Abstract
Stromules are thin projections from plastids that are generally longer and more abundant on non-green plastids than on chloroplasts. Occasionally stromules can be observed to connect two plastid bodies with one another. However, photobleaching of GFP-labeled plastids and stromules in 2000 demonstrated that plastids do not form a network like the endoplasmic reticulum, resulting in the proposal that stromules have major functions other than transfer of material from one plastid to another. The absence of a network was confirmed in 2012 with the use of a photoconvertible fluorescent protein, but the prior observations of movement of proteins between plastids were challenged. We review published evidence and provide new experiments that demonstrate trafficking of fluorescent protein between plastids as well as movement of proteins within stromules that emanate from a single plastid and discuss the possible function of stromules.
Projections from chloroplasts have been reported sporadically in the literature for over a hundred years (reviewed in Gray et al., 2001; Kwok and Hanson, 2004a) and became established as genuine features of plastids when they were observed by the targeting of green fluorescent protein (GFP) to the stromal compartment (Köhler et al., 1997). This study showed that these projections sometimes appeared to connect discrete plastid bodies, and photobleaching experiments demonstrated flow of GFP from one plastid body to another. After GFP in one plastid body was bleached, fluorescence rapidly recovered as a result of flow from GFP from the unbleached plastids. By continuous bleaching of a stromule connecting two plastids, fluorescence was lost from both plastids. This led to the speculation that there could be an interplastid communication system (Köhler et al., 1997). In a follow-up study to test the degree of interplastid connectedness, the term “stromule” was coined to prevent confusion with other tubular structures in the cell (Köhler and Hanson, 2000). The existence of a stromule-based plastid network was ruled out by these experiments, but movement of protein through stromules was confirmed, and it was proposed that stromules might function to facilitate transport of substances in and out of the plastid by increasing surface area and by placing the plastid compartment in close proximity to other organelles or subcellular structures (Köhler and Hanson, 2000). A study by Schattat et al. (2012) confirmed the absence of a plastid network with the use of a photoconvertible fluorescent protein. These authors also describe photoconversion experiments that appear to contradict our prior work demonstrating flow of GFP between two plastid bodies connected by a stromule. Here, we confirm our prior fluorescence recovery after photobleaching (FRAP) results, showing that proteins can move through stromules between individual plastids, and we demonstrate that a red photoconverted protein can also move into a region where photoconversion has not occurred, provided that potentially damaging levels of light are not used during the photoconversion experiment. We review previous studies showing the lack of an interconnected plastid network and consider other functions for stromules, such as facilitating the transport of enzymes and metabolites to and from the plastid to the vicinity of other organelles or regions of the cell.
LACK OF A NETWORK OF INTERCONNECTED PLASTIDS
The existence of a stromule-based network was ruled out by experiments in which localized regions of cultured cells with stroma-localized GFP were photobleached (Figures 1 and 2; Köhler and Hanson, 2000). In fluorescence loss in photobleaching experiments, a portion of a putative network is irradiated in order to determine whether GFP flows from other regions into the target region and becomes photobleached. Had a plastid network existed, most or all of the cell would have lost fluorescence. However, instead, only the plastids and stromules within the zone of photobleaching, plus stromules extending from inside to outside the zone, were photobleached. This led to the conclusion that stromules did not form a network (Köhler and Hanson, 2000). As noted in another study (Köhler et al., 2000), “even in suspension cultured cells, where stromules are abundant, most of the plastids appear to be independent. Optical sections as well as photobleaching experiments have revealed that most of the plastids within the cell are not interconnected.” Other work in 2000 also suggested that most plastids are not interconnected. Izumi et al. (2013) fused wild-type protoplasts with protoplasts from a plant expressing GFP from the plastid genome. After 3 d, the number of plastids expressing GFP had not increased significantly, indicating that few, if any, chloroplasts had become interconnected in the somatic hybrid cell.
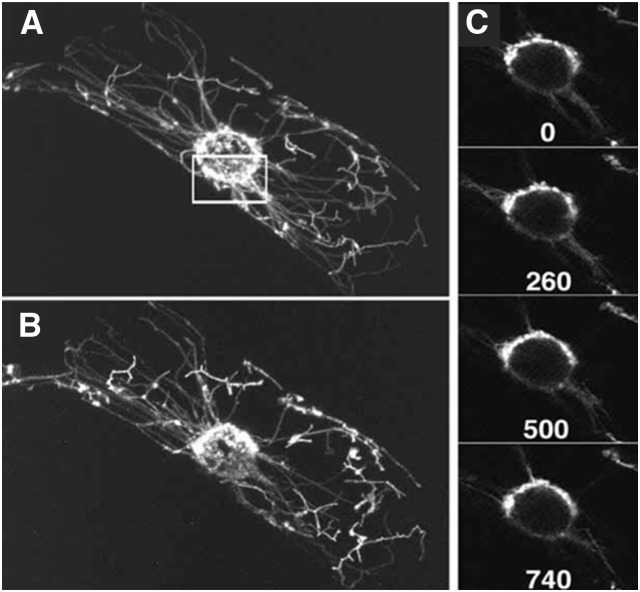
Figure 1.
GFP Photobleaching to Assess Degree of Autonomy of Plastids Clustered around the Nucleus.
Confocal microscopy images of plastids in a tobacco (Nicotiana tabacum) suspension cell expressing chloroplast-targeted GFP.
(A) and (B) Projections of 36 images taken at 1-μm intervals along the optical z axis. Prebleach image (A); postbleach image (B) collected after the time lapse.
(C) Single images of a time-lapse series collected after photobleaching of the area indicated by a box in (A). The area was photobleached with 100 scans at full laser power. The first image was taken 80 s after the beginning of photobleaching. The time-lapse images in (C) were taken at 20-s intervals over a period of 12 min. Shown are selected images with the time after the first image indicated in seconds. (Reprinted from Köhler and Hanson [2000], Figure 5.)
Figure 2.
