Abstract
OBJECTIVES
Olfactory loss is a debilitating symptom of chronic rhinosinusitis. To study the impact of inflammation on the olfactory system, the Inducible Olfactory Inflammation (IOI) transgenic mouse was created in which inflammation can be turned on and off within the olfactory epithelium. In this study, the type II TNF receptor (TNFR2) was knocked out, and the effect on the olfactory loss phenotype was assessed.
METHODS
IOI mice were bred to TNFR2 knockout mice to yield progeny IOI mice lacking the TNFR2 receptor (TNFR2−/−). TNF-α expression was induced within the olfactory epithelium for 6 weeks to generate chronic inflammation. Olfactory function was assayed by electro-olfactogram (EOG), and olfactory tissue was processed for histology and immunohistochemical staining.
RESULTS
Compared to IOI mice with wild type TNFR2, IOI mice lacking the TNFR2 demonstrated similar levels of inflammatory infiltration and enlargement of the subepithelial layer. However, IOI-TNFR2−/− mice differed markedly in that the neuronal layer was largely preserved and active progenitor cell proliferation was present. Odorant responses were maintained in the IOI-TNFR2−/− mice, in contrast to IOI mice.
CONCLUSIONS
TNFR2 is the minor receptor for TNF-α, but appears to play an important role in mediating TNF-induced disruption of the olfactory system. This finding suggests that neuronal death and inhibition of proliferation in CRS may be mediated by TNFR2 on olfactory neurons and progenitor cells. Further studies are needed to elucidate the subcellular pathways involved and develop novel therapies for treating olfactory loss in the setting of CRS.
Keywords: olfactory loss, rhinosinusitis, TNF-alpha, TNFR2, transgenic model
INTRODUCTION/BACKGROUND
Among the symptoms associated with chronic rhinosinusitis (CRS), olfactory loss can have a particularly negative impact on quality of life 1–3. Moreover, the loss of the sense of smell presents a health risk, because of an inability to detect dangers such as smoke, natural gas, or rotting food. The cause of olfactory dysfunction in CRS is incompletely understood. While obstruction of airflow to the olfactory cleft is likely a major contributing factor, chronic sinonasal inflammation can also lead to pathologic alterations in peripheral olfactory structure and physiology 4. Olfactory receptor neurons and their progenitor cells reside within the nasal mucosa in the olfactory cleft 5. They have a remarkable capacity for regeneration, but, nevertheless are susceptible to local immune mediators in the setting of chronic rhinosinusitis 5. It is believed that longstanding tissue damage results in replacement of the specialized olfactory neuroepithelium with respiratory mucosa 4. However, the relatively rapid reversibility of CRS-associated olfactory loss with systemic corticosteroids observed in some patients suggests that other mechanisms, which are perhaps cytokine-mediated, decrease olfactory function.
A transgenic mouse model of inducible olfactory inflammation (IOI) has recently been developed to provide insight into the pathophysiology of the human disease 6. In this IOI mouse model system, which has been previously described by our group 7–9, tissue-specific expression of inflammatory cytokines can be controlled temporally by administration of the antibiotic doxycycline. Our initial studies have employed the CRS-associated cytokine, tumor necrosis factor alpha (TNF-α), for which in vitro evidence had suggested a direct effect on olfactory neurons and their progenitors 10–11. Chronic inflammation induced by TNF-α causes neuronal apoptosis and suppression of normal olfactory regeneration 6. Also, there is a decrease in the electrophysiologic response to odorant stimulation in these mice that occurs before neuronal loss is evident 6, 8. Expression of TNF-α results in a progressive inflammatory infiltrate that mimics mucosal inflammation in CRS. In the IOI mouse, once administration of doxycycline is discontinued, the inflammatory infiltrate resolves rapidly and the olfactory epithelium regenerates. Simultaneous treatment of the mice with systemic corticosteroids inhibits the inflammatory response and preserves the normal architecture in some portions of the olfactory mucosa 9. However, steroids cannot modulate transgene TNF-α expression, and thus the decrease in odorant responses remains.
TNF-α is a pleiotrophic cytokine with diverse roles in multiple cell types and organ systems. It is associated with most infectious and inflammatory diseases, including rhinosinusitis. The binding of TNF-α to its receptors results in recruitment of signal transducers that act on complex signaling cascades and networks. There are two receptors for TNF-α that have been described. Both are members of the TNF receptor superfamily characterized by cysteine-rich pseudorepeats in their extracellular regions 12–13. The type I TNF receptor (TNFR1), or p55 receptor, is the dominant receptor that is expressed in all cell types and believed to responsible for most effects of the cytokine 14. TNFR1 is activated by both membrane-bound and soluable forms of TNF-α. Research on TNF signaling is derived mostly from the TNFR1-dependent pathway. TNF-α activation can initiate one or more of the three pathways: activation of NF-κB, activation of MAPK, and induction of death signaling 13, 15. The NF-κB pathway is primarily responsible for the inflammatory response; the MAPK pathway is primarily responsible for cell differentiation and proliferation; the induction of death signaling pathway is primarily responsible for caspase-induced cell apoptosis. Activation of TNFR1 appears to be sufficient to induce the most common TNF responses 16–18.
The role of the type II TNF receptor (TNFR2), or p75 receptor, is less well characterized and presumed to have an accessory function. Experimental results reported in the literature do not demonstrate a consistent activity of TNFR2 in mediating TNF-α responses. In some cell types, TNFR2 can independently mediate cellular responses like activation of NF-κB 18–19, proliferation 20, and cell death 21–24. TNF-α has been shown to induce apoptosis of mature olfactory receptor neurons in olfactory epithelium explants 11. However, its role in human olfaction physiology is unknown. In this study, we utilize the inducible olfactory inflammation model to explore the importance of the TNFR2 in the development of the olfactory loss phenotype. The findings suggest a previously unrecognized role of this receptor in mediating olfactory neuron death and progenitor cell proliferation in inflammatory olfactory loss.
