Abstract
Lymphedema following treatment for breast cancer can be an irreversible condition with a profound negative impact on quality of life. The lack of consensus regarding standard definitions of clinically significant lymphedema and optimal methods of measurement and quantification are unresolved problems. Inconsistencies persist regarding the appropriate timing of intervention and what forms of treatment should be the standard of care. There are reports that early detection and intervention can prevent progression, however the Level 1 evidence to support this hypothesis has yet to be generated. To assess these controversies, we propose the implementation of a screening program to detect early lymphedema in conjunction with a randomized, prospective trial designed to generate Level 1 evidence regarding the efficacy of early intervention and appropriate treatment strategies. Collaboration among institutions that manage breast cancer patients is essential to establish a standardized approach to lymphedema and to establish guidelines for best practice.
Keywords: lymphedema, screening, perometer, breast cancer
1. INTRODUCTION
Lymphedema is a chronic swelling caused by accumulation of fluid in the interstitial tissues due to the inability of the lymphatic system to adequately transport lymph fluid [1, 2]. In the era when axillary lymph node dissection (ALND) was routine practice, the incidence of lymphedema in patients treated for breast cancer was reported as high [3]. Due to advancements in care and surgical techniques such as the sentinel lymph node biopsy (SLNB), incidence rates have decreased substantially, with a 5–8% incidence in patients who undergo SLNB and a 14–16% incidence in patients who undergo ALND, including only levels I and II [4, 5]. Reports on cohorts with longer follow up report incidence rates as high as 34%–94% [2, 6] depending on the methods of lymphedema measurement and quantification.
The discrepancies in definitions, measurement techniques, and quantification methods of lymphedema have been cited as limitations in multiple previously published reports [7–9]. The various methods of measurement, quantification, and definition that have previously been used and reported in the literature are not interchangeable, preventing the comparison of data across studies [10]. This lack of standardization has created a major hindrance in the interpretation of data that ultimately needs to be applicable to the entire breast cancer population.
The detrimental effects of lymphedema on a breast cancer survivor’s physical and psychosocial health can be overwhelming, particularly because lymphedema is a risk that lasts for the survivor’s life [7, 9, 11]. Physical morbidities caused by lymphedema include recurrent infections, skin changes, and symptoms of heaviness and numbness [12]. While physical distortion is characteristic of advanced lymphedema, even low-level swelling can cause a patient to be symptomatic and result in physical impairment [8] (Figure 1). Quality of life (QOL) may also be compromised due to emotional distress, anxiety and disturbance of body image accompanying the development of lymphedema [11]. The fear of developing lymphedema has been reported to influence survivors’ function and activity, as those at-risk may alter their lifestyle and impose restrictions on their activity in hope of preventing the condition [13]. With survivorship from breast cancer increasing, attention to the QOL challenges that survivors face, such as lymphedema, is also increasing. Furthermore, patients with lymphedema have significantly more medical costs than those who do not have this condition. In a review of medical claims for 1877 breast cancer patients, Shih et al. reported that the costs for those with lymphedema were $14,877– $23,167 higher than were the claims for those patients who did not have lymphedema [14].
Figure 1.
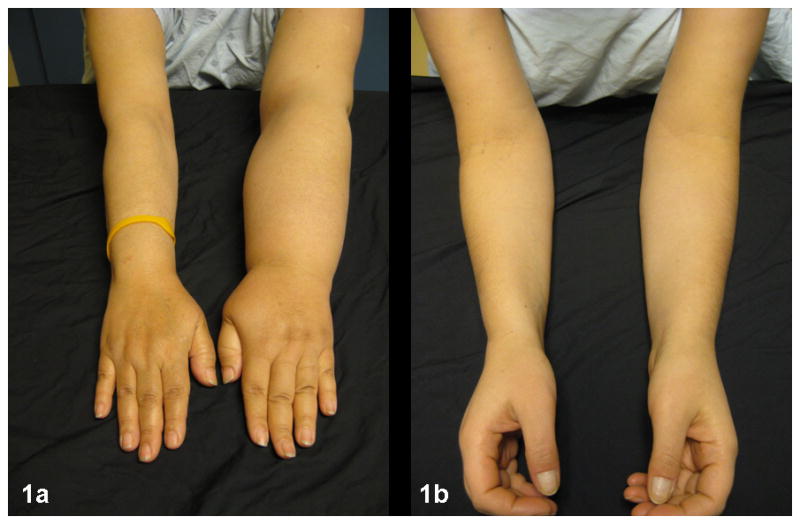
(a) Advanced lymphedema. By perometry, this woman’s left arm is 44% larger than her right and is physically distorted, which makes fitting into clothing with sleeves a challenge. The weight of the arm results in difficulty with functional reaching activities. (b) Low-level edema. This women’s left arm is 9% larger than her right arm. Despite her low-level swelling, she reports symptoms of discomfort from ““fullness and tightness.” She verbalizes a great fear that the swelling will increase.
A surveillance approach to this condition is being strongly advocated for in the literature [15–17]. However, the hypothesis that early detection and early intervention can minimize lymphedema progression presently lacks the Level 1 evidence to support this approach. To date, the lack of a uniform approach to assess lymphedema has limited the level of accurate data necessary to test the premise that a surveillance approach should become the standard of care. This, in turn, has hindered the generation of Level 1 evidence that is needed to determine appropriate intervention for this condition. Standardization is imperative for surveillance programs to be designed in such a manner that data can be shared and generalized.
In this work we sought to emphasize the need for all providers involved in the care of breast cancer patients to be aware of the risk of lymphedema and the negative impact it has on their patients’ QOL. We aimed to explore current controversies in the field of lymphedema following treatment for breast cancer, emphasize the need to generate Level 1 evidence regarding intervention, suggest the implementation of screening programs and encourage collaboration in the effort to develop a standardized approach to this dreaded side effect of breast cancer treatment.
2. METHODS
For the purposes of this critical review, an extensive literature research was conducted. Multiple websites and patient educational brochures were also utilized to assess the recommendations presently available to patients regarding lymphedema prevention, management, and treatment strategies.
3. FACTORS CONTRIBUTING to LYMPHEDEMA FOLLOWING TREATMENT for BREAST CANCER
ALND unequivocally increases the risk of developing lymphedema. Many reports cite that this vulnerability increases with the number of nodes excised, as well as with the number of positive nodes in the dissection [4, 5, 18–21]. Radiation is another treatment-related risk factor for lymphedema, with lymphedema risk rising when more nodal fields are radiated [19, 20, 22, 23, 24]. Post-operative infection is often not included in analyses on risk factors for lymphedema, but when considered, it has been reported as contributing to the risk of developing lymphedema [20, 25]. There has also been reference to lymphedema developing during treatment secondary to chemotherapy regimens [21, 24, 26]. The rationale for this association remains unclear and is a phenomenon that requires further study.