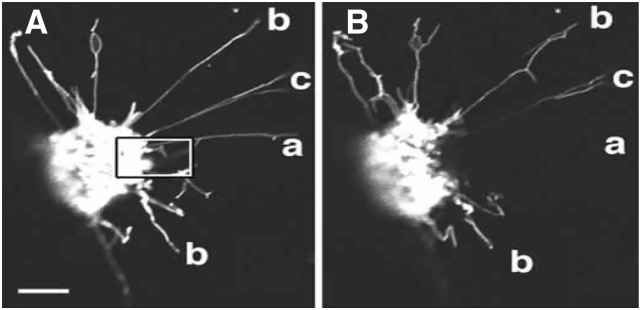
GFP Photobleaching to Assess Flow of GFP through Individual Stromules.
A selected area indicated by a white box in image (A) was bleached by scanning 30 times at full laser power. A portion of stromule (a) exhibits loss of fluorescence outside the zone of bleaching, indicating flow of GFP between the bleached and unbleached region. Stromule (b) does not lose fluorescence in any part. Stromule (c) does not lose fluorescence in the outer part, indicating it is not connected to a stromule in the bleached zone. Prebleach image (A); cell after bleaching (B). Bar = 10 μm. (Reprinted from Köhler and Hanson [2000], Figure 7.)
Since 2000, there has been no dogma that plastids form an interconnected network, though a few rare examples of obsolete or inaccurate statements can be found in the literature. In addition to reviews from our laboratory (Kwok and Hanson, 2004a; Hanson and Sattarzadeh, 2008, 2011), those of other authors also routinely pointed out the absence of a network and the low frequency of connected plastids. For example, Gray et al. (2001) noted that “Köhler and Hanson (2000) also observed that the plastids in the octopus and millipede structures surrounding the nucleus in tobacco suspension culture were not all part of a single communicating network…this indicates that not all plastids are interconnected.” In a subsequent review, Natesan et al. (2005) wrote, “the movement of macromolecules, and presumably lower molecular mass solutes, between plastids cannot be the primary function of stromules, because most stromules do not appear to interconnect plastids.” Later, Gray et al. (2011) pointed out “in many cells, plastids interconnected by stromules are rarely observed, suggesting that stromules have other functions.”
MOVEMENT OF PROTEIN THROUGH STROMULES
The development of monomeric photoconvertible proteins provided a new tool to examine intracellular dynamics (Nienhaus et al., 2006). Schattat et al. (2012) made use of a photoconvertible fluorescent protein named mEosFP (for monomeric Eos fluorescent protein; Wiedenmann et al., 2004) to examine the degree of connection between plastids. Transient or stable expression of mEosFP fused to a plastid transit sequence in Nicotiana benthamiana leaves, cultured tobacco cells, or transgenic Arabidopsis thaliana resulted in labeling of the plastid stroma, including stromules (Schattat et al., 2012). When irradiated, mEosFP fluorescence in plastids and stromules became red instead of green. These authors also performed an experiment comparable to the regional photobleaching experiments described above. When a portion of a suspension culture was irradiated, the plastids and stromules located in that region became red. The stromules that extended outside the zone of photoconversion also became red. However, during a 2-h observation period, other plastids and stromules in the unirradiated zone did not become red (Schattat et al., 2012), indicating lack of connectedness as had been found by the earlier GFP photobleaching experiments (Köhler and Hanson, 2000). In N. benthamiana leaves and Arabidopsis seedlings, stromules with red photoconverted mEosFP also appeared independent of stromules with green mEosFP (Schattat et al., 2012).
Schattat et al. (2012) concluded from their mEosFP photoconversion experiments that individual plastids, “despite conveying a strong impression of interactivity and fusion, maintained well-defined boundaries and did not exchange fluorescent proteins,” which differs from conclusions drawn from the previous GFP photobleaching data. Several images were provided of plastid bodies that appeared to be connected by a stromule prior to photoconversion. However, after photoconversion of the mEosFP in one plastid, the red fluorescence appeared to remain in that plastid body or its attached stromule, while the other plastid body remained green (Figure 1; Schattat et al., 2012). One possible explanation for these observations is breakage of the stromule following irradiation. We often observed that stromules can rupture during prolonged imaging. Suitable imaging conditions are needed to prevent artifacts that can result from strong irradiation. Schattat et al. (2012) performed photoconversion with a mercury arc lamp, sometimes irradiating the cells up to 30 s, and then observed the tissue by confocal microscopy. If some of the stromules did break, evidently they were not able to reconnect during the period of observation. We also occasionally observed striking images of photoconverted mEosFP that is present in only a portion of a stromule, but only when we have used the 405-nm line of an argon laser at 100% intensity to perform photobleaching (Figures 3A and 3B; see Supplemental Movie 1 online). We never observed such images when photoconversion was performed with the laser set at 1% intensity, even though we performed dozens of photoconversions of plastid bodies at 1% power. We do not know whether the irradiation conditions used by Schattat et al. (2012) might have broken some stromules or somehow affected the mEosFP so that it can no longer diffuse freely.
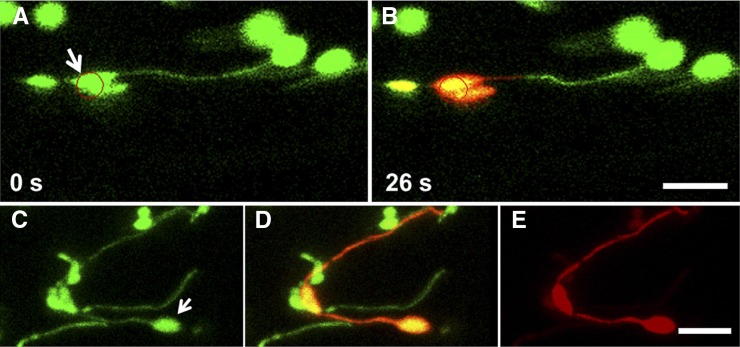
Figure 3.
Photoconversion of mEosFP at High and Low Laser Power.
(A) and (B) The lack of movement of photoconverted mEos (red state) to the end of a plastid stromule when conversion is performed at 100% laser power with 20 iterations in etiolated Arabidopsis hypocotyls expressing mEosFP in plastids and stromules.
