This work shows that plants respond to arsenate by immediately freezing its uptake through the action of a transcriptional repressor of phosphate transporters and that the same transcription factor influences transposon expression in response to arsenate. Plants therefore have an arsenate perception mechanism that controls arsenate uptake and transposon expression, providing an integrated strategy for arsenate tolerance and genome stability.
Abstract
Stress constantly challenges plant adaptation to the environment. Of all stress types, arsenic was a major threat during the early evolution of plants. The most prevalent chemical form of arsenic is arsenate, whose similarity to phosphate renders it easily incorporated into cells via the phosphate transporters. Here, we found that arsenate stress provokes a notable transposon burst in plants, in coordination with arsenate/phosphate transporter repression, which immediately restricts arsenate uptake. This repression was accompanied by delocalization of the phosphate transporter from the plasma membrane. When arsenate was removed, the system rapidly restored transcriptional expression and membrane localization of the transporter. We identify WRKY6 as an arsenate-responsive transcription factor that mediates arsenate/phosphate transporter gene expression and restricts arsenate-induced transposon activation. Plants therefore have a dual WRKY-dependent signaling mechanism that modulates arsenate uptake and transposon expression, providing a coordinated strategy for arsenate tolerance and transposon gene silencing.
INTRODUCTION
Environmental stress is a driving force in evolution. Plants have evolved sophisticated mechanisms to perceive different environmental stresses and activate specific tolerance mechanisms. Early in the earth’s history, volcanic emissions of arsenic to the biosphere comprised a major threat to incipient life forms (Oremland et al., 2009; Dani, 2010); indeed, all life forms have strategies to cope with this metalloid (Rosen, 2002; Tripathi et al., 2007; Mendoza-Cózatl et al., 2011; Ye et al., 2012). When the oxygen concentration increased, arsenate [As(V)] became the most prevalent form of arsenic in the biosphere (Oremland et al., 2009; Dahl et al., 2010; Dani, 2010). This chemical threat was particularly critical for sessile organisms, such as plants, which were forced to evolve rapid tolerance responses when As(V) was detected. An additional challenge related to As(V) in the biosphere is its similarity to the macronutrient Pi; when Pi is limited, Pi transporters are induced and As(V) is incorporated preferentially into plant cells (Raghothama, 1999; Catarecha et al., 2007; Wu et al., 2011).
Modulation of Pi transporter activity could be an efficient strategy for As(V) tolerance. The Arabidopsis thaliana PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 mutant, in which general Pi transporter trafficking to the plasma membrane is altered and As(V) uptake is impaired, shows remarkable As(V) tolerance (González et al., 2005). Of all transporters, the high affinity Pi transporters PHOSPHATE TRANSPORTER1;1 (PHT1;1) and PHOSPHATE TRANSPORTER1;4 (PHT1;4) in Arabidopsis have a major role in As(V) uptake (Shin et al., 2004). The pht1;1 pht1;4 double mutant has a notable As(V) tolerance phenotype, indicating that these two members of the Pi transporter family contribute to As(V) uptake in Arabidopsis (Shin et al., 2004). Some species restrict phosphate uptake as an adaptive response in As(V) tolerance (Meharg and Macnair, 1990; Murota et al., 2012). In the reference plant Arabidopsis, we identified an As(V)-tolerant mutant that harbors a semidominant allele of the Pi transporter PHT1;1 (Catarecha et al., 2007). This mutant has a slow rate of As(V) uptake that allows the arsenic detoxification machinery to cope more efficiently with the metalloid leading to enhanced arsenic accumulation in the plant; nonetheless, any strategy that interrupts As(V) uptake to protect plants from its toxic effects also compromises Pi acquisition and, thus, plant growth in natural soils.
There are few descriptions of the molecular mechanisms involved in plant As(V) perception, although recent efforts have been made to understand the systems underlying As(V) tolerance (Sung et al., 2009; Song et al., 2010; Mendoza-Cózatl et al., 2011; Jobe et al., 2012). In As(V)- or Pi-exposed Arabidopsis, As(V) downregulates expression of the high affinity Pi transporter gene PHT1;1 more efficiently than does Pi, even when the Pi uptake rate is 2 times greater than that of As(V) (Catarecha et al., 2007). By contrast, another set of genes that respond to Pi starvation (such as IPS1) are repressed more efficiently by Pi than by As(V) (Catarecha et al., 2007). Although As(V) is rapidly reduced to arsenite once incorporated into cells, PHT1;1 expression is not repressed by this form or by other heavy metals or even by short-term exposure to Pi (Catarecha et al., 2007), suggesting that PHT1;1 repression is As(V) specific.
Here, we found that As(V) uptake was modulated by the As(V)-responsive transcription factor WRKY6, which rapidly repressed expression of the As(V)/Pi transporter PHT1;1. This repression was accompanied by PHT1;1 delocalization from the plasma membrane. Once As(V) was removed, the system rapidly restored PHT1;1 transporter expression and membrane relocalization. In addition, As(V) induced transcriptional activation of transposons, also restricted by WRKY6. Plants thus have a regulatory mechanism that controls As(V) uptake and transposon expression in an integrated strategy for As(V) tolerance and transposon gene silencing.
RESULTS
As(V) Is Responsible for Rapid Pi Transporter Repression and Its Relocalization
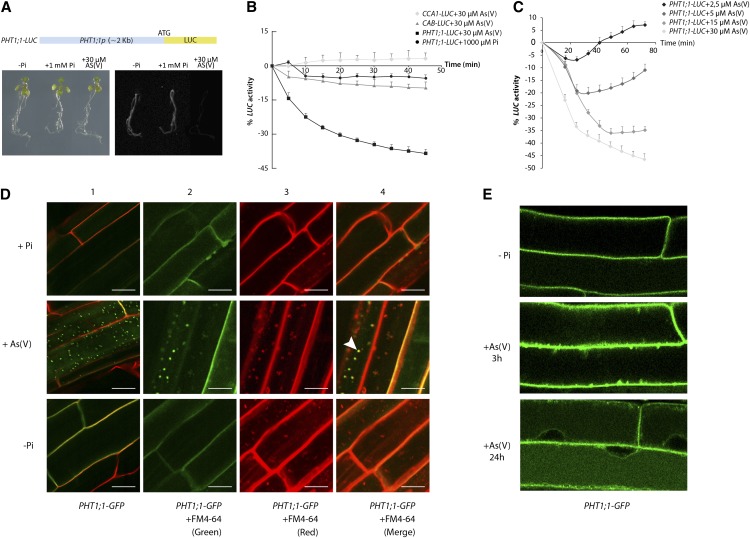
To define the molecular mechanisms that underlie PHT1;1 repression in response to As(V), we generated a transcriptional reporter line that expresses the luciferase (LUC) gene under the control of the PHT1;1 promoter region (PHT1;1-LUC). This line stably expresses luciferase activity in response to Pi starvation. PHT1;1-LUC activity decreased more rapidly in response to As(V) than to Pi (Figure 1A). Time-course analysis showed that the LUC activity of the PHT1;1-LUC construct was reduced by >40% after 45-min exposure to As(V), at which time no repression was observed in response to Pi, even at a 33-fold higher concentration (Figure 1B). As(V) had no effect on unrelated genes such as CCA1-LUC and CAB-LUC promoter fusions. Dose–response experiments using decreasing As(V) doses showed that PHT1;1-LUC repression occurred at low As(V) concentrations (Figure 1C). PHT1;1 repression and dose–response experiments were validated by quantitative RT-PCR (qRT-PCR) (see Supplemental Figure 1 online), indicating that this construct serves as a suitable readout for As(V) repression of PHT1;1. These observations indicate the operation of a sensitive As(V) detection mechanism in Arabidopsis plants.
Figure 1.
As(V) Represses and Delocalizes the Pi Transporter PHT1;1.
(A) Diagram of the 2-kb PHT1;1 promoter region fused to the luciferase reporter gene (PHT1;1-LUC; top). Analysis of LUC activity (right panel) in transgenic plants expressing PHT1;1-LUC grown on 1 mM phosphate medium for 7 d, transferred to -Pi medium for 2 d, and finally to -Pi medium supplemented with 30 μM As(V) or 1 mM Pi for 16 h (left panel).
(B) Kinetic study of LUC activity in response to 30 μM As(V) or 1 mM Pi in PHT1;1-LUC–expressing plants and in control transgenic lines CCA1-LUC and CAB-LUC. Values show mean ± sd.
(C) Kinetic study of LUC activity in response to different As(V) concentrations in PHT1;1-LUC–expressing plants. Values show mean ± sd.
(D) and (E) Confocal analysis of PHT1;1-GFP–expressing Arabidopsis root epidermal cells.
(D) Five-day-old plants grown in -Pi medium were transferred to fresh -Pi medium or to medium containing 1 mM Pi (+Pi) or 30 μM As(V) [+As(V)] for 1.5 h in the dark. Roots were stained with propidium iodide (column 1) or with the endocytic tracer FM4-64 (columns 2 to 4). Arrowhead indicates colocalization of FM4-64 with PHT1;1-GFP in endosomes. Bars = 10 μm.
(E) Five-day-old PHT1;1-GFP–expressing plants grown in -Pi medium were incubated (24 h) (-Pi) or exposed to 30 μM As(V) for 3 h [+As(V) 3 h] and 24 h [+As(V) 24 h] in the dark.