Many lifestyle risk factors appear on pamphlets and websites, cautioning patients with and without lymphedema to avoid repetitive activity, lifting weighted objects, having injections or blood drawn in the arm on the side of surgery and to use a compression sleeve for any air travel. However, to date, there is no robust data to support any of these activities as risk factors for developing lymphedema [27–29]. Although well intentioned, these warnings could potentially result in patients unnecessarily altering their lifestyle and decreasing their overall QOL [30]. In fact, there is an increasing body of data indicating that a gradually progressive exercise program does not increase the risk of lymphedema either for survivors with established lymphedema or for those considered to be at-risk [31–33]. Recent work by Schmidt et al. demonstrated that a slowly advanced weight lifting program did not increase the risk of lymphedema in breast cancer survivors. For those women with lymphedema, exercise was shown to decrease symptoms of lymphedema [34, 35]. This information is important, especially given the many and various health-related and QOL reasons women should exercise. Investigating the lifestyle risk factors that contribute to lymphedema is a research area ripe for study.
A high body mass index (BMI) at diagnosis is consistently cited as a risk factor for lymphedema development [21, 24, 36, 37]. Although BMI at diagnosis cannot be modified, these patients may benefit from close monitoring for lymphedema during their years of follow up. An unresolved question worthy of study is whether losing weight after breast cancer treatment decreases lymphedema risk, as post-treatment weight loss would be something survivors could pursue as a risk reduction strategy.
4. IMPACT of LYMPHEDEMA on QUALITY OF LIFE
Data abounds on the negative impact lymphedema following treatment for breast cancer has on a person’s QOL [11, 38–41]. Lymphedema is frequently referred to as a “dreaded” and “feared” side effect of treatment [9, 42]. Body image can be profoundly affected as the limb becomes distorted, the sleeves in clothing no longer fit properly or a swollen finger prevents a wedding ring from being worn. Symptoms of heaviness in the upper quarter and difficulty using the limb for functional activities are routinely reported. Cormier et al. reported on the association of symptoms and lymphedema in a cohort of 269 breast cancer patients, demonstrating that even a 5% volume difference significantly increased symptoms and compromised QOL [8].
The fear of developing lymphedema has also been reported [13]. This fear can influence how patients who have been treated for breast cancer approach activity during their survivorship years and can be a constant reminder of their disease. Although most lymphedema occurs in the first 3–4 years, long-term follow up reports show that edema development can present many years after that time [43]. In a 6-year follow up, Hayes et al. reported some new cases at that time, albeit much less than in the first 2 years [2]. Data from Armer et al.’s 60-month follow up demonstrated that cases of lymphedema continued to develop at 5 years [6]. The risk of developing lymphedema lasts for a lifetime [6], so this sense of vulnerability can be difficult for many patients to overcome.
5. UNRESOLVED CONTROVERSIES in the LYMPHEDEMA FIELD
Table 1 summarizes the main data from each of the sections described below (5.1 – 5.5).
Table 1.
Summary of the various methods used to define, measure, quantify, and manage lymphedema following treatment for breast cancer, as well as the appropriate timing of assessment.
| Topic | Details | References | |
|---|---|---|---|
| Definition | Circumferential Difference | 1.0 – 3.0 cm | Tsai 2009 [46], Wernicke 2011 [48] |
| Volume Difference | 200 ml | Boccardo 2009 [16], Tsai 2009 [46] | |
| Percent Difference | 3 – 20% | Armer 2009 [7], Tsai 2009 [46], Stout Gergich 2008 [49] | |
| Self-Report | Armer 2010 [6], Norman 2009 [62] | ||
| Measurement | Tape Measurement | Arm circumference | Deltombe 2007 [10], Jain 2010 [53] |
| Water Displacement | Volume | Deltombe 2007 [10], Tewari 2008 [54], Smoot 2011 [55], Fu 2009 [56], Ridner 2007 [57] | |
| Bioimpedance Spectroscopy | Impedance value | Smoot 2011 [55], Ward 2011 [58], Ridner 2009 [59] | |
| Perometry | Volume | Jain 2010 [53], Stanton 1997 [60], Petlund 1991 [61] | |
| Self-Report | McLaughlin 2008 [4], Norman 2009 [62], Czerniec 2010 [63] | ||
| Quantification | Absolute Volume Difference | ml or cm difference | McLaughlin 2008 [4], Ashikaga 2010 [5], Tsai 2009 [46], Wernicke 2011 [48] |
| Relative Volume Difference | Percent difference between arms | Ancukiewicz 2012 [64] | |
| Relative Volume Change (RVC) | Percent change between arms as compared to baseline | Ancukiewicz 2012 [64], Ancukiewicz 2011 [65] | |
| Timing | Pre-operative Arm Volume Measurements | Accounts for any normal asymmetry between arms | Armer 2010 [6], Stout Gergich 2008 [49], Ancukiewicz 2011 [65], Harris 2001 [66], NLN Position Paper 2011 [67] |
| Continuous Post-operative Screening | Prospective surveillance may allow for early detection and treatment, but has not been fully evaluated for its application, cost and outcome | NLN Position Paper 2012 [79], Cheville 2012 [80], Stout 2011 [81] | |
| Management | Complex Decongestive Therapy (CDT) | Recommended and considered effective; lacks Level 1 evidence to support its use; further study needed to determine efficacy as stand-alone treatment | Vignes 2011 [70], Forner-Cordero 2009 [71], Lasinski 2012 [72] |
| Manual Lymphatic Drainage | No signficant impact on edema development when compared to exercise and precautionary advice | Devoogdt 2011 [73] | |
| Compression Sleeve without CDT | Assessed in a small cohort study without randomization; further study needed | Stout Gergich 2008 [49], Partsch 2010 [74] | |
| Pneumatic Compression | No robust evidence about long-term outcomes | Fife 2012 [75], Wilburn 2006 [76] | |
| Low-Level Laser Treatment | Lacks data to support its use | Omar 2012 [77] |
5.1. Definition of Lymphedema Following Treatment For Breast Cancer
A significant and unresolved dilemma in the lymphedema field is the level of swelling considered to be clinically significant for lymphedema [6, 8, 44]. Reports by Cheville et al. and Tsai et al. illustrate this problem [45, 46]. The current lack of consensus regarding lymphedema definition contributes to the difficulty of obtaining uniformity in reports of incidence, risk factors, and intervention strategies [47].