(A) Prephotoconversion image. Red circle indicates the region that will be irradiated.
(B) Postphotoconversion image from the green state to the red state. Time is indicated in seconds. Bar = 10 μm. See also Supplemental Movie 1 online.
(C) to (E) Photoconversion of mEosFP at 1% laser power in one plastid body in an Arabidopsis cultured cell results in transmission of photoconverted protein to a second plastid body and to a stromule attached to the second plastid body. Bar = 5 μm.
(C) Green state of mEosFP. Arrow indicates the plastid that will undergo localized photoconversion.
(E) Red state of mEosFP.
(D) The overlay of images (C) and (E). Postphotoconversion images are maximum projections of confocal images taken along the z axis.
By carefully selecting conditions to avoid damage of plastids and stromules (see Methods), we can readily document flow of photoconverted protein from one plastid to another. In Figures 3C to 3E, we provide images of photoconverted mEosFP that has flowed from one plastid body to another and through a stromule attached to the second plastid body but has not entered a nearby stromule or plastid body. This experiment also demonstrates that the red photoconverted stromule is not continuous with nearby stromules that contain green-state mEosFP, consistent with the report of Schattat et al. (2012). The increase in red-state mEosFP in a photoconverted plastid occurs before the increase in red fluorescence in plastids connected to the irradiated plastid. We reported similar data in 2000 (Köhler and Hanson, 2000), when we wrote “some of our photobleaching experiments revealed that GFP does not always flow from an unbleached tubule into a bleached tubule that appeared to be connected, indicating the plastid tubules were independent rather than connected.” Thus, contrary to the statement of Schattat et al. (2012) that “the use of single-colored proteins does not allow resolving between actual and apparent contact,” our photobleaching experiments in 2000 clearly demonstrated the power of the FRAP method to distinguish between plastid bodies connected by stromule versus plastid bodies that were merely in close proximity to a stromule. If we bleach a plastid body to which a stromule is attached, we invariably see increase in GFP in the photobleached body as a result of GFP moving from the stromule. If the stromule is also attached to a second plastid body, we invariably see that the increase in GFP in the nonfluorescent body is accompanied by a decrease in GFP in the attached nonbleached body as GFP diffuses into the bleached plastid body.
In addition to GFP photobleaching for visualization of movement of protein through stromules, movement of photoconverted protein through stromules can also be readily demonstrated, provided that irradiation conditions are used that do not damage the stromule or viability of the cell (Figure 4; see Supplemental Movies 2 and 3 online). These images illustrate the conversion of green mEosFP to red in the irradiated region marked by a circle. In both examples, there is the expected delay between the time that the irradiated plastid exhibits an increase in red-state mEosFP and the time that the recipient plastid begins to acquire red-state mEosFP (Figures 4J and 4K). In Figure 5, we show an example of a recipient plastid over 40 μm away from a photoconverted plastid that exhibits an increase in the red mEosFP soon after photoconversion is initiated (Figure 5; see Supplemental Movie 4 online). By contrast, two plastids that are nearby but unconnected do not exhibit significant increase in red signal.
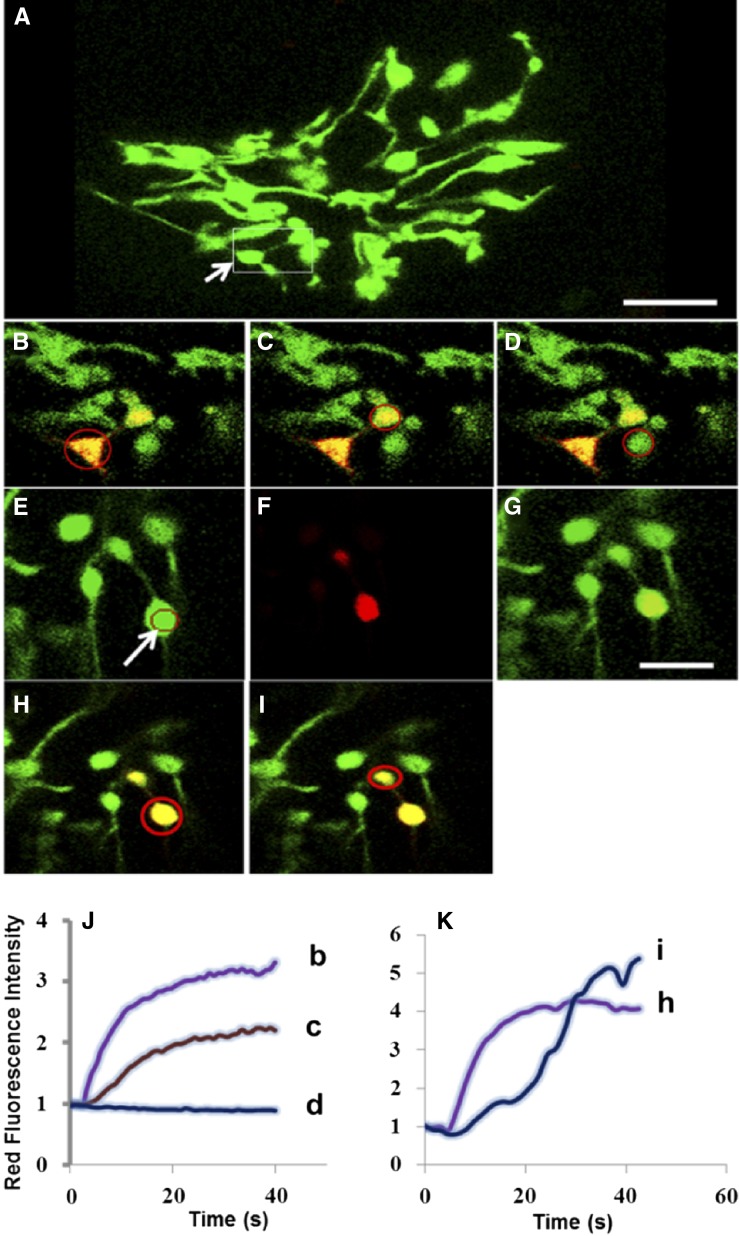
Figure 4.