MATERIALS & METHODS
Inducible Olfactory Inflammation (IOI) Mouse
The creation of the IOI mouse line has been described previously 6. Briefly, the reverse tetracycline transactivator gene was knocked into the olfactory-specific cyp2g1 coding region generating a cyp2g1-rtTA strain. This line was crossed with a line containing the TNF-α gene under the control of a tetracycline-responsive element (TRE-TNF-α) to generate the inducible inflammation model (IOI mouse). The IOI mouse line was bred to a strain deficient in the tumor necrosis factor receptor superfamily, member 1b (Tnfrsf1b or TNFR2) (Jackson Laboratory, Bangor ME), until homozygous progeny were achieved that were homozygous for TNFR2 knockout and carried the IOI genotype (IOI-TNFR2−/−). Doxycycline (DOX) was used to induce TNF-α expression in adult mice between the ages of 6 and 8 weeks old.
Histologic Analyses
After sacrifice by CO2 inhalation, the mice were decapitated and the heads were fixed and decalcified by immersion in TBD2 solution (Shandon, Pittsburgh, PA) for 24 hours. The heads were embedded in paraffin, and 6-μm sections were obtained and collected on glass slides for hemotoxylin and eosin staining. For frozen section analysis, the mice were anesthetized by an intraperitoneal injection of 100 mg/kg of ketamine hydrocholoride/xylazine hydrochloride solution (Sigma, St. Louis, MO), before intracardiac perfusion with 4% paraformaldehyde. The olfactory tissue was then dissected, postfixed in 4% paraformaldehyde overnight, and transferred to a solution of 30% sucrose and 250 mM of EDTA for 48 hours. The decalcified heads were then infiltrated with TFM tissue freezing medium (Triangle Biomedical Sciences, Inc., Durham, NC) and frozen on dry ice into a plastic mold. Sections of mouse olfactory tissue in OCT were cut on a cryostat (10 μm), placed on Super-frost plus slides (Fisher Scientific, Pittsburgh, PA), and dried 4° C overnight. Slides were stored −80°C for future staining.
Immunohistochemistry
Cryostat sections were microwaved at 60% power for 10 minutes in 0.01M Citrate Buffer, pH 6.0, washed in phosphate-buffered saline (PBS) and were blocked for 1 hour in PBS containing 10% normal goat serum. Slides were incubated overnight at 4°C in 5% normal serum containing primary antibody to keratin 5 (1:500) (K5, Covance, Princeton, NJ), NCAM (1:1000) (Millipore, Billerica, MA) or CD-68 (1:1000) (Serotec, Raleigh, NC). Primary antibodies were detected using 1:200 dilution of fluorescent tagged secondary antibodies (Alexa-fluor, Invitrogen, Carlsbad, CA; Dylight, Jackson ImmunoResearch, West Grove, PA). Each sample was counterstained by the nuclear stain, DAPI (Vector Labs, Burlingame, CA). Images were viewed using a LSM510 confocal microscope (Carl Zeiss Micro-imaging). Measurements of K5 fluorescent intensity along a zone 4 turbinate were made using Axiovision 4.8 software.
Bromodeoxyuridine Labeling
Mice were injected intraperitoneally with bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO), 50 μg/g of body weight, 120 minutes before sacrifice. Cryostat sections were then incubated with 3 N HCl for 30 minutes and treated with proteinase K in Tris-Edta buffer for 10 minutes before immunostaining with rat anti-BrdU antibody (1:100) (Abcam, Cambridge, MA). Primary antibodies were detected using a fluorescent tagged secondary antibody (Alexa-fluor, Invitrogen, Carlsbad, CA). Each sample was counterstained by the nuclear stain, DAPI (Vector Labs, Burlingame, CA). Images were viewed using a LSM510 confocal microscope.
Electro-olfactogram (EOG)
EOGs were performed according to previously published methods. 6,7,9 The medial surface of the olfactory turbinates was prepared for recording after the mouse was sacrificed using CO2. Odorant solutions (Aldrich, St. Louis, MO) were prepared in DMSO and diluted with water to the working concentration just before EOG recording. Test odorants for air delivery were prepared at liquid concentrations of 10−3 [final DMSO concentration of 0.2% (v/v)], and diluted to 10−4 and 10−5 M concentrations. Responses to DMSO diluent alone were measured. Odorant stimulation was delivered in the vapor phase as a 100-millisecond pulse by injection into the continuous stream of humidified air. The odorant stimulus pathway was cleaned by air between each stimulus presentation with a minimum interval of 1 minute between two adjacent stimuli. Field potentials were measured with two electrodes, placed on either turbinate IIb or turbinate III, to acquire simultaneous recordings. Data was analyzed with Clampfit (Axon Instruments, Union City, CA). Peak amplitudes were determined from pre-pulse baseline, but no other data normalizations were performed.
RESULTS
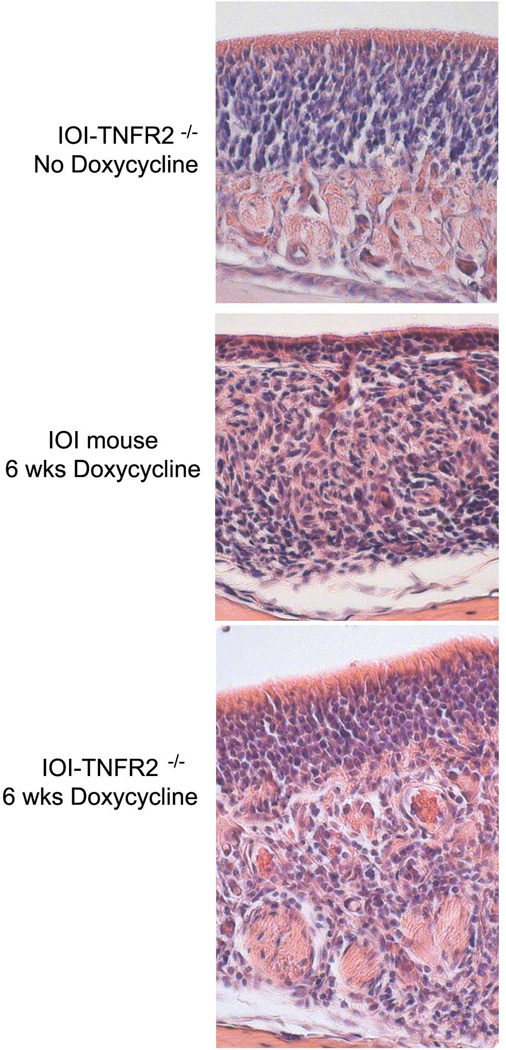
In the absence of inflammation, the olfactory epithelium (OE) demonstrates an apical single layer of sustentacular cells overlying a densely packed layer of olfactory sensory neurons and their progenitors (figure 1, top panel). Multipotent stem cells (horizontal basal cells and globose cells) reside in the most basal aspect of the neuronal layer. Below the basement membrane in the subepithelium are well-demarcated axon bundles. In the absence of doxycycline administration, the gross olfactory histologic phenotypes of the IOI and IOI-TNFR2−/− mice are identical and normal. After 6 weeks of doxycycline-induced TNF-α expression in IOI mice, the OE is significantly thinned (figure 1, middle panel). There is a near complete loss of the population of mature olfactory receptor neurons, although the sustentacular layer remains intact. In the subepithelium, there is disruption in the architecture with inflammatory cell infiltration and absent axon bundles. In the IOI-TNFR2−/− mice after 6 weeks of doxycycline-induced TNF-α expression, there is a subepithelial inflammatory infiltrate that is comparable to IOI mouse (figure 1, bottom panel). However, the OE in many areas is similar in thickness to that of the uninflamed mouse, and axon bundles are present, although compressed and irregular.