Circumferential differences considered indicative of lymphedema vary from 1.0 cm to 3.0 cm [46, 48]. If changes in arm volume are utilized instead, a 200 ml difference is considered indicative of lymphedema [16, 46]. Edemas deemed to be significant by percent change have ranged from 3–20% [7, 46, 49]. The work of Armer et al. demonstrated how edema incidence varies widely within the same cohort when comparing a 2.0 cm circumferential difference, a 200 ml difference, a 10% volume difference and self-report [6].
In addition to defining the impact of lymphedema as an objective change in arm size, the work of many authors illustrates the role of assessing functional compromise and pain when evaluating a patient for lymphedema. Reports by Dawes et al., Tsauo et al. and Hormes et al. clearly illustrate that only addressing arm volume, without managing other symptoms, may not improve QOL [50–52]. Thus, there is a need for universal recognition that upper quarter symptoms, physical function, fear avoidance behavior, and QOL may need to be included in the definition of clinically significant lymphedema, helping to facilitate comparison of outcome data. Hence, it is our recommendation that adopting one of the well-validated questionnaires capable of assessing upper quarter function and pain should be considered in developing a uniform approach to evaluating lymphedema following treatment for breast cancer.
5.2. Measurement Methods
The different methods utilized to measure lymphedema are continually cited as a significant limitation when interpreting data from multiple studies [7–9, 44]. These measurement methods include circumferential tape measurement, water displacement, bioimpedance spectroscopy (BIS), perometry and self-report.
Utilizing a flexible tape to measure a circumferential difference between the affected and unaffected arm is convenient and cost-effective, but is subject to error if the technique of the examiners is not consistent [10]. Circumferential measurement has been referred to as “marginally accurate” [53]. Measurement by water displacement is based on Archimedes’ principle and has been cited as a reliable method [54]. Although it has been referred to as “the gold standard,” reports by Deltombe et al. and Smoot et al. challenge this assumption [10, 55]. It has also been reported as messy and inappropriate for patients with open wounds on their arms [54]. It is also time-consuming because of the need to clean the equipment between patients amid a busy clinical setting [55–57]. BIS utilizes resistance to electrical current to assess interstitial fluid differences between a patient’s arms, with the difference expressed as an impedance value of standard deviations from normative data (Figure 2). BIS is utilized in some settings, reported in several works and is considered a reliable and sensitive measurement method for detecting early changes in interstitial fluid [55, 58, 59]. However, this method may not be optimal for measuring late-stage lymphedema when much of the fluid has become fibrotic tissue. Additionally, BIS involves the continued expense of purchasing the costly electrodes that are required for each use. The Perometer is another instrument that ensures reliable and reproducible data for quantifying lymphedema as a volume change between arms (Figure 3). The utility of the Perometer has been well-documented and it is a device suited for a busy clinical setting that manages a large volume of patients each day [53, 60, 61]. It is, however, an expensive piece of equipment to purchase, requires a properly trained individual to conduct measurements, and necessitates a dedicated space in the clinic.
Figure 2.
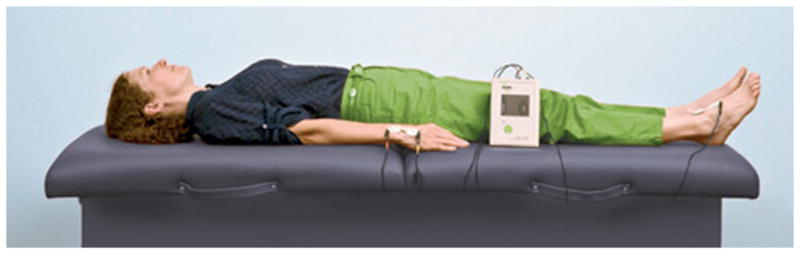
Lymphedema measurement by bioimpedance spectroscopy (BIS). BIS utilizes electrical current to assess interstitial fluid differences between arms and has been shown to be an effective method for detecting early changes in interstitial fluid. Source: http://german.l-dex.com/what-is-l-dex/.
Figure 3.
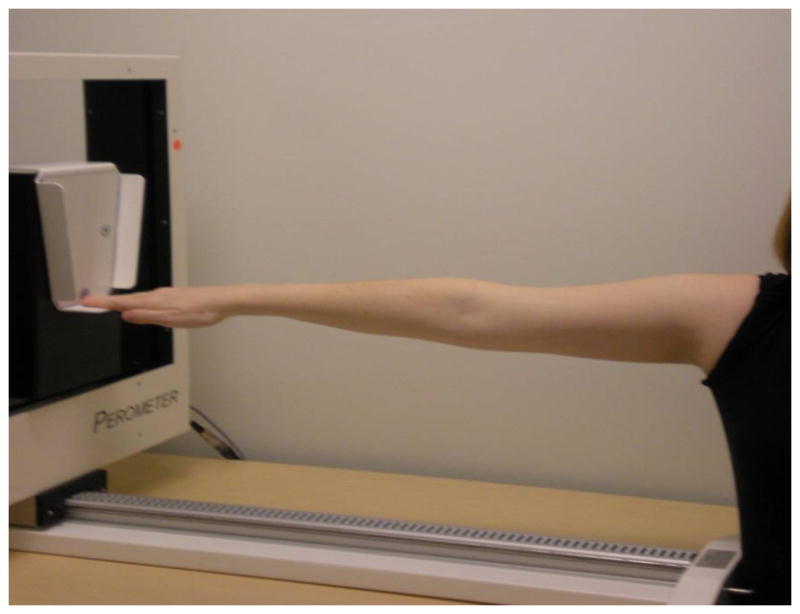
Lymphedema measurement by perometry. The Perometer is a device that measures patient arm volumes with accuracy and inter-rater reliability, allowing for the valid quantification of lymphedema.
Many studies have utilized a patient’s self-report of lymphedema as the method to assess incidence. Norman et al. published their results with confidence in a validated self-reporting tool [62]. Conversely, McLaughlin et al. found a patient’s report of lymphedema “significantly discordant” when compared to objective measurement of lymphedema [4]. The work of Czerniec et al. supports this discrepancy, finding that self-report was only “moderately” reliable when compared to objective measurement [63]. It is clear that, without consensus on a standard approach to measure lymphedema, progress in this field will continue to be thwarted.
