3. [DOI] [PubMed] [Google Scholar]
- 20.Irving DB, Cook JL, Menz HB. Factors associated with chronic plantar heel pain: a systematic review. J Sci Med Sport. 2006;9((1–2)):11–22. doi: 10.1016/j.jsams.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Riddle DL, Pulisic M, Pidcoe P, Johnson RE. Risk factors for plantar fasciitis: a matched case-control study. J Bone Joint Surg Am. 2003;85(5):872–877. doi: 10.2106/00004623-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Backman LJ, Danielson P. Low range of ankle dorsiflexion predisposes for patellar tendinopathy in junior elite basketball players: a 1-year prospective study. Am J Sports Med. 2011;39(12):2626–2633. doi: 10.1177/0363546511420552. [DOI] [PubMed] [Google Scholar]
- 23.Dettori JR, Pearson BD, Basmania CJ, Lednar WM. Early ankle mobilization, part I: the immediate effect on acute, lateral ankle sprains (a randomized clinical trial) Mil Med. 1994;159(1):15–20. [PubMed] [Google Scholar]
- 24.Brantingham JW, Globe G, Pollard H, Hicks M, Korporaal C, Hoskins W. Manipulative therapy for lower extremity conditions: expansion of literature review. J Manipulative Physiol Ther. 2009;32(1):53–71. doi: 10.1016/j.jmpt.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Vicenzino B, Paungmali A, Teys P. Mulligan's mobilization-with-movement, positional faults and pain relief: current concepts from a critical review of literature. Man Ther. 2007;12(2):98–108. doi: 10.1016/j.math.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Wikstrom EA, McKeon PO. Manipulative therapy effectiveness following acute lateral ankle sprains: a systematic review. Athl Train Sports Health Care. 2011;3(6):271–279. [Google Scholar]
- 27.Bleakley CM, McDonough SM, MacAuley DC. Some conservative strategies are effective when added to controlled mobilisation with external support after acute ankle sprain: a systematic review. Aust J Physiother. 2008;54(1):7–20. doi: 10.1016/s0004-9514(08)70061-8. [DOI] [PubMed] [Google Scholar]
- 28.Reid A, Birmingham TB, Alcock G. Efficacy of mobilization with movement for patients with limited dorsiflexion after ankle sprain: a crossover trial. Physiother Can. 2007;59(3):166–172. [Google Scholar]
- 29.Vicenzino B, Branjerdporn M, Teys P, Jordan K. Initial changes in posterior talar glide and dorsiflexion of the ankle after mobilization with movement in individuals with recurrent ankle sprain. J Orthop Sports Phys Ther. 2006;36(7):464–471. doi: 10.2519/jospt.2006.2265. [DOI] [PubMed] [Google Scholar]
- 30.Collins N, Teys P, Vicenzino B. The initial effects of a Mulligan's mobilization with movement technique on dorsiflexion and pain in subacute ankle sprains. Man Ther. 2004;9(2):77–82. doi: 10.1016/S1356-689X(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 31.Green T, Refshauge K, Crosbie J, Adams R. A randomized controlled trial of a passive accessory joint mobilization on acute ankle inversion sprains. Phys Ther. 2001;81(4):984–994. [PubMed] [Google Scholar]
- 32.Sandoval MC, Ramirez C, Camargo DM, Salvini TF. Effect of high-voltage pulsed current plus conventional treatment on acute ankle sprain. Rev Bras Fisioter. 2010;14(3):193–199. doi: 10.1590/s1413-35552010000300012. [DOI] [PubMed] [Google Scholar]
- 33.Borromeo CN, Ryan JL, Marchetto PA, Peterson R, Bove AA. Hyperbaric oxygen therapy for acute ankle sprains. Am J Sports Med. 1997;25(5):619–625. doi: 10.1177/036354659702500506. [DOI] [PubMed] [Google Scholar]
- 34.Youdas JW, McLean TJ, Krause DA, Hollman JH. Changes in active ankle dorsiflexion range of motion after acute inversion ankle sprain. J Sport Rehabil. 2009;18(3):358–374. doi: 10.1123/jsr.18.3.358. [DOI] [PubMed] [Google Scholar]