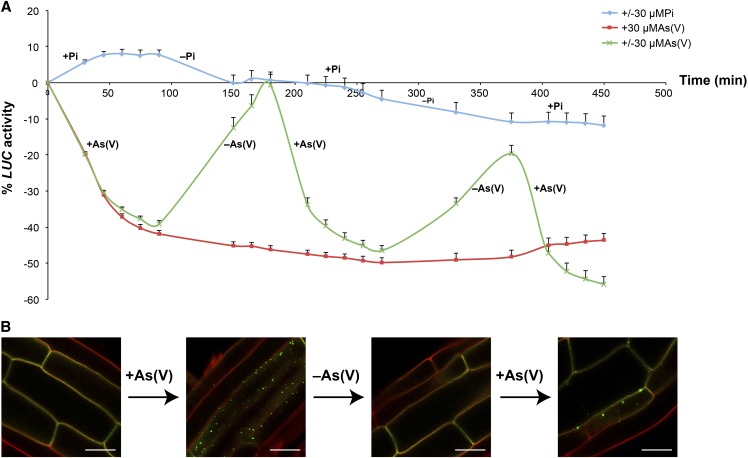
PHT1;1 repression in response to As(V) is also accompanied by endocytosis of the As(V)/Pi transporter from the plasma membrane (Figure 1D). Shortly after plant exposure to As(V), we found PHT1;1-GFP (for green fluorescent protein) fluorescence in punctate cytosolic structures that colocalized with the styryl endocytic tracer FM4-64, indicating transporter internalization into endosomes (Figure 1D). After longer incubation with As(V), GFP fluorescence labeled the vacuole lumen (Figure 1E), indicating PHT1;1-GFP internalization from the plasma membrane and transport through the endocytic pathway for turnover in the vacuole. By contrast, As(V) did not alter PIP1;4-YFP control protein localization at the plasma membrane, showing that Pi transporter relocalization is not a nonspecific response to As(V) toxicity (see Supplemental Figure 2A online). After As(V) exposure, there was an intracellular arsenic accumulation arrest that correlated with PHT1;1 relocalization (see Supplemental Figures 2B and 2C online). We thus propose the existence of a mechanism in plants that restricts As(V) uptake via two parallel responses: downregulation and relocalization of PHT1;1. As(V) removal led to restoration of correct PHT1;1 expression, with kinetics similar to that of repression (Figure 2A). After a second round of high/low As(V) exposure, plants again showed the transcriptional repression/reactivation pattern (Figure 2A) closely linked to As(V)/Pi transporter delocalization/relocalization at the plasma membrane (Figure 2B). qRT-PCR experiments again confirmed PHT1;1-LUC expression mimicry of PHT1;1 transcript accumulation (see Supplemental Figure 3 online). The rapid recovery of the system under Pi-free conditions indicated that both PHT1;1 transcriptional repression and membrane relocalization depend exclusively on As(V).
Figure 2.
PHT1;1 Expression and Its Membrane Localization Depend on As(V) Concentration.
(A) Kinetic study of LUC activity in response to pulses of 30 μM Pi (±Pi; blue line), 30 μM As(V) [±As(V); green line], or to a continuous concentration of 30 μM As(V) (red line) in PHT1;1-LUC–expressing plants. Duration of each pulse and gaps between them were 1.5 h; during the gap, samples were washed with buffer to remove Pi and As(V) from the medium. Values show mean ± sd.
(B) Analysis of PHT1;1-GFP localization after two pulses of 30 μM As(V) [±As(V)] in PHT1;1-GFP–expressing Arabidopsis root cells. Duration of each pulse and gaps between them were 1.5 h in the conditions as in (A). Bars = 10 μm.
WRKY6 Transcription Factor Mediates PHT1;1 Repression in Response to As(V)
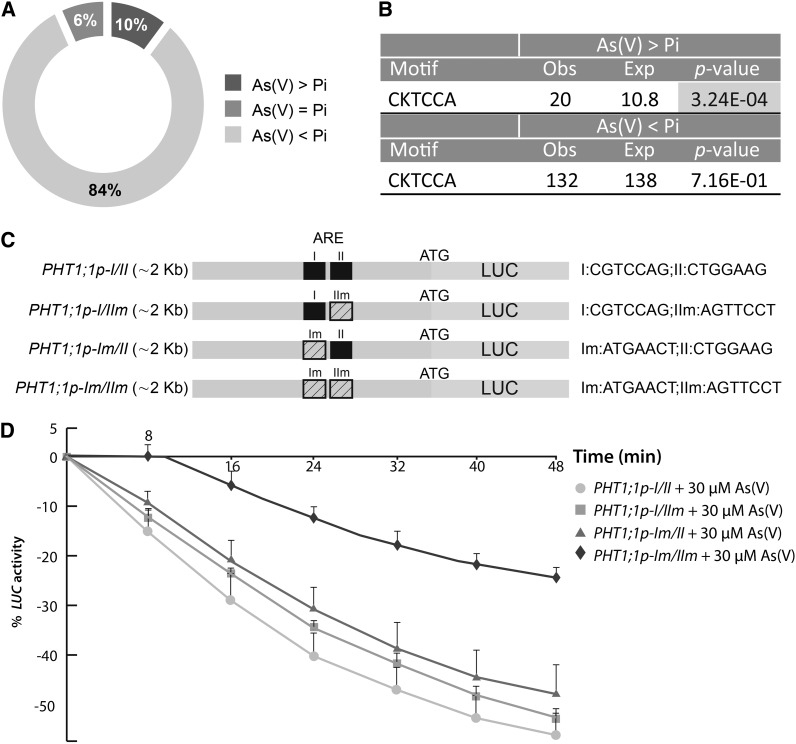
To further study the role of As(V) in the repression of Pi starvation-induced genes similar to PHT1;1, we performed a transcriptomic analysis of Pi-starved plants exposed to As(V) or Pi for 8 h. Only 10% of Pi starvation-upregulated genes were repressed more efficiently by As(V) than by Pi, indicating that few genes show the transcriptional repression pattern of the Pi transporters in response to As(V) (Figure 3A; see Supplemental Data Set 1 online). In silico analysis of enriched regulatory elements in the promoter regions of these genes allowed identification of a cis As(V) repression element (ARE; KTCCAG, K:G/T) also found in the PHT1;1 promoter region (Figures 3B and 3C). Analysis of luciferase activity in transgenic plants expressing ARE-mutated versions of PHT1;1-LUC constructs showed that this element contributes to repression of the Pi transporter in response to As(V) (Figure 3D). This ARE box resembles the TTTTCCAC (WK-box) core motif that, in addition to the classical W-box, is bound by plant WRKY transcription factors (Rushton et al., 2010). As(V) transcriptome profile analysis identified a member of the WRKY family, WRKY6, as an As(V)-responsive gene (see Supplemental Data Set 1 online). WRKY6 is involved in the control of several plant responses, including nutrient starvation (Robatzek and Somssich, 2002; Kasajima et al., 2010), and negatively regulates PHO1, a plasma membrane protein involved in xylem Pi loading (Chen et al., 2009). These observations prompted us to consider WRKY6 involvement in PHT1;1 As(V) repression.
Figure 3.
The ARE Contributes to Downregulation of the Pi Transporter in Response to As(V).
(A) Diagram showing the relative sizes of the three classes of downregulated genes in response to 30 μM As(V) and 30 μM Pi identified in a microarray analysis. As(V) > Pi, genes preferentially downregulated by As(V); As(V) < Pi, genes preferentially downregulated by Pi; As(V) = Pi, genes downregulated equally in response to both. For microarray analysis, wild-type plants were grown on Johnson medium with 1 mM Pi for 7 d, transferred to -Pi for 2 d, and finally to -Pi medium supplemented with 30 μM As(V) or 30 μM Pi (8 h).
(B) Analysis of ARE frequency in 0.5 kb of the promoter regions of genes in the As(V)>Pi and As(V)<Pi classes. The table shows observed and expected ARE numbers assuming random distribution. Significant overrepresentation is highlighted (χ2 test, P < 0.05).
(C) Diagram showing wild-type (PHT1;1p-I/II) and mutated versions (PHT1;1p-I/IIm, PHT1;1p-Im/II, and PHT1;1p-Im/IIm) of the PHT1;1 promoter region fused to luciferase (PHT1;1-LUC). The ARE in the PHT1;1 promoter (I and II; black) was mutated sequentially (Im and IIm; diagonal stripes) by PCR site-directed mutagenesis.
(D) Kinetic analysis of LUC activity in transgenic plants expressing the PHT1;1p-I/II, PHT1;1p-I/IIm, PHT1;1p-Im/II, or PHT1;1p-Im/IIm constructs, in response to 30 μM As(V) (1.5 h). Values represent data from analysis of 12 independent lines of each construct; mean ± sd.