5.3. Quantification Methods
Lymphedema is very frequently quantified as an absolute volume change, typically a 200 ml difference between arms demonstrated by water displacement or a 1.0 – 3.0 cm difference assessed by tape measurement [4, 5, 46, 48]. Absolute volume measurement lacks specificity and is inherently flawed because the magnitude of absolute volume change depends upon the body size of each patient [64]. A relative volume difference between the affected and the unaffected arm expressed as a percent change is a more reliable method as it does not vary with body size and shape [64]. We advocate that volume should only be expressed as a relative volume difference between arms, and that the use of an absolute difference, expressed in centimeters or milliliters should be abandoned.
An ideal quantification method for lymphedema in patients who undergo unilateral breast surgery would utilize percent change between arms with incorporation of pre-operative measurements. Ancukiewicz et al. developed a mathematical model to quantify volume changes in the affected arm as compared to the unaffected arm, known as the relative volume change (RVC) formula [65]. This formula compares the ipsilateral arm to the contralateral arm at follow-up, taking into account a patient’s pre-operative measurements. Specifically, Equation 1: , where A represents the ipsilateral arm, and U represents the contralateral arm which serves as a control for arm volume changes that may be unrelated to lymphedema, including fluctuations in patient weight. The numeral 1 indicates the pre-operative measurement and numeral 2 refers to the follow-up measurement. The use of RVC to quantify lymphedema has been shown to have the reliability and specificity required for accurate reporting [64, 65].
Lymphedema assessment remains particularly challenging for patients with bilateral breast cancer as they may be bilaterally at-risk for lymphedema and lack a control arm to use for comparison. An equation based on weight adjustment has been developed to calculate unilateral arm volume changes for detection and monitoring of lymphedema in patients who undergo bilateral breast surgery. Weight-adjusted arm volume change (WAC) can be calculated according to Equation 2: where A1 is pre-operative arm volume on the affected side and A2 is post-operative arm volume on the affected side, and W1, W2 are the patient’s weights at these time points (unpublished data). This formula is applicable for quantifying lymphedema in patients who undergo bilateral breast surgery since it does not rely on the contralateral arm volume as a control. The research community still needs to come to agreement on this topic. Until consensus is reached regarding methods of measurement and quantification, the problem of data incongruence will continue.
5.4. Appropriate Timing of Measurement
In addition to the varied methods of defining, measuring, and quantifying lymphedema, there is a lack of consistency with regard to the timing and sequencing of screening. The necessity of obtaining pre-operative arm volume measurements has been well demonstrated, as it allows for the normal asymmetry which may exist between arms to be considered when assessing post-operative changes [6, 49, 66]. The analysis of the pre-operative baseline perometer measurements of 677 patients at our institution demonstrated that 11.2% of patients present with a >5% pre-operative difference in arm volume between arms, and 1.5% of patients present with a >10% pre-operative difference in arm volume between arms (Figure 4) [65]. The National Lymphedema Network has recommended that pre-operative assessment be done routinely [67]; however, reports on lymphedema incidence that do not have baseline data continue to be published [68, 69].
Figure 4.
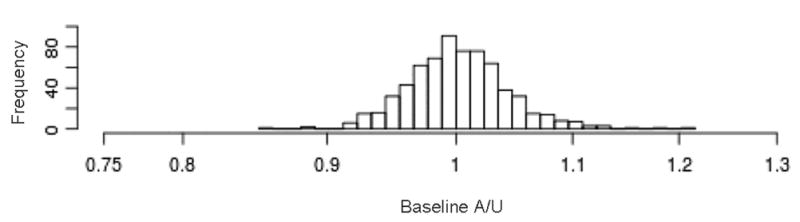
Histogram assessing pre-operative volume differences between arms among a cohort of 677 patients at our institution. 11.2% of patients presented with a >5% pre-operative difference between their arms [59].
5.5. Optimal Management Strategies
Complex Decongestive Therapy (CDT) has been cited as the recommended treatment for lymphedema following treatment for breast cancer [70, 71]. It consists of skin care, manual lymphatic drainage, compression strategies and exercise. Although there have been small trials aimed at assessing this type of intervention, no Level 1 evidence exists to support it. A systematic review by Lasinski et al. demonstrated that although CDT was considered to be effective in reducing swelling, further study was needed to determine if the components of CDT could be effective as stand-alone treatments [72].
Devoogdt et al. pre-operatively enrolled 160 breast cancer patients and randomized them into two groups for post-operative management. They reported that manual drainage did not have a significant impact on edema development when compared to exercise and precautionary advice [73]. Stout Gergich et al. assessed 196 patients pre-operatively and followed them post-operatively using the Perometer to evaluate for lymphedema. The 46 patients whose arm volume increased to 3% postoperatively were reported to be successfully managed with use of a compression sleeve and no CDT. Importantly, this trial utilized a small cohort of patients and did not involve randomization [49]. The recommendations of an expert panel led by Partsch et al. in 2010 were that more clinical trials are required to adequately assess the use of compression to treat lymphedema [74].
Pneumatic compression devices have been another treatment modality utilized and reported over the years. However, the evidence regarding the utility of this device with respect to long-term outcomes is not robust [75, 76]. Low-level laser treatment has also been suggested, but lacks the data to support its use [77]. In 2009, Devoogdt et al. reviewed the treatment options for lymphedema and concluded that high quality, well-powered randomized trials that included symptoms, QOL and large cohorts are needed to inform clinical decision making when developing a plan of care for these patients [78].
6. A PROSPECTIVE SURVEILLANCE APPROACH
The prospective screening of medical conditions to allow for early intervention has become the standard of care for many diseases, such as breast and colorectal cancer. Such surveillance has been increasingly advocated for breast cancer patients who are at-risk for lymphedema. The 2012 National Lymphedema Network Position Paper, “Screening and Early Detection of Breast Cancer-Related Lymphedema: The Imperative,” [79] addresses the rationale for lymphedema screening as a method to detect and subsequently treat lymphedema at an early, even subclinical, stage so as to “reverse the progression to chronic, irreversible lymphedema.” If early identification of increased fluid in the arm (by BIS, volume or circumference) allows a patient to be identified and managed in a manner that minimizes lymphedema progression, then the screening for lymphedema would be advantageous. As outlined in a recent report by Cheville et al. this model has yet to be fully evaluated for its application, cost and outcome [80]. Should it be for all survivors or targeted only for those patients who are at high risk for developing lymphedema? In 2011, Stout et al. reported on the direct costs involved with a surveillance approach, compared to the traditional approach of treating lymphedema after it has developed [81]. Using a 2009 Medicare physicians’ fee schedule, the projected cost of managing early stage lymphedema, per patient per year was $636.19, compared to $3,124.92 per patient per year in a more traditional manner. Thus, a surveillance approach to lymphedema through prospective screening would not only have a positive impact on the patient, but also reduce financial burden on the health care system.
