Abstract
Rationale
Dephosphorylation of extracellular signal-regulated kinase (ERK) and cyclic AMP response element binding protein (CREB) in the dorsomedial prefrontal cortex (dmPFC) at the end of short access (ShA) cocaine self-administration is implicated in cocaine-seeking. However, what receptors and phosphatases mediate this effect and whether ERK/CREB and related phospho-proteins in the dmPFC react similarly during early withdrawal from long access (LgA) cocaine self-administration are unknown.
Objectives
The effects of ShA vs. LgA cocaine self-administration on the phosphorylation of protein phosphatase 2A (PP2A) and striatal-enriched protein tyrosine phosphatase (STEP), as well as GluN and GluA receptor subtype expression in the dmPFC during early withdrawal were compared.
Methods
Rats self-administered cocaine or received saline during 2-hr or 6-hr daily sessions for 10-11 days. Two hr after the final session, the dmPFC was dissected out and processed for immunoblotting.
Results
Similar to previous findings after ShA cocaine, phospho-ERK and phospho-CREB in the dmPFC were decreased after LgA cocaine. Cocaine elevated phospho-PP2A (de-activation) and decreased phosphor-STEP (activation) in both ShA and LgA cocaine rats. GluN1, GluN2B and phospho-GluN2B Tyr1472 in the dmPFC were decreased after ShA and LgA cocaine. Further, a significant reduction of GluA2, GluA1 and phospho-GluA1 Ser845 was found only in LgA rats.
Conclusions
Activation of phospho-STEP may underlie ERK and CREB dephosphorylation in the dmPFC as well as internalization and degradation of GluN complexes during early withdrawal from both ShA and LgA cocaine self-administration whereas differential alteration of AMPA receptor subunits after ShA and LgA cocaine self-administration depends on cocaine intake.
Keywords: CREB, ERK, Glutamate receptors, Phosphatases, Prefrontal cortex, Self-administration, STEP
Introduction
Relapse to drug seeking after withdrawal is the major clinical challenge to the treatment of cocaine addiction (Mendelson and Mello 1996; O’Brien 1997). The susceptibility to drug relapse and other addictive behaviors is dependent on long-term neuroadaptations in several brain regions in the mesocorticolimbic system including the dorsomedial prefrontal cortex (dmPFC- Koob et al. 1997; Wang and McGinty 1999; White and Kalivas 1998). The PFC is the executive center that inhibits impulses or unwanted behaviors and evidence indicates a dysregulation in the PFC after chronic cocaine usage. For example, clinical studies have demonstrated reduced cerebral blood flow, metabolic activity, gray matter volume and white matter integrity in the PFC of human cocaine addicts (Fein et al. 2002; Franklin et al. 2002; Volkow et al. 1992, 1998). Pre-clinically, in a rat cocaine self-administration model, reduced basal activity and increased excitability in the PFC has been reported (Sun and Rebec 2006). Previously, we also showed that the phosphorylation of ERK (p-ERK) and CREB (p-CREB) as well as brain-derived neurotrophic factor (Bdnf) mRNA were downregulated in the dmPFC 2 hr and 22 hr, respectively, after the end of the last cocaine self-administration session (Berglind et al. 2007; McGinty et al. 2010; Whitfield et al. 2011). Further, a single BDNF infusion into dmPFC abrogated the decrease of p-ERK and p-CREB in the dmPFC and normalized a disturbance in glutamate transmission in the nucleus accumbens that resulted in long-term inhibition of cocaine seeking behaviors (Berglind et al. 2007, 2009; Whitfield et al. 2011). Together, the results suggest that attenuated neuronal activity in the dmPFC during early withdrawal plays a critical role in cocaine relapse. However, the molecular mechanisms underlying the p-ERK and p-CREB dephosphorylation during early withdrawal from cocaine are still unknown.
There are several families of ERK-selective phosphatases. Protein phosphatase 2A (PP2A) and striatal-enriched protein tyrosine phosphatase (STEP) are the best characterized. PP2A is a major serine/threonine phosphatase containing two regulatory subunits and one catalytic subunit (PP2Ac). The phosphorylation at Tyr307 of PP2Ac negatively regulates PP2A activity (Chung et al. 1999). PP2A mediates a rapid inactivation of p-ERK in vitro (Alessi et al. 1995). Additionally, PP2A has been implicated in cocaine-mediated molecular changes, cocaine’s rewarding effect, and stress-induced hyperlocomotion in cocaine-sensitized animals (Bauman et al. 2000; Maeda et al. 2006; Taniguchi et al. 2012). Alternatively, through the activation of Raf-1, an upstream activator of MEK, PP2A may induce MEK/ERK pathway activation (Abraham et al. 2000; Wassarman et al. 1996). Recently, Raf-1 was implicated in microRNA-212-mediated CREB induction and cocaine intake of self-administering rats (Hollander et al. 2010). Therefore, PP2A-Raf-1 may indirectly modulate ERK/CREB activation.
STEP is another phosphatase that regulates ERK activation. Although it is enriched in the striatum, STEP is expressed abundantly in the mesocorticolimbic system (Boulanger et al. 1995; Lombroso et al. 1993). Through direct interaction of a kinase-interacting motif, STEP and its non-neuronal homologues have been shown to dephosphorylate p-ERK and prevent its nuclear translocation (Nika et al. 2004; Zuniga et al. 1999). Phosphorylation of STEP (p-STEP) reduces its activity and its capacity to inhibit p-ERK (Nika et al. 2004; Paul et al. 2000). In addition, STEP dephosphorylates and triggers endocytosis of the NMDA receptor subunit, GluN2B, and the AMPA receptor subunits, GluA1 and GluA2 (Goebel-Goody et al. 2010), indicating that tyrosine phosphorylation regulates the surface expression of NMDA and AMPA receptors responsible for synaptic activity. Furthermore, STEP has been implicated in the effects of psychostimulants. Concurrent elevation of p-ERK and p-STEP was documented in the striatum after acute amphetamine or cocaine injection (Sun et al. 2007; Valjent et al. 2005). Further, intra-striatal infusion of a substrate-trapping STEP protein prevented the development of repeated amphetamine-induced stereotypies (Tashev et al. 2009).
