Abstract
The bile salt export pump (BSEP, ABCB11) is the primary transporter of bile acids from the hepatocyte to the biliary system. This rate-limiting step in bile formation is essential to the formation of bile salt dependent bile flow, the enterohepatic circulation of bile acids, and the digestion of dietary fats. Mutations in BSEP are associated with cholestatic diseases such as progressive familial intrahepatic cholestasis type 2 (PFIC2), benign recurrent intrahepatic cholestasis type 2 (BRIC2), drug-induced cholestasis, and intrahepatic cholestasis of pregnancy. Development of clinical therapies for these conditions necessitates a clear understanding of the cell biology of biosynthesis, trafficking, and transcriptional and translational regulation of BSEP. This chapter will focus on the molecular and cell biological aspects of this critical hepatic membrane transporter.
Keywords: Bile salt secretion, ATP-binding cassette transporter, Cholestasis
1. Introduction
Bile is a largely (~95%) aqueous fluid that is produced by the hepatocyte in the liver and released into the biliary system of ducts and, if present, the gallbladder. It is a complex mixture of endogenous solid constituents, including bile salts, bilirubin, phospholipids, cholesterol, amino acids, steroids, enzymes, porphyrins, vitamins, and heavy metals, as well as exogenous drugs, xenobiotics and environmental toxins. After ingestion of a meal, bile, in the form of mixed micelles of bile salts and phospholipids, gets released into the duodenum where its detergent properties act to solubilize lipids, thus aiding in their absorption by the intestine. Bile salts also stimulate intestinal immunity and function as ligands for G-coupled protein receptors to regulate thermogenesis (Keitel et al., 2008). The rate limiting step in the secretion of bile salts by the liver is achieved by an ABC transporter known as the bile salt export pump (BSEP, ABCB11). This member of the ATP-binding cassette (ABC) family of proteins is localized to the hepatocyte canalicular membrane and is critical to the formation of bile salt dependent bile flow and the normal enterohepatic circulation of bile salts from the distal intestine back to the liver. Thus, defects in the biosynthesis or trafficking to the apical plasma membrane due to mutations in the gene result in a deficiency in bile flow that is known as cholestasis. BSEP mutations have been associated with progressive familial intrahepatic cholestasis type 2 (PFIC2) (Strautnieks et al., 1998), benign recurrent intrahepatic cholestasis type 2 (BRIC2) (van Mil et al., 2004), drug-induced cholestasis (Modica et al., 2009), hormone-dependent intrahepatic cholestasis of pregnancy (ICP), biliary lithiasis (Davit-Spraul et al., 2010) and transient neonatal cholestasis (Hermeziu et al., 2006). This pivotal role in liver function in normal and disease conditions has made it essential to understand the regulation of BSEP in order to develop therapies directed toward treating cholestasis. This review will focus on the cell biology of the synthesis and trafficking of BSEP/Bsep, and how this information is being utilized in treating patients suffering from different forms of cholestasis related to BSEP.
2. Structure and function of BSEP
An ATP dependent bile salt transporter was initially described in 1991 by Nishida et al in canalicular membrane vesicles (Nishida et al., 1991) and rapidly confirmed by 3 other groups (Adachi et al., 1991; Muller et al., 1991; Stieger et al., 1992). It was first partially cloned from a pig liver cDNA library based on its similarity to the multidrug resistance protein, P-glycoprotein (MDR1, ABCB1) (Childs et al., 1995). Therefore, it was originally named sister of P-glycoprotein (spgp). The full-length coding region was identified in 1998 by Gerloff et al (Gerloff et al., 1998) from rat liver and characterized as the bile salt export pump, Bsep. BSEP consists of 1321 amino acids in humans and has a molecular mass of ~160 kDa. The gene is located to chromosome 2q24-31 in humans (Strautnieks et al., 1997) and mouse Bsep is located in a region of chromosome 2 syntenic to 2q24-31 (Lecureur et al., 2000). Rodent Bseps are ~80% identical to human BSEP, whereas Bsep in lower vertebrates such as the marine skate (Leucoraja erinacea) has 69% identity to human BSEP (Cai et al., 2001). The crystal structure of BSEP/Bsep has not yet been determined, but as a member of the ABC superfamily, it is predicted to have two segments of six transmembrane domains (TMD) separated by an ~350 amino acid cytoplasmic nucleotide binding domain (NBD) that contains Walker A and B signatures. A molecular model of the structure of BSEP/Bsep has been proposed based on the crystal structure of the multidrug transporter from S. aureus, Sav1866 (Kubitz et al., 2012). Both the N- and C-terminal portions of the molecule are located to the cytoplasmic face of the plasma membrane. The first extracellular loop contains four N-glycosylation sites which act in the stability, trafficking and function of BSEP (Mochizuki et al., 2007). Additionally, BSEP/Bsep can be modified by phosphorylation and ubiquitinylation (Hayashi and Sugiyama, 2009; Kubitz et al., 2004; Noe et al., 2001; Wang et al., 2008).
BSEP/Bsep is responsible for bile salt dependent bile flow. Transport of bile salts is dependent on ATP hydrolysis. It cannot be stimulated by any other nucleotide and is not driven by ATP-dependent pH gradient or an inside positive membrane potential (Adachi et al., 1991; Muller et al., 1991; Stieger et al., 1992). BSEP/Bsep transports primarily monovalent bile salt species, including taurine and glycine conjugates of primary bile salts, cholic acid (CA) and chenodeoxycholic acid (CDCA), and the secondary bile salt, deoxycholic acid (DCA), as well as ursodeoxycholic acid (UDCA) (Stieger, 2011). Substrate specificity does not vary greatly among species, although differences in the native bile acid pool in different species are likely responsible for the variability (Byrne et al., 2002; Stieger, 2011). The order of affinity for conjugated bile salts for human and rodent BSEP/Bsep is taurochenodeoxycholic acid (TCDCA)= taurocholic acid (TCA)>taurodeoxycholic acid (TDCA)>tauroursodeoxycholic acid (TUDCA)~glycocholic acid (GCA) (Byrne et al., 2002; Gerloff et al., 1998; Green et al., 2000; Noe et al., 2002).
BSEP also has low affinity for certain drugs that are also substrates for MDR1, but pravastatin is the only non-bile salt solute that has been confirmed as a substrate for BSEP (Hirano et al., 2005). However, in vitro studies with human and rodent BSEP/Bsep-expressing cells have shown that some drugs, notably cyclosporine, rifamycin and glibenclamide, can competitively inhibit BSEP/Bsep function (see review (Stieger, 2011) and (Byrne et al., 2002)). It is still unclear whether these drugs directly cause liver injury in humans through their inhibition of BSEP, or whether another “hit”, such as a susceptibility mutation in BSEP, predisposes to these drug induced liver diseases (Morgan et al., 2010). See further discussion in Section 5 below.
3. Plasma Membrane Localization of BSEP/Bsep
3.1 Constitutive Expression
BSEP/Bsep is almost exclusively expressed in the liver on the hepatocyte apical, canalicular membrane, evenly distributed throughout the lobular domains. Although there are reports of low levels of mRNA expression in non-hepatic tissue, including the kidney of the sea lamprey (Cai et al., 2012), the protein has never been detected in these tissues (Stieger, 2011). Immunolabeling experiments have shown that Bsep is predominantly expressed on the canalicular plasma membrane on the microvillar, but not the intermicrovillar, membrane (Gerloff et al., 1998). Smaller amounts of the protein are localized to subapical vesicles (Dombrowski et al., 2006; Gerloff et al., 1998). This partitioning of the membrane is believed to reflect lipid microdomains, or rafts, which are enriched in caveolin-1, cholesterol, and sphingomyelin. These domains are resistant to extraction by Lubrol WX (Ismair et al., 2009) and bile salts (Guyot and Stieger, 2011), thus protecting the canalicular membrane from the high concentration of secreted bile salts. Furthermore, these cholesterol rich microdomains are important for the activity of Bsep (Paulusma et al., 2009) and over-expression of caveolin in mice increases bile salt secretion (Moreno et al., 2003).
It is still unclear how BSEP/Bsep traffics to the apical membrane after its synthesis and post-translational modification in the Golgi. Biosynthetic labeling of newly synthesized rat Bsep suggests that it traffics out of the Golgi directly into a subapical, vesicular compartment where it can reside for several hours before moving to the apical plasma membrane (Kipp and Arias, 2000). This subapical compartment consists of rab11-positive endosomes (Wakabayashi et al., 2004). Interestingly, other apical ABC transport proteins, multidrug resistance protein 1 and 2 (Mdr1, Mdr2), also appear to traffic directly to the canalicular membrane from the Golgi, but they do not linger in a subapical vesicular compartment (Kipp and Arias, 2000; Sai et al., 1999). This direct trafficking is in contrast to apical membrane ectoenzymes, such as dipeptidyl peptidase IV, aminopepdidase N, and the cell adhesion molecule, cCAM105, which first traffic from the ER and Golgi to the sinusoidal membrane before being transcytosed to the apical membrane. However, we have shown that Bsep can reside in the same intracellular vesicle as the transcytotic marker, pIgARec (Soroka et al., 1999). Furthermore, when alloantibodies to BSEP found in some children with PFIC2 after liver transplantation were incubated with isolated rat hepatocytes they were found at the apical, unexposed, plasma membrane within 30 minutes, presumably by binding at the basolateral membrane and trafficking to the apical membrane (Keitel et al., 2009).