It has been our experience that a lymphedema screening program can be successfully implemented even in a busy clinical environment. Such a screening program, in which patients are continuously monitored for arm volume changes via perometer measurements pre-operatively and every few months post-operatively, was implemented at our institution in 2005. Since the screening program’s initiation in 2005, we have screened 3110 patients at their pre-operative baseline. Data from the first few years allowed us to assess the natural history of lymphedema, evaluate rates of progression, and determine the relative volume change to test for appropriate intervention. With this necessary information, we were able to design and implement a prospective clinical lymphedema screening trial in tandem with a randomized-controlled Phase III intervention trial (ClinicalTrials.gov Identifiers: NCT01521741 and NCT00959985, respectively).
The primary objective of the lymphedema screening trial is to identify patients with early lymphedema who can subsequently be enrolled in the randomized intervention trial. The intervention trial is designed to generate Level 1 evidence regarding early intervention and optimal treatment strategies. Subjects on the clinical lymphedema screening trial are assessed for lymphedema objectively via perometry to assess limb volume changes and subjectively via a questionnaire to evaluate symptoms, arm function and QOL. The questionnaire is a compilation of questions from the following previously validated assessment tools: the Lymphedema Breast Cancer Questionnaire (LBCQ) [12]; the Disability of the Arm, Hand and Shoulder (DASH) [82]; the Survey of Arm Care Following Breast Cancer [83]; and the Functional Assessment of Cancer Therapy for Breast Cancer (FACT-B) [84]. Assessments occur pre-operatively, at the completion of chemotherapy and/or radiation therapy, and every 3–8 months thereafter. This framework will allow us to analyze data on symptoms, function, fear of edema and QOL in a large sample size.
When a patient on the clinical lymphedema screening trial exhibits a RVC≥5% at two consecutive visits that are one to two months apart, they are recruited into a Phase III randomized-controlled intervention trial designed to produce Level 1 evidence by testing the hypothesis that early treatment of low-level increases in arm volume after treatment for breast cancer will reduce the likelihood of lymphedema progression. One of the primary objectives of the trial is to evaluate the efficacy of early intervention using compression garments for low-level arm volume changes (RVC 5–<10%) and using compression garments with or without night compression bandaging for moderate-level arm volume changes (RVC 10–<20%). Another primary objective is to assess symptom clusters, treatment adherence, upper extremity function, fear avoidance behavior, and QOL as they are associated with varying degrees of lymphedema.
Specifically, participants in Group 1 are randomized to either exercise or exercise with compression. Participants in Group 2 are randomized to either exercise with compression or exercise with compression plus night bandaging. The study endpoints for Groups 1 and 2 are time to progression of RVC ≥10% and failure to regression of RVC<10% at weeks 8 and 12. Figure 5 displays the intervention trial schema.
Figure 5.
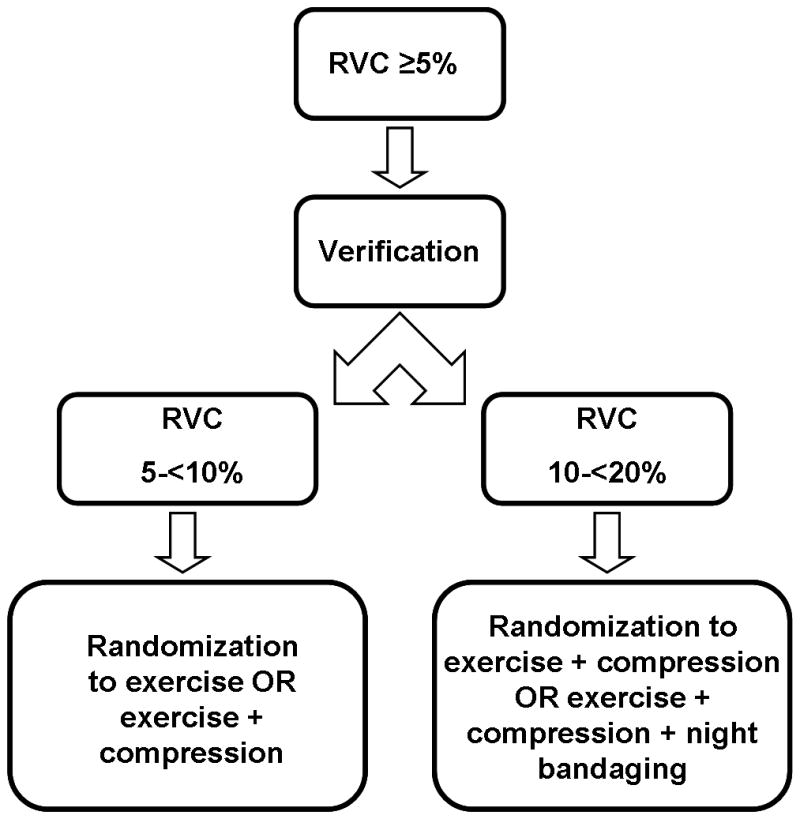
Schema for a prospective, randomized Phase III lymphedema intervention trial (ClinicalTrials.gov Identifier: NCT00959985). This trial is being conducted at our institution and is testing the hypothesis that early treatment of low-level increases in arm volume after treatment for breast cancer reduces the likelihood of lymphedema progression.
The sample size for Groups 1 and 2 of the intervention trial was calculated to provide 85% power to detect treatment response differences using 2-sided Fisher exact test, with an overall type I error of 0.05. The sample size for the 5–<10% group is 208 subjects. The sample size for the 10–<20%” group is 128 subjects. Thus, the total sample size for the intervention trial is 336 subjects. Based upon screening data collected thus far, 7446 patients must be screening in order to achieve a total sample size of N=336 on the intervention trial. To date, since its initiation in 2009, the screening trial has accrued 1028 subjects, with a 72% accrual rate for 2012 (293 enrolled of 408 eligible). The 2012 accrual rate most accurately represents current accrual, since inclusion criteria have been modified since trial initiation. As the screening trial serves as a conduit for the intervention trial, the sample size for the screening trial was calculated based upon the sample size for the intervention trial. We have not yet reached target accrual for the intervention trial, but have recently expanded to multiple other institutions and continue to actively accrue eligible participants.