Trafficking of Photoconverted mEos (Red State) through Plastid Stromules in Arabidopsis Cultured Cells Expressing mEosFP.
(A) Prephotoconversion image is a maximum projection of confocal images taken along the z axis. Arrow indicates the plastid that will undergo localized photoconversion with a 405-nm laser. Photoconversion was performed at 1% laser power, for 10 iterations.
(B) to (D) The fluorescence intensity was measured in the area indicated by a red circle in the irradiated plastid body (B), a directly connected plastid body that contains red mEosFP despite not being irradiated (C), and an unconnected plastid (D).
(E) Prephotoconversion image. Arrow indicates the plastid that will undergo localized photoconversion.
(F) Postphotoconversion image of red state of mEosFP.
(G) Overlay of the green and red state of mEosFP after photoconversion. Bar = 5 μm.
(H) and (I) Measurement of the fluorescence intensity in the area indicated by a red circle in irradiated plastid (H) and in a directly connected but unirradiated plastid (I).
(J) and (K) Red fluorescence intensity in the area indicated by a red circle in (B) to (D) and (H) and (I), respectively, was normalized by dividing the measurements by the intensity at the initial time point at the region. Time is indicated in seconds. See also Supplemental Movies 2 and 3 online.
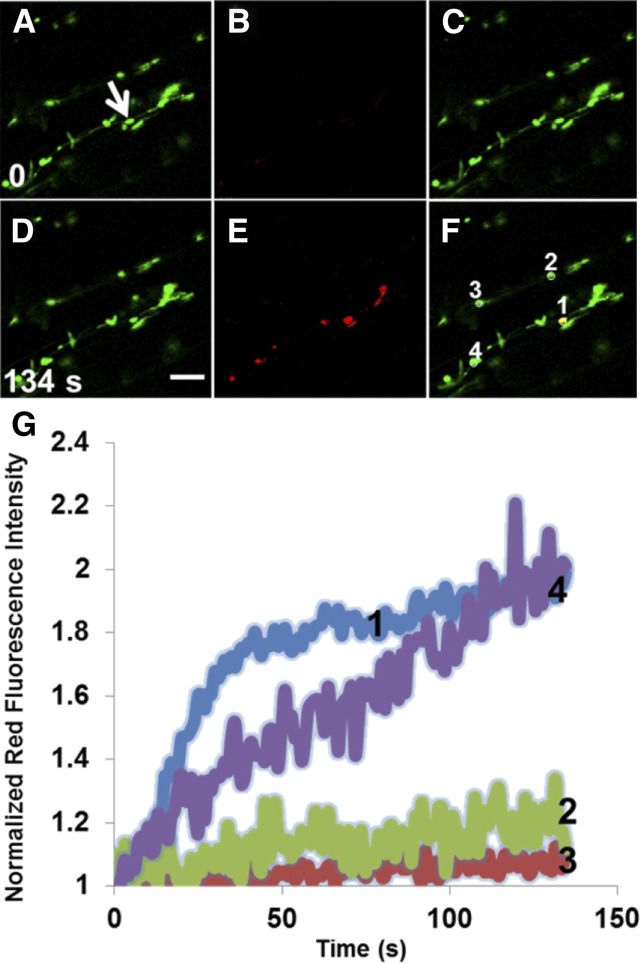
Figure 5.
Movement of Photoconverted mEos (Red State) through Plastid Stromules and Quantitative Analysis of Photoconversion Experiments after Photoconversion from the Green State to the Red State of mEosFP Expressed in Plastids and Stromules of a Arabidopsis Hypocotyl Cell.
(A) to (C) Single snapshots from the prephotoconversion from the time-lapse series.
(D) and (E) Single snapshots from the time-lapse series indicating photoconversion from mEos (green state) (D) to mEosFP (red state) (E).
(F) Overlay of the green and red states of mEosFP after photoconversion.
(G) Red fluorescence intensity was normalized by dividing the measurements by the intensity at the initial time point at the region.
A 405-nm laser applied at 100% power but only two iterations of irradiation were used for photoconversion. Ten prephotoconversion scans were recorded before starting the photoconverting irradiation in order to establish the baseline fluorescence intensity and distribution. Signal is noisy because plastids are moving at the time of imaging. Bar = 20 μm. See also Supplemental Movie 4 online.
THE QUESTION OF STROMULE FUSION
The concept that stromules can sometimes, though infrequently, fuse with one another and with other plastids, arose from several lines of indirect evidence. Foremost are observations of two plastids bodies at some distance from each other with a linear stromule between them, as observed in experiments described above. Another line of evidence derives from the video microscopy of Wildman et al. (1962) and Gunning (2005, 2009), in which actively moving stromules were visualized. Stromules sometimes appeared to reach out and touch and stick to each other or other plastids; stromules extending from a single plastid also appeared to flip back onto the envelope membrane, forming loop structures. Gunning (2005) provides an example of two plastids in a tomato (Solanum lycopersicum) trichome connected by a stromule that separate and then become “bridged” together again and move away together. Furthermore, in GFP-visualized stromules, two plastid bodies at some distance from each other are sometimes observed to travel together, tethered by a stromule between them (Figure 6; see Supplemental Movie 5 online). When the actin cytoskeleton is disrupted, stromules appear to flip back attach to the main plastid body (Kwok and Hanson, 2003), forming loops, as also observed by Gunning (2005, 2009).
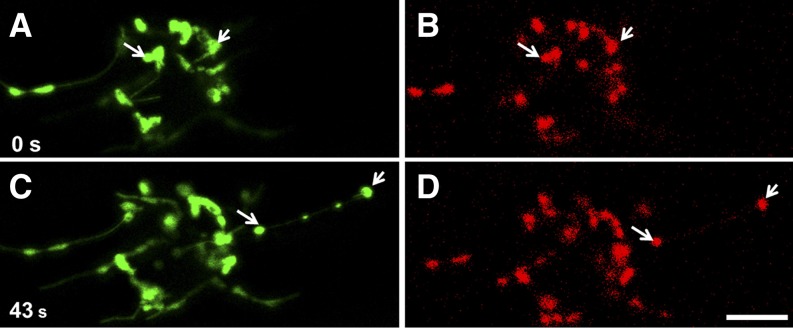
Figure 6.