The half-life of BSEP/Bsep in the apical membrane is ~4-6 days and the protein is believed to constitutively recycle between the plasma membrane and subapical vesicles (endosomes) containing Rab11 and requiring myosin Vb (Lam et al., 2007; Wakabayashi et al., 2005). In WIF-B9 cells expressing BSEP-YFP this constitutive recycling was microtubule dependent and sensitive to actin inhibitors, but unaffected by brefeldin A, cAMP, TCA, or phosphatidylinositol 3-kinase inhibitors (Wakabayashi et al., 2004). This pathway has been proposed to provide a rapid response for canalicular transporters when bile acid secretion is needed (Kipp et al., 2001). Although this study suggests that BSEP/Bsep transporters are constantly cycling on and off the apical plasma membrane, other research has identified additional proteins that associate with BSEP/Bsep and aid in the “anchoring” of the transporter at it’s site of action. The motor protein myosin II regulatory light chain (MLC2) has been shown to interact with the NBD region of BSEP and to be important in BSEP trafficking to the plasma membrane (Chan et al., 2005). Expression of a dominant negative, non-phosphorylatable form of MLC2 reduced BSEP levels in the apical membrane of MDCK cells and treatment with the MLC2 inhibitor, Blebbistatin, reduced delivery of BSEP to the apical plasma membrane (Chan et al., 2005). Furthermore, Kruglov et al found that the type II inositol 1,4,5 trisphosphate receptor (InsP3R2) colocalizes with Bsep at the canalicular membrane, and loss of this Ca++ regulator results in endocytosis of Bsep into an intracellular, pericanalicular compartment with a resultant decrease in bile salt secretion (Kruglov et al., 2011).
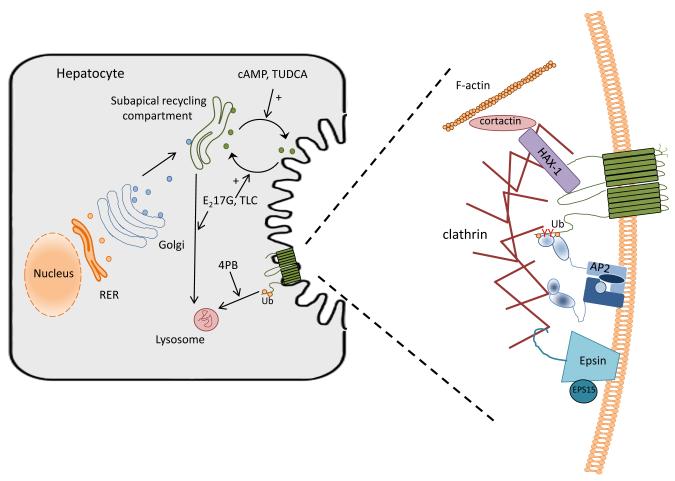
The NBD region has also been shown to bind to the cytoskeletal-associated protein, HCLS1-associated protein X-1 (Hax-1), which also interacts with cortactin (Ortiz et al., 2004). Depletion of Hax-1 or over-expression of the dominant negative forms of cortactin or EPS15 leads to the increased level of BSEP in the apical plasma membrane of MDCK cells, suggesting that these proteins are important in internalization of BSEP from the plasma membrane. EPS15 specifically interacts with epsin (Chen et al., 1998) and the AP-2 adaptor (Benmerah et al., 1995), both important in clathrin-dependent endocytosis (Benmerah et al., 1998; Chen et al., 1998). More recently we have shown that the C-terminal tail of the Bsep molecule contains a tyrosine motif (1310YYKLV1314) that is critical to the constitutive endocytosis of Bsep. This motif is conserved among closely related members of the ABCB subfamily of proteins that mediate ATP-dependent transport of substrates of broad substrate specificity. At the same time Hayashi et al (Hayashi et al., 2012) showed that the C-terminal tyrosine motif in BSEP directly interacts with AP-2, an adaptor protein associated with dynamin- and clathrin- dependent endocytosis. Thus, all these data indicate that endocytosis of BSEP/Bsep from the plasma membrane is controlled by its interactions with complexes of cytoskeletal-associated proteins and suggest that plasma membrane localization, and therefore function, may be regulated through these interactions. Figure 1 summarizes these interactions.
Fig 1.
Biosynthesis, trafficking and regulation of BSEP in the hepatocyte. (A) After post-translational modification in the Golgi, BSEP is trafficked to a subapical recycling compartment. Traffic to and from this endosomal compartment is highly regulated through various kinase pathways (Roma et al., 2008). Notably, cAMP and tauroursodeoxycholate treatment result in increased movement to the canalicular plasma membrane, whereas estradiol 17-glucuride and taurolithocholate treatment lead to increased endocytosis back to the recycling endosome and, perhaps eventually, to the lysosome for degradation. Phenylbutyrate (PB) may act by altering the rate of ubiquitination (Ub) and lysosomal degradation of BSEP. (B) Internalization of BSEP from the plasma membrane is a clathrin-dependent process involving many interacting partners. AP-2 has been shown to bind to a tyrosine containing motif in the C-terminus of Bsep which allows for endocytosis by a clathrin dependent mechanism (Hayashi et al., 2012; Lam et al., 2012). 4-PB has been shown to decrease the expression of the α-adaptin subunit of AP2, thus altering the ability of AP2 to interact with BSEP and decreasing endocytosis of the transporter (Hayashi et al., 2012). Ubiquination of the C-terminus of BSEP has also been shown to influence the cell surface expression of the transporter (Hayashi and Sugiyama, 2009). HAX-1 binds to the NBD domain of BSEP and with cortactin and has been shown to be involved in BSEP internalization from the plasma membrane (Ortiz et al., 2004). Epsin and EPS15 are important to the formation of the clathrin coated vesicle and, thus can influence the endocytosis of BSEP from the canalicular membrane (Ortiz et al., 2004). Regulation of these complexes through signaling pathways and by such compounds as phenylbutyrate may provide mechanisms for therapeutic intervention in various forms of cholestasis.
3.2 Regulated Expression
The ability to regulate the amount of BSEP/Bsep in the hepatocyte canalicular membrane is a critical step in controlling the function of BSEP/Bsep in health and disease. Cyclic AMP, TUDCA, and TCA all stimulate insertion of Bsep into the canalicular membrane (Stieger, 2011). Ursodeoxycholic acid has been used in humans to treat cholestasis and its taurine conjugate, TUDCA, has been shown in experimental animal models of cholestasis to stimulate insertion of Mrp2 (Abcc2) and Bsep transporters into the apical plasma membrane (Beuers et al., 2001; Dombrowski et al., 2006; Kurz et al., 2001; Micheline et al., 2002). Subsequent studies have shown that p38 MAP kinase and the protein kinase C (PKC) signal transduction pathways are important in this stimulated exocytosis of Bsep by TUDCA from a vesicular compartment to the plasma membrane (Kubitz et al., 2004; Kurz et al., 2001). Hypotonic swelling of cells has also been shown to result in stimulated bile acid excretion and probably acts through activation of Erk-1/2 and p38 MAP kinase (Haussinger et al., 2000; Noe et al., 1996; Schmitt et al., 2001). A phosphoinositide 3-kinase (PI3K) pathway has been shown to be involved in regulated exocytosis of ATP-dependent canalicular transporters by taurocholate, although BSEP was not identified at that time (Misra et al., 1998). Cyclic AMP stimulated secretion of bile salts in isolated perfused rat livers is blocked by microtubule inhibitors, indicating the need for an intact cytoskeleton in this regulation (Hayakawa et al., 1990). Rapid microtubular dependent targeting of Bsep and other canalicular proteins is lost in liver kinase B1 (LKB1) null mice (Woods et al., 2011). LKB1 is an upstream kinase that is activated by bile acids and facilities the establishment of apical polarity in the hepatocyte (Fu et al., 2010; Fu et al., 2011). The LKB1/AMPK pathway is postulated to act at AMPK sites on the plus end of microtubules.