Our two-part screening and intervention trial is an example of how a surveillance approach can serve as a conduit for a Phase III trial to assess the efficacy of early intervention and identify optimal treatment interventions. To our knowledge, there are no other prospective randomized-controlled trials in a cohort of this size assessing early intervention. More screening programs and clinical trials are needed to respond to the call of researchers and organizations such as the National Lymphedema Network to generate rigorous research that will yield Level 1 evidence for “Best Practices in surveillance and early intervention for post-breast cancer lymphedema” [79].
7. CONCLUSION
Lymphedema following treatment for breast cancer is a major concern for most patients who experience it, and for many patients who fear its development. The challenges that patients with lymphedema face on a daily basis are further exacerbated by the lack of a standard approach to measure, quantify and define their condition. Inconsistencies also persist regarding the appropriate timing and type of treatment intervention that should be the standard of care. Not only do such discrepancies significantly inhibit the ability to compare data across studies and to develop a universal understanding of lymphedema incidence, but they also limit our ability to effectively and optimally treat patients who suffer from lymphedema. We endorse a unified effort among providers caring for breast cancer patients to work together to arrive at a consensus regarding these dilemmas.
Early detection and intervention have been increasingly advocated to prevent lymphedema progression; however, Level 1 evidence to support this hypothesis has yet to be generated. We support the design and implementation of prospective surveillance programs in conjunction with prospective, randomized Phase III trials capable of generating Level 1 evidence regarding the efficacy of early intervention and appropriate treatment strategies. To accrue a sample size of significance and to include patients from diverse populations, national and/or international collaboration is pivotal. Such collaboration will allow healthcare professionals to provide breast cancer patients who suffer from or who are at-risk for lymphedema with clinical decisions grounded in robust evidence that should ultimately guide clinical practice on a universal level.
Acknowledgments
The project described was supported by Award Number R01CA139118 (AGT), Award Number P50CA089393 (AGT) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
9. CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to disclose and all authors have read and approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg JI, Wiechmann LI, Riedel ER, Morrow M, Van Zee KJ. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol. 2010 Dec;17(12):3278–86. doi: 10.1245/s10434-010-1155-4. [DOI] [PubMed] [Google Scholar]
- 2.Hayes S, Sipio TD, Rye S, JALP, Saunders C, Pyke C, et al. Prevalence and prognostic significance of secondary lymphedema following breast cancer. Lymphat Res Biol. 2011;9(3):135–41. doi: 10.1089/lrb.2011.0007. [DOI] [PubMed] [Google Scholar]
- 3.Gerber L, Lampert M, Wood C, Duncan M, D’Angelo T, Schain W, et al. Comparison of pain, motion, and edema after modified radical mastectomy vs. local excision with axillary dissection and radiation. Breast Cancer Res Treat. 1992;21(2):139–45. doi: 10.1007/BF01836960. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008 Nov 10;26(32):5213–9. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010 Aug 1;102(2):111–8. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010 Sep;43(3):118–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Armer JM, Stewart BR, Shook RP. 30-Month Post-Breast Cancer Treatment Lymphoedema. J Lymphoedema. 2009 Apr 1;4(1):14–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009 Dec;42(4):161–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Bernas MJAR, Armer JM, Cormier JN. Lymphedema: How do we diagnose and reduce the risk of this dreaded complication of breast cancer treatment? Curr Breast Cancer Rep. 2010;2:53–8. [Google Scholar]
- 10.Deltombe T, Jamart J, Recloux S, Legrand C, Vandenbroeck N, Theys S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007 Mar;40(1):26–34. [PubMed] [Google Scholar]
- 11.Chachaj A, Malyszczak K, Pyszel K, Lukas J, Tarkowski R, Pudelko M, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2009 Mar;19(3):299–305. doi: 10.1002/pon.1573. [DOI] [PubMed] [Google Scholar]
- 12.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003 Nov-Dec;52(6):370–9. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lee TS, Kilbreath SL, Sullivan G, et al. Factors That Affect Intention to Avoid Strenuous Arm Activity After Breast Cancer Surgery. Oncol Nurs Forum. 2009;36(4):454– 62. doi: 10.1188/09.ONF.454-462. [DOI] [PubMed] [Google Scholar]
- 14.Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009 Apr 20;27(12):2007–14. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 15.Soran A, Finegold DN, Brufsky A. Lymphedema prevention and early intervention: a worthy goal. Oncology (Williston Park) 2012 Mar;26(3):249, 54, 56. [PubMed] [Google Scholar]
- 16.Boccardo FM, Ansaldi F, Bellini C, Accogli S, Taddei G, Murdaca G, et al. Prospective evaluation of a prevention protocol for lymphedema following surgery for breast cancer. Lymphology. 2009 Mar;42(1):1–9. [PubMed] [Google Scholar]
- 17.Stout NL, Binkley JM, Schmitz KH, Andrews K, Hayes SC, Campbell KL, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012 Apr 15;118(8 Suppl):2191–200. doi: 10.1002/cncr.27476. [DOI] [PubMed] [Google Scholar]
- 18.Bar Ad V, Cheville A, Solin LJ, Dutta P, Both S, Harris EE. Time course of mild arm lymphedema after breast conservation treatment for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2010 Jan 1;76(1):85–90. doi: 10.1016/j.ijrobp.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 19.van der Veen P, De Voogdt N, Lievens P, Duquet W, Lamote J, Sacre R. Lymphedema development following breast cancer surgery with full axillary resection. Lymphology. 2004 Dec;37(4):206–8. [PubMed] [Google Scholar]
- 20.Bevilacqua JL, Kattan MW, Changhong Y, Koifman S, Mattos IE, Koifman RJ, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012 Aug;19(8):2580–9. doi: 10.1245/s10434-012-2290-x. [DOI] [PubMed] [Google Scholar]
