In 1997, a radical idea challenged the individuality of plastids and their discrete nature in vascular plants. The idea was based on the flow of green fluorescent protein (GFP) between interconnected plastids through narrow tubules (Köhler et al., 1997), later called stromules (Köhler and Hanson, 2000). The initial observations suggested an interconnected-plastid network, but by 2000 it was made clear that such a network did not exist. However, the underlying concept of protein flow between interconnected plastids remained unchallenged until our publication in 2012 (Schattat et al., 2012a). In a commentary on the subject, Hanson and Sattarzadeh (2013) note that they had previously negated the idea of a plastid network and suggest that our investigations using a photoconvertible protein merely confirmed their earlier findings. We agree with several points made in the commentary, namely, that stromules emerging from a single plastid rarely form a connection to other independent plastids, and as such, protein movement between interconnected plastids likely does not have major biological significance to the plant cell.
We note that we are largely in agreement with Hanson and Sattarzadeh as to the major function of stromules. It is also gratifying that their observations have reaffirmed that “plastids do not form a network like the endoplasmic reticulum” in plant cells, a conclusion originally arrived at by the Hanson lab more than a decade ago (Köhler and Hanson, 2000; Köhler et al., 2000). According to their earlier observations made through photobleaching of plastid-targeted GFP, “most of the plastids appear to be independent” and “most of the plastids within the cell are not interconnected” (Köhler et al., 2000). However, the commentary fails to address some important issues about stromules and plastid connectivity raised by Schattat et al. (2012a).
Schattat et al. (2012a) defined the problem as an attempt to find “details of the precise mechanism by which two or more independent plastids can interconnect for exchanging proteins.” We believed this question to be important because many publications and textbook discussions on stromules discuss interconnected plastids without providing adequate explanation of how and when the interconnections are formed. For example, the commentary by Hanson and Sattarzadeh maintains that “occasionally stromules can be observed to connect two plastid bodies with one another,” but does not inform us how or when the connection might take place. Hanson and Köhler (2006) describe stromules as “long thin protuberances [that] sometimes form and extend from the main plastid body into the cytosol, occasionally touching and fusing with projections extending from other chloroplasts.” We wished to discover how such plastid fusion occurs. Is it, for example, a process similar to mitochondrial fusion?
Köhler and Hanson (2000) presented evidence that “most of the plastids within the cell are not interconnected.” Nevertheless, the authors stated that “our studies have conclusively shown that one plastid can be connected by a tubular extension to one or a few other plastid bodies within the same cell at a single moment in time” and that “GFP can flow through a stromule that connects two root plastids” (Köhler and Hanson, 2000). This left the reader with the impression that free-ended stromules might be able to connect plastids; as the authors pointed out, “Until longer time-lapse studies are performed, we will not know whether there is a point during development or the cell cycle when all plastids of a particular cell type are connected simultaneously ” (Köhler et al., 2000). In the experiments reported by (Schattat et al., 2012a), we followed this thought and performed relatively longer time-lapse observations on photoconverted plastids but still did not find plastids connecting with each other. Hanson and Sattarzadeh (2013) note that “direct evidence for complete fusion would be the observation of a stromule that is initially independent that then touches a different plastid, followed by photoconversion or photobleaching of one of the plastids to demonstrate that there is exchange of proteins through the stromule. Such an experiment is extremely difficult technically. It requires finding a stromule in the act of attaching to another one or to another plastid body.” We agree with this statement and think that one of the challenges in this field is to tackle this technical difficulty, which is what we attempted to do by creating a photoconvertible probe. As reported by Schattat et al., (2012a), we were unable to find evidence for plastid fusion.
In Schattat et al. (2012a), we suggested that the isthmus of dividing plastids, as well as elongated etioplasts and leucoplasts, might in some cases be misinterpreted as stromules. In their commentary, Hanson and Sattarzadeh (2013) clearly explain why the isthmus between plastids undergoing division cannot be considered as a stromule. While we agree with this clarification about the isthmus, we do not agree with the statement, that two plastid bodies both containing “chlorophyll (and therefore thylakoid membranes) and… well separated from one another… should be considered to be individual chloroplasts connected by a stromule” (Hanson and Sattarzadeh, 2013). This statement assumes that at some time two independent chlorophyll-containing plastids have become connected. However, no evidence exists so far for interplastid fusion under normal conditions. Furthermore, it does not consider the case of single pleomorphic etioplasts that contain fluorescent protochlorophyllide but not thylakoid membranes (Solymosi and Schoefs, 2010) and, therefore, as suggested by Schattat et al. (2012a), might be misinterpreted as two or more fluorescent plastids connected by a stroma filled region.
Hanson and Sattarzadeh (2013) raised an interesting point about possible flaws in our imaging technique causing breakage of stromules and preventing flow between previously interconnected plastids. Hanson and Sattarzadeh observed apparent breakage of stromules, as measured by lack of flow of photoconverted monomeric Eos fluorescent protein (mEosFP), when employing high-intensity laser power. We can only humbly assure that our photoconversion method, using UV light generated by a mercury arc lamp, usually requires 7 to 10 s but can be extended up to 30 s without photobleaching chlorophyll. Thus, we consider it to be very mild in comparison to procedures using intense lasers. Moreover, we are convinced that our method does not stop stromal flow since the localized photoconversion is performed either on the main plastid body or in a portion of a stromule. The photoconverted protein invariably flows into the entire plastid delineated by its bounding envelope.