In contrast, induction of cholestasis in rodents by numerous agents including estradiol-17β-glucuronide, lithocholate, LPS and hypoxia result in internalization of Bsep into subapical membrane vesicles and loss of staining of the protein at the canalicular membrane (Crocenzi et al., 2003a; Crocenzi et al., 2003b; Elferink et al., 2004; Fouassier et al., 2007; Micheline et al., 2002; Zinchuk et al., 2005). BSEP is also internalized in cholestatic liver disease in humans (Elferink et al., 2004; Roma et al., 2008). Although the mechanism of regulation in these cholestatic models remains somewhat unclear, multiple pathways have been implicated. Taurolithocholate was shown to act on Bsep membrane localization through protein kinase C epsilon, in a PI3K-dependent manner (Beuers et al., 2003). In addition, lithocholate has been shown to be an antagonist of FXR, thus perhaps also acting through down-regulation of BSEP gene expression (Yu et al., 2002). Estradiol 17-glucuronide induces cholestasis through signaling pathways that include the Ca++-dependent protein kinase C (cPKC) and PI3K (Boaglio et al., 2010; Crocenzi et al., 2008). Sepsis associated cholestasis is mediated by lipopolysaccharide (LPS) and has been shown to act through cytokines, such as Il-6, IL-1α and TNFα (Hartmann et al., 2002; Siewert et al., 2004).
4. Transcriptional regulation of BSEP expression
The level of human BSEP expression varies significantly between individuals (Ho et al., 2010; Tirona, 2011) and its expression is highly regulated by transcriptional mechanisms. The main transcriptional regulator of BSEP/Bsep is the nuclear receptor, fanesoid X receptor (FXR, NH1H4)(Makishima et al., 1999; Trauner and Boyer, 2003). FXR/Fxr transactivates the proximal promoter of BSEP/Bsep in humans (Ananthanarayanan et al., 2001) and in rodents (Gerloff et al., 2002). Bile acids are the physiologic ligand for FXR and thus can regulate the transcription of their own transporter (Makishima et al., 1999). Chenodeoxycholic acid (CDCA) is the major endogenous physiological ligand for FXR (Wang et al., 1999). The critical role of Fxr in Bsep expression is demonstrated by its low basal expression in Fxr−/− mice and the lack of an induced response to bile salts (Marschall et al., 2006). This mechanism, together with FXR mediated down-regulation of bile salt synthesis and hepatic uptake transporters, assures that hepatic levels of bile salts should not reach toxic levels. Even in cholestatic liver, BSEP/Bsep expression is relatively sustained (Geier et al., 2007; Lee et al., 2001). Because of this homeostatic mechanism, FXR is a major target for nuclear receptor regulation of cholestatic liver disease (Boyer, 2005; Cai and Boyer, 2006; Stedman et al., 2006). A potent FXR ligand, obeticholic acid, has ~ 100 times affinity for FXR than CDCA and is now in phase lll clinical trials for the treatment of primary biliary cirrhosis.
FXR regulates the expression of target genes by acting together as a heterodimer with retinoid X receptor, RXRα (Forman et al., 1995; Mangelsdorf and Evans, 1995). The human FXR promoter also contains receptors for retinoic acid and vitamin D. Activation of the vitamin D receptor with vitamin D3 suppresses FXR activation in in-vitro luciferase reporter assays (Honjo et al., 2006). In addition to FXR, the BSEP promoter is also induced by the hepatocyte-specific liver receptor homologue-1 (LRH-1, NR5A2) (Song et al., 2008). The BSEP promoter is positively regulated by the nuclear erythroid 2 p45-regulated factor 2 (NRF2) that plays a significant role in responses to oxidative stress. NRF2 binds to response elements that regulate many hepatic phase I and II enzymes and efflux transporters such as MRP3 and MRP4 (Weerachayaphorn et al., 2009).
5. BSEP Mutations and Disease
The sequencing of the human genome led to the discovery that mutations in BSEP caused a form of progressive familial intrahepatic cholestasis in infancy known as PFIC2. This discovery provided the final confirmation that BSEP was indeed the determinant of bile salt dependent bile flow. As additional mutations and polymorphisms in BSEP were discovered, it became apparent that genetic mutations in BSEP can result in altered BSEP function and subsequent liver disease. These mutations result in a range of mild to severe, progressive forms of intrahepatic cholestasis known as the BSEP deficiency syndrome (Lam et al., 2006; Pauli-Magnus et al., 2005). Patients with PFIC2 mutations are also at risk for hepatocellular carcinoma (Davit-Spraul et al., 2010; Knisely et al., 2006; Strautnieks et al., 2008). A few patients with PFIC2 who have received liver transplants have developed antibody mediated recurrent disease when the newly synthesized BSEP protein is recognized as foreign by the recipient (Jara et al., 2009; Keitel et al., 2009; Maggiore et al., 2010; Siebold et al., 2010). Common mutations can be grouped into missense mutations, nonsense mutations, deletions and insertions and splice site mutations. In one study of 109 families of patients, most of the mutations were shown to result in decreased or absent BSEP expression on the canalicular plasma membrane (Strautnieks et al., 2008). Another study analyzed 20 mutations/single nucleotide polymorphisms (SNPs) that resulted in reduced wild-type splicing and levels of mRNA in vitro (Byrne et al., 2009). All these mutations can result in truncated or misfolded BSEP proteins that would be subject to quality control mechanisms in the endoplasmic reticulum (ER), thus preventing their expression on the plasma membrane. The most common mutation of the cystic fibrosis transmembrane conductance regulator (CFTR) in patients with cystic fibrosis, ΔF508, is a well known example where an ABC transporter is subjected to defective protein folding and processing in the ER (Denning et al., 1992b; Du et al., 2005; Thomas et al., 1992). When seven PFIC2 missense mutations were expressed in MDCK cells, five of these common mutations (G238V, E297G, G982R, R1153C and R1268Q) were unable to traffic to the apical membrane (Wang et al., 2002). Of these, four also lacked the ability to transport taurocholate when expressed in Sf9 cells, again suggesting misfolding and retention by the ER quality control. Two common mutations in human BSEP, E297G and D482G, have been reported to have both reduced (Noe et al., 2005; Wang et al., 2002) and normal (Hayashi et al., 2005; Lam et al., 2007) transport activity.
The D482G mutation has also been found to have enhanced aberrant mRNA splicing, perhaps providing an explanation for the variable expression, function and severity of disease (Byrne et al., 2009). This mutation (D482G) is the most common PFIC missense mutation in the European population and 16% of BSEP deficiency patients who develop malignancy have this mutation (Knisely et al., 2006; Strautnieks et al., 2008). Aberrant mRNA splicing in this mutation would result in a truncated protein that would not be able to be detected in immunological assays, thus possibly explaining the lack of detectable BSEP at the canalicular membrane. In addition, Bryne et al have reported that the D482G-containing mRNA is unstable (Byrne et al., 2009). The variability in the clinical phenotype of the E297G mutation, on the other hand, may be due to the ability of the mRNA to be stabilized by splicing factors present in the hepatocyte (Byrne et al., 2009).
Some BSEP mutations are associated more frequently with a particular form of cholestasis, suggesting a possible correlation with disease severity (see(Byrne et al., 2009) for a review). We examined differences in protein maturation, plasma membrane localization and transport activity in mutants of rat Bsep representing two PFIC2 (D482G and E297G), two BRIC2 (A570T and R1050C) and one ICP (N591) mutation (Lam et al., 2007). It was found that all but the PFIC2 mutation, E297G, retained the ability to transport taurocholate. The reduction in plasma membrane expression correlated equally with reduction in the total amount of Bsep protein, suggesting a defect in protein stability rather than in trafficking. In general, the membrane Bsep expression ranked in the order ICP>BRIC2>PFIC2 (Lam et al., 2007). A similar study also found differences between BRIC2 and PFIC2 in Bsep localization and function after transfection into the polarized cell line, MDCKII (Kagawa et al., 2008).
Young patients with severe BSEP deficiency syndrome are at risk to subsequently develop hepatocellular carcinoma (HCC) and cholangiocarcinoma (Knisely et al., 2006; Scheimann et al., 2007; Strautnieks et al., 2008), with the reported cases of HCC being much greater than cholangiocarcinoma. These patients frequently present with splice site changes, deletion, insertions, and nonsense mutations that result in the absence of functional protein. This absence results in elevated levels of intracellular bile salts that have been shown to influence many aspects of cell function, including mitochondrial function, cell cycle, DNA repair, homeobox gene activation, and cell polarization and differentiation (Ng et al., 2000; Palmeira and Rolo, 2004; Sokol et al., 2006; Souza et al., 2008; Woolbright and Jaeschke, 2012). Recently it has been shown in HCC patients that the decrease in BSEP expression is associated with an alteration in FXR isoform expression induced by inflammation (Chen et al., 2013). The FXRα2 isoform has more potent activity than FXRα1. In HCC, the ratio of FXRα1/FXRα2 was altered due to the absence of the FXRα2 isoform in one-third of the tumors. The cytokines Il-6 and TNFα increased FXRα1/FXRα2 ratios in Huh7 cells with a subsequent reduction in BSEP expression. These findings raise the possibility that suppression of inflammation in the liver in HCC patients might increase BSEP expression and re-establish bile acid homeostasis.
