This work examines the functional differences among different PPR motifs of PROTON GRADIENT REGULATION3 by introducing a charged or nonpolar amino acid into the fourth position of the PPR and testing the effect on the functions in RNA stability and translation of two target genes.
Abstract
Pentatricopeptide repeat (PPR) proteins bind RNA and act in multiple eukaryotic processes, including RNA editing, RNA stability, and translation. Here, we investigated the mechanism underlying the functional versatility of Arabidopsis thaliana PROTON GRADIENT REGULATION3 (PGR3), a chloroplast protein harboring 27 PPR motifs. Previous studies suggested that PGR3 acts in (1) stabilization of photosynthetic electron transport L (petL) operon RNA, (2) translation of petL, and (3) translation of ndhA. We showed here that replacement of the 4th amino acid of the 12th PPR with nonpolar or charged amino acids abolished functions (1) and (2) but not (3) of PGR3 by compromising the function of this specific PPR. This discovery enabled us to knock out the RNA binding ability of individual PPR motifs. Consequently, we showed that the 16 N-terminal PPRs were sufficient for function (1) via sequence-specific RNA binding, whereas the 11 C-terminal motifs were essential for functions (2) and (3) by activating translation. We also clarified that the 14th amino acid of the 12th PPR should be positively charged to make the PPR functionally active. Our finding opens up the possibility of selectively manipulating the functions of PPR proteins.
INTRODUCTION
Pentatricopeptide repeat (PPR) proteins form a large family of helical repeat proteins ubiquitously found in eukaryotes and are deeply involved in coevolution of the nucleus with the mitochondria and plastids (Small and Peeters, 2000; Schmitz-Linneweber and Small, 2008). Each PPR motif consists of 35–amino acid degenerate consensus related to tetratricopeptide motifs (Small and Peeters, 2000). A number of recent studies proved the direct RNA binding activity of the PPR motifs (Pfalz et al., 2009; Cai et al., 2011; Prikryl et al., 2011; Kobayashi et al., 2012; Okuda and Shikanai, 2012). PPR proteins harbor between two and 30 PPR motifs, and their tandem alignment allows the modular recognition of specific RNA sequences. Many studies have found that PPR mutants have compromised functions of specific genes in mitochondria or plastids; PPR proteins can promote versatile molecular processes involving RNA, such as editing, splicing, stabilization, and translation (reviewed in Schmitz-Linneweber and Small, 2008; Fujii and Small, 2011; Nakamura et al., 2012). As a consequence, the majority of PPR proteins are essential for important cellular processes, such as respiration and photosynthesis. The expansive evolution of PPR proteins in terrestrial plant species (ranging from 100 to over 1000 genes per genome) also suggests the plasticity of these proteins in adapting to a wide range of RNA targets (Delannoy et al., 2007; O’Toole et al., 2008; Fujii and Small, 2011). These findings suggest that it may be possible to redirect or even synthesize PPR proteins to bind to desired RNA targets in a manner similar to that of transcription activator-like effectors, a class of protein motif that binds to specific DNA sequences. Transcription activator-like effectors have already been applied to biotechnology via the rapid computational design of repeat arrays that bind to virtually any DNA sequence (Boch et al., 2009; Moscou and Bogdanove, 2009). With the unveiling of the primary version of the PPR code, manipulation of PPRs to bind to target RNAs is within reach (Barkan et al., 2012). Biochemical (Barkan et al., 2012; Kobayashi et al., 2012), statistical (Barkan et al., 2012; Yagi et al., 2013), and evolutionary constraint (Fujii et al., 2011) studies have postulated that the 1st, 3rd, and 6th amino acids within the PPR consensus govern RNA base recognition specificity. However, to put the final polish on the code for de novo prediction of PPR binding RNA sequences, we still need a better understanding of how PPR tracts recognize and bind to RNA.
PROTON GRADIENT REGULATION3 (PGR3) is a PPR protein that was identified through the screening of high-chlorophyll-fluorescence mutants at high light intensity in Arabidopsis thaliana (Shikanai et al., 1999). All the results obtained so far are consistent with a model in which PGR3 involves three distinct functions: (i) stabilization of photosynthetic electron transport L (petL) operon RNA, (ii) translational activation of petL, and (iii) translational activation of ndhA (Yamazaki et al., 2004; Cai et al., 2011; Figure 1). For the first two functions, PGR3 should bind to the 5′untranslated region (UTR) of petL operon RNA, as has been confirmed by in vitro RNA binding assay (Cai et al., 2011). As for the third function, although recombinant PGR3 also bound to the 5′UTR of ndhA, in a polysome analysis we were unable to detect the defect, probably because of technical limitations (Cai et al., 2011). We therefore still cannot definitely conclude that the phenotype observed in the chloroplast NADH dehydrogenase-like (NDH) complex is due to a defect in ndhA translation (Cai et al., 2011). In pgr3-1 and pgr3-2 mutant alleles, lack of the petL operon RNA stabilization results in the absence of both PetL and PetG proteins (Yamazaki et al., 2004). pgr3-1 is further defective in the ndhA translation, whereas pgr3-2 retains function. Although pgr3-3 mutants did not accumulate NDH, the level of cytochrome f was not affected; the phenotype of this mutant was explained by an inability to translate both petL and ndhA. In pgr3-3, PetG is likely translated (Yamazaki et al., 2004), resulting in a clear phenotypic difference from pgr3-1 and supporting our hypothesis that PetG, rather than PetL, is required to stabilize cytochrome f.
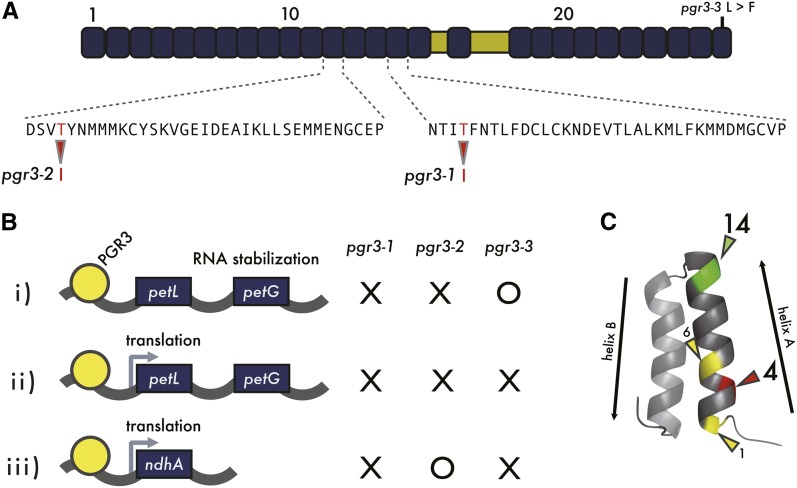
Figure 1.
Summary of the Results of Previous Studies on PGR3.
(A) The three pgr3 mutant alleles carry the amino acid point mutations indicated. pgr3-1 and pgr3-2 carry T-to-I replacements at the 4th amino acid position of the 15th and 12th PPR motifs, respectively. pgr3-3 carries an L-to-F substitution in the last PPR motif.