- 21.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007 Apr;16(4):775–82. doi: 10.1158/1055-9965.EPI-06-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes SB, Freedman GM, Li T, Anderson PR, Ross E. Does axillary boost increase lymphedema compared with supraclavicular radiation alone after breast conservation? Int J Radiat Oncol Biol Phys. 2008 Dec 1;72(5):1449–55. doi: 10.1016/j.ijrobp.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 23.Ozcinar B, Guler SA, Kocaman N, Ozkan M, Gulluoglu BM, Ozmen V. Breast cancer related lymphedema in patients with different loco-regional treatments. Breast. 2012 Jun;21(3):361–5. doi: 10.1016/j.breast.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Swenson KK, Nissen MJ, Leach JW, Post-White J. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009 Mar;36(2):185–93. doi: 10.1188/09.ONF.185-193. [DOI] [PubMed] [Google Scholar]
- 25.Soran A, Wu WC, Dirican A, Johnson R, Andacoglu O, Wilson J. Estimating the probability of lymphedema after breast cancer surgery. Am J Clin Oncol. 2010 Oct;34(5):506–10. doi: 10.1097/COC.0b013e3181f47955. [DOI] [PubMed] [Google Scholar]
- 26.Ohsumi S, Shimozuma K, Ohashi Y, Takeuchi A, Suemasu K, Kuranami M, et al. Subjective and objective assessment of edema during adjuvant chemotherapy for breast cancer using taxane-containing regimens in a randomized controlled trial: The National Surgical Adjuvant Study of Breast Cancer 02. Oncology. 2012;82(3):131–8. doi: 10.1159/000336480. [DOI] [PubMed] [Google Scholar]
- 27.Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg. 2011 Oct;213(4):543–51. doi: 10.1016/j.jamcollsurg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin SA. Lymphedema: separating fact from fiction. Oncology (Williston Park) 2012 Mar;26(3):242–9. [PubMed] [Google Scholar]
- 29.Nielsen I, Gordon S, Selby A. Breast cancer-related lymphoedema risk reduction advice: a challenge for health professionals. Cancer Treat Rev. 2008 Nov;34(7):621–8. doi: 10.1016/j.ctrv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Showalter SL, Brown JC, Cheville AL, Fisher CS, Sataloff D, Schmitz KH. Lifestyle Risk Factors Associated with Arm Swelling Among Women with Breast Cancer. Ann Surg Oncol. 2012 Oct 3; doi: 10.1245/s10434-012-2631-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol. 2009;48(8):1102–10. doi: 10.3109/02841860903061683. [DOI] [PubMed] [Google Scholar]
- 32.Hayes SC, Reul-Hirche H, Turner J. Exercise and secondary lymphedema: safety, potential benefits, and research issues. Med Sci Sports Exerc. 2009 Mar;41(3):483–9. doi: 10.1249/MSS.0b013e31818b98fb. [DOI] [PubMed] [Google Scholar]
- 33.Kwan ML, Cohn JC, Armer JM, Stewart BR, Cormier JN. Exercise in patients with lymphedema: a systematic review of the contemporary literature. J Cancer Surviv. 2011 Dec;5(4):320–36. doi: 10.1007/s11764-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009 Aug 13;361(7):664–73. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010 Dec 22;304(24):2699–705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 36.Mahamaneerat WK, Shyu CR, Stewart BR, Armer JM. Breast cancer treatment, BMI, postop swelling/lymphoedema. J Lymphoedema. 2008 Oct 1;3(2):38–44. [PMC free article] [PubMed] [Google Scholar]
- 37.Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011 Jun;19(6):853–7. doi: 10.1007/s00520-011-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green T. Understanding body image in patients with chronic oedema. Br J Community Nurs. 2008 Oct;13(10):S15–8. doi: 10.12968/bjcn.2008.13.Sup5.31191. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008 Dec 10;26(35):5689–96. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keeley V, Crooks S, Locke J, Veigas D, Riches K, Hilliam R. A quality of life measure for limb limphoedema (LYMQOL) Journal of Lymphoedema. 2010;5(1):26–37. [Google Scholar]
- 41.Ridner SH. The psycho-social impact of lymphedema. Lymphat Res Biol. 2009;7(2):109–12. doi: 10.1089/lrb.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008 Jul 20;26(21):3536–42. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 43.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001 Sep 15;92(6):1368–77. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Hayes S, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema secondary to breast cancer: how choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology. 2008 Mar;41(1):18–28. [PubMed] [Google Scholar]
- 45.Cheville AL, McGarvey CL, Petrek JA, Russo SA, Thiadens SR, Taylor ME. The grading of lymphedema in oncology clinical trials. Semin Radiat Oncol. 2003 Jul;13(3):214–25. doi: 10.1016/S1053-4296(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 46.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009 Jul;16(7):1959–72. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 47.Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat. 2005 Feb;89(3):221–6. doi: 10.1007/s10549-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 48.Wernicke AG, Shamis M, Sidhu KK, Turner BC, Goltser Y, Khan I, et al. Complication Rates in Patients With Negative Axillary Nodes 10 Years After Local Breast Radiotherapy After Either Sentinel Lymph Node Dissection or Axillary Clearance. Am J Clin Oncol. 2011 Nov 29; doi: 10.1097/COC.0b013e3182354bda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008 Jun 15;112(12):2809–19. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 50.Dawes DJ, Meterissian S, Goldberg M, Mayo NE. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med. 2008 Aug;40(8):651–8. doi: 10.2340/16501977-0232. [DOI] [PubMed] [Google Scholar]
- 51.Tsauo JY, Hung HC, Tsai HJ, Huang CS. Can ICF model for patients with breast-cancer-related lymphedema predict quality of life? Support Care Cancer. 2010 May;19(5):599–604. doi: 10.1007/s00520-010-0857-2. [DOI] [PubMed] [Google Scholar]
- 52.Hormes JM, Bryan C, Lytle LA, et al. Impact of Lymphedema and Arm Symptoms on Quality of Life in Breast Cancer Survivors. Lymphology. 2010;43:1– 13. [PubMed] [Google Scholar]
- 53.Jain MS, Danoff JV, Paul SM. Correlation between bioelectrical spectroscopy and perometry in assessment of upper extremity swelling. Lymphology. 2010 Jun;43(2):85–94. [PMC free article] [PubMed] [Google Scholar]
- 54.Tewari N, Gill PG, Bochner MA, Kollias J. Comparison of volume displacement versus circumferential arm measurements for lymphoedema: implications for the SNAC trial. ANZ J Surg. 2008 Oct;78(10):889–93. doi: 10.1111/j.1445-2197.2008.04686.x. [DOI] [PubMed] [Google Scholar]
- 55.Smoot BJ, Wong JF, Dodd MJ. Comparison of diagnostic accuracy of clinical measures of breast cancer-related lymphedema: area under the curve. Arch Phys Med Rehabil. 2011 Apr;92(4):603–10. doi: 10.1016/j.apmr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu MR, Ridner SH, Armer J. Post-breast cancer. Lymphedema: part 1. Am J Nurs. 2009 Jul;109(7):48–54. doi: 10.1097/01.NAJ.0000357172.94131.58. [DOI] [PubMed] [Google Scholar]