Some forms of drug induced liver injury (DILI) can be attributed to inhibition of BSEP (Dawson et al., 2012; Fattinger et al., 2001; Pauli-Magnus and Meier, 2006). Animal models of drug-induced cholestasis indicate that cholestatic drugs can inhibit bile secretion and bile acid transport at many levels, including uptake and efflux across the sinusoidal membrane, as well as canalicular efflux. For example, rifampicin, cyclosporine A, rifamycin SV, bosentan, troglitazone, erythromycin estolate, and glibenclamide have all been shown to inhibit Bsep in rats in a dose-dependent fashion (Fattinger et al., 2001; Funk et al., 2001a; Funk et al., 2001b; Stieger et al., 2000). Sulindac also competitively inhibits canalicular bile acid transport (Bolder et al., 1999). Ethinylestradiol-17β- glucuronide is secreted into bile by Mrp2 and then trans-inhibits Bsep from the luminal side of the canalicular membrane (Stieger et al., 2000). Cyclosporine, an MDR1 substrate, is a prototypical drug that can cause cholestatic liver injury by competitively inhibiting ATP dependent transporters such as BSEP and MRP2 (Bohme et al., 1993; Bohme et al., 1994; Kadmon et al., 1993) and it can inhibit intrahepatic vesicle transport, targeting, and function of BSEP to the canalicular membrane (Roman et al., 2003; Roman et al., 1990). Other drugs that can be associated with cholestasis, such as the endothelin antagonist bosentan, also inhibit the bile salt export pump, an effect that is enhanced by co-administration of the oral hypoglycemic agent glibenclamide (Fattinger et al., 2001). Troglitazone and troglitazone sulfate, the main troglitazone metabolites eliminated in bile, competitively cis-inhibit Bsep, which could lead to troglitazone-induced intrahepatic cholestasis and liver toxicity (Funk et al., 2001a; Funk et al., 2001b). Male rats are more susceptible to liver injury than female rats, probably due to higher formation rates of troglitazone sulfate (Kostrubsky et al., 2001). Although DILI is a clinically important and serious problem, the frequency of adverse drug reactions is low enough to suggest that these patients may also have genetic susceptibility factors. The BSEP variants, V444A and M677V, have been reported to consistently occur with frequencies of greater that 50% (Lang et al., 2006; Saito et al., 2002). The V444A variant has been found in patients with DILI (Lang et al., 2007) and intrahepatic cholestasis of pregnancy (Dixon et al., 2009; Meier et al., 2008) with greater frequency than controls. In contrast, preliminary study from Japan reported a lower frequency of the V444A variant in DILI (Kagawa et al., 2012). Larger studies will be needed to clearly demonstrate an association of BSEP deficiency syndromes with a common BSEP variant.
6. Clinical Therapy
As described in the previous section and reviewed by Jacquemin (Jacquemin, 2012), BSEP mutations can fall into various classes, including nonsense mutations leading to truncated protein and missense mutations leading to endoplasmic reticulum-associated degradation (ERAD), mistrafficked protein, or protein with reduced functional activity. Ideally, clinical therapy should be guided by an individual’s clinical presentation as assessed by immunohistochemistry, liver function tests, and genetic analysis (Jacquemin, 2012). Studies utilizing in vitro cell systems expressing different BSEP/Bsep mutations have provided a clearer understanding of how these genetic mutations affect the synthesized protein, thus allowing the development of targeted therapy. For example, the very low plasma membrane expression of the PFIC2 mutant, D482G, could be increased by treatment with low temperature, sodium butyrate (Figure 2) and sodium 4-phenylbutyrate (4-PB) in MDCK II, HEK293, or HepG2 cells (Hayashi and Sugiyama, 2007; Lam et al., 2007; Plass et al., 2004). These are believed to stabilize misfolded proteins which otherwise are subjected to ERAD and get trapped in the ER. Low temperature treatment had previously been shown to stabilize the CFTR ΔF508 mutant (Denning et al., 1992a). In vitro studies using rat Bsep mutants of the human mutations G238V, D482G, G982R, R1153C, and R1268Q all resulted in retention of Bsep in the ER to different extents (Wang et al., 2008). Ubiquitinylation with over expression of E3 ubiquitin ligases shortened the half life of both the wild type protein and the already short half life of the PFIC2 mutant, D482G, however small amounts of the mutant protein was still able to reach the plasma membrane (Wang et al., 2008). This and other studies also suggest that the residence time on the cell surface of the common D482G and E297G mutant proteins is greatly reduced due to accelerated internalization, reduced recycling or targeting of the endocytosed protein for degradation. These observations provide the rational for attempts to “rescue” the mutant proteins with small molecules (Hayashi and Sugiyama, 2009; Wang et al., 2008). However, it should be noted that additional studies would need to verify that an increase in plasma membrane localization of BSEP by drug chaperones would also translate to an increase in transporter function. Indeed, in vitro studies demonstrate that 4-phenylbutyrate (4-PBA) can increase the cell surface expression of PFIC2 mutant proteins D482G and E297G in MDCK cells and stimulate bile salt secretion and Bsep expression in vivo in rats (Hayashi and Sugiyama, 2007). This suggests that therapeutic intervention may aid in stabilizing BSEP at the plasma membrane. This was recently shown to be true in a child suffering from PFIC2 (Gonzales et al., 2012a). Phenylbutryate therapy was started as a final option for stabilizing the disease in this 10 year old child who had suffered since the age of one. After 5 months of therapy serum bile acid concentrations and pruritus had improved, as well as liver function tests. A liver biopsy performed after 3 months demonstrated canalicular localization of BSEP which had not been present before therapy was initiated (Figure 3). Discontinuation of the therapy resulted in reversion of the improvement. A preliminary report from the same group finds that 4-PBA decreases pruritus and serum bile acid concentrations, and improves liver function within 3 months of treatment in 3 PFIC2 children harboring a least one missense mutation (A257V, G982R and T1210P) (Gonzales et al., 2012b).
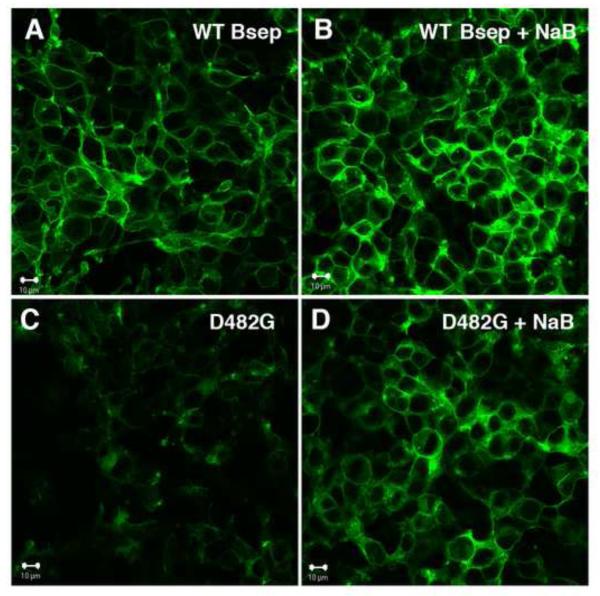
Figure 2.
Sodium butyrate increases the plasma membrane localization of Bsep-GFP. HEK293 cells were transfected with WT Bsep-GFP (A and B) or D482G-GFP (C and D) and confocal microscopy was used to monitor the plasma membrane expression of the constructs after treatment with sodium butyrate (NaB) for 24 hr. (A) DMSO control treated cells express WT Bsep-GFP primarily on the plasma membrane. (B) Similar localization was seen after NaB treatment. (C) Cells transfected with mutant D482G Bsep-GFP show little GFP fluorescence due to degradation of the protein (see details in (Lam et al., 2007). (D) Treatment with NaB resulted in stabilization of the protein and cells show increased plasma membrane expression of the D482G mutant of Bsep-GFP.
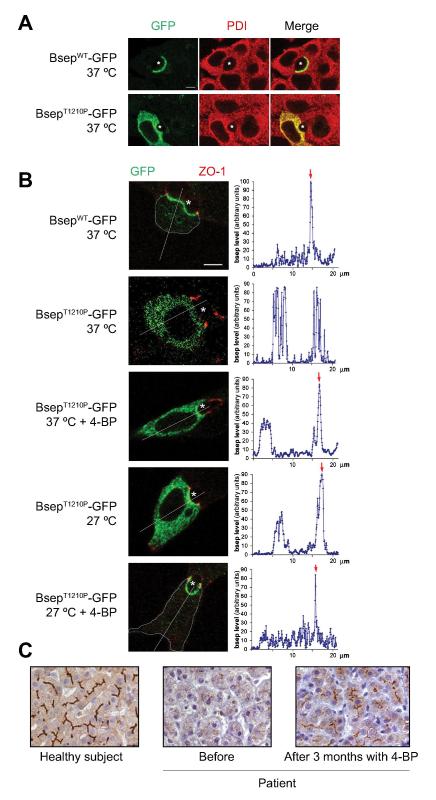
Figure 3.