(B) PGR3 is required for three functions in two distinct targets: stabilization of petL operon RNA i), and translation of ii) petL and iii) ndhA. pgr3-1 is deficient in all three functions. pgr3-2 is defective in functions i) and ii), and pgr3-3 is defective in functions ii) and iii). It is still not certain that ndhA is the sole direct target of PGR3 among the 11 plastid-encoded ndh genes (Cai et al., 2011).
(C) Spatial demonstration of the 4th amino acid within 35 amino acids of a PPR motif. Known RNA recognition determination residues (1st and 6th amino acids) are indicated in yellow. X-ray structure of the PPR motif was retrieved from human mitochondrial RNA polymerase harboring two PPR motifs (PDB 3SPA) (Ringel et al., 2011).
pgr3-2 carries a Thr-to-Ile alteration at the 4th amino acid of the 12th PPR motif, resulting in specific loss of petL operon accumulation, but normal ndhA translation. Thus, the single amino acid alteration changes the RNA binding specificity. More specifically, the chemical properties of the amino acid at the 4th position of the PPR may be critical for PGR3 RNA binding affinity. We took advantage of the distinct phenotypes of these mutant alleles to evaluate the contribution of each PPR motif to PGR3 specificity and function.
RESULTS
Uncharged Polar Amino Acids Can Functionally Replace Thr at the 4th Amino Acid Position
Because the pgr3-2 mutation did not affect ndhA translation (function iii) (Cai et al., 2011), we reasoned that this amino acid replacement (Thr to Ile) at the 4th amino acid position of the PPR motif consensus did not seriously affect the entire protein structure (which would have led to instability of PGR3), but instead specifically affected the RNA affinity of the motif in question. In addition, the 1st and 6th amino acids have been identified as RNA base recognition residues (Barkan et al., 2012). The residues proximal to these amino acids may also be required for RNA binding (Figure 1C); indeed, the 4th amino acid has also been proposed as an important site for RNA binding affinity (Kobayashi et al., 2012). This 4th amino acid is located on the solvent surface of the first α-helix in a PPR motif (Ringel et al., 2011; Kobayashi et al., 2012). However, these residues may not directly influence binding specificity (Fujii et al., 2011; Barkan et al., 2012; Yagi et al., 2013), since statistical test inferred that the 1st and the 6th amino acids serve as principle components for RNA recognition.
To gain an understanding of the chemical properties of the residues required to retain RNA affinity, we introduced a series of point mutations at the 4th amino acid of the 12th PPR of PGR3 (the pgr3-2 mutation site). Mutant PGR3 genes encoding nine different amino acid substitutions were introduced into the pgr3-1 mutant, which lacks all three functions, under the control of the native PGR3 promoter (Figure 2). Constructs carrying the amino acid replaced with Cys, Asn, or Ser complemented petL operon RNA accumulation and consequently cytochrome f accumulation (Figures 2A and 2B), although Cys was less preferential for full activity. Other substitutions at the site did not complement the function (Figure 2A), suggesting that the 4th position of the PPR motif should contain uncharged and polar amino acids for binding to the 5′UTR of petL operon RNA. By contrast, all of the constructs complemented the accumulation of NdhK (Figure 2B). All attempts to directly detect PGR3 in vivo have failed, but our results suggest that substitution with other amino acids at this site did not seriously affect the stability of PGR3.
Figure 2.
Investigation of Compensatory Amino Acids at the 4th Amino Acid.
(A) RNA gel blot analysis detecting petL operon RNA. The 4th amino acid of the 12th PPR motif was replaced by each amino acid indicated and then introduced into pgr3-1. The amino acid at this position was replaced by Ile in pgr3-2. WT, the wild type.
(B) Immunoblot detection of cytochrome f, a subunit of the cytochrome b6f complex. Cytochrome f stability depends on PetG, rather than PetL, in Arabidopsis (Yamazaki et al., 2004); the analysis thus monitored function (i) rather than function (ii). NdhK is a subunit of chloroplast NDH and was detected instead of NdhA because the absence of NdhA destabilized the entire NDH complex (Peng et al., 2009). Note that all the PGR3 variants complemented the accumulation of NdhA, suggesting that any substitutions did not dramatically affect PGR3 stability. PsaA abundance is displayed as the loading control.
Scanning of PPR Motifs Conferring Versatility on PGR3 Function
Which specific PPR motifs of PGR3 contribute to each of the three hallmark functions (Figure 1)? To answer this question, we systematically substituted the 4th amino acid of each PPR motif (Thr) with Ile, a nonpolar amino acid; this abolished the function of this PPR motif but was unlikely to affect the stability of PGR3 (Figure 2). The series of PGR3 variants was introduced into pgr3-1 under the control of the PGR3 promoter.
To monitor function (i), petL operon RNA was detected by RNA gel blots (Figure 3B). Because petG is also transcribed from the same promoter as petL, petG expression depends on function (i). The absence of PetG but not PetL destabilizes the cytochrome b6f complex (Yamazaki et al., 2004), whose accumulation was monitored by measuring the cytochrome f level (Figure 3C). Because the reduced level of the cytochrome b6f complex restricts linear electron transport, function (i) can be quantitatively evaluated as quantum yields of photosystem II (Figure 3D). Activity of translation was assessed using the antibody against PetL for function (ii) and NdhK and NdhL for function (iii) (Figure 3C). Since absence of NdhA totally destabilizes the chloroplast NDH complex (Peng et al., 2009), function (iii) can be monitored by the antibodies against NdhK and NdhL (Figure 3C).
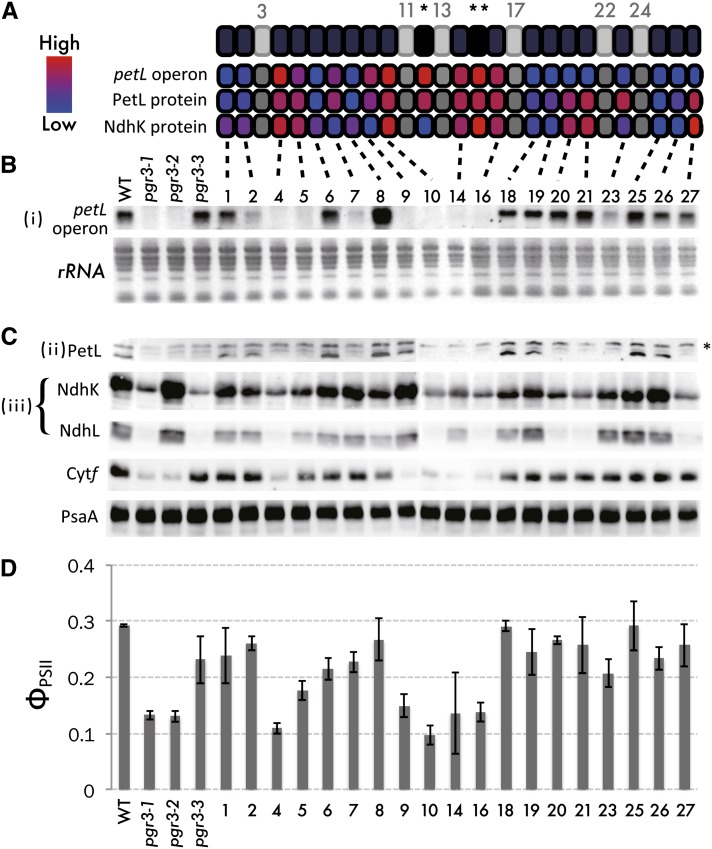
Figure 3.