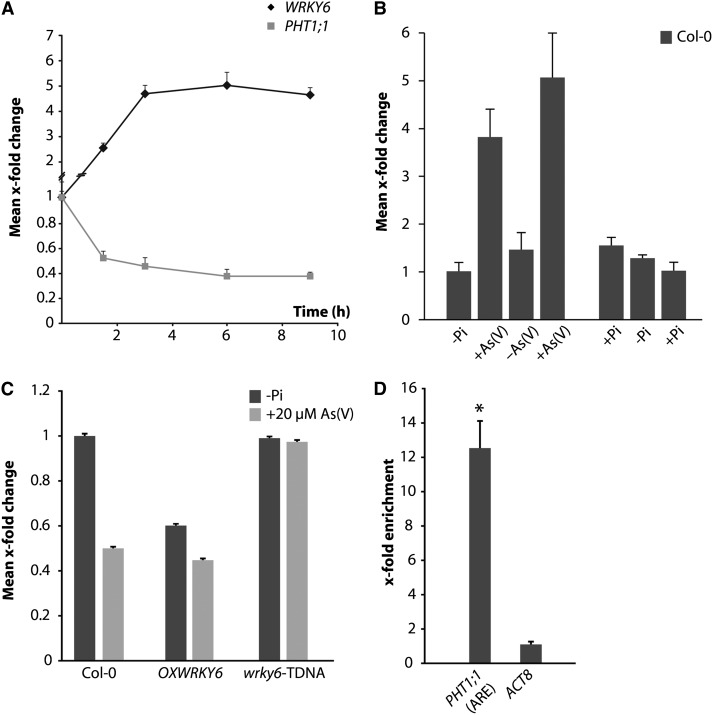
To test whether WRKY6 is responsible for PHT1;1 repression following As(V) stress, we first evaluated WRKY6 kinetic responsiveness to As(V). The kinetics of WRKY6 transcript accumulation in response to As(V) was inverse to that of As(V)/Pi transporter PHT1;1 repression by As(V) (Figure 4A). In response to As(V) pulses, the WRKY6 activation/repression pattern was again inverse to that of PHT1;1, as predicted for a transport repressor-mediated mechanism, whereas WRKY6 transcript accumulation was unaltered in response to Pi pulses (Figure 4B).
Figure 4.
WRKY6 Responds to As(V) and Represses the Pi Transporter PHT1;1.
(A) Kinetic study of PHT1;1 and WRKY6 expression by qRT-PCR in wild-type plants exposed to 30 μM As(V). Values show mean ± sd.
(B) qRT-PCR expression analysis of WRKY6 in wild-type plants in response to 30 μM As(V) pulses [±As(V)] or in response to 30 μM Pi pulses (±Pi); duration of each pulse and gap was 1.5 h. Values show mean ± sd.
(C) qRT-PCR of PHT1;1 transcript in wild-type plants (Col-0), in the WRKY6-GFP–overexpressing line (OXWRKY6), and in wrky6-TDNA line grown in +Pi medium for 7 d, transferred to -Pi for 2 d and then to -Pi medium alone or with 20 μM As(V) (1.5 h). In the case of WRKY6-overexpressing lines, values show data from analysis of 10 independent lines. Values show mean ± sd.
(D) ChIP assay of WRKY6-GFP seedlings and PHT1;1 promoter PCR amplification analysis. qPCR of ARE-containing fragments of the PHT1;1 promoter. Enrichment was calculated relative to wild-type plants. ACT8 was used as negative control. Values show mean ± sd. *P < 0.05 (Student’s t test).
In a transgenic Arabidopsis line expressing WRKY6-GFP at a level similar to that of endogenous WRKY6 in response to As(V) (see Supplemental Figure 4A online; WRKY6-GFP-L10), PHT1;1 expression was reduced in As(V)-exposed wild-type plants (Figure 4C). By contrast, expression of the Pi starvation-induced gene SQD1, which is not preferentially repressed by As(V), was unaltered in the WRKY6-GFP–overexpressing line (see Supplemental Figure 4B online). To test whether WRKY6 interacts directly with the PHT1;1 promoter region in vivo, we used chromatin immunoprecipitation (ChIP) assays in the WRKY6-GFP–overexpressing line. Quantitative PCR (qPCR) of chromatin fragments from this line immunoprecipitated with anti-GFP showed enrichment in ARE-bearing fragments of the PHT1;1 promoter compared with wild-type plants and the ACTIN8 (ACT8) control (Figure 4D). Transient LUC activity expression assays in Nicotiana benthamiana leaf discs agroinfiltrated with PHT1;1-LUC or PHT1;1-LUC ARE mutants showed that this cis motif mediates WRKY6 repression of the Pi transporter (Figure 5A). ChIP assays using N. benthamiana plants infiltrated with wild-type and PHT1;1 ARE mutant promoters showed that WRKY6 requires an intact ARE to bind the PHT1;1 promoter region (Figure 5B). In our experiments, we included a wrky6-TDNA insertion line that behaves as a null mutant, with no detectable WRKY6 expression (see Supplemental Figure 4A online). The wrky6-TDNA line showed no PHT1;1 repression in response to As(V) (Figure 4C). These results reinforce the idea that WRKY6 mediates PHT1;1 transcriptional repression.
Figure 5.
The ARE Mediates WRKY6 Repression of the Pi Transporter PHT1;1 and Confers the As(V) Tolerance Phenotype.
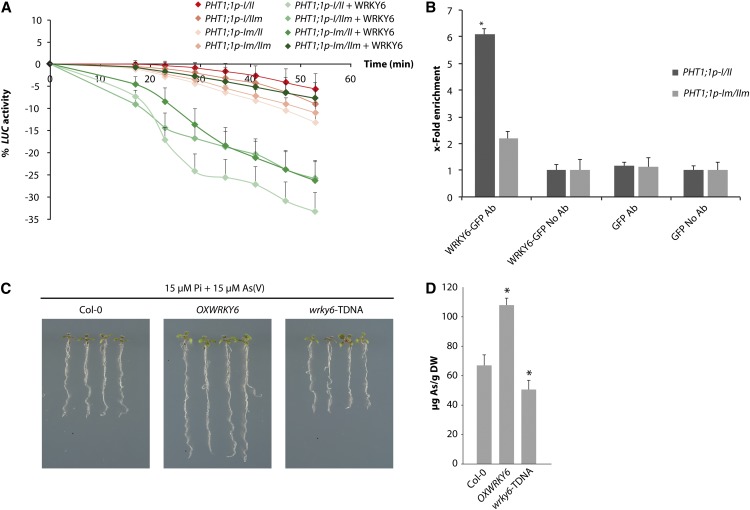
(A) Kinetic analysis of transient LUC activity in N. benthamiana leaf discs agroinfiltrated with PHT1;1-LUC wild type or the mutated versions alone or with a WRKY6-GFP–overexpressing construct. Leaf discs were incubated in medium with 30 μM As(V) (1.5 h). Values show mean ± sd.
(B) ChIP assay of WRKY6-GFP followed by qPCR of the PHT1;1 promoter. ChIP assays were performed in N. benthamiana leaf discs agroinfiltrated with PHT1;1-LUC wild type and the PHT1;1-LUC mutated version (PHT1;1p-Im/IIm), with WRKY6-GFP or GFP-overexpressing constructs. Values represent the x-fold enrichment of WRKY6-bound DNA of the PHT1;1 promoter in immunoprecipitated samples relative to total input DNA. ARE (PHT1;1p-I/II-LUC) or mutated ARE-containing fragments (PHT1;1p-Im/IIm-LUC) in the PHT1;1 promoter were amplified by qPCR using specific primers. Values show mean ± sd. *P < 0.01 (Student’s t test). Values show mean ± sd.
(C) As(V) tolerance phenotype of wild-type (Col-0), OXWRKY6-GFP–overexpressing line (OXWRKY6), and wrky6-TDNA plants grown on 15 μM Pi supplemented with 15 μM As(V) for 7 d.
(D) Intracellular arsenic concentration in Col-0, OXWRKY6, and wrky6-TDNA plants exposed to 5 μM As(V) (1 h). Values show mean ± sd. *p < 0.05 (Student’s t test). DW, dry weight.
In the absence of As(V), the WRKY6-GFP–overexpressing line and wrky6-TDNA insertion line showed no visible differences in root length compared with wild-type plants (see Supplemental Figures 4C and 4D online). By contrast, As(V) tolerance was markedly enhanced in the WRKY6-GFP–overexpressing line (Figure 5C); quantification of root length showed a significant increase compared with wild-type plants in the presence of As(V) (see Supplemental Figure 4D online). No differences in root length were observed in the wrky6-TDNA insertion line relative to wild-type plants, probably due to functional redundancy among WRKY gene family members (Figure 5C; see Supplemental Figure 4D online). As noted above, however, PHT1;1 expression was not repressed in response to As(V) in the wrky6-TDNA background, indicating incomplete functional redundancy.
Arsenic accumulation was increased in the WRKY6-overexpressing line compared with wild-type controls, whereas levels in the wrky6-TDNA mutant were significantly lower (Figure 5D). Reduction in As(V) uptake is associated with enhanced arsenic accumulation (Catarecha et al., 2007). These findings thus indicate that WRKY6 modulates As(V) uptake through transcriptional repression of the As(V)/Pi transporters.
As(V) Causes a Transposon Burst Modulated by WRKY6
In our experiments, >80% of the PHT1;1 repression in response to As(V) was observed as early as 1.5 h after treatment (Figure 1B). For detailed study of the expression profile of the As(V) response, we thus performed transcriptome analysis on plants exposed to As(V) for 1.5 h. As(V) induces marked transcriptional repression of 1519 genes (see Supplemental Figure 5 and Supplemental Data Set 2 online), similar to a previous report (Abercrombie et al., 2008). Less than 2% of the As(V)-downregulated transcripts overlapped with Pi starvation-inducible genes, based on data from the 8-h array experiment (Figure 6A; see Supplemental Figure 5 online). As(V) repression of the As(V)/Pi transporters is thus a specific short-term response.
Figure 6.
WRKY6 Restricts Transposon Expression.