In vitro studies of mutant BsepT1210P–GFP in a polarize cell line and the effect of 4-PB in a PFIC2 child expressing this mutation. (A) Immunofluorescent localization of GFP constructs of WT and mutant BSEP (green) and protein disulfide isomerase (PDI) (red) in Can 10 cells. (B) 4-PB and low temperature increase canalicular membrane expression of BsepT1210P–GFP (green). The apical membrane was delineated with immunofluorescent labeling of the tight junction protein, zonula occludens 1 (ZO-1) (red). Quantification of Bsep–GFP levels is shown in graphs to the right. Red arrows indicate canalicular fluorescence reinforcement, when present. A thin blue-grey line was drawn on confocal images to delineate cell limits, when they were hardly visible. * canaliculus; bar: 5 μm. (C) BSEP immunostaining of human liver sections: healthy subject (left), patient before (middle) and after 3 months with 4-PB therapy (right). Bar: 30 μm. (Reproduced with permission from (Gonzales et al., 2012a)).
Additional therapeutic interventions could include drugs such as aminoglycosides and PTC124 to induce read through premature stop codons and treatment with nuclear receptor agonists (6-ethyl CDCA, fibrates statins) might be used to increase gene transcription of BSEP (Boyer, 2005; Gonzales and Jacquemin, 2010; Jacquemin, 2012; Trauner et al., 2005). The search for additional pharmacological chaperones that correct protein folding and trafficking defects in BSEP mutants continues.
One major hurdle that the pharmaceutical industry faces is the frequency with which drugs can cause drug-induced liver injury (DILI), where one of the major risk factors is inhibition of BSEP (Dawson et al., 2012; Greer et al., 2010). A recent study utilized a computational approach combined with an in vitro membrane vesicle assay to build a BSEP inhibition model (Warner et al., 2012). Although the algorithms are not perfect, their results show that lipophilicity and molecular size are significantly correlated with BSEP inhibition. In addition, Vertex Pharmaceuticals have investigated the ability of VX-809, a CFTR corrector, to partially correct the ΔF508 mutation commonly seen in cystic fibrosis (Van Goor et al., 2011). This drug improved chloride secretion and ER processing of CFTR in cultured human bronchial cells. Perhaps similar drugs can be developed to improve the misfolding of BSEP in common mutations leading to PFIC2.
Conclusions
The discovery of an ATP dependent bile salt transporter at the canalicular domain and its subsequent cloning (ABCB11) have provided the foundation for studies of BSEP’s molecular regulation and cellular trafficking. While much has been learned of the effects of genetic mutations and polymorphisms, a more thorough understanding of the cellular determinants of the apical expression of this transporter both in health and in cholestatic liver disease remains to be elucidated. New therapeutic strategies for both genetic and acquired cholestatic liver diseases will depend on these future advances.
Acknowlegements
The authors are supported by USPHS grants R37 DK25636 (JLB) and P30 DK 34989.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi Y, Kobayashi H, Kurumi Y, Shouji M, Kitano M, Yamamoto T. ATP-dependent taurocholate transport by rat liver canalicular membrane vesicles. Hepatology. 1991;14(4 Pt 1):655–659. doi: 10.1016/0270-9139(91)90053-x. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276(31):28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- Benmerah A, Gagnon J, Begue B, Megarbane B, Dautry-Varsat A, Cerf-Bensussan N. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J Cell Biol. 1995;131(6 Pt 2):1831–1838. doi: 10.1083/jcb.131.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Lamaze C, Begue B, Schmid SL, Dautry-Varsat A, Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol. 1998;140(5):1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33(5):1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- Beuers U, Denk GU, Soroka CJ, Wimmer R, Rust C, Paumgartner G, Boyer JL. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol 3-kinase-dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J Biol Chem. 2003;278(20):17810–17818. doi: 10.1074/jbc.M209898200. [DOI] [PubMed] [Google Scholar]
- Boaglio AC, Zucchetti AE, Sanchez Pozzi EJ, Pellegrino JM, Ochoa JE, Mottino AD, Vore M, Crocenzi FA, Roma MG. Phosphoinositide 3-kinase/protein kinase B signaling pathway is involved in estradiol 17beta-D-glucuronide-induced cholestasis: complementarity with classical protein kinase C. Hepatology. 2010;52(4):1465–1476. doi: 10.1002/hep.23846. [DOI] [PubMed] [Google Scholar]
- Bohme M, Buchler M, Muller M, Keppler D. Differential inhibition by cyclosporins of primary-active ATP-dependent transporters in the hepatocyte canalicular membrane. FEBS letters. 1993;333(1-2):193–196. doi: 10.1016/0014-5793(93)80403-h. [DOI] [PubMed] [Google Scholar]
- Bohme M, Muller M, Leier I, Jedlitschky G, Keppler D. Cholestasis caused by inhibition of the adenosine triphosphate-dependent bile salt transport in rat liver. Gastroenterology. 1994;107(1):255–265. doi: 10.1016/0016-5085(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Bolder U, Trang NV, Hagey LR, Schteingart CD, Ton-Nu HT, Cerre C, Elferink RP, Hofmann AF. Sulindac is excreted into bile by a canalicular bile salt pump and undergoes a cholehepatic circulation in rats. Gastroenterology. 1999;117(4):962–971. doi: 10.1016/s0016-5085(99)70356-2. [DOI] [PubMed] [Google Scholar]
- Boyer JL. Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology. 2005;129(2):735–740. doi: 10.1016/j.gastro.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Strautnieks SS, Ihrke G, Pagani F, Knisely AS, Linton KJ, Mieli-Vergani G, Thompson RJ. Missense mutations and single nucleotide polymorphisms in ABCB11 impair bile salt export pump processing and function or disrupt pre-messenger RNA splicing. Hepatology. 2009;49(2):553–567. doi: 10.1002/hep.22683. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123(5):1649–1658. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- Cai SY, Boyer JL. FXR: a target for cholestatic syndromes? Expert opinion on therapeutic targets. 2006;10(3):409–421. doi: 10.1517/14728222.10.3.409. [DOI] [PubMed] [Google Scholar]
- Cai SY, Lionarons DA, Hagey L, Soroka CJ, Mennone A, Boyer JL. Adult sea lamprey tolerates biliary atresia by altering bile salt composition and renal excretion. Hepatology. 2012 doi: 10.1002/hep.26161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai SY, Wang L, Ballatori N, Boyer JL. Bile salt export pump is highly conserved during vertebrate evolution and its expression is inhibited by PFIC type II mutations. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G316–322. doi: 10.1152/ajpgi.2001.281.2.G316. [DOI] [PubMed] [Google Scholar]
- Chan W, Calderon G, Swift AL, Moseley J, Li S, Hosoya H, Arias IM, Ortiz DF. Myosin II regulatory light chain is required for trafficking of bile salt export protein to the apical membrane in Madin-Darby canine kidney cells. J Biol Chem. 2005;280(25):23741–23747. doi: 10.1074/jbc.M502767200. [DOI] [PubMed] [Google Scholar]
- Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394(6695):793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Chen Y, Song X, Valanejad L, Vasilenko A, More V, Qiu X, Chen W, Lai Y, Slitt A, Stoner M, Yan B, Deng R. Bile salt export pump is dysregulated with altered farnesoid x receptor isoform expression in patients with hepatocellular carcinoma tissues. Hepatology. 2013;57(4):1530–1541. doi: 10.1002/hep.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S, Yeh RL, Georges E, Ling V. Identification of a sister gene to P-glycoprotein. Cancer research. 1995;55(10):2029–2034. [PubMed] [Google Scholar]
- Crocenzi FA, Mottino AD, Cao J, Veggi LM, Pozzi EJ, Vore M, Coleman R, Roma MG. Estradiol-17beta-D-glucuronide induces endocytic internalization of Bsep in rats. Am J Physiol Gastrointest Liver Physiol. 2003a;285(2):G449–459. doi: 10.1152/ajpgi.00508.2002. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Mottino AD, Sanchez Pozzi EJ, Pellegrino JM, Rodriguez Garay EA, Milkiewicz P, Vore M, Coleman R, Roma MG. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut. 2003b;52(8):1170–1177. doi: 10.1136/gut.52.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocenzi FA, Sanchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, Vore M. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48(6):1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, Bernard O, Jacquemin E. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51(5):1645–1655. doi: 10.1002/hep.23539. [DOI] [PubMed] [Google Scholar]
- Dawson S, Stahl S, Paul N, Barber J, Kenna JG. In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos. 2012;40(1):130–138. doi: 10.1124/dmd.111.040758. [DOI] [PubMed] [Google Scholar]