Systematic Replacement of the 4th Thr with Ile in Each PPR Motif.
(A) to (C) Protein structure of PGR3 (A) and summary of results ([B] and [C]).
(A) Light gray indicates PPR motifs that were not mutated, as they harbored amino acids other than Thr at the 4th position. Single and double asterisks indicate the PPR motifs mutated in pgr3-2 and pgr3-1, respectively. The contribution of each PPR motif to the three functions was estimated from the RNA and protein abundance relative to that in the wild type, as digitized from the experiments below. When a point mutation severely affects PGR3 function, the color is shown as red (high contribution), but when a point mutation does not cause any defect the color is shown as blue (low contribution).
(B) RNA gel blot analysis of the petL operon RNA in transgenic pgr3-1 lines expressing PGR3 variants carrying Thr-to-Ile replacements at the 4th position of each PPR motif indicated. WT, the wild type.
(C) Immunoblot detection of PetL, two subunits of the chloroplast NDH complex NdhK and NdhL, cytochrome f, and PsaA. Asterisk indicates nonspecific signals.
(D) Quantum yields of PSII determined at a light intensity of 500 µmol photons m−2 s−1. Data represent means of three biological replicates, with standard deviations. The cytochrome f level and PSII yield are linked to the PetG level, which could not be tested directly (Yamazaki et al., 2004; Cai et al., 2011); the analysis thus is equivalent to monitoring petL operon accumulation.
Three PGR3 variants (20, 21, and 27) stabilized the petL operon RNA but did not complement the translation of petL or ndhA (Figure 3). This result suggests that these PPR motifs are required for translation but are not required for binding to the 5′UTR of petL operon RNA. Another two lines, with PGR3 variant 7 or 23, accumulated NdhK but not PetL (Figure 3). Although the stability of petL operon RNA was slightly complemented by introducing variants 7 and 23, the cytochrome f level was fully complemented (Figure 3), suggesting that low levels of RNA were sufficient for wild-type levels of petG translation. These results suggest that the 7th and 23th PPR motifs are not required for ndhA translation but are partially required for petL operon stabilization. Because petL translation requires its transcript accumulation, we were unable to conclude whether these PPR motifs could be specifically required for petL translation (see Discussion). The petL RNA level did not recover upon introduction of variant 9, resulting in a reduction in cytochrome f level (Figure 3). Puzzlingly, this line accumulated a level of PetL protein equivalent to that in the wild type (Figure 3); the reason for this is discussed later. The mutation at the 8th PPR motif resulted in overaccumulation of petL mRNA, implying that this PPR motif partly suppresses the activity to stabilize petL operon RNA in the wild type. Photosystem II (PSII) yields reflected the cytochrome f levels (Figures 3C and 3D) and were used to analyze multiple transgenic lines quantitatively for the evaluation of function (i) (see Methods for details).
Because we cannot evaluate the protein levels of PGR3 variants, it is possible that the observed differences in phenotypes depended on the level of PGR3 variants in vivo, rather than the function of each PPR motif. If this is the case, the mutant phenotypes depending on the defect in each function should be correlated because all the phenotypes depend on the protein levels. To study this possibility, petL RNA accumulation and PetL and NdhK protein levels were plotted against each other (see Supplemental Figure 1 online). We found no significant correlation between petL RNA and NdhK protein accumulation (see Supplemental Figure 1B online; r = 0.28, P = 0.09). This indicates that functional partitioning of PGR3 is not just visualizing the artifact resulting from the different stability among PGR3 variants. By contrast, there were correlations between petL RNA and PetL protein abundance, especially when plots were limited to N-terminal 16 PPR motifs (squared plots in Supplemental Figure 1A online). As discussed later, the N-terminal 16 PPR motifs are required for target recognition, whereas the C-terminal 11 PPR motifs likely function in translation. Meanwhile, correlation between PetL and NdhK protein abundances was also observed (r = 0.60, P = 0.001), and this was probably because the C-terminal 11 PPR motifs functions in a similar manner upon translation of both petL and ndhA (triangular plots in Supplemental Figure 1C online). We do not eliminate the effects of stability of PGR3 variants on the phenotypes completely, but the majority of observed phenotypes are explained by our model on the functional partitioning of PGR3.
Charges in the 14th Amino Acid Influence the Function of a PPR Motif
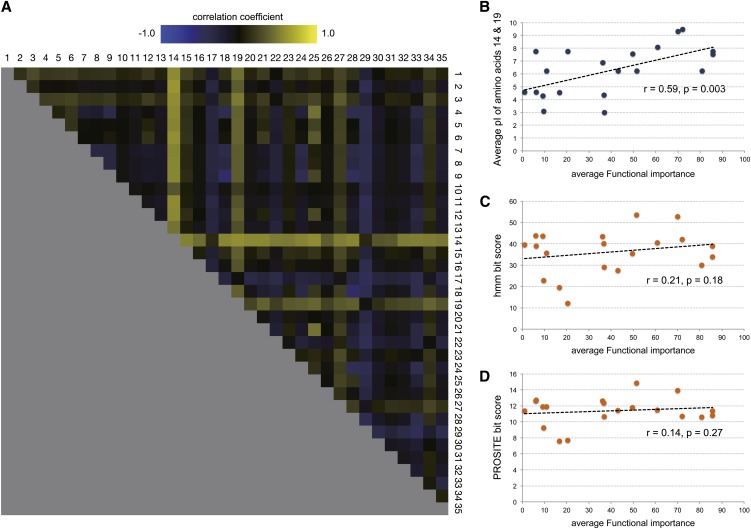
We established the method to evaluate the function of a PPR motif in question, leading to the conclusion that the 4th amino acid should be uncharged polar to make the motif functionally active (Figure 2). This strategy may be also efficient to determine other amino acid residue(s) or amino acid chemical properties that determine the contribution of each motif to RNA binding or translation. First, we surveyed if there is any correlation between amino acid charges and the accumulation levels of RNA or protein in PGR3 variants. We calculated the average isoelectric points for every two pair of residues in a PPR motif (595 amino acid combinations), and their correlation coefficient was calculated with the levels of petL RNA, PetL protein, and NdhK protein and also with the average functional importance estimated (see Methods for details) from all the three functions. As a result, the average isoelectric points of combinations that included the 14th amino acid were prone to have positive correlation with the average functional importance of each motif (Figure 4) and negative correlation with the individual RNA or protein accumulation level (see Supplemental Figure 2 online), suggesting that the residue should be positively charged to make the PPR motif functionally active. Precisely, the functional contributions of the PPR motifs were higher when a positively charged amino acid (e.g., Lys or Arg) was located at the 14th position in PGR3 (Figure 4B). Functional importance of each PPR motif did not have a significant correlation with the bit scores from the HMM search (r = −0.21, P = 0.18) or with that from the PROSITE motif scanning (r = −0.14, P = 0.27) (Figure 4B). This indicates that soundness of the PPR motif, or closeness to the PPR consensus, did not have great influence on its functional importance in PGR3. We also used the molecular weights and the Kyte-Dolittle hydrophobicity scale (Kyte and Doolittle, 1982) of the amino acids as the parameters to correlate with average functional importance (see Supplemental Figure 3 online). The correlation patterns calculated from these parameters were more obscure than that with the pI of the amino acids (Figure 4), showing a mosaic coloring structure that indicates the absence of a particular amino acid position with strong effect.