Coordinated Movement of Two Plastid Bodies Connected by a Stromule in a Hypocotyl Cell of Transgenic Tobacco Plants Carrying a Plastid-Targeted GFP Expressed from a Nuclear Transgene Carrying a Double 35S Promoter and the recA Transit Sequence on S65T GFP (Köhler et al., 1997).
GFP fluorescence is shown as green and chlorophyll autofluorescence as red. Images are single snapshots from the Supplemental Movie 5 online. The 488-nm line of an argon laser was used to excite GFP and chlorophyll. Bar = 10 μm.
(A) and (B) The two plastid bodies indicated with arrows begin moving together to the right.
(C) and (D) Position of the two plastid bodies shown in (A) and (B) after 43 s.
While stromules appear to be “sticky” in video and epifluorescence microscopy, such images cannot distinguish strong contacts and partial fusion mediated by the outer envelope membrane from complete fusion that results in functional connection of the two plastid compartments. Direct evidence for complete fusion would be the observation of a stromule that is initially independent that then touches a different plastid, followed by photoconversion or photobleaching of one of the plastids to demonstrate that there is exchange of proteins through the stromule. Such an experiment is extremely difficult technically. It requires finding a stromule in the act of attaching to another one or to another plastid body. Even if complete fusions do occur, there would be many instances where the stromules enter into close contacts with one another but do not fuse completely, as seen by the photobleaching experiments in 2000 and the photoconversion experiments in 2012. Evidence against the existence of occasional complete fusion is virtually impossible to obtain, given the number of apparent contacts that might need to be assayed before concluding that fusion never occurs. The relative rarity of apparently connected plastids and chloroplasts that we observed over the course of many experiments led us to conclude that “transmission of molecules from one plastid to another is not likely to be the primary function of stromules” (Hanson and Köhler, 2001).
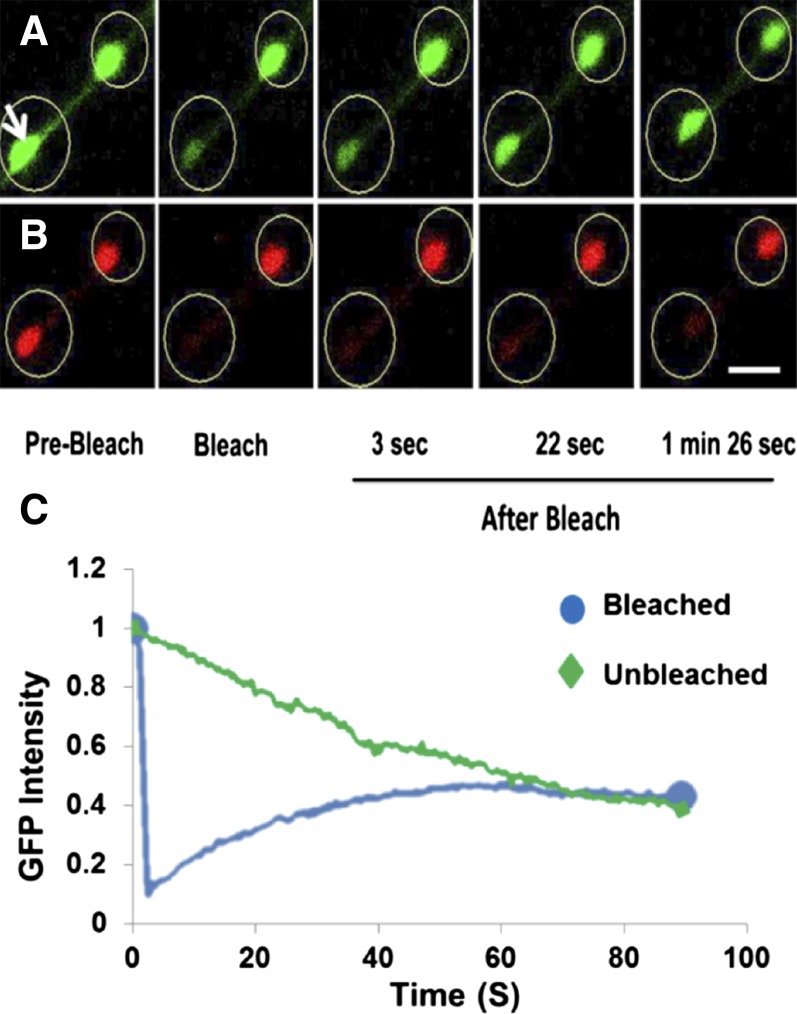
Schattat et al. (2012) raised the possibility that the plastid bodies interconnected by a stromule that have been imaged are daughter plastids that have not completely separated from one another or are single pleomorphic plastids. However, the definition of a stromule was not intended to include the short narrow structure (isthmus) sometimes seen between plastids in the late stages of division. Stromules are clearly distinguished from the isthmus of dividing plastids by their length. Stromules much longer than the length of a typical plastid have been observed between two plastid bodies, and photobleaching experiments have previously shown transfer of GFP between them (Hanson and Sattarzadeh, 2011). Although stromules in green tissue sometimes exhibit bulbous ends, the absence of chlorophyll and thylakoid membranes in such bulbs readily distinguishes them from plastid bodies (Holzinger et al., 2008). An example of two distinct chloroplasts connected by a stromule is shown in Figure 7 and Supplemental Movie 6 online. Following photobleaching, GFP is clearly being transferred from the unbleached plastid to the bleached plastid, while chlorophyll, evidently due to its membrane association, is not transferred. If two plastid bodies both contain chlorophyll (and therefore thylakoid membranes) and are well separated from one another, we maintain that they should be considered to be individual chloroplasts connected by a stromule.
Figure 7.
Photobleaching Demonstrates Movement of GFP through a Stromule Connecting Two Chloroplasts in a Hypocotyl Cell of Tobacco Plants Carrying a Nuclear Transgene Encoding Chloroplast-Targeted GFP.