Figure 1.
Histopathologic features of the olfactory epithelium in the IOI mouse and TNFR2 knockout. Top panel: In the absence of dox induction of TNF-α, the olfactory epithelium has a normal appearance with apical sustentacular cells, a thick layer olfactory neurons and their basal progenitors, and a loose subepithelial space below the basement membrane with blood vessels, glands, and axon bundles. Middle panel: Within 6 weeks of doxycycline-induced TNF-α expression in IOI mice, the olfactory neuronal layer becomes dramatically thinned due to widespread death of olfactory neurons. The sustentacular cells remain intact. There is prominent subepithelial infiltration by inflammatory cells and axon bundles are largely absent. Bottom panel: In IOI-TNFR2−/− mice, 6 weeks of doxycycline-induced TNF-α expression, the olfactory neuron layer remains relatively intact, despite a subepithelial infiltration of inflammatory cells comparable to that observed in the IOI mouse. Axon bundles appear reduced in size and irregular, likely secondary to crowding by subepithelial inflammatory cells.
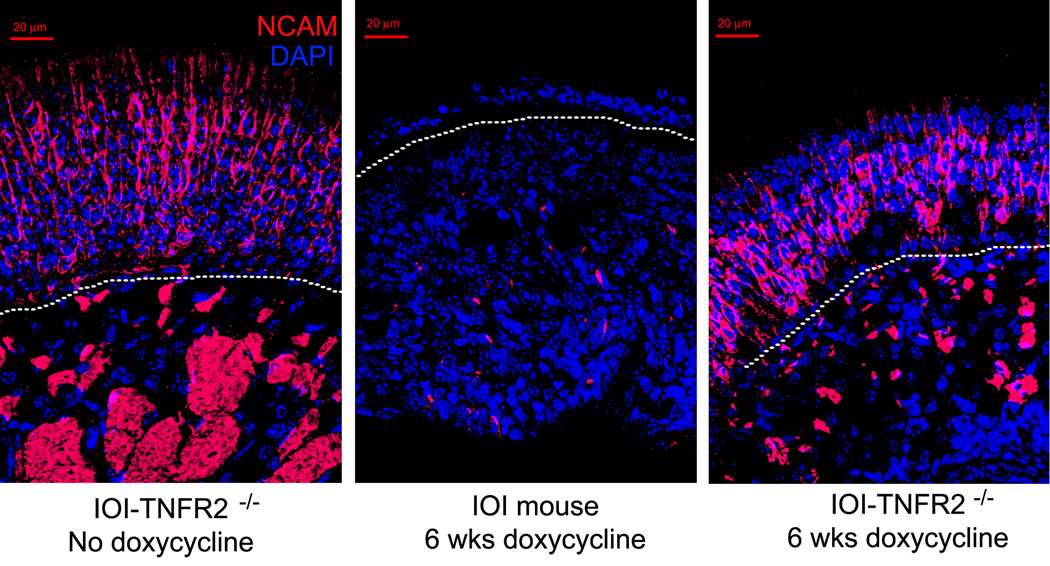
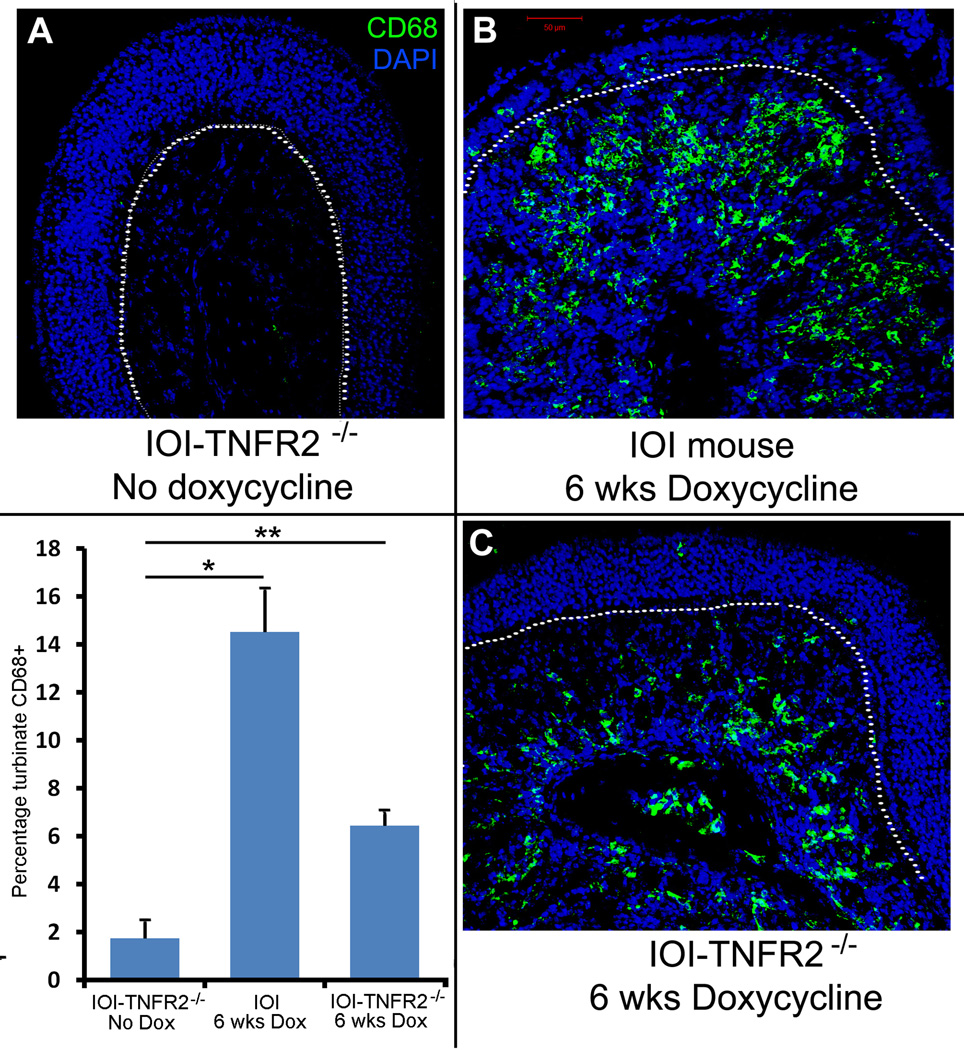
To better visualize the changes suggested by the histologic sections, immunohistochemical staining was performed for a series of markers. First, the population of olfactory neurons and their axon bundles were labeled with an antibody to neural cell adhesion molecules (NCAM). As seen in figure 2, in the absence of inflammation, NCAM is highly expressed in the cytoplasm of the olfactory neurons within the OE and in the axon bundles within the subepithelium. NCAM staining is almost completely absent in the IOI mouse after 6 weeks of doxycycline. In the IOI-TNFR2−/− mice, the neurons are preserved, although the axon bundles are smaller. Using an antibody to CD68, a protein heavily expressed by tissue macrophages 25, the inflammatory cell infiltrate was better demarcated (figure 3). This reveals that the changes in the axon bundle size and shape in IOI-TNFR2−/− mice are due to inflammatory cell infiltration and crowding within the subepithelium. Taken together, these findings suggest that loss of TNFR2 does not significantly impact the inflammatory response to chronic TNF-α, but does prevent the destruction of the olfactory neuron layer that we have reported in IOI mice.
Figure 2.