Figure 4.
Calculation of Correlation between the pI of Amino Acids and the Functional Importance of Each PPR Motif.
(A) Pearson correlation coefficient calculated for every possible combination of two amino acid positions (595 pairs) and average functional importance equivalent to the average of contribution value of three functions from Figure 3A.
(B) Plots between average pI of amino acid positions, 14 and 19, and the average functional importance. Shown as the example of significant positive correlation between pI of amino acid 14 and average functional importance.
(C) Plots between hmmsearch bit score and the average functional importance.
(D) Plots between PROSITE scan bit score and the average functional importance.
For panels (B) to (D), data points from 18 out of 19 PGR3 point mutation transgenic lines generated in this study, pgr3-1 and pgr3-2 were used. Data points for PGR3 variants in the last 27th PPR motif (including pgr3-3) were not used because the motif is truncated and amino acids 14 and 19 are absent.
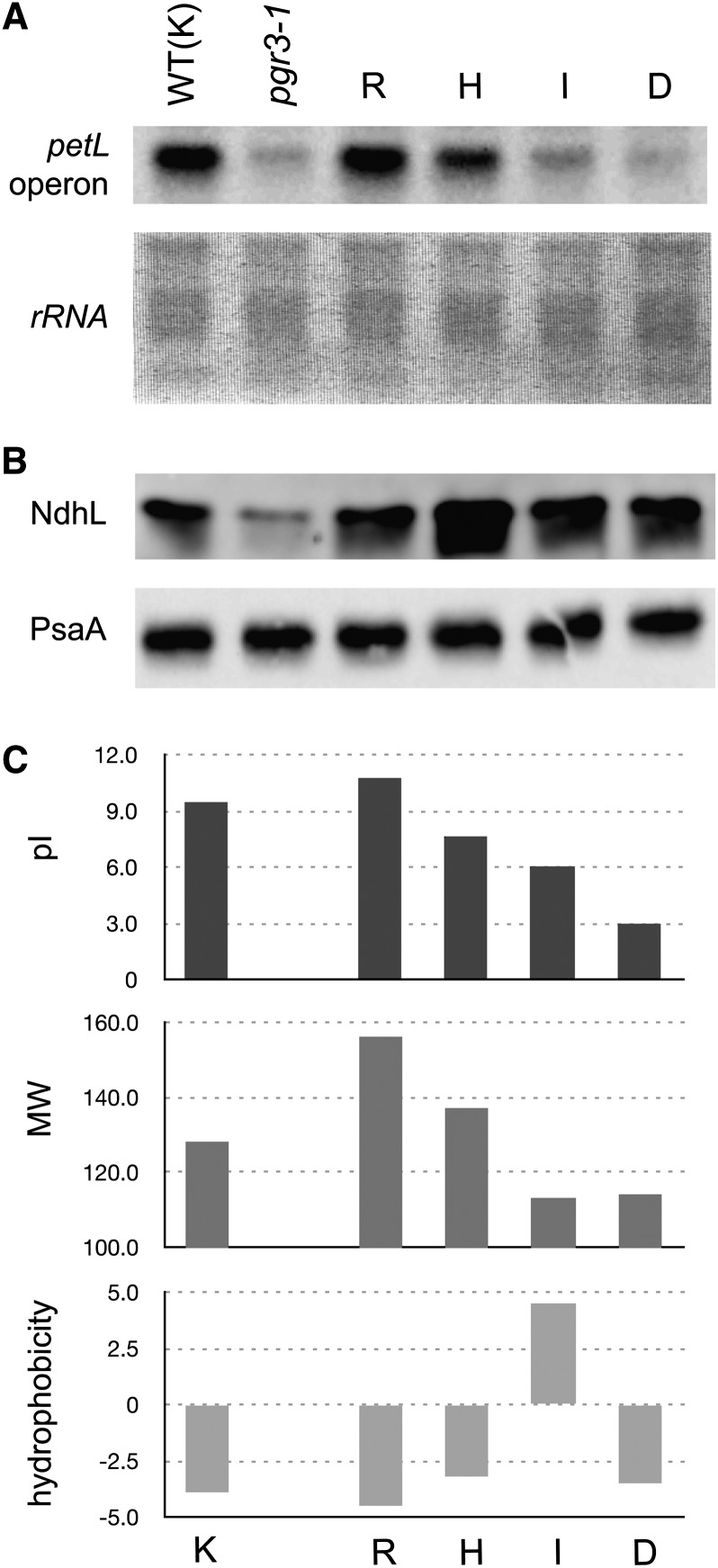
We attempted to verify the influence experimentally by introducing amino acids with different charges into the 14th amino acid position of the 12th PPR, which is required for the stabilization of petL operon RNA (Figure 2). As a result, petL operon RNA was equally stabilized by the substitution of this site (Lys in the wild type) with positively charged Arg, slightly less effectively when replaced with His, and much less effectively when nonpositively charged amino acids Ile and Asp (Figure 5). Also importantly, differences of the molecular weights or the hydrophobicities of the replaced amino acids do not adequately explain the effectiveness of the petL RNA stabilization function of the PGR3 variants (Figure 5C).
Figure 5.
Influence of pI at the 14th Amino Acid of the 12th PPR.
(A) RNA gel blot analysis detecting petL operon RNA. The 14th amino acid of the 12th PPR motif was replaced by each amino acid indicated and then introduced into pgr3-1. WT, the wild type.
(B) Immunoblot detection of NdhL and PsaA.
(C) pI, molecular weight (MW), and hydrophobicity of each amino acid replaced. pI at pH 7.0 is indicated. Molecular weight is in daltons, hydrophobicity according to the Kyte-Doolittle scale (Kyte and Doolittle, 1982).