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992a;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Denning GM, Ostedgaard LS, Welsh MJ. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol. 1992b;118(3):551–559. doi: 10.1083/jcb.118.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon PH, van Mil SW, Chambers J, Strautnieks S, Thompson RJ, Lammert F, Kubitz R, Keitel V, Glantz A, Mattsson LA, Marschall HU, Molokhia M, Moore GE, Linton KJ, Williamson C. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut. 2009;58(4):537–544. doi: 10.1136/gut.2008.159541. [DOI] [PubMed] [Google Scholar]
- Dombrowski F, Stieger B, Beuers U. Tauroursodeoxycholic acid inserts the bile salt export pump into canalicular membranes of cholestatic rat liver. Laboratory investigation; a journal of technical methods and pathology. 2006;86(2):166–174. doi: 10.1038/labinvest.3700371. [DOI] [PubMed] [Google Scholar]
- Du K, Sharma M, Lukacs GL. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nature structural & molecular biology. 2005;12(1):17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- Elferink MG, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJ, Meijer DK, Groothuis GM. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol. 2004;287(5):G1008–1016. doi: 10.1152/ajpgi.00071.2004. [DOI] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clinical pharmacology and therapeutics. 2001;69(4):223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- Fouassier L, Beaussier M, Schiffer E, Rey C, Barbu V, Mergey M, Wendum D, Callard P, Scoazec JY, Lasnier E, Stieger B, Lienhart A, Housset C. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G25–35. doi: 10.1152/ajpgi.00175.2006. [DOI] [PubMed] [Google Scholar]
- Fu D, Wakabayashi Y, Ido Y, Lippincott-Schwartz J, Arias IM. Regulation of bile canalicular network formation and maintenance by AMP-activated protein kinase and LKB1. J Cell Sci. 2010;123(Pt 19):3294–3302. doi: 10.1242/jcs.068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci U S A. 2011;108(4):1403–1408. doi: 10.1073/pnas.1018376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology. 2001a;167(1):83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- Funk C, Ponelle C, Scheuermann G, Pantze M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol. 2001b;59(3):627–635. [PubMed] [Google Scholar]
- Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773(3):283–308. doi: 10.1016/j.bbamcr.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Gerloff T, Geier A, Roots I, Meier PJ, Gartung C. Functional analysis of the rat bile salt export pump gene promoter. European journal of biochemistry / FEBS. 2002;269(14):3495–3503. doi: 10.1046/j.1432-1033.2002.03030.x. [DOI] [PubMed] [Google Scholar]
- Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273(16):10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- Gonzales E, Grosse B, Cassio D, Davit-Spraul A, Fabre M, Jacquemin E. Successful mutation-specific chaperone therapy with 4-phenylbutyrate in a child with progressive familial intrahepatic cholestasis type 2. J Hepatol. 2012a;57(3):695–698. doi: 10.1016/j.jhep.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Gonzales E, Grosse B, Davit-Spraul A, Schuller B, Fabre M, Cassio D, Jacquemin E. In vitro and in vivo effects of chaperone therapy with 4-phenylbutryrate on ABCB11 missense mutations involved in progressive familial intrahepatic cholestasis type 2 (PFIC2) Hepatology. 2012b;56(4 (Suppl)):204A. doi: 10.1016/j.jhep.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Gonzales E, Jacquemin E. Mutation specific drug therapy for progressive familial or benign recurrent intrahepatic cholestasis: a new tool in a near future? J Hepatol. 2010;53(2):385–387. doi: 10.1016/j.jhep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Green RM, Hoda F, Ward KL. Molecular cloning and characterization of the murine bile salt export pump. Gene. 2000;241(1):117–123. doi: 10.1016/s0378-1119(99)00460-6. [DOI] [PubMed] [Google Scholar]
- Greer ML, Barber J, Eakins J, Kenna JG. Cell based approaches for evaluation of drug-induced liver injury. Toxicology. 2010;268(3):125–131. doi: 10.1016/j.tox.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Guyot C, Stieger B. Interaction of bile salts with rat canalicular membrane vesicles: evidence for bile salt resistant microdomains. J Hepatol. 2011;55(6):1368–1376. doi: 10.1016/j.jhep.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther. 2002;303(1):273–281. doi: 10.1124/jpet.102.039404. [DOI] [PubMed] [Google Scholar]
- Haussinger D, Schmitt M, Weiergraber O, Kubitz R. Short-term regulation of canalicular transport. Seminars in liver disease. 2000;20(3):307–321. doi: 10.1055/s-2000-9386. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Bruck R, Ng OC, Boyer JL. DBcAMP stimulates vesicle transport and HRP excretion in isolated perfused rat liver. Am J Physiol. 1990;259(5 Pt 1):G727–735. doi: 10.1152/ajpgi.1990.259.5.G727. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Inamura K, Aida K, Naoi S, Horikawa R, Nagasaka H, Takatani T, Fukushima T, Hattori A, Yabuki T, Horii I, Sugiyama Y. AP2 adaptor complex mediates bile salt export pump internalization and modulates its hepatocanalicular expression and transport function. Hepatology. 2012;55(6):1889–1900. doi: 10.1002/hep.25591. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sugiyama Y. 4-phenylbutyrate enhances the cell surface expression and the transport capacity of wild-type and mutated bile salt export pumps. Hepatology. 2007;45(6):1506–1516. doi: 10.1002/hep.21630. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sugiyama Y. Short-chain ubiquitination is associated with the degradation rate of a cell-surface-resident bile salt export pump (BSEP/ABCB11) Mol Pharmacol. 2009;75(1):143–150. doi: 10.1124/mol.108.049288. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Takada T, Suzuki H, Akita H, Sugiyama Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology. 2005;41(4):916–924. doi: 10.1002/hep.20627. [DOI] [PubMed] [Google Scholar]
- Hermeziu B, Sanlaville D, Girard M, Leonard C, Lyonnet S, Jacquemin E. Heterozygous bile salt export pump deficiency: a possible genetic predisposition to transient neonatal cholestasis. Journal of pediatric gastroenterology and nutrition. 2006;42(1):114–116. doi: 10.1097/01.mpg.0000184429.34001.68. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther. 2005;314(2):876–882. doi: 10.1124/jpet.105.084830. [DOI] [PubMed] [Google Scholar]
- Ho RH, Leake BF, Kilkenny DM, Meyer Zu Schwabedissen HE, Glaeser H, Kroetz DL, Kim RB. Polymorphic variants in the human bile salt export pump (BSEP; ABCB11): functional characterization and interindividual variability. Pharmacogenetics and genomics. 2010;20(1):45–57. doi: 10.1097/FPC.0b013e3283349eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo Y, Sasaki S, Kobayashi Y, Misawa H, Nakamura H. 1,25-dihydroxyvitamin D3 and its receptor inhibit the chenodeoxycholic acid-dependent transactivation by farnesoid X receptor. The Journal of endocrinology. 2006;188(3):635–643. doi: 10.1677/joe.1.06105. [DOI] [PubMed] [Google Scholar]
- Ismair MG, Hausler S, Stuermer CA, Guyot C, Meier PJ, Roth J, Stieger B. ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology. 2009;49(5):1673–1682. doi: 10.1002/hep.22807. [DOI] [PubMed] [Google Scholar]
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clinics and research in hepatology and gastroenterology. 2012;36(Suppl 1):S26–35. doi: 10.1016/S2210-7401(12)70018-9. [DOI] [PubMed] [Google Scholar]
- Jara P, Hierro L, Martinez-Fernandez P, Alvarez-Doforno R, Yanez F, Diaz MC, Camarena C, De la Vega A, Frauca E, Munoz-Bartolo G, Lopez-Santamaria M, Larrauri J, Alvarez L. Recurrence of bile salt export pump deficiency after liver transplantation. The New England journal of medicine. 2009;361(14):1359–1367. doi: 10.1056/NEJMoa0901075. [DOI] [PubMed] [Google Scholar]
- Kadmon M, Klunemann C, Bohme M, Ishikawa T, Gorgas K, Otto G, Herfarth C, Keppler D. Inhibition by cyclosporin A of adenosine triphosphate-dependent transport from the hepatocyte into bile. Gastroenterology. 1993;104(5):1507–1514. doi: 10.1016/0016-5085(93)90363-h. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Hirose S, Arase Y, Nagata N, Shiraishi K, Inoko H, Mine T. Impact of ABCB11 V444A polymorphism and HLA genotype on suseptibility to drug-induced cholestasis in Japan. Hepatology. 2012;56:550A. [Google Scholar]
- Kagawa T, Watanabe N, Mochizuki K, Numari A, Ikeno Y, Itoh J, Tanaka H, Arias IM, Mine T. Phenotypic differences in PFIC2 and BRIC2 correlate with protein stability of mutant Bsep and impaired taurocholate secretion in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G58–67. doi: 10.1152/ajpgi.00367.2007. [DOI] [PubMed] [Google Scholar]