(A) Venn diagram showing relative proportion of As(V)-downregulated genes [Col+As(V) versus Col-As(V)] and Pi starvation-upregulated genes (Col-Pi versus Col+Pi) and degree of overlap. For comparison, only genes of the 12x135K platform (NimbleGen) that coincide with those in the ATH1 platform (Affymetrix) were considered (twofold [2x], FDR < 0.1, and twofold [2x], FDR <0.05, respectively).
(B) Comparative transcriptome analysis of As(V)-responsive transposons in wild-type plants and in the WRKY6-GFP–overexpressing line (OXWRKY6). Total number and percentage is indicated of transposons induced and repressed in wild-type [Col+As(V) versus Col-As(V)] and in WRKY6-GFP–overexpressing plants [OXWRKY6+As(V) versus Col+As(V)] in response to As(V). Cutoff values for analysis of As(V)-responsive transposons were twofold (2x), FDR < 0.1, and for OXWRKY6-responsive transposons, 1.5-fold (1.5x), FDR < 0.2. The table shows the size of observed overlap and expected size, assuming random distribution. Significant overlaps are in bold (χ2 test, P < 0.05).
(C) qRT-PCR time-course analysis of transposon expression in wild-type plants exposed to 30 μM As(V).
(D) qRT-PCR analysis of transposon expression in wild-type (Col-0), OXWRKY6, and wrky6-TDNA plants in response to 30 μM As(V) (1.5 h). Values show mean ± sd; *P < 0.05 (Student’s t test).
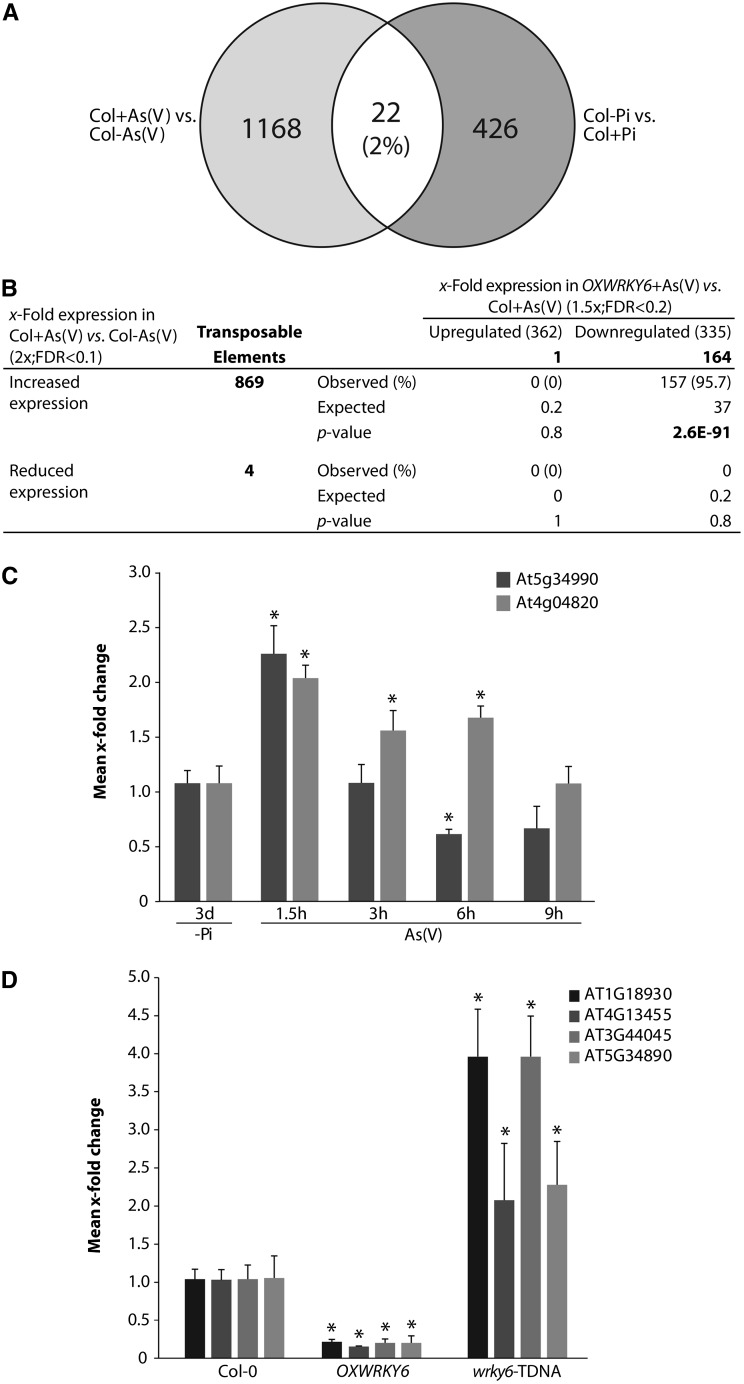
This gene expression analysis also identified 1936 genes upregulated in response to As(V) (see Supplemental Figure 5 and Supplemental Data Set 2 online); of these, 869 (44.9%) encoded transposons (Figure 6B). qRT-PCR analysis confirmed transposon induction (see Supplemental Figure 6A online); As(V) stress therefore induced a notable transposon burst (Figure 6B; see Supplemental Data Set 3 online). As(V) induction of transposons was transient, with the highest expression at 1.5 h after As(V) exposure (Figure 6C). Maximum WRKY6 transcript accumulation was delayed compared with that observed for transposons; however, its expression peak correlated with intense transposon downregulation (Figures 4A and 6C), in accordance with a possible role of WRKY6 in transposon repression. Transcriptome analysis of the As(V)-exposed WRKY6-GFP–overexpressing line showed that close to 50% of transcripts downregulated in this line encoded transposons (164 of 335; see Supplemental Data Set 4 online) and nearly all (95.7%) corresponded to As(V)-induced transposons (Figure 6B; see Supplemental Data Set 4 online). qRT-PCR experiments confirmed that WRKY6 overexpression downregulated transposon expression, which was enhanced in wrky6-TDNA (Figure 6D; see Supplemental Figure 6B online). WRKY6 activation thus has an important function in limiting As(V)-induced transposon activation.
Transposon downregulation by WRKY6 could be, at least in part, an indirect effect of reduced As(V) uptake due to PHT1;1 repression. To explore this possibility, we tested whether Pi starvation was able to activate transposon expression independently of As(V)/Pi transport. Five of 12 As(V)-responsive transposons were induced by Pi starvation (see Supplemental Figure 6C online); in all cases, WRKY6 overexpression reduced transposon induction in response to Pi starvation (see Supplemental Figure 6D online). Transposon downregulation in the WRKY6-overexpressing lines is thus not merely a consequence of stress alleviation due to the arrest of intracellular arsenic accumulation.
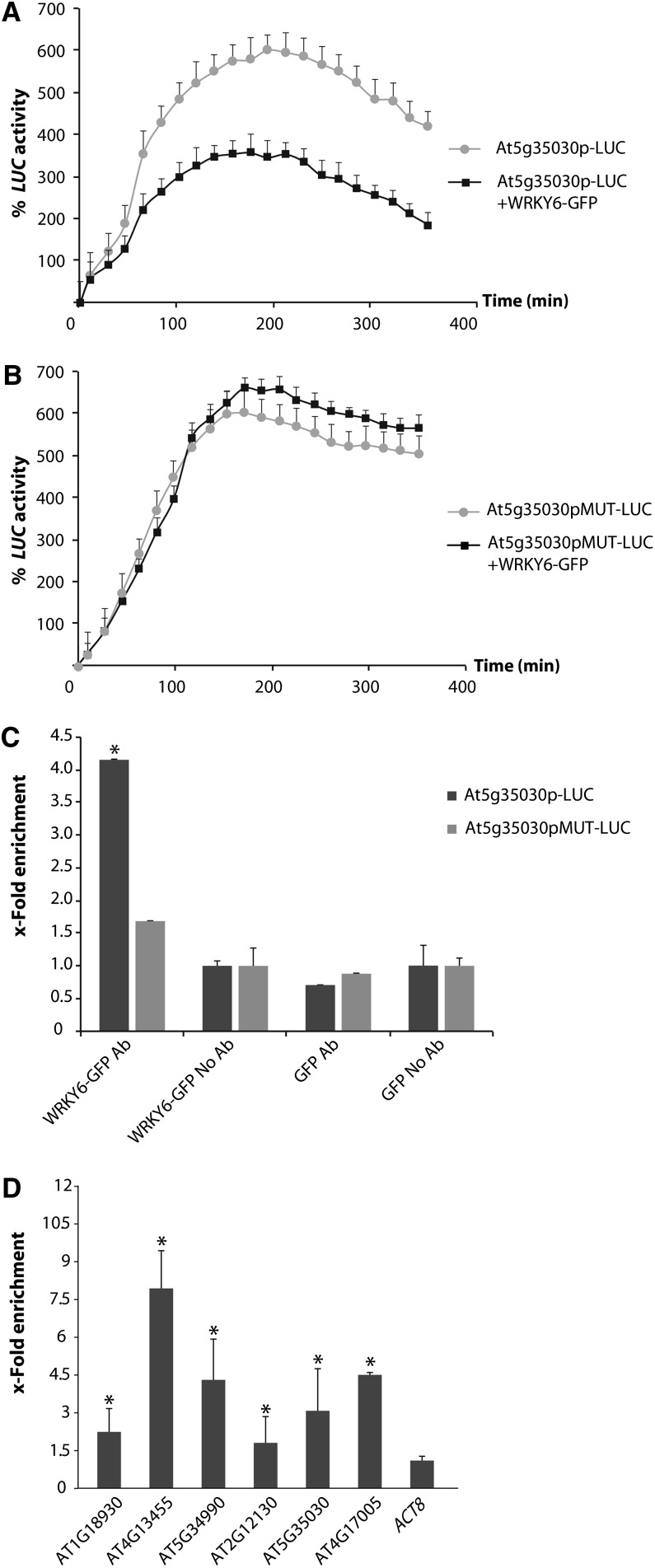
We found WRKY canonical cis-acting elements (W-box) in the promoter regions of As(V)-responsive transposons. To study W-box function, we performed kinetic analyses of luciferase activity in N. benthamiana leaves agroinfiltrated with WRKY6-GFP and the luciferase-fused promoter region of the transposable element At5g35030 or of the At5g35030 mutated W-box version; WRKY6-GFP repressed luciferase activity efficiently in the former (Figure 7A), but not in the latter (Figure 7B). Downregulation of transposon expression thus depends on an intact W-box at the promoter region. ChIP assays on N. benthamiana plants using At5g35030 transposon promoters and the mutated version of the At5g35030 W-box confirmed that WRKY6 repression of transposable elements operates via direct WRKY6 interaction with its cis-acting binding site (Figure 7C). We also performed ChIP assays in the WRKY6-GFP transgenic plants. qPCR of chromatin fragments from WRKY6-GFP–overexpressing lines immunoprecipitated with anti-GFP showed enrichment in fragments of six transposon promoters with the W-box, compared with wild-type plants and the ACT8 control (Figure 7D). WRKY6 therefore interacts in vivo with these six promoter regions, as also observed for PHT1;1. These experiments clearly indicate that WRKY6 acts directly as a transcriptional repressor of both the Pi transporter PHT1;1 and As(V)-responsive transposable elements.