- 35.Christakou A, Zervas Y, Lavallee D. The adjunctive role of imagery on the functional rehabilitation of a grade II ankle sprain. Hum Mov Sci. 2007;26(1):141–154. doi: 10.1016/j.humov.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Peer KS, Barkley JE, Knapp DM. The acute effects of local vibration therapy on ankle sprain and hamstring strain injuries. Phys Sportsmed. 2009;37(4):31–38. doi: 10.3810/psm.2009.12.1739. [DOI] [PubMed] [Google Scholar]
- 37.PEDro Scale. http://www.pedro.org.au/english/downloads/pedro-scale/. Accessed September 29, 2010. [Google Scholar]
- 38.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 39.Oxford Centre for Evidence-based Medicine Levels of Evidence. http://www.cebm.net/index.aspx?o=1025. Accessed October 2010. [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates;; 1988. pp. 20–40. [Google Scholar]
- 41.Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people's published data: general procedures for research consumers. Psychol Methods. 1996;1(4):331–340. [Google Scholar]
- 42.Riemann BL, DeMont RG, Ryu K, Lephart SM. The effects of sex, joint angle, and the gastrocnemius muscle on passive ankle joint complex stiffness. J Athl Train. 2001;36(4):369–375. [PMC free article] [PubMed] [Google Scholar]
- 43.Chesworth BM, Vandervoort AA. Comparison of passive stiffness variables and range of motion in uninvolved and involved ankle joints of patients following ankle fractures. Phys Ther. 1995;75(4):253–261. doi: 10.1093/ptj/75.4.253. [DOI] [PubMed] [Google Scholar]
- 44.Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain: a systematic review. Arch Orthop Trauma Surg. 2001;121(8):462–471. doi: 10.1007/s004020100283. [DOI] [PubMed] [Google Scholar]
- 45.Gribble PA, Guskiewicz KM, Prentice WE, Shields EW. Effects of static and hold-relax stretching on hamstring range of motion using the FlexAbility LE1000. J Sport Rehabil. 1999;8(3):195–208. [Google Scholar]
- 46.Johnson E, Bradley B, Witkowski K, et al. Effect of a static calf muscle–tendon unit stretching program on ankle dorsiflexion range of motion of older women. J Geriatr Phys Ther. 2007;30(2):49–52. doi: 10.1519/00139143-200708000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Radford JA, Burns J, Buchbinder R, Landorf KB, Cook C. Does stretching increase ankle dorsiflexion range of motion? A systematic review. Br J Sports Med. 2006;40(10):870–875. doi: 10.1136/bjsm.2006.029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Etnyre BR, Abraham LD. Gains in range of ankle dorsiflexion using three popular stretching techniques. Am J Phys Med. 1986;65(4):189–196. [PubMed] [Google Scholar]
- 49.Mulligan BR. Manual Therapy: “NAGS,” “SNAGS,” “MWMS,” etc. 4th ed. Wellington, NZ: Plane View Services;; 1999. pp. 110–115. [Google Scholar]
- 50.Hubbard TJ, Olmsted-Kramer LC, Hertel J, Sherbondy P. Anterior-posterior mobility of the talus in subjects with chronic ankle instability. Phys Ther Sport. 2005;6(3):146–152. [Google Scholar]
- 51.Watson AD. Ankle instability and impingement. Foot Ankle Clin. 2007;12(1):177–195. doi: 10.1016/j.fcl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Landrum EL, Kelln CB, Parente WR, Ingersoll CD, Hertel J. Immediate effects of anterior-to-posterior talocrural joint mobilization after prolonged ankle immobilization: a preliminary study. J Man Manip Ther. 2008;16(2):100–105. doi: 10.1179/106698108790818413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulligan BR. Mobilisation with movement (MWM's) J Man Manip Ther. 1993;1(4):154–156. [Google Scholar]
- 54.Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251–261. doi: 10.1177/0363546503260757. [DOI] [PubMed] [Google Scholar]
- 55.Ranganathan VK, Siemionow V, Liu JZ, Sahgal V, Yue GH. From mental power to muscle power: gaining strength by using the mind. Neuropsychologia. 2004;42(7):944–956. doi: 10.1016/j.neuropsychologia.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 56.Denegar CR. Therapeutic Modalities for Athletic Injuries. Champaign, IL: Human Kinetics;; 2000. pp. 48–71. [Google Scholar]