- Keitel V, Burdelski M, Vojnisek Z, Schmitt L, Haussinger D, Kubitz R. De novo bile salt transporter antibodies as a possible cause of recurrent graft failure after liver transplantation: a novel mechanism of cholestasis. Hepatology. 2009;50(2):510–517. doi: 10.1002/hep.23083. [DOI] [PubMed] [Google Scholar]
- Keitel V, Kubitz R, Haussinger D. Endocrine and paracrine role of bile acids. World journal of gastroenterology : WJG. 2008;14(37):5620–5629. doi: 10.3748/wjg.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J Biol Chem. 2000;275(21):15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276(10):7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikci B, Ozcay F, Laszlo A, Tiszlavicz L, Moore L, Raftos J, Arnell H, Fischler B, Nemeth A, Papadogiannakis N, Cielecka-Kuszyk J, Jankowska I, Pawlowska J, Melin-Aldana H, Emerick KM, Whitington PF, Mieli-Vergani G, Thompson RJ. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44(2):478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- Kostrubsky VE, Vore M, Kindt E, Burliegh J, Rogers K, Peter G, Altrogge D, Sinz MW. The effect of troglitazone biliary excretion on metabolite distribution and cholestasis in transporter-deficient rats. Drug Metab Dispos. 2001;29(12):1561–1566. [PubMed] [Google Scholar]
- Kruglov EA, Gautam S, Guerra MT, Nathanson MH. Type 2 inositol 1,4,5-trisphosphate receptor modulates bile salt export pump activity in rat hepatocytes. Hepatology. 2011;54(5):1790–1799. doi: 10.1002/hep.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitz R, Droge C, Stindt J, Weissenberger K, Haussinger D. The bile salt export pump (BSEP) in health and disease. Clinics and research in hepatology and gastroenterology. 2012 doi: 10.1016/j.clinre.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Kubitz R, Sutfels G, Kuhlkamp T, Kolling R, Haussinger D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology. 2004;126(2):541–553. doi: 10.1053/j.gastro.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kurz AK, Graf D, Schmitt M, Vom Dahl S, Haussinger D. Tauroursodesoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology. 2001;121(2):407–419. doi: 10.1053/gast.2001.26262. [DOI] [PubMed] [Google Scholar]
- Lam CW, Cheung KM, Tsui MS, Yan MS, Lee CY, Tong SF. A patient with novel ABCB11 gene mutations with phenotypic transition between BRIC2 and PFIC2. J Hepatol. 2006;44(1):240–242. doi: 10.1016/j.jhep.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Lam P, Pearson CL, Soroka CJ, Xu S, Mennone A, Boyer JL. Levels of plasma membrane expression in progressive and benign mutations of the bile salt export pump (Bsep/Abcb11) correlate with severity of cholestatic diseases. Am J Physiol Cell Physiol. 2007;293(5):C1709–1716. doi: 10.1152/ajpcell.00327.2007. [DOI] [PubMed] [Google Scholar]
- Lam P, Xu S, Soroka CJ, Boyer JL. A C-terminal tyrosine-based motif in the bile salt export pump directs clathrin-dependent endocytosis. Hepatology. 2012;55(6):1901–1911. doi: 10.1002/hep.25523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenetics and genomics. 2007;17(1):47–60. doi: 10.1097/01.fpc.0000230418.28091.76. [DOI] [PubMed] [Google Scholar]
- Lang T, Haberl M, Jung D, Drescher A, Schlagenhaufer R, Keil A, Mornhinweg E, Stieger B, Kullak-Ublick GA, Kerb R. Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11) Drug Metab Dispos. 2006;34(9):1582–1599. doi: 10.1124/dmd.105.008854. [DOI] [PubMed] [Google Scholar]
- Lecureur V, Sun D, Hargrove P, Schuetz EG, Kim RB, Lan LB, Schuetz JD. Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol. 2000;57(1):24–35. [PubMed] [Google Scholar]
- Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121(6):1473–1484. doi: 10.1053/gast.2001.29608. [DOI] [PubMed] [Google Scholar]
- Maggiore G, Gonzales E, Sciveres M, Redon MJ, Grosse B, Stieger B, Davit-Spraul A, Fabre M, Jacquemin E. Relapsing features of bile salt export pump deficiency after liver transplantation in two patients with progressive familial intrahepatic cholestasis type 2. J Hepatol. 2010;53(5):981–986. doi: 10.1016/j.jhep.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Marschall H-U, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, Silbert D, Fuchsbichler A, Sjovall J, Trauner M. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. Journal of Lipid Research. 2006;47(3):582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- Meier Y, Zodan T, Lang C, Zimmermann R, Kullak-Ublick GA, Meier PJ, Stieger B, Pauli-Magnus C. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World journal of gastroenterology : WJG. 2008;14(1):38–45. doi: 10.3748/wjg.14.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheline D, Emmanuel J, Serge E. Effect of Ursodeoxycholic Acid on the Expression of the Hepatocellular Bile Acid Transporters (Ntcp and bsep) in Rats With Estrogen-Induced Cholestasis. Journal of pediatric gastroenterology and nutrition. 2002;35(2):185–191. doi: 10.1097/00005176-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Misra S, Ujhazy P, Gatmaitan Z, Varticovski L, Arias IM. The role of phosphoinositide 3-kinase in taurocholate-induced trafficking of ATP-dependent canalicular transporters in rat liver. J Biol Chem. 1998;273(41):26638–26644. doi: 10.1074/jbc.273.41.26638. [DOI] [PubMed] [Google Scholar]
- Mochizuki K, Kagawa T, Numari A, Harris MJ, Itoh J, Watanabe N, Mine T, Arias IM. Two N-linked glycans are required to maintain the transport activity of the bile salt export pump (ABCB11) in MCDK II cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G818–G828. doi: 10.1152/ajpgi.00415.2006. [DOI] [PubMed] [Google Scholar]
- Modica S, Bellafante E, Moschetta A. Master regulation of bile acid and xenobiotic metabolism via the FXR, PXR and CAR trio. Front Biosci. 2009;14:4719–4745. doi: 10.2741/3563. [DOI] [PubMed] [Google Scholar]
- Moreno M, Molina H, Amigo L, Zanlungo S, Arrese M, Rigotti A, Miquel JF. Hepatic overexpression of caveolins increases bile salt secretion in mice. Hepatology. 2003;38(6):1477–1488. doi: 10.1016/j.hep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Trauner M, van Staden CJ, Lee PH, Ramachandran B, Eschenberg M, Afshari CA, Qualls CW, Jr., Lightfoot-Dunn R, Hamadeh HK. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010;118(2):485–500. doi: 10.1093/toxsci/kfq269. [DOI] [PubMed] [Google Scholar]
- Muller M, Ishikawa T, Berger U, Klunemann C, Lucka L, Schreyer A, Kannicht C, Reutter W, Kurz G, Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991;266(28):18920–18926. [PubMed] [Google Scholar]
- Ng KH, Le Goascogne C, Amborade E, Stieger B, Deschatrette J. Reversible induction of rat hepatoma cell polarity with bile acids. J Cell Sci. 2000;113(Pt 23):4241–4251. doi: 10.1242/jcs.113.23.4241. [DOI] [PubMed] [Google Scholar]
- Nishida T, Gatmaitan Z, Che M, Arias IM. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci U S A. 1991;88(15):6590–6594. doi: 10.1073/pnas.88.15.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe B, Schliess F, Wettstein M, Heinrich S, Haussinger D. Regulation of taurocholate excretion by a hypo-osmolarity-activated signal transduction pathway in rat liver. Gastroenterology. 1996;110(3):858–865. doi: 10.1053/gast.1996.v110.pm8608896. [DOI] [PubMed] [Google Scholar]
- Noe J, Hagenbuch B, Meier PJ, St-Pierre MV. Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology. 2001;33(5):1223–1231. doi: 10.1053/jhep.2001.24171. [DOI] [PubMed] [Google Scholar]
- Noe J, Kullak-Ublick GA, Jochum W, Stieger B, Kerb R, Haberl M, Mullhaupt B, Meier PJ, Pauli-Magnus C. Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J Hepatol. 2005;43(3):536–543. doi: 10.1016/j.jhep.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Noe J, Stieger B, Meier PJ. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 2002;123(5):1659–1666. doi: 10.1053/gast.2002.36587. [DOI] [PubMed] [Google Scholar]
- Ortiz DF, Moseley J, Calderon G, Swift AL, Li S, Arias IM. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells. J Biol Chem. 2004;279(31):32761–32770. doi: 10.1074/jbc.M404337200. [DOI] [PubMed] [Google Scholar]
- Palmeira CM, Rolo AP. Mitochondrially-mediated toxicity of bile acids. Toxicology. 2004;203(1-3):1–15. doi: 10.1016/j.tox.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 2006;44(4):778–787. doi: 10.1002/hep.21359. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol. 2005;43(2):342–357. doi: 10.1016/j.jhep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. J Biol Chem. 2009;284(15):9947–9954. doi: 10.1074/jbc.M808667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plass JR, Mol O, Heegsma J, Geuken M, de Bruin J, Elling G, Muller M, Faber KN, Jansen PL. A progressive familial intrahepatic cholestasis type 2 mutation causes an unstable, temperature-sensitive bile salt export pump. J Hepatol. 2004;40(1):24–30. doi: 10.1016/s0168-8278(03)00483-5. [DOI] [PubMed] [Google Scholar]