- 57.Ridner SH, Montgomery LD, Hepworth JT, Stewart BR, Armer JM. Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology. 2007 Mar;40(1):35–46. [PubMed] [Google Scholar]
- 58.Ward LC. Assessment of lymphedema by bioelectrical impedance spectroscopy. Jpn J Nurs Sci. 2011 Jun;8(1):108. doi: 10.1111/j.1742-7924.2010.00165.x. author reply 9. [DOI] [PubMed] [Google Scholar]
- 59.Ridner SH, Dietrich MS, Deng J, Bonner CM, Kidd N. Bioelectrical impedance for detecting upper limb lymphedema in nonlaboratory settings. Lymphat Res Biol. 2009;7(1):11–5. doi: 10.1089/lrb.2008.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer) Lymphology. 1997 Jun;30(2):77–97. [PubMed] [Google Scholar]
- 61.Petlund C. Volumetry of Limbs. Boston: Olszewski & Waldeman; 1991. Lymph Stasis: Pathophysiology, Diagnosis and Treatment; pp. 443–51. [Google Scholar]
- 62.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009 Jan 20;27(3):390–7. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czerniec SA, Ward LC, Refshauge KM, Beith J, Lee MJ, York S, et al. Assessment of breast cancer-related arm lymphedema--comparison of physical measurement methods and self-report. Cancer Invest. 2010 Jan;28(1):54–62. doi: 10.3109/07357900902918494. [DOI] [PubMed] [Google Scholar]
- 64.Ancukiewicz M, Miller CL, Skolny MN, O’Toole J, Warren LE, Jammallo LS, et al. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: the flaws in current studies and need for universal methodology. Breast Cancer Res Treat. 2012 Aug;135(1):145–52. doi: 10.1007/s10549-012-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ancukiewicz M, Russell TA, Otoole J, Specht M, Singer M, Kelada A, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011 Apr 1;79(5):1436–43. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris SR, Hugi MR, Olivotto IA, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: 11. Lymphedema. CMAJ. 2001 Jan 23;164(2):191–9. [PMC free article] [PubMed] [Google Scholar]
- 67.National Lymphedema Network. Position Statement of National Lymphedema Network: Screening and Measurement for Early Detection of Breast Cancer Related Lymphedema. Available at: http://www.lymphnet.org/pdfDocs/nlnBCLE.pdf.
- 68.Lee KT, Mun GH, Lim SY, Pyon JK, Oh KS, Bang SI. The impact of immediate breast reconstruction on post-mastectomy lymphedema in patients undergoing modified radical mastectomy. Breast. 2012 May 15; doi: 10.1016/j.breast.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Crosby MA, Card A, Liu J, Lindstrom WA, Chang DW. Immediate breast reconstruction and lymphedema incidence. Plast Reconstr Surg. 2012 May;129(5):789e–95e. doi: 10.1097/PRS.0b013e31824a2ab1. [DOI] [PubMed] [Google Scholar]
- 70.Vignes S, Porcher R, Arrault M, Dupuy A. Factors influencing breast cancer-related lymphedema volume after intensive decongestive physiotherapy. Support Care Cancer. 2011 Jul;19(7):935–40. doi: 10.1007/s00520-010-0906-x. [DOI] [PubMed] [Google Scholar]
- 71.Forner-Cordero I, Munoz-Langa J, Forner-Cordero A, DeMiguel-Jimeno JM. Predictive factors of response to decongestive therapy in patients with breast-cancer-related lymphedema. Ann Surg Oncol. 2009 Mar;17(3):744–51. doi: 10.1245/s10434-009-0778-9. [DOI] [PubMed] [Google Scholar]
- 72.Lasinski BB, McKillip Thrift K, Squire D, Austin MK, Smith KM, Wanchai A, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R. 2012 Aug;4(8):580–601. doi: 10.1016/j.pmrj.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Devoogdt N, Christiaens MR, Geraerts I, Truijen S, Smeets A, Leunen K, et al. Effect of manual lymph drainage in addition to guidelines and exercise therapy on arm lymphoedema related to breast cancer: randomised controlled trial. BMJ. 2011;343:d5326. doi: 10.1136/bmj.d5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Partsch H, Stout N, Forner-Cordero I, et al. Clinical trials needed to evaluate compression therapy in breast cancer related lymphedema (BCRL). Proposals from an expert group. Int Angiol. 2010;29(5):442–53. [PubMed] [Google Scholar]
- 75.Fife CE, Davey S, Maus EA, Guilliod R, Mayrovitz HN. A randomized controlled trial comparing two types of pneumatic compression for breast cancer-related lymphedema treatment in the home. Support Care Cancer. 2012 Dec;20(12):3279–86. doi: 10.1007/s00520-012-1455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilburn O, Wilburn P, Rockson SG. A pilot, prospective evaluation of a novel alternative for maintenance therapy of breast cancer-associated lymphedema [ISRCTN76522412] BMC Cancer. 2006;6:84. doi: 10.1186/1471-2407-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omar MT, Shaheen AA, Zafar H. A systematic review of the effect of low-level laser therapy in the management of breast cancer-related lymphedema. Support Care Cancer. 2012 Nov;20(11):2977–84. doi: 10.1007/s00520-012-1546-0. [DOI] [PubMed] [Google Scholar]
- 78.Devoogdt N, Van Kampen M, Geraerts I, Coremans T, Christiaens MR. Different physical treatment modalities for lymphoedema developing after axillary lymph node dissection for breast cancer: a review. Eur J Obstet Gynecol Reprod Biol. 2009 Mar;149(1):3–9. doi: 10.1016/j.ejogrb.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 79.National Lymphedema Network. Supplement to National Lymphedema Network Position Statement Breast Cancer Screening. Screening and Early Detection of Breast Cancer-Related Lymphedema: The Imperative. Available at: http://www.lymphnet.org/pdfDocs/PP_Lymphedema_BC_Supplement.pdf.
- 80.Cheville AL, Nyman JA, Pruthi S, Basford JR. Cost considerations regarding the prospective surveillance model for breast cancer survivors. Cancer. 2012 Apr 15;118(8 Suppl):2325–30. doi: 10.1002/cncr.27473. [DOI] [PubMed] [Google Scholar]
- 81.Stout NL, Pfalzer LA, Springer B, Levy E, McGarvey CL, Danoff JV, et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2011 Jan;92(1):152–63. doi: 10.2522/ptj.20100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001 Apr-Jun;14(2):128–46. [PubMed] [Google Scholar]
- 83.Lee TS, Kilbreath SL, Sullivan G, Refshauge KM, Beith JM. The development of an arm activity survey for breast cancer survivors using the Protection Motivation Theory. BMC Cancer. 2007;7:75. doi: 10.1186/1471-2407-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001 Aug;68(3):273–82. doi: 10.1023/a:1012278023233. [DOI] [PubMed] [Google Scholar]