Figure 7.
WRKY6 Interacts with the W-Box in the Promoter Region of As(V)-Responsive Transposable Elements.
(A) and (B) Kinetic analysis of LUC activity in N. benthamiana leaves agroinfiltrated with the promoter region of a transposable element At5g35030 fused to LUC (At5g35030p-LUC) (A) or At5g35030p-LUC in which the W-box was mutated (At5g35030pMUT-LUC) (B), alone or with a WRKY6-GFP–overexpressing construct. Leaf discs were incubated in medium with 30 μM As(V) (1.5 h).
(C) ChIP assay of WRKY6-GFP followed by qPCR of the At5g35030 promoter. ChIP assays were performed in N. benthamiana leaf discs agroinfiltrated with At5g35030p-LUC and the At5g35030pMUT-LUC version, with WRKY6-GFP– or GFP-overexpressing constructs. Values represent x-fold enrichment of WRKY6-bound DNA of the At5g35030 promoter in immunoprecipitated samples relative to total input DNA. W-box–containing fragments (At5g35030p-LUC) or mutated W-box–containing fragments (At5g35030pMUT-LUC) of the At5g35030 promoter were qPCR amplified using specific primers. *P < 0.01 (Student’s t test).
(D) ChIP assay of WRKY6-GFP–overexpressing plants, followed by qPCR of the W-box–bearing fragments of transposon promoters. Enrichment was calculated relative to wild-type plants. ACT8 was used as negative control. *P < 0.05 (Student’s t test). Values show mean ± sd.
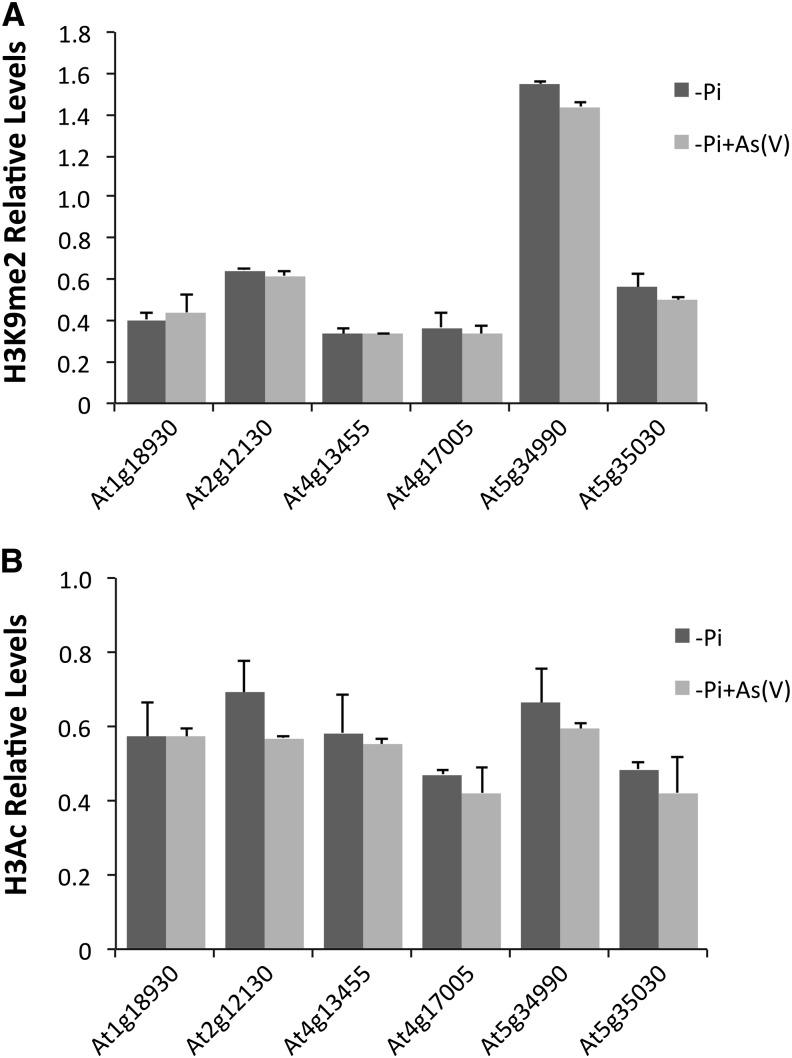
Transposable elements of Arabidopsis are under epigenetic regulation (Tsukahara et al., 2009; Mirouze and Paszkowski, 2011; Bucher et al., 2012). We thus assessed whether transcriptional activation of transposons in response to As(V) occurs through changes in histone modifications. We used ChIP to analyze chromatin in the genomic regions of six As(V)-responsive transposable elements in As(V)-treated plants, to determine histone H3 dimethylation levels at Lys-9 (H3K9me2), a hallmark of heterochromatin and transposable element repression (Johnson et al., 2002), and H3Ac, a histone modification associated with transcriptional activation (Kouzarides, 2007). As predicted, we found high H3K9me2 and low H3Ac levels in these transposons (see Supplemental Figure 7 online). This pattern is similar to that of the Ta3 transposon, a classical target of epigenetic silencing (Johnson et al., 2002), and differs from the constitutively expressed TUB8 (see Supplemental Figure 7 online). There were no differences in the amount of immunoprecipitated H3K9me2 between As(V)-treated and untreated plants, suggesting that at least in the short term, transposons are activated without modifications of this epigenetic mark, commonly associated with transposon silencing (Figure 8A). H3Ac levels were also unchanged, indicating that transcriptional activation of As(V)-responsive transposable elements is independent of changes in this histone mark (Figure 8B).
Figure 8.
Activation of As(V)-Responsive Transposable Elements Is Independent of Changes in H3K9me2 and H3Ac Levels.
ChIP analysis of As(V)-induced transposable elements in Arabidopsis Col-0 plants grown on 1 mM Pi medium for 7 d, transferred to -Pi medium for 2 d and finally to liquid -Pi medium, alone or supplemented with 30 μM As(V). The assay was performed using antibodies specific for H3K9me2 (A) or H3Ac (B), histone marks associated with repressive and active transcription, respectively. Levels of histone modifications in the genomic regions of As(V)-responsive transposable elements are represented relative to Ta3 in the case of H3K9me2 or to TUB8 for H3Ac. Values show mean ± sd.
DISCUSSION
In this study, we show that in response to As(V), WRKY6 is involved in the rapid repression of the As(V)/Pi transporter PHT1;1, which is coordinated with its delocalization from the plasma membrane. When As(V) was removed, the system quickly restored PHT1;1 expression and membrane localization. We show that As(V) provoked a notable transposon burst, which was also counteracted by WRKY6 action. A classical repressor is therefore involved in a dual protection mechanism that restricts As(V) uptake and transposon expression.
Arabidopsis Plants Have a Sensitive As(V) Perception Mechanism
In plants, the expression of genes that encode transporters for nutrients, including Pi, is altered in response to nutrient deficiencies (Maruyama-Nakashita et al., 2004; Bustos et al., 2010; Kiba et al., 2012). The regulation of Pi transporter expression in response to nutrient starvation and the underlying components have been studied in detail (Bustos et al., 2010; Bayle et al., 2011); by contrast, the mechanisms involved in Pi perception are mostly unknown. Phosphate transporters are precisely regulated by Pi amount. In plants and yeast, exposure to Pi or nonmetabolized phosphate analogs triggers Pi transporter degradation (Mouillon and Persson, 2005; Bayle et al., 2011). Here we show that in response to As(V), Pi transporters are also delocalized from the plasma membrane to the vacuole, in parallel with the arrest of As(V) uptake. The kinetics of transcriptional repression and plasma membrane delocalization is nonetheless more rapid in response to As(V) than in response to resupply of Pi (or Pi analogs). Furthermore, Pi and its analogs cause broad repression of the Pi starvation response (Carswell et al., 1996, 1997; Rubio et al., 2001; Ticconi et al., 2001; Varadarajan et al., 2002; Bustos et al., 2010), whereas As(V) induced short-term repression specific mainly for the Pi transporter, with no marked alteration in the overall phosphate starvation response. Both As(V) and Pi thus act as signals in the control of Pi transporter expression, but specific regulatory elements must be involved for each of these environmental cues.