Truncation of the Last 11 PPR Motifs Does Not Substantially Affect the petL Operon Stabilization Function of PGR3
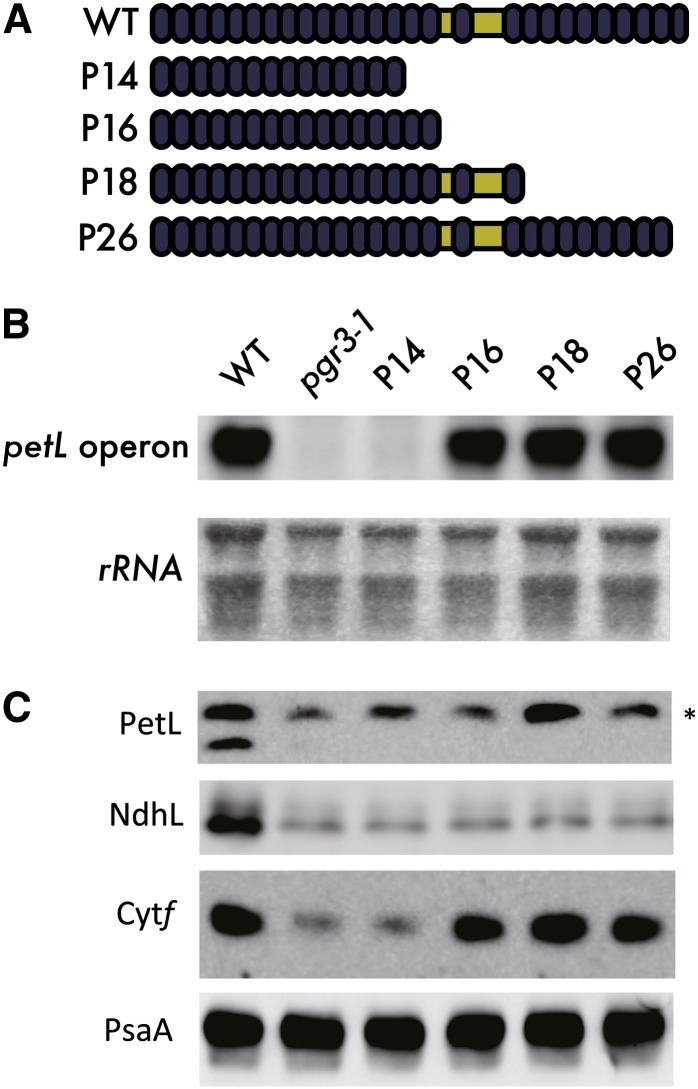
We noticed that none of the 4th amino acid point mutations within the last 10 PPR motifs (18 to 27) affected the petL operon accumulation, although the 23rd PPR was required for full activity (Figure 3). This result suggests that the N-terminal 16 PPR motifs are sufficient for stabilizing petL operon RNA. To test this possibility, several versions of truncated PGR3 were expressed in pgr3-1. Consistent with our hypothesis, P16, the PGR3 variant carrying only the first 16 N-terminal PPR motifs, showed recovery of petL operon RNA accumulation to the same extent as P18 and P26 (Figure 6). However, further truncation of two PPR motifs (P14) impaired this function to the level of pgr3-1 (Figure 6), suggesting that the first 16 PPR motifs of PGR3 are required, and are sufficient, for petL operon stabilization.
Figure 6.
Investigation of the PGR3 Region Essential for Stabilizing the petL Operon RNA.
(A) Introduction of truncated PGR3 series into the pgr3-1 mutant (e.g., P14 indicates the line expressing the 14 N-terminal PPR motifs). WT, the wild type.
(B) and (C) Levels of petL operon RNA (B) and also cytochrome f protein (C) were determined to monitor the complementation of petL transcript stabilization. None of the truncated series recovered the PetL or NdhL accumulation. PsaA level was used as the internal control. Asterisk indicates nonspecific signals.
[See online article for color version of this figure.]
To test our hypothesis further, we expressed the truncated versions (P14 and P16) of recombinant PGR3 and tested whether they possess the affinity against the petL RNA ligand (see Supplemental Figure 4 online). As expected from the in vivo phenotype, P16 retained the ability to bind to the petL RNA with only a slight compromise compared with the full-length PGR3, but P14 did not, suggesting that the functional partitioning structure was reproducible in vitro.
DISCUSSION
By introducing a charged or nonpolar amino acid into the 4th position, we succeeded in specifically impairing the RNA binding affinity of a particular motif without affecting the stability of the whole protein for most of the PPR motifs in PGR3. This approach is supported by the fact that the 4th amino acid is exposed to the putative RNA binding surface of a PPR, as predicted by the PPR-RNA docking model (Fujii et al., 2011) and by in vitro experimental evidence (Kobayashi et al., 2012). A mutation in such a residue is unlikely to affect the protein structure drastically. Furthermore, if amino acid substitutions at the site affect PGR3 function via a reduction in protein stability, we should observe high correlations among the extents of functional disruption in all the three PGR3 functions. This was not the case (see Supplemental Figure 1 online), indicating that the functional partitioning of PGR3 cannot be explained by the different stability of PGR3 variants.
We introduced systematic mutations into the 4th site of a PPR motif, which revealed that some PPR motifs contribute to only one or two of the three proposed functions of PGR3 (Yamazaki et al., 2004; Cai et al., 2011). Not all of the PPR motifs participate in the same function. For instance, the 7th PPR motif was required for petL RNA accumulation, or petL translation, but the motif was not essential for ndhA translation (Figure 3). For several point mutations that severely impaired all three functions of PGR3 (PPR motifs 4, 10, and 16), we cannot exclude the possibility that these mutations destabilized PGR3. More probably, however, these PPR motifs are required for all three functions.
We found that PPR motifs carrying positively charged amino acids at position 14 (Figure 1C) contributed more to PGR3 function (Figure 4; see Supplemental Figure 2 online). This amino acid is located at the bottom of the central groove of the two α-helices of a PPR motif that may interact with the phosphate skeleton of RNA (Small and Peeters, 2000). Yeast PET309 with point mutations in basic amino acids in the proximity of this site was only partially functional; more importantly, double point mutations in basic residues in this region gave stronger mutant phenotype (Tavares-Carreón et al., 2008). Our study suggests, in agreement with the previous studies, that surface charge of the amino acid at the bottom of the central groove provides quantitative information on RNA binding strength. Notably, the statistical analysis also highlighted some other amino acids that may contribute to PPR function (Figure 4). Although not as strong as the 14th amino acid, positive charges on the 19th amino acid might also contribute to RNA binding by a similar mechanism, as they are located nearby. The PPR motifs with negatively charged amino acids at the 29th amino acid were prone to have a stronger influence on PGR3 function (Figure 4). It will be worth further investigating if the charges on these amino acids might also influence PGR3 function, as do the amino acids at position 14.
