Phlorotannins are a diverse set of polymers synthesized by brown algae, but their physiological functions are not well understood. They are structurally analogous to terrestrial plant tannins and are proposed to be important in cell wall structure, UV protection, and defense against herbivores (Koivikko et al., 2005). They are also being studied for potential benefits to human health (Thomas and Kim, 2011). Similar to the well-characterized biosynthesis of terrestrial plant tannins from alcoholic monomers, phlorotannins are produced by polymerization of phloroglucinol (1,3,5-trihydroxybenzene) monomer units in a variety of combinations (see figure). However, the biosynthetic pathway for phlorotannin biosynthesis is poorly characterized and has been variously proposed to be via condensation of acetate and malonate units, by the shikimate pathway, or by the phenylpropanoid pathway.
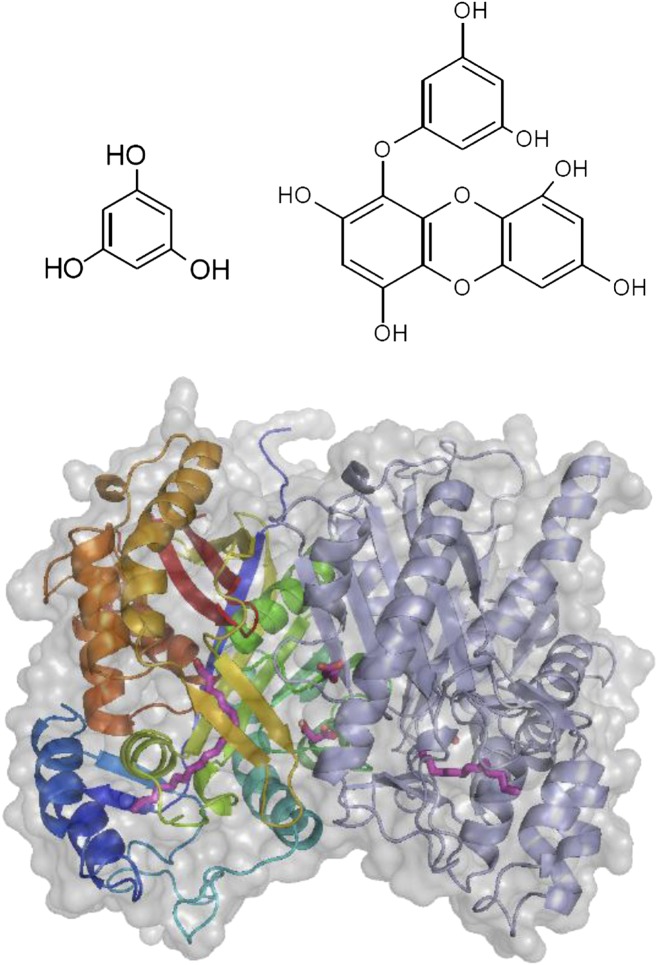
Brown algal phlorotannins are produced by polymerization of phloroglucinol monomers (top left) into a variety of structures, such as eckol (top right). PKS1 (bottom panel) catalyzes the formation of phloroglucinol monomers from malonyl-CoA, a key step in phlorotannin biosynthesis. (Reprinted from Meslet-Cladière et al. [2013], Figures 1 [top panels] and 4 [bottom panel].)
Meslet-Cladière et al. (pages 3089–3103) have made a major advance in our understanding of phlorotannin biosynthesis by identifying and characterizing PKS1, a type III polyketide synthase in the brown alga Ectocarpus siliculosus, an upcoming model system for algal biology (Cock and Coelho, 2011). They show that PKS1 catalyzes the synthesis of phloroglucinol monomers from malonyl-CoA, thus identifying a major step in phlorotannin biosynthesis. Phylogenetic analysis of the type III PKS gene family in Stramenopiles suggested a lateral gene transfer event from an ancestral actinobacterium, also proposed as a major event in the origin of alginate synthesis and the emergence of multicellular brown algae (Michel et al., 2010). The authors identified three potential PKS loci in the recently published E. siliculosus genome. They expressed one of these, PKS1, in Escherichia coli and showed it to produce a protein of the expected size of 40 kD and verified its identity as a PKS protein by mass spectrometry.
Using malonyl-CoA as the sole substrate, recombinant PKS1 synthesized a single product confirmed by liquid chromatography–mass spectrometry to be phloroglucinol. Further biochemical analysis suggested the ability of PKS1 to discriminate between fatty acyl substrates differing in length by only two carbon atoms. To investigate the structural basis for this substrate specificity, the authors determined the crystal structure of PKS1 to a resolution of 2.85 Å and showed it to be a homodimer (see figure). They identified three key amino acid residues in the active site that comprise a catalytic triad and discovered two pockets for initiation and elongation of the product. They also found a long tunnel that can accommodate long-chain fatty acyl substrates and identified two residues that affect the volume of the active site and likely are responsible for the observed substrate specificity.
They further show that the levels of PKS1 expression and phloroglucinol in a freshwater strain of E. siliculosus are low compared with a marine strain, but rise dramatically when the freshwater strain is transferred to sea water, suggesting a role for PKS1 in acclimatization to high salinity. In summary, the authors provide genetic, functional, and structural evidence that significantly advances our knowledge of phlorotannin biosynthesis in brown algae, not only demonstrating phloroglucinol synthesis from malonyl-CoA but also revealing structural evidence for substrate specificity in the synthesis of long-chain acylated phloroglucinols.
References
- Cock J.M., Coelho S.M. (2011). Algal models in plant biology. J. Exp. Bot. 62: 2425–2430 [DOI] [PubMed] [Google Scholar]
- Meslet-Cladière L., Delage L., Leroux C., Goulitquer S., Leblanc C., Creis E., Gall E.A., Stiger-Pouvreau V., Czjzek M., Potin P. (2013). Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 25: 3089–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel G., Tonon T., Scornet D., Cock J.M., Kloareg B. (2010). The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 188: 82–97 [DOI] [PubMed] [Google Scholar]
- Koivikko R., Loponen J., Honkanen T., Jormalainen V. (2005). Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 31: 195–212 [DOI] [PubMed] [Google Scholar]
- Thomas N.V., Kim S.K. (2011). Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 32: 325–335 [DOI] [PubMed] [Google Scholar]