WRKY6 Mediates As(V) Repression of As(V)/Pi Transporters
Here, we found that WRKY6 expression is activated in response to As(V) and is an essential component of the As(V) repression of As(V)/Pi transporters. WRKY transcription factors are involved in plant responses to a wide variety of environmental stresses (Rushton and Somssich, 1998; Robatzek and Somssich, 2002; Rushton et al., 2010). WRKY6 was initially implicated in the control of gene expression during plant senescence and more recently in Pi and boron starvation (Robatzek and Somssich, 2002; Chen et al., 2009; Kasajima et al., 2010). Members of the WRKY family can act as activators or repressors of distinct biological functions including Pi homeostasis. WRKY6 is described as a repressor of PHO1, which encodes a plasma membrane protein involved in Pi loading into xylem (Chen et al., 2009). The ability of WRKY6 to repress both extracellular Pi uptake and vascular Pi loading suggests that it operates in an integral strategy to prevent arsenic accumulation in the plant. Once As(V) is incorporated into cells, however, it is rapidly reduced to As(III) and is transported to the aerial part of the plant (Dhankher et al., 2006). In addition, arsenic amount in pho1 mutant shoots is similar to or slightly higher than that observed in wild-type shoots (Quaghebeur and Rengel, 2004), suggesting that arsenic content in the aerial part of the plant is at least partially independent of PHO1 regulation and, thus, of WRKY6. WRKY6 transcriptional repression of the Pi transporter nonetheless leads to a clear As(V) tolerance phenotype. These findings reinforce the importance of WRKY6 as an essential component in the regulation of As(V)/Pi transporters in response to As(V) and dependent on its concentration.
WRKY6 Restricts Transposon Activation in Response to As(V)
Our studies show that As(V) induces a rapid transposon burst in plants. Transposon activation in response to stress can be responsible for deleterious effects, such as gene deletion or insertion, chromosome rearrangement, and alterations in gene expression (Ma and Bennetzen, 2006; Ito, 2012). Of the many stress factors in the early environment, high arsenic concentration was a serious impediment to the establishment of life on earth (Oremland et al., 2009; Dani, 2010); suppression of transposon bursts might thus be a valuable plant strategy for survival in the presence of arsenic. Stress-induced activation of transposons is usually suppressed by host epigenetic mechanisms, including methylation and histone tail modifications (Tittel-Elmer et al., 2010; Ito, 2012; Saze et al., 2012). It is thus assumed that stress-induced transposon activation takes place through changes in these epigenetic marks. Our data indicate that WRKY6 contributes to the restriction of transposon-induced activation in response to As(V). Although there is evidence that WRKY transcriptional activity can be regulated by interaction with histone modifiers (Kim et al., 2008), we show here that WRKY6 acts directly as a transcriptional repressor of As(V)-responsive transposable elements. More than 90% of transposons repressed in the WRKY6-overexpressing L10 line are As(V) induced, indicating that transcriptional repression contributes to transposon silencing in response to As(V). We also show that transposons are upregulated with no modification of two classical histone marks commonly associated with transposon silencing release. Our results coincide with data for the heat stress response, in which silencing is released with no obvious changes in these marks; this suggests that other mechanisms operate in transposon silencing (Pecinka et al., 2010; Tittel-Elmer et al., 2010; Saze et al., 2012). Although we cannot rule out the involvement of other epigenetic mechanisms in the activation of As(V)-responsive transposable elements, our results show that classical repression contributes to transposon silencing in response to As(V).
In this study, we found that Arabidopsis plants have a rapid detection mechanism that restricts As(V) uptake and transposon expression. When As(V) was removed, plants recovered PHT1;1 expression and membrane localization, indicating a sensitive mechanism for perception of As(V). In addition, we show that the WRKY6 transcription factor is involved in PHT1;1 repression and in preventing transposon expression in response to As(V). Both responses are coordinated temporally with the delocalization of the As(V)/Pi transporter PHT1;1, although it is currently not known whether PHT1;1 relocalization is under WRKY6 control. The coordination of these two responses under the control of a single repressor is a unique strategy that allows the plant to overcome As(V) toxicity and transposon activation. These findings provide evidence of a major role for classical transcription repressors in restoring transposon silencing after stress-induced activation. We identify a dual As(V) signaling mechanism that, depending on As(V) amounts, regulates stress-induced transposon expression and As(V) uptake.
METHODS
Materials and Growth Conditions
We used the T-DNA mutant of gene WRKY6 SALK_012997C that bears an insertion in the third exon, as well as several Arabidopsis thaliana transgenic lines, including 35S:PHT1;1-GFP (González et al., 2005), CCA1-LUC, CAB-LUC (a kind gift of Salomé Prat [Centro Nacional de Biotecnología–Consejo Superior de Investigaciones Cientificas]), and PIP1;4-YFP (wave 138) (Geldner et al., 2009).
Seedlings were grown on Johnson medium supplemented with distinct Pi (KH2PO4) concentrations depending on the experiment; 1 mM Pi was used for complete medium (+Pi), 30 μM for low-Pi medium, and 5 μM for phosphate starvation (-Pi). In general, for treatment with As(V) (NaH2AsO4·7H2O), plants were grown on Johnson medium +Pi for 7 d, then transferred to -Pi medium for 2 d, and finally to liquid -Pi medium alone or supplemented with 30 μM As(V). For all molecular and biochemical analyses, Arabidopsis plants were grown in a culture chamber in a 16-h-light/8-h-dark regime (24°C/21°C).
Physiological Measurements
For As(V) tolerance analysis, plants were grown on Johnson medium with 15 μM Pi, alone or with 15 μM As(V), in a horizontal position for 10 d. Main root length was then measured using ImageJ software (Barboriak et al., 2005). For arsenic accumulation experiments, plants were grown on plates containing half-strength Bates and Lynch medium solidified with 0.4% bacto-agar with 30 μM Pi and covered with 0.4-mm-pore nylon mesh. Seeds were sown onto the mesh and cultured for 7 d. Plants were treated with PI and PII buffers (Narang et al., 2000). Arsenic accumulation experiments were performed in 50-mL pots using 5 μM As(V). Plants were dried (60°C, 5 d), mineralized with HNO4-H2O2 in a pressure digester, and analyzed for total arsenic content by inductively coupled plasma–mass spectrometry at the Servicio Interdepartamental de Investigación (Universidad Autónoma de Madrid, Spain).
Binary Construct and Plant Transformation
To generate the WRKY6-overexpressing lines, full-size WRKY6 cDNA was amplified with primers Wrky6-GWF and Wrky6-GWR (see Supplemental Table 1 online), cloned into the pDNR207 vector, and inserted into the pGWB5 binary vector containing the constitutive 35S promoter, using the LR recombination reaction (Invitrogen). This binary construct was introduced into Agrobacterium tumefaciens strain C58C1 and transformed in wild-type Columbia-0 (Col-0) plants using the floral dip transformation method (Clough and Bent, 1998). Transformants were selected on 40 mg/L hygromycin-containing medium. Homozygous lines were selected for strong expression.
Approximately 2 kb of the PHT1;1 and 1.3 kb of the transposable element (At5g35030) promoter regions were amplified using their respective primer pairs, PHT1;1p-I/II-F, PHT1;1p-I/II-R and At5g35030p-I-F, At5g35030p-I-R (see Supplemental Table 1 online). The products were excised with HindIII and BamHI and cloned into the pLUC vector (Ulm et al., 2004). Using these constructs as templates, three versions of the PHT1;1 and one of the At5g35030 promoters were generated by PCR site-directed mutagenesis to alter the sequence of the ARE and W-box, respectively; primers used are listed in Supplemental Table 1 online. All binary constructs were introduced into Agrobacterium strain C58C1. Agrobacterium-mediated transient expression assays in Nicotiana benthamiana plants were performed as described (Jiménez et al., 2006). The PHT1;1 binary constructs were transformed in wild-type Col-0 plants by floral dip transformation. Transformants were selected on 50 mg/L kanamycin-containing medium. Homozygous lines were selected for strong expression.
Confocal Imaging
For intracellular fluorescence, we used a confocal multispectral system TCS SP5 with LAS AF v.2.3.6 software (Leica Microsystems) and a ×63.0 1.20 water-immersion objective. To avoid emission spectra overlap, the sequential mode was used. GFP, propidium iodide, and FM4-64 were excited with a 488-nm argon laser line. Fluorescence was detected using a photomultiplier (GFP 505 to 546 nm, propidium iodide, and FM4-64 625 to 670 nm).