Mutagenesis within the C-terminal 11 PPR motifs, or even complete depletion of these motifs, did not impair the PGR3 function to stabilize the petL operon RNA (Figures 3 and 6). At least PPR motifs 20, 21, and 27 were definitely required for the other two functions, petL and ndhA translation (Figure 3). A number of PPR proteins have been reported to target multiple RNA sequences (Schmitz-Linneweber et al., 2005; Chateigner-Boutin et al., 2008; Hammani et al., 2009; Pfalz et al., 2009; Zehrmann et al., 2009; Bentolila et al., 2010; Okuda et al., 2010); here, we report of the partitioning of a single PPR protein into regions harboring different functions. A straightforward question arises: What is the function of each region? The first 16 PPR motifs are likely essential for RNA binding; this hypothesis was supported by the results of our in vitro RNA binding assay of PGR3-2 (Cai et al., 2011). Recent transcriptomic studies reported the presence of PPR footprints in chloroplast RNA: binding of PPR proteins protects short RNA sequences from exonucleases (Ruwe and Schmitz-Linneweber, 2012; Zhelyazkova et al., 2012). The footprints included the predicted binding sites of PGR3 present in petL and ndhA 5′UTRs. N-terminal 16 PPR motifs most likely bind to the leader sequence of the petL operon and protect the entire transcript from a 5′→3′ exonuclease. This was also supported by this study, demonstrating the correlation between the levels of petL RNA and PetL protein when the analyzed region was restricted to the N-terminal 16 PPR motifs rather than the entire PGR3 (see Supplemental Figure 1 online). The N-terminal PPRs likely contribute to petL translation via the stabilization of petL operon RNA. Because petG translation also depends on the stabilization of petL operon RNA, the defect in function (i) results in a reduction in cytochrome f levels and consequently reduced PSII yields (Figures 3C and 3D). By contrast, the C-terminal 11 PPR motifs have functions specifically required for petL and ndhA translation (see Supplemental Figure 1C online). How could the 11 C-terminal PPR motifs be involved in translation? A likely scenario is that the C terminus of PGR3 binds the target RNA with less sequence specificity compared with the N terminus, thereby promoting translation via a mechanism such as conformational alteration of RNA secondary structure. For example, PPR10 can release the RNA secondary structure flanking the Shine-Dalgarno sequence of the plastid atpH gene, likely facilitating the access of ribosomes to initiate translation (Prikryl et al., 2011). It is also possible that the C terminus sequence recruits cofactors required for translational activation. The Pumilio and FBF homology repeat proteins contain a class of RNA binding motifs, each of which binds a single base in modular fashion, as do PPRs (Wang et al., 2002; Wickens et al., 2002; Quenault et al., 2011). Pumilio and FBF homology proteins can recruit other proteins to destabilize or repress the translation of target RNAs (Goldstrohm et al., 2006). From an evolutionary perspective, these C-terminal sequences could be modules of PPR motifs that are redirected to acquire novel functions (e.g., protein translation) other than specific RNA recognition. This is reminiscent of the function of the E domain, which consists of C-terminal peptides present in a subgroup of PPR genes known as the PLS subfamily (Lurin et al., 2004; Shikanai, 2006; Schmitz-Linneweber and Small, 2008; Fujii and Small, 2011). The sequence structure of the E domain is similar to that of the PPR motif because they conceivably share the same origin, except that the E domain is considered to participate in the core RNA editing mechanism rather than in RNA recognition (Shikanai, 2006; Schmitz-Linneweber and Small, 2008; Fujii and Small, 2011). The correlation between PetL and NdhK protein abundance within the PGR3 variants was stronger in the C-terminal 11 PPR motifs than in the entire PGR3 (see Supplemental Figure 1 online), suggesting that the region is required for the translational mechanism shared by petL and ndhA. Introduction of the C-terminal 16 PPR motifs of PGR3 fused to its chloroplast targeting peptides into the pgr3-1 phenotype did not result in complementation of any of the three phenotypes (data not shown). This suggests that the C terminus is unlikely to be able to function on its own without the N terminus.
Our model cannot explain the phenotype of the line with PGR3 variant 9; in this line, PetL accumulated normally without recovery of the petL operon RNA level (Figures 3B and 3C). The defect in function (i) resulted in reduced cytochrome f level and lower PSII yield, probably via the reduced PetG level (Figures 3C and 3D). Comparison with the results for the line harboring variant 7, which accumulated slightly more petL operon RNA but less PetL protein than did the line with variant 9 (Figures 3B and 3C), reveals that the RNA level does not simply determine the protein level. The 9th PPR motif is essential for petL RNA stabilization, but its mutation may activate petL translation: This PPR motif suppresses petL translation in the wild-type protein. Further analysis of polysome-associated RNA in these variant transgenic lines should address this question in the future. Also puzzlingly, petL RNA abundance was only partially recovered in the line with variant 23, although truncation of the last 11 PPR motifs did not affect the level of petL operon RNA (Figures 3B and 4). Other than the possibility that the mutation in the 23th PPR partially destabilizes PGR3, the mutation may cause the aberrant interference of the motif with the 16 N-terminal PPR motifs upon PGR3–petL RNA interaction. We propose that the N-terminal (PPR1 to PPR16) and C-terminal (PPR17 to PPR27) halves of PGR3 have distinct functions, but to fully explain these results we must speculate that there is yet identified intramolecular communication between the two regions.
METHODS
Plant Materials and Growth Conditions
All pgr3 alleles were obtained by ethyl methanesulfonate mutagenesis, as described in a previous study (Yamazaki et al., 2004). Plants were grown in Metromix potting soil under controlled conditions (light intensity of 40 µmol photons m−2 s−1, 16-h-light/8-h-dark cycle at 23°C).
Transgenic Arabidopsis thaliana Plants
All site-directed mutagenesis was performed by fusion PCR. Specifically, two DNA fragments carrying the desired point mutations at the overlapping position were amplified with PCR using KOD Plus Neo DNA polymerase (Toyobo). Two fragments were gel extracted, mixed, and subjected to a second PCR amplification to obtain the mutagenized single fragment. DNA fragments harboring the PGR3 (At4g31850) promoter and the mutagenized PGR3 coding region sequences were cloned into pDONR-zeo (Life Technologies) or pENTER/D-TOPO (Life Technologies). The PGR3 promoter corresponds to the 689-bp DNA fragment immediately upstream of the PGR3 start codon, which reaches to the last exon of the adjacent gene (At4g31840). All of the primers used in this study are listed in Supplemental Table 1 online. These gene fragments were further introduced into pGWB-NB1 (Nakagawa et al., 2007) by homologous recombination using LR clonase II (Life Technologies). Mutations were only introduced into the 19 PPR motifs that carried Thr in the 4th amino acid. Transgenic plants were obtained using an Agrobacterium tumefaciens transfection method with Basta resistance selection on Murashige and Skoog medium (Clough and Bent, 1998). We confirmed that the presence of the original mutation of pgr3-1 and the additional copy of the mutant PGR3 in the transgenic lines by PCR. For all of the constructs used in this study, at least three independent transgenic lines were used for chlorophyll fluorescence analysis that monitors PSII yield and NDH activity. As observed in small standard deviations, PSII yield was not divergent among lines (Figure 3D), indicating that the gene expression driven by the PGR3 native promoter was sufficient and stable enough. For each construct, representative lines with intermediate values of PSII yield were selected to further quantify protein or RNA. Chlorophyll fluorescence analysis of NDH activity (Okegawa et al., 2008) did not provide quantitative information, but no lines showed a phenotype inconsistent with the others. In summary, a single line represented each construct for the molecular phenotypes (protein and RNA), but the electron transport phenotypes were confirmed in multiple lines.