Neural cell adhesion molecule (NCAM) staining of olfactory epithelium. Left panel: At baseline, control mice express NCAM diffusely within the cytoplasm of olfactory sensory neurons as well as within the axon bundles. Center panel: After 6 weeks of doxycycline-induced TNF-α expression, IOI mice show almost no NCAM expression. Right panel: After 6 weeks of doxycycline-induced TNF-α expression, IOI-TNFR2−/− mice demonstrate NCAM expression within the olfactory neuroepithelium and axon bundles. Note the smaller size of the axon bundles due to compression by infiltrating inflammatory cells.
Figure 3.
CD68 staining of olfactory epithelium. CD68 is a glycoprotein that is expressed on monocytes and macrophages. A: At baseline, control mice show no expression of CD68+ cells. B&C: After 6 weeks of doxycycline-induced TNF-α expression, IOI mice (B) and IOI-TNFR2−/− mice (C) demonstrate diffuse CD68 expression within the subepithelium, representing an inflammatory cell infiltrate. As depicted in the graph lower left panel, there is a statistically significant increase in the percentage of turbinate tissue with CD68 staining in both IOI and IOITNFR2−/− mice as compared to untreated mice. *p=0.009, **p=0.035. Error bars represent standard error of the mean.
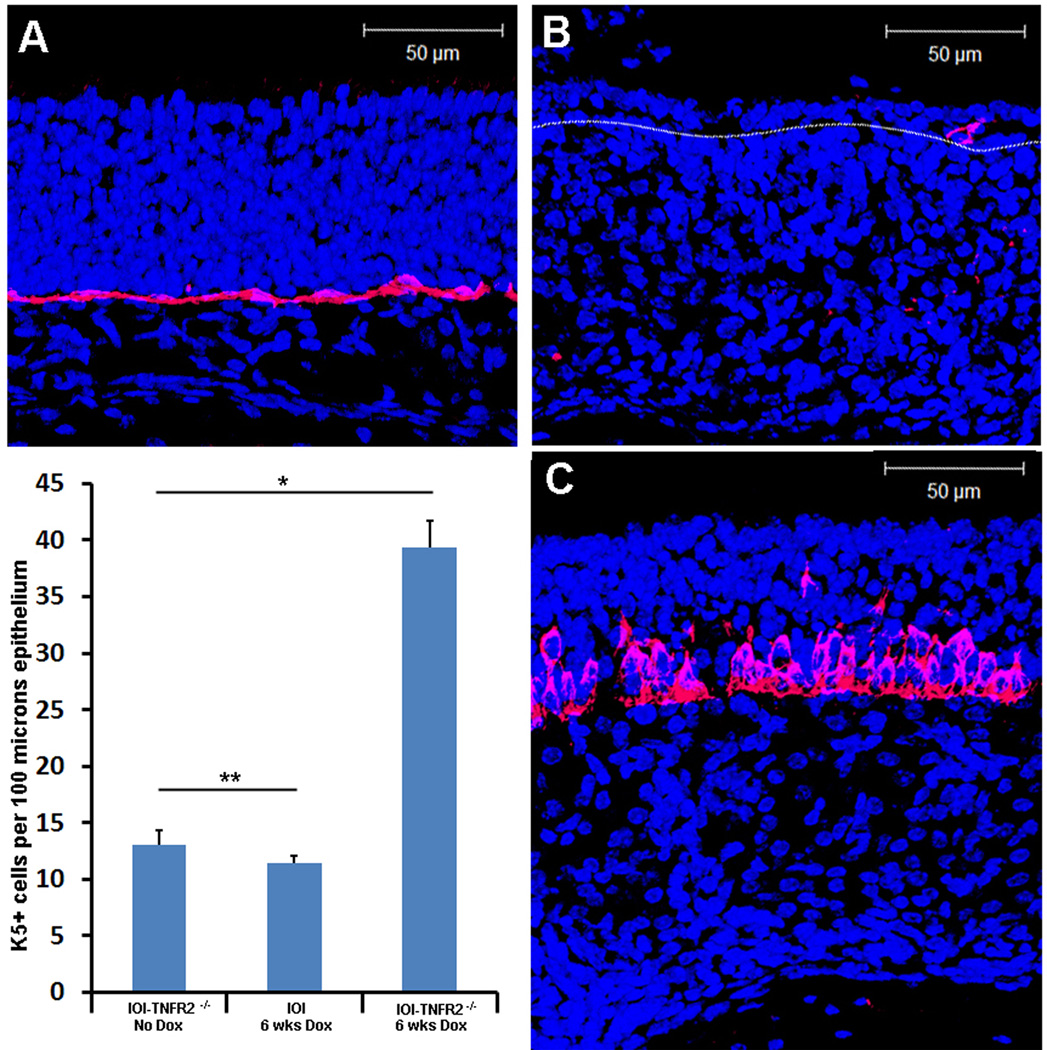
Horizontal basal cells (HBC) lie in the basal layer of the OE and contribute to normal turn-over and injury-induced neurogenesis 26. These cells are normally quiescent but undergo a proliferative burst in the setting of extensive olfactory neuron depletion to repopulate all olfactory epithelial cell compartments 27. Keratin 5 (K5) is specifically expressed on HBCs and it used as a identifying marker in immunohistochemical analysis 28. We employed K5 immunohistochemical staining to identify HBC in the OE (figure 4). In uninflamed mice, the basal aspect of the OE shows a uniform layer of HBC directly above the basement membrane (figure 4A). After 6 weeks of doxycycline-induced TNF-α expression in IOI mice, there is diminished expression of K5 in the thinned epithelium (figure 4B). This suggests that the HBC population in inflamed regions was destroyed, depleted, or lost K5 expression. However, in IOI-TNFR2−/− mice after 6 weeks of doxycycline-induced TNF-α expression, K5+ cells persist above the basement membrane, and in some places appear to form multiple layers (figure 4C). The increased number of K5+ cells after TNF- α induction is highly statistically significant (p=0.006). This unexpected finding suggests that TNFR2 may be important in mediating the loss of olfactory stem cells in the setting of prolonged inflammation.