(A) GFP (green) in two chloroplasts with a stromule between was imaged before and during recovery after bleaching of the chloroplast indicated with an arrow.
(B) Chlorophyll autofluorescence (red) before and after photobleaching. Bar = 5 μm.
(C) Change in GFP intensity in the bleached chloroplast and unbleached chloroplast over time indicates decreasing fluorescence in unbleached plastid due to transfer to the unbleached chloroplast, which acquires GFP. A 488-nm argon laser applied at 100% power was used to photobleach GFP. See also Supplemental Movie 6 online.
WHAT IS THE FUNCTION OF STROMULES?
Stromules are longer and more prevalent in nongreen cells where there are fewer plastids/cell than in leaf cells (Köhler and Hanson, 2000; Kwok and Hanson, 2004a; Waters et al., 2004; Shaw and Gray, 2011; Gray et al., 2012). In the absence of stromules, the biosynthetic reactions that occur in the plastid, such as amino acid,fatty acid, and starch synthesis, would be present in a more limited region of the cell’s cytoplasm. Knowing whether proteins, and therefore other molecules, are present and move through stromules is therefore of interest with regard to the possible functions of stromules. We have previously shown that ribulose-1,5-bisphosphate carboxylase/oxygenase (550 kD) and Asp aminotransferase can traffic within stromules, as well as GFP (Kwok and Hanson, 2004b).
Earlier, we proposed that one of the likely functions of stromules is to place the components of the plastid compartment in close proximity to other subcellular structures to reduce diffusion distance of molecules that enter or are released from the stromule (Köhler and Hanson, 2000; Kwok and Hanson, 2004a; Hanson and Sattarzadeh, 2008, 2011). Stromules also increase the surface area of the envelope membrane, which may enhance import and export. Stromules are often seen next to mitochondria, other plastids, the endoplasmic reticulum (ER), and the plasma membrane, and can even enter grooves and channels within the nucleus (Gray et al., 2001; Hanson and Köhler, 2001; Kwok and Hanson, 2004c; Holzinger et al., 2007, 2008; Hanson and Sattarzadeh, 2008, 2011; Schattat et al., 2011). In a recent report, Mehrshahi et al. (2013) describe the use of transorganellar complementation to show exchange of nonpolar metabolites between the chloroplast lumen and ER. These authors propose a mechanism, involving membrane hemifusion or plastid-associated membranes, that would allow bidirectional transport between plastids and other organelles and provide ER-resident enzymes access to nonpolar metabolites located in the plastid envelope. This study highlights the importance of close contacts between plastid envelopes and other organelles to facilitate biosynthetic processes.
We demonstrated that the movement of GFP by diffusion approximates the rate of movement of GFP in the red blood cell membrane due to the high viscosity of the stroma (Köhler et al., 2000). Fluorescence correlation spectroscopy indicates that GFP can move in small packets of multiple molecules within stromules (Köhler et al., 2000). While such small packets are often too small to be imaged by the confocal microscope, occasionally larger GFP globules with stromules are visible by fluorescence microscopy. A few times we have observed directional movement of GFP globules through a long stromule (Hanson and Sattarzadeh, 2008). Two such globules can be seen in Supplemental Movie 5 online, although the globules do not seem to be moving within the stromule, unlike one documented by Hanson and Sattarzadeh (2008). The mechanism that causes directional movement in a stromule is unknown.
While this report has focused on the possible role of stromules in placing the chloroplast stromal compartment in close proximity to other organelles, stromules may also have an additional important role in chloroplast function. In collaboration with Hiroyuki Ishida and his colleagues (Ishida et al., 2008), we demonstrated that fluorescently labeled stroma-containing bodies, possibly resulting from breakage of stromules from the main plastid body, enter vacuoles for degradation during nutrient stress. The stroma-containing bodies that entered the vacuole were identified as autophagosomes, due to their absence in an autophagy mutant and by labeling of the bodies by an AUTOPHAGY GENE8-GFP fusion (Ishida et al., 2008). A role for stromules in recycling of chloroplast contents fits with the observation that stromule and/or chloroplast protrusion frequency increases in stressful environments, such as arctic growth conditions (Holzinger et al., 2007), abscisic acid treatment (Gray et al., 2012), or growth in a saline hydroponic culture (Yamane et al., 2012). Ishida’s group later demonstrated that the carbohydrate rather than nitrogen status of the plant influenced whether stromal contents were recycled (Izumi and Ishida, 2011). Thus, stromules may play a role in trafficking of stromal contents to the vacuole through the autophagic pathway to facilitate growth in stressful conditions (Izumi et al., 2013).
In addition to considering the variety of possible functions of stromules, we should also consider which hypothetical functions are unlikely. At the time of our first report of GFP labeling of stromules (Köhler et al., 1997), we pointed out that chlorophyll and, therefore, thylakoid membranes, do not enter stromules. Because chloroplast nucleoids are thought to be bound to thylakoid membranes (Liu and Rose, 1992), chloroplast genomes are likewise unlikely to be present in stromules. Furthermore, if DNA flowed between plastids, recombination could occur between variant chloroplast genomes present in different plastids. However, the rarity of plastid genome recombination following fusion of parental protoplasts carrying two different plastid genomes indicates that DNA transfer is rare (Medgyesy et al., 1985; Clark et al., 1986; Gray et al., 2012). This genetic data is consistent with a recent report by Newell et al. (2012), who observed that neither nucleoids nor ribosomes, which are presumably nucleoid-associated, enter stromules.
In conclusion, in contrast with statements made by Schattat et al. (2012), the absence of an interconnected network of plastids has been known since the GFP photobleaching experiments of Köhler and Hanson (2000). Nevertheless, pairs of plastid or chloroplast bodies that are a significant distance from one another have been observed to be functionally connected through stromules by FRAP and fluorescence loss in photobleaching experiments. Both photobleaching and photoconversion experiments can demonstrate trafficking of fluorescent protein from one plastid or chloroplast to another. Using appropriate conditions for photoconversion, transfer of photoconverted protein from one plastid to another and to the tip of a connected stromule can be readily visualized. However, connection of plastid bodies by stromules resulting in movement of protein between them may be limited in significance because of the relativity rarity of such connections within most plant cells. Instead, movement of molecules within stromules is potentially important as a means of allowing the plastid stromal compartment to be located in the far reaches of the cell, not merely confined to small plastid bodies present in a limited area. Stromule-mediated distribution of enzymes, reactants, and products to other subcellular compartments, such as the ER, potentially has major significance to the biosynthetic and metabolic processes of the plant cell.