RNA Preparation and RNA Gel Blot Analysis
RNA was isolated from the leaves of 3-week-old plants using RNAiso (TaKaRa-Bio). Three micrograms of total RNA was electrophoresed and then transferred onto a nylon membrane. The membrane was hybridized with a digoxigenin-labeled DNA probe corresponding to the petL-petG genic region (primers used are listed in Supplemental Table 1 online), and the signals were detected with a Gene Image CDP-Star detection kit (GE Healthcare). rRNA was visualized by staining with either ethidium bromide or methylene blue. Band intensities were quantified and digitized using ImageJ (Schneider et al., 2012).
Immunoblot Analysis
Immunoblot analysis was performed as described previously (Yamazaki et al., 2004). Protein corresponding to 2.0 µg of chlorophyll was loaded to detect NdhK and NdhL; 0.4 µg was loaded to detect cytochrome f, 0.75 µg to detect PetL, and 0.5 µg to detect PsaA. The chlorophyll contents were used to normalize signal intensities. Band intensities of each PGR3 variant line were quantified using relative protein abundance in pgr3-1, pgr3-2, and pgr3-3, which was measured in the previous study using a dilution series (Yamazaki et al., 2004). Signals were digitized using ImageJ (Schneider et al., 2012). Signal saturation was avoided using the saturated-coloring function implemented in LAS-3000 (Fuji-Film).
Chlorophyll Fluorescence Analysis
Quantum yields of PSII were determined with a Mini-PAM fluorometer (Walz) as described previously (Shikanai et al., 1999).
Statistical Analysis to Correlate Amino Acid Chemical Properties with PPR Functions
All of the bioinformatic analysis was done using custom PERL scripts. PPR motifs were identified by hmmsearch (Finn et al., 2011) or by the stand-alone version of PROSITE (Gattiker et al., 2002). In order to evaluate the possible combinatorial effects of independent positions, we calculated the average isoelectric points of two amino acids. Average functional importance of individual PPR motifs was calculated based on the quantification of petL RNA, PetL protein, and the NdhK protein abundance of each transgenic line (Figure 3). Specifically, reduction rate of RNA or protein was subtracted from 100%, and the remaining value was considered as the functional contribution rate, as indicated in Figure 3A. The functional contribution rates of all three functions were averaged to obtain the average functional importance. Pearson correlation coefficients between the average functional importance and the amino acid chemical property parameters were calculated using R (R Development Core Team, 2013).
Expression and Purification of Recombinant PGR3 Proteins
The sequences encoding mature PGR3, or the two truncated forms of PGR3 (P14 and P16), were cloned into the NotI-EcoRI sites of pMAL-c5x (New England Biolabs). Primers used are listed in Supplemental Table 1 online. Constructs were transformed into Rosetta (DE3) pLysS (Novagen). Expression of the maltose binding protein–fused PGR3 fragments was induced by the addition of 0.3 mM isopropyl thio-β-galactoside and incubation at 16°C for 16 h. The cells were disrupted in column buffer (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 10 mM β-mercaptoethanol) by sonication. The lysate was centrifuged for 30 min at 18,000g, and the supernatant was filtered through a 0.45-µm filter (Millipore). The flow-through was incubated with Amylose Resin (New England Biolabs) for 2 h at 4°C, and the resin was washed with wash buffer (20 mM Tris-HCl, pH 7.4, 1 M NaCl, and 10 mM β-mercaptoethanol) three times. The fused protein was eluted with the wash buffer containing 10 mM maltose.
RNA Gel Mobility Shift Assays
The purified recombinant PGR3 protein fragments were dialyzed against buffer containing 100 mM NaCl, 40 mM Tris-HCl, pH 7.5, 4 mM DTT, and 10% glycerol. RNA binding reaction with designated concentrations of the recombinant proteins were performed under 25°C for 20 min, with the 25-μL scale reaction mixture containing 100 mM NaCl, 40 mM Tris-HCl, pH 7.5, 4 mM DTT, 10% glycerol, 0.1 mg/mL BSA, 0.5 mg/mL heparin, 10 units of RNase inhibitors (Life Technologies), and 1.0 nM petL RNA probe labeled with digoxigenin at both 5′ and 3′ ends. RNA sequence of the petL RNA probe was 5′- UUAGGGAAGUACUUUAAGAAACAUAUGUAUAA-3′, which harbors the entire RNA footprint observed in the petL 5′UTR (Ruwe and Schmitz-Linneweber, 2012) and thus is most likely the minimal PGR3 binding site. Gel electrophoresis under native conditions was performed as described previously (Cai et al., 2011), except that 10% acrylamide gels were used. RNA was electroblotted onto a nylon membrane in 4°C for 1 h. Chemical luminescence was detected using the equivalent method to that used for the RNA gel blot analysis described above.
Accession Number
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession number At4g31840 (PGR3).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Linear Plots of petL RNA Abundance (Function i), PetL Protein Abundance (Function ii), and NdhK Protein Abundance (Function iii) in the Transgenic Lines with T-to-I Point Mutations at Each PPR Motif.
Supplemental Figure 2. Correlation between the Isoelectric Points of Amino Acids and the Accumulation Levels of RNA or Proteins in Each T-to-I Mutation Transgenic Line.
Supplemental Figure 3. Calculation of Correlation between the Molecular Weights and Hydrophobicities of Amino Acids and the Functional Importance of Each PPR Motif.
Supplemental Figure 4. In Vitro RNA Gel-Shift Assay Using the Full-Length Recombinant PGR3 (MBP-FL) and the Truncated Forms (MBP-P16 and MBP-P14).
Supplemental Table 1. List of Primers Used in This Study.
Acknowledgments
We thank Asako Tahara for her excellent technical assistance. We thank Yuichiro Takahashi and Amane Makino for giving us antibodies. T.S. was supported by Grants 22114509 and 22247005 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation; GPN0008). S.F. was supported by the Grant-in-Aid for Japan Society for the Promotion of Science fellows.
AUTHOR CONTRIBUTIONS
S.F. designed research, performed research, analyzed data, and wrote the article. N.S. performed research. T.S. designed research, analyzed data, and wrote the article.