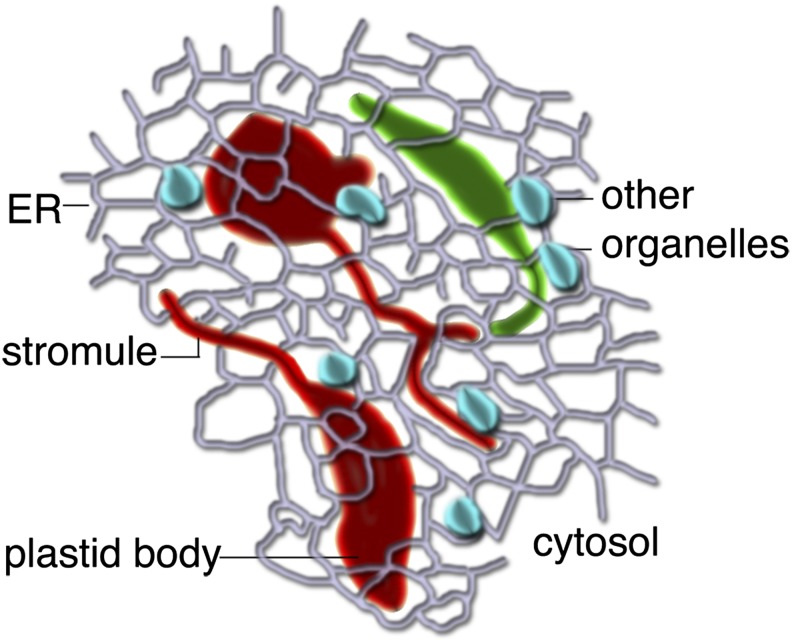
We agree with the conclusion of Hanson and Sattarzadeh that transfer of proteins between individual plastids is not likely to be a major function of stromules. Even if other researchers are able to demonstrate flow of protein between connected plastids in rare instances (which we were unable to confirm), this observation may have little biological relevance to the plant cell, except possibly under certain limited conditions of cell culture. We believe that stromules should be considered as thin projections emanating from single plastids (Figure 1), and we also agree with Hanson and Sattarzadeh that their major role is likely to be in increasing the interactive surface of a plastid with the rest of the cytoplasm. In other recent work, we have shown the frequency of stromules (i.e., as thin tubules emanating from single plastids) in Arabidopsis thaliana epidermal cells is strongly dependent upon illumination, and stromule formation can be induced by Suc application in dark-adapted leaves, suggesting that they might be formed by physiological changes within the cell (Schattat et al., 2012b).
Figure 1.
A Diagrammatic Representation of the Possible Role of Plastid Stromules.
As extensions from independent plastids (red or green), stromules likely allow more effective communication and interaction with other cellular components, such as the endoplasmic reticulum (ER); small organelles, such as mitochondria and peroxisomes (depicted in blue); and the cytosol in general.
Despite the differences in opinion on stromule fusion and interconnected plastids, we hold the considerable body of work by Hanson and colleagues in high esteem and hope that future endeavors from both our labs will lead to many more insights on stromules and their role in the plant cell.
Acknowledgments
We thank Ian Tetlow for critical comments. J.M. and K.A.B. acknowledge research funding by the Natural Sciences and Engineering Research Council of Canada.
AUTHOR CONTRIBUTIONS
All authors contributed equally to writing this article.
References
- Hanson M.R., Köhler R.H. (2006). A novel view of chloroplast structure, Web Essay 7.1. In Plant Physiology V, L. Taiz and E. Zeiger, eds (Sunderland, MA: Sinauer Associates). http://5e.plantphys.net/article.php?ch=7&id=122
- Hanson M.R., Sattarzadeh A. (2013). Trafficking of proteins through plastid stromules. Plant Cell 25: 2774–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler R.H., Cao J., Zipfel W.R., Webb W.W., Hanson M.R. (1997). Exchange of protein molecules through connections between higher plant plastids. Science 276: 2039–2042 [DOI] [PubMed] [Google Scholar]
- Köhler R.H., Hanson M.R. (2000). Plastid tubules of higher plants are tissue-specific and developmentally regulated. J. Cell Sci. 113: 81–89 [DOI] [PubMed] [Google Scholar]
- Köhler R.H., Schwille P., Webb W.W., Hanson M.R. (2000). Active protein transport through plastid tubules: Velocity quantified by fluorescence correlation spectroscopy. J. Cell Sci. 113: 3921–3930 [DOI] [PubMed] [Google Scholar]
- Schattat M.H., Griffiths S., Mathur N., Barton K., Wozny M.R., Dunn N., Greenwood J.S., Mathur J. (2012a). Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell 24: 1465–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat M.H., Klösgen R.B., Mathur J. (2012b). New insights on stromules: Stroma filled tubules extended by independent plastids. Plant Signal. Behav. 7: 1132–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymosi K., Schoefs B. (2010). Etioplast and etio-chloroplast formation under natural conditions: The dark side of chlorophyll biosynthesis in angiosperms. Photosynth. Res. 105: 143–166 [DOI] [PubMed] [Google Scholar]