Figure 4.
K5+ olfactory progenitor cells are abundantly present in IOI-TNFR2−/− mice after chronic TNF-α expression. A: The K5+ cell population (horizontal basal cells) are normally present as a uniform single layer of immediately above the basal lamina. B: In IOI mice expressing TNF-α for 6 weeks, there is a near complete absence of K5 expression in the markedly thinned olfactory epithelium. C: In IOI-TNFR2−/− mice, horizontal basal cells are not only present, but appear to be in multiple layers with heterogeneous cell sizes. Differences in K5-expressing cells are represented graphically in the lower left panel. *p=006, **p=0.043. Error bars represent standard error of the mean.
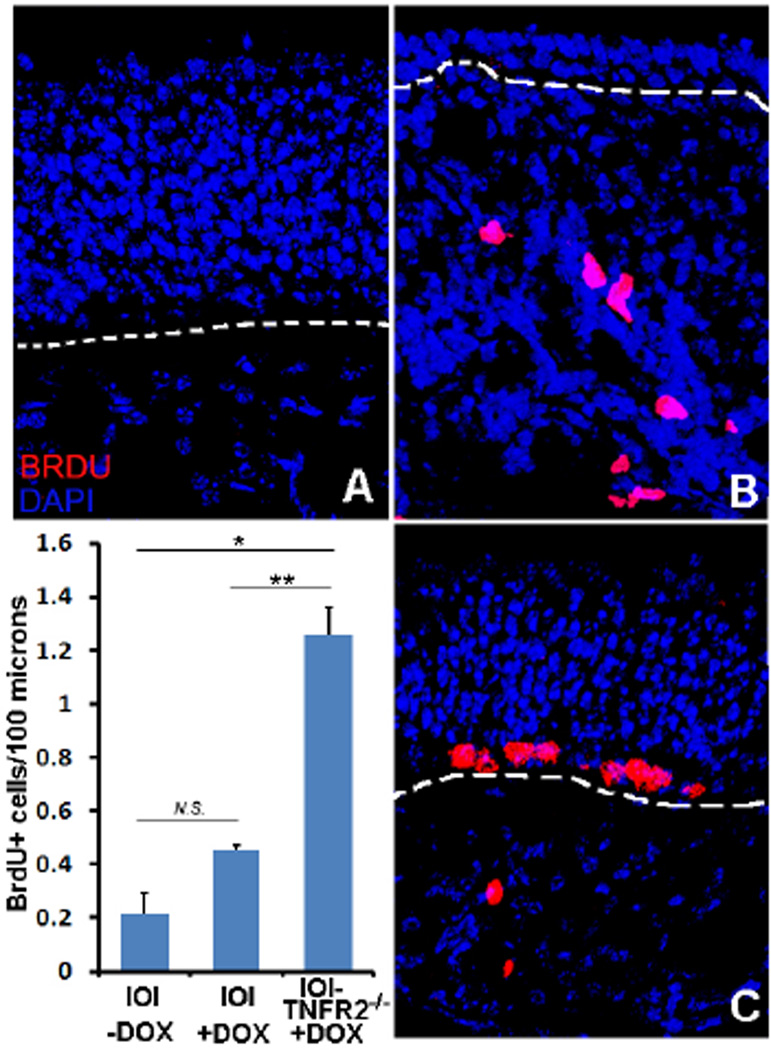
A key feature of the IOI mouse is the absence of progenitor cell proliferation and regeneration of the OE during active inflammation. This is notable, because other experimental insults to the OE result in very robust expansion of progenitor cells and rapid reconstitution of the OE. In order to determine whether the maintenance of stem cells in IOI-TNFR2−/− mice was associated with ongoing OE regeneration, we utilized bromodeoxyuridine (BrdU) staining. 29. Dividing cells in the OE were labeled with BrdU and visualized with a fluorescent anti-BrdU antibody (figure 5). As previously reported, the normal level of cell division in the OE is very low in the uninflamed state (figure 5A) 6. After 6 weeks of doxycycline induction, BrdU+ cells above the basement membrane remain rare in the IOI mouse (figure 5B, center panel), although dividing cells may be seen in the lamina propria. However, after 6 weeks of doxycycline-induced TNF-α expression in the IOI-TNFR2−/− mice, there is active proliferation in the basal layer of the epithelium, with seven to eight BrdU+ cells observed per high power field (figure 5C). The increased number of proliferating cells in the doxycycline-treated IOI-TNFR2−/− mice versus IOI mice is statistically significant (p=0.03). Our previous studies had suggested that TNF-α directly inhibited progenitor cell proliferation 7, and these current results further support a TNFR2-dependent pathway.