METHODS
Seeds of transgenic Arabidopsis thaliana (provided by Jaideep Mathur) expressing transit peptide ferredoxin-NADP(+) oxidoreductase (tpFNR):mEosFP (Schattat et al., 2012) were treated with bleach and grown on Murashige and Skoog media with agar and 3% Suc in the dark to obtain etiolated hypocotyls. An Arabidopsis cell culture was produced by placing explants of green seedlings expressing tpFNR:mEosFP (Schattat et al., 2012) onto the Gamborg B5 callus-inducing medium described by May and Leaver (1993). Callus was then transferred into the same medium lacking agar in order to produce suspension cultures.
A Zeiss laser scanning microscope 710 confocal microscope equipped with a ×40 water immersion objective was used for the imaging and photoconversion of tpFNR:mEosFP. The Zeiss automated bleaching mode was used for photoconversion. A 405-nm laser applied at 1% with 10 iterations of “bleaching” is recommended for photoconversion in order to visualize movement of fluorescent protein. When the laser was applied at 50% or 100% power at 10 or 20 iterations, off-target photoconversion was observed as well as lack of flow of protein through stromules, possibly due to photodamage. Five or 10 prephotoconversion scans were recorded before starting the photoconverting irradiation to establish the baseline fluorescence intensity and distribution. The preconversion, converted, and subsequent images scanned with pixel dwell between 0.5 and 2 μs with scan time completed in <1 s between each frame. Imaging in mEosFP photoconversion experiments was done in the sequential mode to exclude crosstalk due to spectral overlap of the fluorophores with linewise switching. mEosFP (green state) was excited with the 488-nm line of an argon laser, and the 561-nm line of a He/Ne laser was used to excite mEosFP (red state). Emission wavelengths for mEos (green) was 500 to 525 nm and 585 to 680 nm for mEos (red). Images were recorded and processed using Zeiss Zen software 2009. The relative fluorescence intensity of the region of interest from Zeiss software was collected and imported to Excel data and normalized based on initial value (time point).
The 488-nm line of an argon laser at 100% power on a Zeiss LSM 701 confocal microscope was used to photobleach GFP in hypocotyl cells of transgenic tobacco plants carrying a plastid-targeted S65T GFP expressed from a nuclear transgene carrying a double 35S promoter and the recA transit sequence (Köhler et al., 1997).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Movie 1. The Lack of Flow of Photoconverted mEosFP (Red State) through a Plastid Stromule When Conversion Is Performed at High Laser Power.
Supplemental Movies 2 and 3. The Trafficking of Photoconverted mEos (Red State) through Plastid Stromules When Conversion Is Performed at Low Laser Power.
Supplemental Movie 4. Photoconversion Demonstrates the Trafficking of Photoconverted mEos (Red State) through Plastid Stromules.
Supplemental Movie 5. Coordinated Movement of Two Plastid Bodies Connected by a Stromule.
Supplemental Movie 6. Photobleaching Demonstrates Trafficking of GFP through a Stromule Connecting Two Chloroplasts.
Acknowledgments
Recent work on stromules was supported by grants from the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy to M.R.H. (especially Grant DE-FG02-09ER16070). Carol Bayles at the Cornell Life Sciences Core Laboratories Center for Microscopy and Imaging and Warren Zipfel (Department of Biomedical Engineering, Cornell University) provided numerous helpful suggestions. We thank Jaideep Mathur (Department of Molecular and Cellular Biology, University of Guelph) for providing seeds of Arabidopsis expressing mEosFP. We appreciate many helpful suggestions and editorial comments by Nan Eckardt of the American Society of Plant Biologists.
AUTHOR CONTRIBUTIONS
M.R.H. conceived of the study, designed research, analyzed data, reviewed the literature, and wrote the article. A.S. performed research, developed the technique, analyzed data, and edited the article.