- Roma MG, Crocenzi FA, Mottino AD. Dynamic localization of hepatocellular transporters in health and disease. World journal of gastroenterology : WJG. 2008;14(44):6786–6801. doi: 10.3748/wjg.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman ID, Fernandez-Moreno MD, Fueyo JA, Roma MG, Coleman R. Cyclosporin A induced internalization of the bile salt export pump in isolated rat hepatocyte couplets. Toxicol Sci. 2003;71(2):276–281. doi: 10.1093/toxsci/71.2.276. [DOI] [PubMed] [Google Scholar]
- Roman ID, Monte MJ, Gonzalez-Buitrago JM, Esteller A, Jimenez R. Inhibition of hepatocytary vesicular transport by cyclosporin A in the rat: relationship with cholestasis and hyperbilirubinemia. Hepatology. 1990;12(1):83–91. doi: 10.1002/hep.1840120114. [DOI] [PubMed] [Google Scholar]
- Sachs CW, Chambers TC, Fine RL. Differential phosphorylation of sites in the linker region of P-glycoprotein by protein kinase C isozymes alpha, betaI, betaII, gamma, delta, epsilon, eta, and zeta. Biochemical pharmacology. 1999;58(10):1587–1592. doi: 10.1016/s0006-2952(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Sai Y, Nies AT, Arias IM. Bile acid secretion and direct targeting of mdr1-green fluorescent protein from Golgi to the canalicular membrane in polarized WIF-B cells. J Cell Sci. 1999;112(Pt 24):4535–4545. doi: 10.1242/jcs.112.24.4535. [DOI] [PubMed] [Google Scholar]
- Saito S, Iida A, Sekine A, Miura Y, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y. Three hundred twenty-six genetic variations in genes encoding nine members of ATP-binding cassette, subfamily B (ABCB/MDR/TAP), in the Japanese population. Journal of human genetics. 2002;47(1):38–50. doi: 10.1007/s10038-002-8653-6. [DOI] [PubMed] [Google Scholar]
- Scheimann AO, Strautnieks SS, Knisely AS, Byrne JA, Thompson RJ, Finegold MJ. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. The Journal of pediatrics. 2007;150(5):556–559. doi: 10.1016/j.jpeds.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Kubitz R, Lizun S, Wettstein M, Haussinger D. Regulation of the dynamic localization of the rat Bsep gene-encoded bile salt export pump by anisoosmolarity. Hepatology. 2001;33(3):509–518. doi: 10.1053/jhep.2001.22648. [DOI] [PubMed] [Google Scholar]
- Siebold L, Dick AA, Thompson R, Maggiore G, Jacquemin E, Jaffe R, Strautnieks S, Grammatikopoulos T, Horslen S, Whitington PF, Shneider BL. Recurrent low gamma-glutamyl transpeptidase cholestasis following liver transplantation for bile salt export pump (BSEP) disease (posttransplant recurrent BSEP disease) Liver transplantation. 2010;16(7):856–863. doi: 10.1002/lt.22074. [DOI] [PubMed] [Google Scholar]
- Siewert E, Dietrich CG, Lammert F, Heinrich PC, Matern S, Gartung C, Geier A. Interleukin-6 regulates hepatic transporters during acute-phase response. Biochemical and biophysical research communications. 2004;322(1):232–238. doi: 10.1016/j.bbrc.2004.07.102. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Devereaux M, Dahl R, Gumpricht E. “Let there be bile”--understanding hepatic injury in cholestasis. Journal of pediatric gastroenterology and nutrition. 2006;43(Suppl 1):S4–9. doi: 10.1097/01.mpg.0000226384.71859.16. [DOI] [PubMed] [Google Scholar]
- Song X, Kaimal R, Yan B, Deng R. Liver receptor homolog 1 transcriptionally regulates human bile salt export pump expression. J Lipid Res. 2008;49(5):973–984. doi: 10.1194/jlr.M700417-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroka CJ, Pate MK, Boyer JL. Canalicular export pumps traffic with polymeric immunoglobulin A receptor on the same microtubule-associated vesicle in rat liver. J Biol Chem. 1999;274(37):26416–26424. doi: 10.1074/jbc.274.37.26416. [DOI] [PubMed] [Google Scholar]
- Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G211–218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci U S A. 2006;103(30):11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handbook of experimental pharmacology. 2011;(201):205–259. doi: 10.1007/978-3-642-14541-4_5. [DOI] [PubMed] [Google Scholar]
- Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118(2):422–430. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- Stieger B, O’Neill B, Meier PJ. ATP-dependent bile-salt transport in canalicular rat liver plasma-membrane vesicles. Biochem J. 1992;284(Pt 1):67–74. doi: 10.1042/bj2840067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Nemeth A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20(3):233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerova D, Rayner A, Dutton L, Meier Y, Antoniou A, Stieger B, Arnell H, Ozcay F, Al-Hussaini HF, Bassas AF, Verkade HJ, Fischler B, Nemeth A, Kotalova R, Shneider BL, Cielecka-Kuszyk J, McClean P, Whitington PF, Sokal E, Jirsa M, Wali SH, Jankowska I, Pawlowska J, Mieli-Vergani G, Knisely AS, Bull LN, Thompson RJ. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134(4):1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Kagalwalla AF, Tanner MS, Knisely AS, Bull L, Freimer N, Kocoshis SA, Gardiner RM, Thompson RJ. Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. American journal of human genetics. 1997;61(3):630–633. doi: 10.1086/515501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PJ, Ko YH, Pedersen PL. Altered protein folding may be the molecular basis of most cases of cystic fibrosis. FEBS letters. 1992;312(1):7–9. doi: 10.1016/0014-5793(92)81399-7. [DOI] [PubMed] [Google Scholar]
- Tirona RG. Molecular mechanisms of drug transporter regulation. Handbook of experimental pharmacology. 2011;(201):373–402. doi: 10.1007/978-3-642-14541-4_10. [DOI] [PubMed] [Google Scholar]
- Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiological reviews. 2003;83(2):633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- Trauner M, Wagner M, Fickert P, Zollner G. Molecular regulation of hepatobiliary transport systems: clinical implications for understanding and treating cholestasis. Journal of clinical gastroenterology. 2005;39(4 Suppl 2):S111–124. doi: 10.1097/01.mcg.0000155551.37266.26. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011;108(46):18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mil SW, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull LN, van den Berg IE, Berger R, Houwen RH, Klomp LW. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127(2):379–384. doi: 10.1053/j.gastro.2004.04.065. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Dutt P, Lippincott-Schwartz J, Arias IM. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci U S A. 2005;102(42):15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15(7):3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Dong H, Soroka CJ, Wei N, Boyer JL, Hochstrasser M. Degradation of the bile salt export pump at endoplasmic reticulum in progressive familial intrahepatic cholestasis type II. Hepatology. 2008;48(5):1558–1569. doi: 10.1002/hep.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Soroka CJ, Boyer JL. The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J Clin Invest. 2002;110(7):965–972. doi: 10.1172/JCI15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DJ, Chen H, Cantin LD, Kenna JG, Stahl S, Walker CL, Noeske T. Mitigating the inhibition of human bile salt export pump by drugs: opportunities provided by physicochemical property modulation, in silico modeling, and structural modification. Drug Metab Dispos. 2012;40(12):2332–2341. doi: 10.1124/dmd.112.047068. [DOI] [PubMed] [Google Scholar]
- Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression. Hepatology. 2009;50(5):1588–1596. doi: 10.1002/hep.23151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Heslegrave AJ, Muckett PJ, Levene AP, Clements M, Mobberley M, Ryder TA, Abu-Hayyeh S, Williamson C, Goldin RD, Ashworth A, Withers DJ, Carling D. LKB1 is required for hepatic bile acid transport and canalicular membrane integrity in mice. Biochem J. 2011;434(1):49–60. doi: 10.1042/BJ20101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World journal of gastroenterology : WJG. 2012;18(36):4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lo JL, Huang L, Zhao A, Metzger E, Adams A, Meinke PT, Wright SD, Cui J. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002;277(35):31441–31447. doi: 10.1074/jbc.M200474200. [DOI] [PubMed] [Google Scholar]
- Zinchuk V, Zinchuk O, Okada T. Experimental LPS-induced cholestasis alters subcellular distribution and affects colocalization of Mrp2 and Bsep proteins: a quantitative colocalization study. Microscopy research and technique. 2005;67(2):65–70. doi: 10.1002/jemt.20184. [DOI] [PubMed] [Google Scholar]