Figure 5.
Active proliferation of basal cells in IOI-TNFR2−/− mice. BrdU labeling was used to visualilze proliferating progenitor cells in the OE. A: Dividing cells are rare in adult mice in the absence of injury or perturbation. B: After 6 weeks of doxycycline-induced TNF-α expression in IOI mice, there are also no BrdU expression in the OE, although dividing inflammatory cells in the subepithelium may be seen. C: After doxycycline-induced TNF-α expression for 6 weeks in the IOI-TNFR2−/− mice, BrdU+ cells can be seen in the basal layer of the olfactory epithelium. Lower left panel: graph depicting differences in BrdU labeling. *p= 0.02, **p=0.03. Error bars represent standard error of the mean.
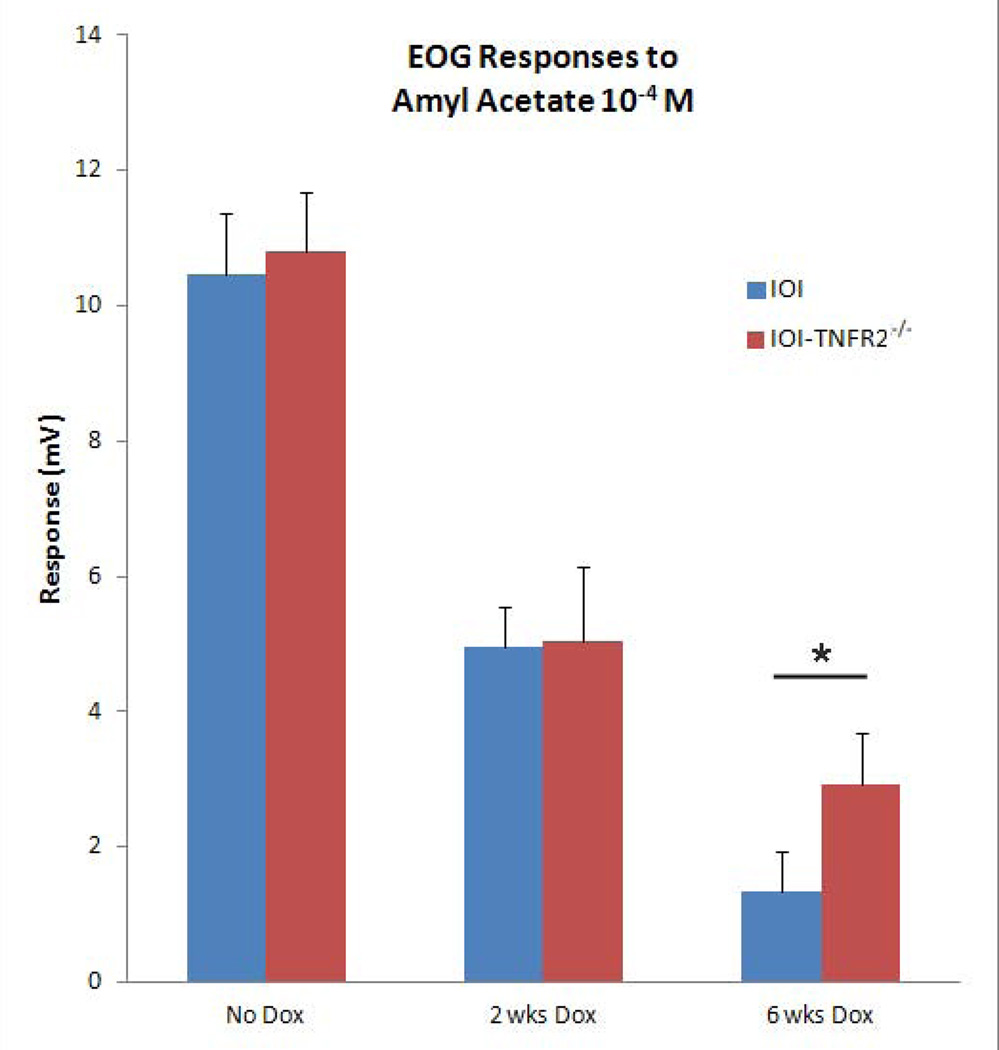
Lastly, we examined the consequences of TNF-α on sensory function. The responses of mature olfactory neurons to odorants were assessed by electro-olfactogram (EOG) recordings (figure 6). Odorant responses were reduced approximately 60% in both IOI and IOI-TNFR2−/− groups at 2 weeks. Of note, our previous study showed that the histological changes seen in IOI mice were not present at this time point 6. After 6 weeks of doxycycline, there is further reduction of odorant responses in both groups. The IOI-TNFR2−/− mice had slightly higher EOG amplitudes at 6 weeks compared to EOG responses in IOI mice, although this did not achieve statistical significance (p=0.06). These results are consistent with our previous hypothesis that TNF-α expression directly results in a desensitization of the odorant response. While the olfactory loss in the IOI mouse becomes profound due to a near total loss of neurons at 6 weeks, the olfactory neuron dysfunction in the IOI-TNFR2−/− mouse is less complete because the neurons remain intact. This suggests that TNF-α modulation of olfactory neuron function is not mediated completely via TNFR2.
Figure 6.
Effect of TNFR2 knockout on functional odorant responses in TNF-α-induced olfactory inflammation. Normal baseline electro-olfactogram (EOG) responses in uninflamed IOI and IOI-TNFR2−/− mice are shown in the first column. After 2 weeks of doxycycline treatment, the EOG responses are reduced in both groups. There is further reduction in odorant response at 6 weeks in both groups, with the IOI-TNFR2−/− group demonstrating slightly higher response amplitudes than the IOI group. This difference did not achieve statistical significance (p=0.06, Student's T-test). The data represent average responses from a minimum of 4 independent recordings. Error bars represent SEM.