Glossary
- UTR
untranslated region
- NDH
NADH dehydrogenase-like
- PSII
photosystem II
References
- Barkan A., Rojas M., Fujii S., Yap A., Chong Y.S., Bond C.S., Small I. (2012). A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet. 8: e1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila S., Knight W., Hanson M. (2010). Natural variation in Arabidopsis leads to the identification of REME1, a pentatricopeptide repeat-DYW protein controlling the editing of mitochondrial transcripts. Plant Physiol. 154: 1966–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. (2009). Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326: 1509–1512 [DOI] [PubMed] [Google Scholar]
- Cai W., Okuda K., Peng L., Shikanai T. (2011). PROTON GRADIENT REGULATION 3 recognizes multiple targets with limited similarity and mediates translation and RNA stabilization in plastids. Plant J. 67: 318–327 [DOI] [PubMed] [Google Scholar]
- Chateigner-Boutin A.L., Ramos-Vega M., Guevara-García A., Andrés C., de la Luz Gutiérrez-Nava M., Cantero A., Delannoy E., Jiménez L.F., Lurin C., Small I., León P. (2008). CLB19, a pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J. 56: 590–602 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Delannoy E., Stanley W.A., Bond C.S., Small I.D. (2007). Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem. Soc. Trans. 35: 1643–1647 [DOI] [PubMed] [Google Scholar]
- Finn R.D., Clements J., Eddy S.R. (2011). HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 39 (Web Server issue): W29–W37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Bond C.S., Small I.D. (2011). Selection patterns on restorer-like genes reveal a conflict between nuclear and mitochondrial genomes throughout angiosperm evolution. Proc. Natl. Acad. Sci. USA 108: 1723–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Small I. (2011). The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 191: 37–47 [DOI] [PubMed] [Google Scholar]
- Gattiker A., Gasteiger E., Bairoch A. (2002). ScanProsite: A reference implementation of a PROSITE scanning tool. Appl. Bioinformatics 1: 107–108 [PubMed] [Google Scholar]
- Goldstrohm A.C., Hook B.A., Seay D.J., Wickens M. (2006). PUF proteins bind Pop2p to regulate messenger RNAs. Nat. Struct. Mol. Biol. 13: 533–539 [DOI] [PubMed] [Google Scholar]
- Hammani K., Okuda K., Tanz S.K., Chateigner-Boutin A.L., Shikanai T., Small I. (2009). A study of new Arabidopsis chloroplast RNA editing mutants reveals general features of editing factors and their target sites. Plant Cell 21: 3686–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Kawabata M., Hisano K., Kazama T., Matsuoka K., Sugita M., Nakamura T. (2012). Identification and characterization of the RNA binding surface of the pentatricopeptide repeat protein. Nucleic Acids Res. 40: 2712–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157: 105–132 [DOI] [PubMed] [Google Scholar]
- Lurin C., et al. (2004). Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou M.J., Bogdanove A.J. (2009). A simple cipher governs DNA recognition by TAL effectors. Science 326: 1501. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., et al. (2007). Improved Gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71: 2095–2100 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Yagi Y., Kobayashi K. (2012). Mechanistic insight into pentatricopeptide repeat proteins as sequence-specific RNA-binding proteins for organellar RNAs in plants. Plant Cell Physiol. 53: 1171–1179 [DOI] [PubMed] [Google Scholar]
- Okegawa Y., Kagawa Y., Kobayashi Y., Shikanai T. (2008). Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol. 49: 825–834 [DOI] [PubMed] [Google Scholar]
- Okuda K., Hammani K., Tanz S.K., Peng L., Fukao Y., Myouga F., Motohashi R., Shinozaki K., Small I., Shikanai T. (2010). The pentatricopeptide repeat protein OTP82 is required for RNA editing of plastid ndhB and ndhG transcripts. Plant J. 61: 339–349 [DOI] [PubMed] [Google Scholar]
- Okuda K., Shikanai T. (2012). A pentatricopeptide repeat protein acts as a site-specificity factor at multiple RNA editing sites with unrelated cis-acting elements in plastids. Nucleic Acids Res. 40: 5052–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole N., Hattori M., Andres C., Iida K., Lurin C., Schmitz-Linneweber C., Sugita M., Small I. (2008). On the expansion of the pentatricopeptide repeat gene family in plants. Mol. Biol. Evol. 25: 1120–1128 [DOI] [PubMed] [Google Scholar]
- Peng L., Fukao Y., Fujiwara M., Takami T., Shikanai T. (2009). Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell 21: 3623–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J., Bayraktar O.A., Prikryl J., Barkan A. (2009). Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J. 28: 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikryl J., Rojas M., Schuster G., Barkan A. (2011). Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein. Proc. Natl. Acad. Sci. USA 108: 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenault T., Lithgow T., Traven A. (2011). PUF proteins: Repression, activation and mRNA localization. Trends Cell Biol. 21: 104–112 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2013). R: A Language and Environment for Statistical Computing. (Vienna, Austria: R Foundation for Statistical Computing).
- Ringel R., Sologub M., Morozov Y.I., Litonin D., Cramer P., Temiakov D. (2011). Structure of human mitochondrial RNA polymerase. Nature 478: 269–273 [DOI] [PubMed] [Google Scholar]
- Ruwe H., Schmitz-Linneweber C. (2012). Short non-coding RNA fragments accumulating in chloroplasts: Footprints of RNA binding proteins? Nucleic Acids Res. 40: 3106–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber C., Small I. (2008). Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 13: 663–670 [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C., Williams-Carrier R., Barkan A. (2005). RNA immunoprecipitation and microarray analysis show a chloroplast Pentatricopeptide repeat protein to be associated with the 5′ region of mRNAs whose translation it activates. Plant Cell 17: 2791–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T. (2006). RNA editing in plant organelles: Machinery, physiological function and evolution. Cell. Mol. Life Sci. 63: 698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T., Munekage Y., Shimizu K., Endo T., Hashimoto T. (1999). Identification and characterization of Arabidopsis mutants with reduced quenching of chlorophyll fluorescence. Plant Cell Physiol. 40: 1134–1142 [DOI] [PubMed] [Google Scholar]
- Small I.D., Peeters N. (2000). The PPR motif - A TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 25: 46–47 [DOI] [PubMed] [Google Scholar]
- Tavares-Carreón F., Camacho-Villasana Y., Zamudio-Ochoa A., Shingú-Vázquez M., Torres-Larios A., Pérez-Martínez X. (2008). The pentatricopeptide repeats present in Pet309 are necessary for translation but not for stability of the mitochondrial COX1 mRNA in yeast. J. Biol. Chem. 283: 1472–1479 [DOI] [PubMed] [Google Scholar]
- Wang X., McLachlan J., Zamore P.D., Hall T.M. (2002). Modular recognition of RNA by a human pumilio-homology domain. Cell 110: 501–512 [DOI] [PubMed] [Google Scholar]
- Wickens M., Bernstein D.S., Kimble J., Parker R. (2002). A PUF family portrait: 3’UTR regulation as a way of life. Trends Genet. 18: 150–157 [DOI] [PubMed] [Google Scholar]
- Yagi Y., Hayashi S., Kobayashi K., Hirayama T., Nakamura T. (2013). Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PLoS ONE 8: e57286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Tasaka M., Shikanai T. (2004). PPR motifs of the nucleus-encoded factor, PGR3, function in the selective and distinct steps of chloroplast gene expression in Arabidopsis. Plant J. 38: 152–163 [DOI] [PubMed] [Google Scholar]
- Zehrmann A., Verbitskiy D., van der Merwe J.A., Brennicke A., Takenaka M. (2009). A DYW domain-containing pentatricopeptide repeat protein is required for RNA editing at multiple sites in mitochondria of Arabidopsis thaliana. Plant Cell 21: 558–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelyazkova P., Hammani K., Rojas M., Voelker R., Vargas-Suárez M., Börner T., Barkan A. (2012). Protein-mediated protection as the predominant mechanism for defining processed mRNA termini in land plant chloroplasts. Nucleic Acids Res. 40: 3092–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]