Determination of Luciferase Activity
Luciferase activity (LA) levels were detected using the LB 960 microplate luminometer center system (Berthold Technologies) with MikronWin 2000 software. Plants were grown in +Pi medium for 7 d and transferred to -Pi medium for 2 d, and single plants were transferred to a microplate (Costar) containing Johnson medium. d-luciferin substrate (Sigma-Aldrich) was added to each well and the plate incubated (1 h) before As(V) supplementation. The plate was covered with optical film. The percentage of luciferase activity (%LUC activity) was calculated as (%LUC activity = [(LAn − LA0)/LA0)*100]. Luciferase activity was determined in N. benthamiana using 1-cm discs of agroinfiltrated leaves transferred to a microplate as above.
qRT-PCR Expression Analysis
qRT-PCR was performed on three independent biological samples as described (Aguilar-Martínez et al., 2007). Each sample was normalized using EF1α. The primer pairs used are described in Supplemental Table 1 online.
ChIP Assay
ChIP assays in the WRKY6-GFP–overexpressing line and N. benthamiana were as described (Lee et al., 2007). OXWRKY6-GFP seedlings were grown on +Pi medium for 7 d and transferred to -Pi medium for 2 d; plants were then treated with 30 μM As(V) for 1.5 h. Leaves of N. benthamiana were agroinfiltrated with PHT1;1p-LUC wild type, a mutated version of PHT1;1p-Im/IIm-LUC and WRKY6-GFP–overexpressing construct or with At5g35030p-LUC, a mutated version of At5g35030pMUT-LUC and a WRKY6-GFP–overexpressing construct. As a negative control in both cases, we used leaves of N. benthamiana agroinfiltrated as above, replacing the WRKY6-GFP–overexpressing construct with a GFP-overexpressing construct.
Extracts of WRKY6-GFP–overexpressing seedlings (∼2 g) or N. benthamiana leaves (∼4 g) were immunoprecipitated with 40 μL anti-GFP Affinity Matrix (Abcam). Precipitated DNA was dissolved in 40 μL water, and 1 μL was used for qPCR. ACT2 was used as internal control for the OXWRKY6-GFP–overexpressing line experiment.
Histone modification was analyzed by ChIP using antidimethyl-histone H3 Lys 9 (CS200550; Millipore) and antiacetyl-histone H3 Lys 9 and 14 (06-599; Millipore) antibodies as described (De Lucia et al., 2008). Briefly, Arabidopsis Col-0 plants were grown on 1 mM Pi medium for 7 d, transferred to -Pi medium for 2 d and then to liquid -Pi medium alone or supplemented with 30 μM As(V). Tissue was fixed, and immunoprecipitated DNA was recovered using Chelex 100 resin (Bio-Rad; 10 g/100 mL double distilled water). All ChIP experiments were quantified by qPCR. Ta3 (Johnson et al., 2002) or TUB8 (Tittel-Elmer et al., 2010) was used as an internal control for H3K9me2 or H3Ac ChIP experiments, respectively. Primers used to amplify the target gene fragments are shown in Supplemental Table 1 online.
Transcriptome Analyses of As(V) Response
Transcriptomic analyses were performed using the Affymetrix ATH1 and NimbleGen Gene Expression 12x135K platforms. For the Affymetrix ATH1 array, wild-type plants were grown on Johnson medium with 1 mM Pi for 7 d, transferred to -Pi medium for 2 d and then to medium supplemented with 30 μM As(V) or 30 μM Pi (8 h). RNA was isolated from three independent biological samples for each condition using the RNeasy plant mini kit (Qiagen). RNA amplification, microarray hybridization, and scanning were as reported (Bustos et al., 2010).
Data were analyzed using GeneChip Operating Software and the affylmGUIR package (Wettenhall et al., 2006). Background correction and normalization of expression data were as reported (Bustos et al., 2010). Differential expression analysis was performed with the Bayes t-statistics from the linear models for Microarray data (limma). P values were corrected for multiple testing using the Benjamini-Hochberg method (false discovery rate) (Reiner et al., 2003).
For the NimbleGen Gene Expression 12x135K platform (Roche), wild-type plants and the WRKY6-GFP–overexpressing line were grown in +Pi solid medium for 7 d, transferred to -Pi medium for 2 d and then to liquid medium supplemented with 30 μM As(V) for 1.5 h. RNA from three independent biological samples was isolated as above. cDNA was synthesized with 10 μg RNA using the double-stranded cDNA synthesis kit (Invitrogen), and cDNA was Cy3-labeled with the NimbleGen one-color DNA labeling kit (Roche). Three replicates for each condition were hybridized independently to the 100718:Athal_TAIR9_exp_H12 genome array (Roche). Each microarray was hybridized with the NimbleGen hybridization kit, washed using the NimbleGen wash buffer kit (both from Roche), and scanned at 2-μm double-pass at 16-bit resolution. Data were analyzed through robust multiarray analysis with NimbleGen Scan software (Roche). Differential expression analysis and P value correction were performed as above.
Identification of cis-Regulatory Elements by in Silico Analysis
To identify putative cis-acting regulatory elements, we identified promoter sequences (0.5 kb) of genes preferentially downregulated by As(V) in The Arabidopsis Information Resource (http://www.Arabidopsis.org/). This group of promoter sequences was submitted for overrepresented motifs analysis to Element (http://element.mocklerlab.org/). The motifs identified with P < 0.05 were used in further analysis.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: WRKY6, AT1G62300; PHT1;1, AT5G43350. Array data from this article can be found in the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) database under accession number GSE49037.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. qRT-PCR Assays of PHT1;1 Expression Kinetics and Dose Response to As(V).
Supplemental Figure 2. Relocalization of the Pi Transporter Is an As(V)-Specific Response That Correlates with Arrest of Intracellular Arsenic Accumulation.
Supplemental Figure 3. qRT-PCR Kinetics Expression Analysis of PHT1;1 Gene in Response to As(V) and Pi Pulses.
Supplemental Figure 4. WRKY6 Mediates PHT1;1 Transcriptional Repression and Confers As(V) Tolerance.
Supplemental Figure 5. Comparative Transcriptome Analysis of As(V) Response and Pi Starvation-Upregulated Genes.
Supplemental Figure 6. WRKY6 Overexpression Restricts As(V) Induction of Transposons.
Supplemental Figure 7. Levels of H3K9me2 and H3Ac Immunoprecipitated from the Genomic Regions of As(V)-Induced Transposable Elements.
Supplemental Table 1. Primers Used for Binary Constructs, qRT-PCR, and Chromatin Immunoprecipitation Assays.
Supplemental Data Set 1. Data set of ATH1 Probes with Significantly Altered Expression in Wild-Type Plants Grown on 1 mM Pi versus -Pi Plants, 30 μM As(V)-Treated versus -Pi Plants, and 30 μM Pi-Treated versus -Pi Plants.
Supplemental Data Set 2. Data Set of NimbleGen Probes with Significantly Altered Expression in Wild-Type Plants Exposed to As(V) versus Untreated Plants.
Supplemental Data Set 3. Data Set of As(V)-Induced Transposable Elements, Ordered by Families.
Supplemental Data Set 4. Data Set of NimbleGen Probes with Significantly Altered Expression in WRKY6-Overexpressing Plants Exposed to As(V) versus Wild-Type Plants Treated with As(V).
Acknowledgments
We thank Salomé Prat, Roberto Solano, Cathie Martin, and Carmen L. Torán for critical reading of the article. We appreciate the support of Alonso Rodriguez-Navarro (Centro de Biotecnología y Genómica de Plantas Universidad Politécnica de Madrid-Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria). We also thank Sylvia Gutiérrez for her help with confocal microscopy studies, José Manuel Franco and the Centro Nacional de Biotecnología Genomics Facility for microarray hybridization and analysis, Cristihan González and Luis Calderón for technical assistance, and Catherine Mark for editorial assistance. This work was supported by fellowships (to L.D., P.C., and E.S.-B.) from the Spanish Ministry of Science and Innovation and from La-Caixa/Centro Nacional de Biotecnología International PhD program (to M.T.C.) as well as research grants (BIO2004-03759, BIO2007-66104, BIO2010-16687, AGL2007-61705, AGL2010-15151, CSD2007-00057, EUI2009-03993, and BIO2011-29085) from the Spanish Ministry of Science and Innovation.
AUTHOR CONTRIBUTIONS
G.C. performed the research, was responsible for most work reported in the article, and contributed to experimental design. E.S.-B. performed RT-PCR and phenotypic analyses. L.D. performed all ChIP experiments. P.C. did arsenic quantification. A.F.-E. collaborated in phenotypic analyses. M.T.C. performed most RT-PCR experiments. P.C. performed the 8h-As(V) transcriptomic analysis. A.M. and J.S.-P. performed histone modification analyses. S.O. did RT-PCR and phenotypic analyses. Y.L.D. collaborated in transcriptomic analysis and confocal microscopy. I.M. performed 1.5h-As(V) transcriptomic analysis. E.R. did all confocal analyses. L.E.H. carried out arsenic quantification. J.A.J. and M.P. did histone modification and data analysis. J.P.-A. analyzed the data. A.L. designed experiments and wrote the article.