DISCUSSION
The IOI mouse model provides a robust system to study inflammation-induced olfactory loss. In the present study, we show that IOI mice lacking the TNFR2 receptor (IOI-TNFR2−/−) develop similar levels of inflammatory cell infiltration and enlargement of the subepithelial layer as do IOI mice with wild type TNFR2 (IOI-TNFR2+/+). However, the IOI-TNFR2−/− mice demonstrate comparative preservation of the neuronal layer and active progenitor cell proliferation, while the IOI mice display progressive death of olfactory receptor neurons. Odorant responses were maintained to a greater extent in the IOI-TNFR2−/− mice, in contrast to the IOI mice, although both were significantly affected, suggesting that TNFR2 does not mediate TNF-α-induced dysfunction of mature olfactory receptor neurons.
TNF-α is a cytokine generally associated with infection, injury, and inflammation throughout the body in diverse disease states, including CRS. The specific mechanisms of TNF-α expression and signaling on olfactory dysfunction have yet to be fully elucidated. Much of the research on TNF-α and inflammation have focused on the TNFR1 receptor, which is believed to be the dominant receptor in inducing TNF responses 16–18. The function of TNFR2 is less well understood. Experimental results reported in the literature do not demonstrate a defined consistent activity of TNFR2 in mediating TNF-α responses 13. TNF-α signaling pathways are complex, and the recent literature suggests functional cross-talk may occur between the two receptors as well as autoregulation of the TNFR2 receptor 13.
There has been growing evidence for an independent role of TNFR2 in immune and inflammatory reactions 30–33. TNFR2 has been shown to maintain and exert tumor cytolytic activity in vitro and induce tumor necrosis in vivo in studies using receptor-specific agonist antibodies that selectively bind TNFR1 and TNFR2 34–36. Recent work with TNFR knock-out mice has highlighted the once overlooked effects of the TNFR2 receptor. Using both single and double knock-out TNFR mice, Zhao et al. showed that TNFR2 expressed on host innate immune cells is sufficient to mediate the antitumor effect of TNF 37. TNFR2 has been shown to play important role in inflammation in the kidney 38, gastrointestinal system 39–40, and lung 13. Our study is the first to use TNFR2 knock-out mice to study the olfactory system, and also the first to implicate a role of TNFR2 in inflammation of the olfactory epithelium.
Our previous investigation into the role of TNF-α in inflammatory olfactory loss provided evidence that TNF-α has both direct and indirect mechanisms that lead to olfactory dysfunction 9. The addition of prednisolone to IOI mouse model diminishes the indirect effects that occur via downstream mediators, thus isolating the direct effects of transgene-mediated TNF-α expression on the olfactory epithelium. Prednisolone resulted in the preservation of large areas of olfactory epithelium, suggesting that neuronal apoptosis was not directly mediated by TNF-α. At the same time, diminished olfactory function remained despite steroid treatment, which may indicate a direct effect of TNF-α. A possible mechanism would be increased neuronal intracellular calcium levels due to TNF-α signaling, leading to feedback desensitization of the odorant electrical response. These findings led us to our current study which attempts to isolate the individual roles of the two TNF-α receptors. We hypothesized that differential effects of TNFR1 and TNFR2 would exist in TNF-α-mediated olfactory loss. The results of the present study are consistent with the concept that chronic TNF-α signaling results in desensitization of olfactory neuron odorant responses via TNFR1, whereas TNFR2 mediates suppression of basal progenitor cell proliferation. It remains unclear whether TNFR2 on progenitor cells are directly responsible, or whether TNF-α acts through TNFR2 on another cell type, which in turn secretes mediators that impact progenitor cells via different receptor types. Future studies will be necessary using steroid treatment in the IOI-TNFR2−/− mice, as well as employing conditional knockouts in progenitor cell populations, in order to clarify this issue.
Olfactory loss in the setting of CRS is difficult to treat, and patients often find this symptom to be particularly debilitating. The use of systemic steroids to reduce inflammation has shown some benefit in improving olfaction 41, but it is not a feasible long-term therapy because of significant side-effects and potentially serious health complications. Functional endoscopic sinus surgery, used to remove polyps and allow for more effective delivery of topical anti-inflammatory agents, has limited benefit in improving olfaction 42. Further research is needed to understand the pathophysiology of inflammation-induced olfactory dysfunction in order to develop new therapies that target the specific pathways involved.
CONCLUSION
The IOI transgenic mouse provides a unique model to study mechanisms underlying human olfactory dysfunction. TNF-α is a potent inflammatory cytokine that provokes severe inflammation and olfactory loss in the IOI mouse. Genetic ablation of the type 2 TNF receptor permits new insights into subcellular pathways underlying TNF-α-induced inflammatory olfactory loss. Although TNFR2 has traditionally been considered the minor receptor for TNF-α, findings in the IOI mouse suggest that TNFR2, either through direct or indirect pathways, has an important function in mediating CRS-associated olfactory loss. Neuronal death and inhibition of proliferation in inflammation appears to be dependent in part on TNFR2 expressed by olfactory neurons and their progenitor cells. Further investigations are needed to fully elucidate this complex pathway in animal models and CRS patients. Insights into the role of TNF-α will promote development of novel specific therapies for treating olfactory loss in the setting of CRS.
Acknowledgments
Research supported by NIH DC009026 (A.P.L.).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Presented at the Annual Meeting of the American Rhinologic Society, September 8, 2012, Washington, DC.