References
- Clark E., Schnuabelrauch L., Hanson M.R., Sink K.C. (1986). Differential fate of plastid and mitochondrial genomes in Petunia somatic hybrids. Theor. Appl. Genet. 72: 748–755 [DOI] [PubMed] [Google Scholar]
- Gray J.C., Hansen M.R., Shaw D.J., Graham K., Dale R., Smallman P., Natesan S.K., Newell C.A. (2012). Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J. 69: 387–398 [DOI] [PubMed] [Google Scholar]
- Gray J.C., Sullivan J.A., Hibberd J.M., Hansen M.R. (2001). Stromules: Mobile protrusions and interconnections between plastids. Plant Biol. (Stuttg.) 3: 223–233 [Google Scholar]
- Gray J.C., Sullivan J.A., Newell C.A. (2011). Visualisation of stromules on Arabidopsis plastids. Methods Mol. Biol. 774: 73–85 [DOI] [PubMed] [Google Scholar]
- Gunning B.E. (2005). Plastid stromules: Video microscopy of their outgrowth, retraction, tensioning, anchoring, branching, bridging, and tip-shedding. Protoplasma 225: 33–42 [DOI] [PubMed] [Google Scholar]
- Gunning B.E.S. (2009). Plant Cell Biology on DVD. (Heidelberg, Germany: Springer-Verlag; ). [Google Scholar]
- Hanson M.R., Köhler R.H. (2001). GFP imaging: Methodology and application to investigate cellular compartmentation in plants. J. Exp. Bot. 52: 529–539 [PubMed] [Google Scholar]
- Hanson M.R., Sattarzadeh A. (2008). Dynamic morphology of plastids and stromules in angiosperm plants. Plant Cell Environ. 31: 646–657 [DOI] [PubMed] [Google Scholar]
- Hanson M.R., Sattarzadeh A. (2011). Stromules: Recent insights into a long neglected feature of plastid morphology and function. Plant Physiol. 155: 1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A., Kwok E.Y., Hanson M.R. (2008). Effects of arc3, arc5 and arc6 mutations on plastid morphology and stromule formation in green and nongreen tissues of Arabidopsis thaliana. Photochem. Photobiol. 84: 1324–1335 [DOI] [PubMed] [Google Scholar]
- Holzinger A., Wasteneys G.O., Lütz C. (2007). Investigating cytoskeletal function in chloroplast protrusion formation in the arctic-alpine plant Oxyria digyna. Plant Biol. (Stuttg.) 9: 400–410 [DOI] [PubMed] [Google Scholar]
- Ishida H., Yoshimoto K., Izumi M., Reisen D., Yano Y., Makino A., Ohsumi Y., Hanson M.R., Mae T. (2008). Mobilization of Rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 148: 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M., Hidema J., Makino A., Ishida H. (2013). Autophagy contributes to nighttime energy availability for growth in Arabidopsis. Plant Physiol. 161: 1682–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M., Ishida H. (2011). The changes of leaf carbohydrate contents as a regulator of autophagic degradation of chloroplasts via Rubisco-containing bodies during leaf senescence. Plant Signal. Behav. 6: 685–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler R.H., Cao J., Zipfel W.R., Webb W.W., Hanson M.R. (1997). Exchange of protein molecules through connections between higher plant plastids. Science 276: 2039–2042 [DOI] [PubMed] [Google Scholar]
- Köhler R.H., Hanson M.R. (2000). Plastid tubules of higher plants are tissue-specific and developmentally regulated. J. Cell Sci. 113: 81–89 [DOI] [PubMed] [Google Scholar]
- Köhler R.H., Schwille P., Webb W.W., Hanson M.R. (2000). Active protein transport through plastid tubules: velocity quantified by fluorescence correlation spectroscopy. J. Cell Sci. 113: 3921–3930 [DOI] [PubMed] [Google Scholar]
- Kwok E.Y., Hanson M.R. (2003). Microfilaments and microtubules control the morphology and movement of non-green plastids and stromules in Nicotiana tabacum. Plant J. 35: 16–26 [DOI] [PubMed] [Google Scholar]
- Kwok E.Y., Hanson M.R. (2004a). Stromules and the dynamic nature of plastid morphology. J. Microsc. 214: 124–137 [DOI] [PubMed] [Google Scholar]
- Kwok E.Y., Hanson M.R. (2004b). GFP-labelled Rubisco and aspartate aminotransferase are present in plastid stromules and traffic between plastids. J. Exp. Bot. 55: 595–604 [DOI] [PubMed] [Google Scholar]
- Kwok E.Y., Hanson M.R. (2004c). Plastids and stromules interact with the nucleus and cell membrane in vascular plants. Plant Cell Rep. 23: 188–195 [DOI] [PubMed] [Google Scholar]
- Liu J.W., Rose R.J. (1992). The spinach chloroplast chromosome is bound to the thylakoid membrane in the region of the inverted repeat. Biochem. Biophys. Res. Commun. 184: 993–1000 [DOI] [PubMed] [Google Scholar]
- May M.J., Leaver C.J. (1993). Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 103: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medgyesy P., Fejes E., Maliga P. (1985). Interspecific chloroplast recombination in a Nicotiana somatic hybrid. Proc. Natl. Acad. Sci. USA 82: 6960–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrshahi P., Stefano G., Andaloro J.M., Brandizzi F., Froehlich J.E., Dellapenna D. (2013). Transorganellar complementation redefines the biochemical continuity of endoplasmic reticulum and chloroplasts. Proc. Natl. Acad. Sci. USA 110: 12126–12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan S.K., Sullivan J.A., Gray J.C. (2005). Stromules: A characteristic cell-specific feature of plastid morphology. J. Exp. Bot. 56: 787–797 [DOI] [PubMed] [Google Scholar]
- Newell C.A., Natesan S.K., Sullivan J.A., Jouhet J., Kavanagh T.A., Gray J.C. (2012). Exclusion of plastid nucleoids and ribosomes from stromules in tobacco and Arabidopsis. Plant J. 69: 399–410 [DOI] [PubMed] [Google Scholar]
- Nienhaus G.U., et al. (2006). Photoconvertible fluorescent protein EosFP: Biophysical properties and cell biology applications. Photochem. Photobiol. 82: 351–358 [DOI] [PubMed] [Google Scholar]
- Schattat M., Barton K., Baudisch B., Klösgen R.B., Mathur J. (2011). Plastid stromule branching coincides with contiguous endoplasmic reticulum dynamics. Plant Physiol. 155: 1667–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat M.H., Griffiths S., Mathur N., Barton K., Wozny M.R., Dunn N., Greenwood J.S., Mathur J. (2012). Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell 24: 1465–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D.J., Gray J.C. (2011). Visualisation of stromules in transgenic wheat expressing a plastid-targeted yellow fluorescent protein. Planta 233: 961–970 [DOI] [PubMed] [Google Scholar]
- Waters M.T., Fray R.G., Pyke K.A. (2004). Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. Plant J. 39: 655–667 [DOI] [PubMed] [Google Scholar]
- Wiedenmann J., Ivanchenko S., Oswald F., Schmitt F., Röcker C., Salih A., Spindler K.D., Nienhaus G.U. (2004). EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA 101: 15905–15910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman S.G., Hongladarom T., Honda S.I. (1962). Chloroplasts and mitochondria in living plant cells: Cinephotomicrographic studies. Science 138: 434–436 [DOI] [PubMed] [Google Scholar]
- Yamane K., Mitsuya S., Taniguchi M., Miyake H. (2012). Salt-induced chloroplast protrusion is the process of exclusion of ribulose-1,5-bisphosphate carboxylase/oxygenase from chloroplasts into cytoplasm in leaves of rice. Plant Cell Environ. 35: 1663–1671 [DOI] [PubMed] [Google Scholar]