Glossary
- As(V)
arsenate
- qRT-PCR
quantitative RT-PCR
- ARE
As(V) repression element
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative PCR
- Col-0
Columbia-0
References
- Abercrombie J.M., Halfhill M.D., Ranjan P., Rao M.R., Saxton A.M., Yuan J.S., Stewart C.N., Jr (2008). Transcriptional responses of Arabidopsis thaliana plants to As(V) stress. BMC Plant Biol. 8: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martínez J.A., Poza-Carrión C., Cubas P. (2007). Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboriak D.P., Padua A.O., York G.E., Macfall J.R. (2005). Creation of DICOM—aware applications using ImageJ. J. Digit. Imaging 18: 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle V., Arrighi J.F., Creff A., Nespoulous C., Vialaret J., Rossignol M., Gonzalez E., Paz-Ares J., Nussaume L. (2011). Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23: 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher E., Reinders J., Mirouze M. (2012). Epigenetic control of transposon transcription and mobility in Arabidopsis. Curr. Opin. Plant Biol. 15: 503–510 [DOI] [PubMed] [Google Scholar]
- Bustos R., Castrillo G., Linhares F., Puga M.I., Rubio V., Pérez-Pérez J., Solano R., Leyva A., Paz-Ares J. (2010). A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 6: e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell C., Grant B.R., Theodorou M.E., Harris J., Niere J.O., Plaxton W.C. (1996). The fungicide phosphonate disrupts the phosphate-starvation response in Brassica nigra seedlings. Plant Physiol. 110: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell M.C., Grant B.R., Plaxton W.C. (1997). Disruption of the phosphate-starvation response of oilseed rape suspension cells by the fungicide phosphonate. Planta 203: 67–74 [DOI] [PubMed] [Google Scholar]
- Catarecha P., Segura M.D., Franco-Zorrilla J.M., García-Ponce B., Lanza M., Solano R., Paz-Ares J., Leyva A. (2007). A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell 19: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.F., Li L.Q., Xu Q., Kong Y.H., Wang H., Wu W.H. (2009). The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21: 3554–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dahl T.W., Hammarlund E.U., Anbar A.D., Bond D.P., Gill B.C., Gordon G.W., Knoll A.H., Nielsen A.T., Schovsbo N.H., Canfield D.E. (2010). Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proc. Natl. Acad. Sci. USA 107: 17911–17915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani S.U. (2010). Gold, coal and oil. Med. Hypotheses 74: 534–541 [DOI] [PubMed] [Google Scholar]
- De Lucia F., Crevillen P., Jones A.M., Greb T., Dean C. (2008). A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. USA 105: 16831–16836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhankher O.P., Rosen B.P., McKinney E.C., Meagher R.B. (2006). Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc. Natl. Acad. Sci. USA 103: 5413–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Dénervaud-Tendon V., Hyman D.L., Mayer U., Stierhof Y.D., Chory J. (2009). Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E., Solano R., Rubio V., Leyva A., Paz-Ares J. (2005). PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17: 3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H. (2012). Small RNAs and transposon silencing in plants. Dev. Growth Differ. 54: 100–107 [DOI] [PubMed] [Google Scholar]
- Jiménez I., López L., Alamillo J.M., Valli A., García J.A. (2006). Identification of a plum pox virus CI-interacting protein from chloroplast that has a negative effect in virus infection. Mol. Plant Microbe Interact. 19: 350–358 [DOI] [PubMed] [Google Scholar]
- Jobe T.O., Sung D.Y., Akmakjian G., Pham A., Komives E.A., Mendoza-Cózatl D.G., Schroeder J.I. (2012). Feedback inhibition by thiols outranks glutathione depletion: A luciferase-based screen reveals glutathione-deficient γ-ECS and glutathione synthetase mutants impaired in cadmium-induced sulfate assimilation. Plant J. 70: 783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Cao X., Jacobsen S. (2002). Interplay between two epigenetic marks. DNA methylation and histone H3 lysine 9 methylation. Curr. Biol. 12: 1360–1367 [DOI] [PubMed] [Google Scholar]
- Kasajima I., Ide Y., Yokota Hirai M., Fujiwara T. (2010). WRKY6 is involved in the response to boron deficiency in Arabidopsis thaliana. Physiol. Plant. 139: 80–92 [DOI] [PubMed] [Google Scholar]
- Kiba T., Feria-Bourrellier A.B., Lafouge F., Lezhneva L., Boutet-Mercey S., Orsel M., Bréhaut V., Miller A., Daniel-Vedele F., Sakakibara H., Krapp A. (2012). The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 24: 245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.C., Lai Z., Fan B., Chen Z. (2008). Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 20: 2357–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. (2007). Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Bennetzen J.L. (2006). Recombination, rearrangement, reshuffling, and divergence in a centromeric region of rice. Proc. Natl. Acad. Sci. USA 103: 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama-Nakashita A., Nakamura Y., Yamaya T., Takahashi H. (2004). Regulation of high-affinity sulphate transporters in plants: Towards systematic analysis of sulphur signalling and regulation. J. Exp. Bot. 55: 1843–1849 [DOI] [PubMed] [Google Scholar]
- Meharg A.A., Macnair M.R. (1990). An altered phosphate uptake system in arsenate-tolerant Holcus lanatus L. New Phytol. 116: 29–35 [Google Scholar]
- Mendoza-Cózatl D.G., Jobe T.O., Hauser F., Schroeder J.I. (2011). Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 14: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze M., Paszkowski J. (2011). Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 14: 267–274 [DOI] [PubMed] [Google Scholar]
- Mouillon J.M., Persson B.L. (2005). Inhibition of the protein kinase A alters the degradation of the high-affinity phosphate transporter Pho84 in Saccharomyces cerevisiae. Curr. Genet. 48: 226–234 [DOI] [PubMed] [Google Scholar]
- Murota C., Matsumoto H., Fujiwara S., Hiruta Y., Miyashita S., Shimoya M., Kobayashi I., Hudock M.O., Togasaki R.K., Sato N., Tsuzuki M. (2012). Arsenic tolerance in a Chlamydomonas photosynthetic mutant is due to reduced arsenic uptake even in light conditions. Planta 236: 1395–1403 [DOI] [PubMed] [Google Scholar]
- Narang R.A., Bruene A., Altmann T. (2000). Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol. 124: 1786–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R.S., Saltkov C.W., Wolfe-Simon F., Stolz J.F. (2009). Arsenic in the evolution of earth and extraterrestrial ecosystems. Geomicrobiol. J. 26: 522–536 [Google Scholar]
- Pecinka A., Dinh H.Q., Baubec T., Rosa M., Lettner N., Mittelsten Scheid O. (2010). Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 22: 3118–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaghebeur M., Rengel Z. (2004). Arsenic uptake, translocation and speciation in pho1 and pho2 mutants of Arabidopsis thaliana. Physiol. Plant. 120: 280–286 [DOI] [PubMed] [Google Scholar]
- Raghothama K.G. (1999). Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Reiner A., Yekutieli D., Benjamini Y. (2003). Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19: 368–375 [DOI] [PubMed] [Google Scholar]
- Robatzek S., Somssich I.E. (2002). Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 16: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B.P. (2002). Biochemistry of arsenic detoxification. FEBS Lett. 529: 86–92 [DOI] [PubMed] [Google Scholar]
- Rubio V., Linhares F., Solano R., Martín A.C., Iglesias J., Leyva A., Paz-Ares J. (2001). A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton P.J., Somssich I.E. (1998). Transcriptional control of plant genes responsive to pathogens. Curr. Opin. Plant Biol. 1: 311–315 [DOI] [PubMed] [Google Scholar]
- Rushton P.J., Somssich I.E., Ringler P., Shen Q.J. (2010). WRKY transcription factors. Trends Plant Sci. 15: 247–258 [DOI] [PubMed] [Google Scholar]
- Saze H., Tsugane K., Kanno T., Nishimura T. (2012). DNA methylation in plants: Relationship to small RNAs and histone modifications, and functions in transposon inactivation. Plant Cell Physiol. 53: 766–784 [DOI] [PubMed] [Google Scholar]
- Shin H., Shin H.S., Dewbre G.R., Harrison M.J. (2004). Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 39: 629–642 [DOI] [PubMed] [Google Scholar]
- Song W.Y., et al. (2010). Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA 107: 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung D.Y., Kim T.H., Komives E.A., Mendoza-Cózatl D.G., Schroeder J.I. (2009). ARS5 is a component of the 26S proteasome complex, and negatively regulates thiol biosynthesis and arsenic tolerance in Arabidopsis. Plant J. 59: 802–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticconi C.A., Delatorre C.A., Abel S. (2001). Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiol. 127: 963–972 [PMC free article] [PubMed] [Google Scholar]
- Tittel-Elmer M., Bucher E., Broger L., Mathieu O., Paszkowski J., Vaillant I. (2010). Stress-induced activation of heterochromatic transcription. PLoS Genet. 6: e1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R.D., Srivastava S., Mishra S., Singh N., Tuli R., Gupta D.K., Maathuis F.J. (2007). Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 25: 158–165 [DOI] [PubMed] [Google Scholar]
- Tsukahara S., Kobayashi A., Kawabe A., Mathieu O., Miura A., Kakutani T. (2009). Bursts of retrotransposition reproduced in Arabidopsis. Nature 461: 423–426 [DOI] [PubMed] [Google Scholar]
- Ulm R., Baumann A., Oravecz A., Máté Z., Adám E., Oakeley E.J., Schäfer E., Nagy F. (2004). Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan D.K., Karthikeyan A.S., Matilda P.D., Raghothama K.G. (2002). Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 129: 1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettenhall J.M., Simpson K.M., Satterley K., Smyth G.K. (2006). affylmGUI: A graphical user interface for linear modeling of single channel microarray data. Bioinformatics 22: 897–899 [DOI] [PubMed] [Google Scholar]
- Wu Z., Ren H., McGrath S.P., Wu P., Zhao F.J. (2011). Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol. 157: 498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Rensing C., Rosen B.P., Zhu Y.G. (2012). Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 17: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]