REFERENCES
- 1.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125(2):116–121. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- 2.Seo HS, Jeon KJ, Hummel T, Min BC. Influences of olfactory impairment on depression, cognitive performance, and quality of life in Korean elderly. Eur Arch Otorhinolaryngol. 2009;266(11):1739–1745. doi: 10.1007/s00405-009-1001-0. [DOI] [PubMed] [Google Scholar]
- 3.Wrobel BB, Leopold DA. Olfactory and sensory attributes of the nose. Otolaryngol Clin North Am. 2005;38(6):1163–1170. doi: 10.1016/j.otc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope. 2001;111(3):409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doty RL. The olfactory system and its disorders. Semin Neurol. 2009;29(1):74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- 6.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010;30(6):2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner JH, Liang KL, May L, Lane AP. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. Am J Rhinol Allergy. 2010;24(5):336–340. doi: 10.2500/ajra.2010.24.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy. 2010;24(3):192–196. doi: 10.2500/ajra.2010.24.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultan B, May LA, Lane AP. The role of TNF-alpha in inflammatory olfactory loss. Laryngoscope. 2011;121(11):2481–2486. doi: 10.1002/lary.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farbman AI, Buchholz JA, Suzuki Y, Coines A, Speert D. A molecular basis of cell death in olfactory epithelium. J Comp Neurol. 1999;414(3):306–314. doi: 10.1002/(sici)1096-9861(19991122)414:3<306::aid-cne2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Farbman AI. Tumor necrosis factor-alpha-induced apoptosis in olfactory epithelium in vitro: possible roles of caspase 1 (ICE), caspase 2 (ICH-1), and caspase 3 (CPP32) Exp Neurol. 2000;165(1):35–45. doi: 10.1006/exnr.2000.7465. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B, Van Huffel C. An evolutionary and functional approach to the TNF receptor/ligand family. Ann N Y Acad Sci. 1994;730:118–133. doi: 10.1111/j.1749-6632.1994.tb44244.x. [DOI] [PubMed] [Google Scholar]
- 13.Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24(6):1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Englaro W, Bahadoran P, Bertolotto C, et al. Tumor necrosis factor alpha-mediated inhibition of melanogenesis is dependent on nuclear factor kappa B activation. Oncogene. 1999;18(8):1553–1559. doi: 10.1038/sj.onc.1202446. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296(5573):1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 16.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 18.Laegreid A, Medvedev A, Nonstad U, et al. Tumor necrosis factor receptor p75 mediates cell-specific activation of nuclear factor kappa B and induction of human cytomegalovirus enhancer. J Biol Chem. 1994;269(10):7785–7791. [PubMed] [Google Scholar]
- 19.Mukhopadhyay A, Suttles J, Stout RD, Aggarwal BB. Genetic deletion of the tumor necrosis factor receptor p60 or p80 abrogates ligand-mediated activation of nuclear factor-kappa B and of mitogen-activated protein kinases in macrophages. J Biol Chem. 2001;276(34):31906–31912. doi: 10.1074/jbc.M105252200. [DOI] [PubMed] [Google Scholar]
- 20.Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28(1):257–263. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Heller RA, Song K, Fan N, Chang DJ. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992;70(1):47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- 22.Medvedev AE, Sundan A, Espevik T. Involvement of the tumor necrosis factor receptor p75 in mediating cytotoxicity and gene regulating activities. Eur J Immunol. 1994;24(11):2842–2849. doi: 10.1002/eji.1830241139. [DOI] [PubMed] [Google Scholar]
- 23.Grell M, Zimmermann G, Gottfried E, et al. Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J. 1999;18(11):3034–3043. doi: 10.1093/emboj/18.11.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter GT, Kuo RC, Jupp OJ, Vandenabeele P, MacEwan DJ. Tumor necrosis factoralpha mediates both apoptotic cell death and cell proliferation in a human hematopoietic cell line dependent on mitotic activity and receptor subtype expression. J Biol Chem. 1999;274(14):9539–9547. doi: 10.1074/jbc.274.14.9539. [DOI] [PubMed] [Google Scholar]
- 25.Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW. Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry. Ann Rheum Dis. 2004;63(7):774–784. doi: 10.1136/ard.2003.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calof AL, Bonnin A, Crocker C, et al. Progenitor cells of the olfactory receptor neuron lineage. Microsc Res Tech. 2002;58(3):176–188. doi: 10.1002/jemt.10147. [DOI] [PubMed] [Google Scholar]
- 27.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10(6):720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 28.Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363(1):129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- 29.Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457(1):44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 30.Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol. 2004;173(7):4500–4509. doi: 10.4049/jimmunol.173.7.4500. [DOI] [PubMed] [Google Scholar]
- 31.Turner SJ, La Gruta NL, Stambas J, Diaz G, Doherty PC. Differential tumor necrosis factor receptor 2-mediated editing of virus-specific CD8+ effector T cells. Proc Natl Acad Sci U S A. 2004;101(10):3545–3550. doi: 10.1073/pnas.0307347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng GM, Zheng L, Chan FK, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11(10):1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 33.Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280(43):36099–36109. doi: 10.1074/jbc.M505291200. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan KC, Pinckard JK, Arthur CD, Dehner LP, Goeddel DV, Schreiber RD. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J Exp Med. 1995;181(2):607–617. doi: 10.1084/jem.181.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss M, Buhl R, Medve M, Schneider EM. Tumor necrosis factor-alpha modulates the selective interference of hypnotics and sedatives to suppress N-formyl-methionyl-leucyl-phenylalanine-induced oxidative burst formation in neutrophils. Crit Care Med. 1997;25(1):128–134. doi: 10.1097/00003246-199701000-00024. [DOI] [PubMed] [Google Scholar]
- 36.Marr RA, Hitt M, Gauldie J, Muller WJ, Graham FL. A p75 tumor necrosis factor receptor-specific mutant of murine tumor necrosis factor alpha expressed from an adenovirus vector induces an antitumor response with reduced toxicity. Cancer Gene Ther. 1999;6(5):465–474. doi: 10.1038/sj.cgt.7700068. [DOI] [PubMed] [Google Scholar]
- 37.Zhao X, Mohaupt M, Jiang J, Liu S, Li B, Qin Z. Tumor necrosis factor receptor 2-mediated tumor suppression is nitric oxide dependent and involves angiostasis. Cancer Res. 2007;67(9):4443–4450. doi: 10.1158/0008-5472.CAN-07-0185. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285(4):F610–F618. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- 39.Mizoguchi E, Mizoguchi A, Takedatsu H, et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122(1):134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 40.Kim WK, Park JS, Sul OJ, et al. Role of TNFR-related 2 mediated immune responses in dextran sulfate sodium-induced inflammatory bowel disease. Mol Cells. 2011;31(2):99–104. doi: 10.1007/s10059-011-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens MH. Steroid-dependent anosmia. Laryngoscope. 2001;111(2):200–203. doi: 10.1097/00005537-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Jiang RS, Lu FJ, Liang KL, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. Am J Rhinol. 2008;22(4):445–448. doi: 10.2500/ajr.2008.22.3195. [DOI] [PubMed] [Google Scholar]