Functional AMPA receptors are tetramers with GluA1/2 being the predominant subtypes in many neurons (Lu et al. 2009). Similarly, functional NMDA receptors are most commonly composed of GluN1/2 heterodimers (Ishii et al 1993). The phosphorylation and trafficking of AMPA and NMDA receptors determine the level of excitatory synaptic strength. Phosphorylation of GluA1 Ser845 by cAMP-dependent protein kinase A (PKA) increases the peak open probability and enhanced AMPA receptor-mediated excitatory current by promoting GluA1 plasma membrane insertion (Banke et al. 2000; Oh et al. 2006; Roche et al. 1996). Dephosphorylation triggered by a calcineurin/inhibitor-1 cascade that disinhibits PP1/2A results in endocytosis of GluA1 and long-term depression (Lee et al. 2000; Mulkey et al. 1994). In contrast, the membrane surface expression of GluN2B is regulated by the phosphorylation of Tyr1472 which depends on a balance between the activity of Src family kinases and STEP. Once dephosphorylated at this site, GluN2B associates with the clathrin adaptor protein, promoting its endocytosis (Marsh and McMahon 1999; Nakazawa et al. 2006).
Long access (LgA; 6hr/session) self-administration has been used as a preclinical model to mimic the transition from recreational use of cocaine to addiction in humans. It is characterized by escalation of cocaine intake over time and compulsive drug seeking, despite adverse consequences (Ahmed and Koob 1998; Belin et al. 2008; Mantsch et al. 2004; Paterson and Markou 2003). Although distinct neuroadaptations have been detected at different times in several brain regions, particularly the nucleus accumbens, after LgA vs. short access (ShA) cocaine self-administration, only a few investigations of molecular changes in the PFC after LgA have been reported. Of these studies, most have focused on changes in dopamine and glutamate receptor expression after one or more days of abstinence or extinction (Ben-Shahar et al. 2006, 2007, 2009, 2013; Briand et al. 2008; Crespo et al. 2002).
Based on this background, we first evaluated whether LgA cocaine self-administration results in similar p-ERK and p-CREB dephosphorylation in the dmPFC during early withdrawal (2 hr after the end of the last self-administration session) as detected in our previous ShA experiments (Whitfield et al. 2011). Further, we explored whether phosphorylation of PP2Ac and STEP as well as STEP-regulated GluN2B, GluN1, GluA1, and GluA2 after ShA and LgA cocaine administration is associated with the ERK/CREB dephosphorylation in the dmPFC after cocaine withdrawal.
Experimental procedures
Subjects
Adult male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC; 301-325 g at the beginning of each experiment) were housed individually in a temperature- and humidity-controlled animal facility on a reversed 12-hr light/dark cycle (light off at 7:00 AM). Animals were given 20-25 g of standard rat chow (Harlan, Indianapolis, IN) with ad libitium access to water daily. Experimental procedures were performed during the dark cycle. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and complied with National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996). All efforts were made to minimize animal suffering and reduce the number of animals used.
Surgery
Animals were given at least a 5-day acclimation period. On the day of surgery, rats were anesthetized with a mixture of ketamine (Vedco Inc., St. Joseph, MO) and xylazine (Lloyd Laboratories, Shenandoah, IA) at 66 and 1.33 mg/kg i.p., respectively, followed by equithesin (0.5 ml/kg i.p.) and ketorolac (Sigma-Aldrich, St. Louis, MO at 2 mg/kg i.p.). A silastic catheter was implanted into the right jugular vein and exited subcutaneously on the back to connect to an infusion harness (Instech Laboratories, Plymouth Meeting, PA) as an external port for intravenous infusions. After surgeries, daily 0.1 ml intravenous infusions of the antibiotics, Cefazolin (10 mg/ml) or Timentin (2.4 mg/ml), and 0.1 ml of heparinized saline (100 U/ml) to maintain the catheter patency were administered during surgical recovery and maintained throughout self-administration. Catheter patency was verified as needed with an intravenous infusion of 0.1 ml of methohexital sodium (Eli Lilly & Co., Indianapolis, IN) which produces a rapid loss of muscle tone.
Cocaine self-administration
After 5 days of recovery, one cohort of animals was subjected to 2-hr daily cocaine saline self-administration sessions under a FR1 reinforcement schedule in self-administration chambers (30 × 20 × 20 cm; MED Associates, East Fairfield, VT) for 10 or 11 days (ShA group). Each chamber contained two retractable levers (7 cm above floor) and a circular stimulus light above each lever. A Tygon infusion line within the spring leash was mounted to a balanced metal arm and attached to a liquid swivel (Instech, Polymouth Meeting, PA). Before each session, the catheter was secured to the infusion line. Presses on the active lever resulted in a 2-s cocaine hydrochloride (0.2 mg/50 μl) (National Institute on Drug Abuse, Research Triangle Park, NC) infusion via a computer-controlled pump. Cocaine infusions were paired with 5-s presentation of a conditioned-stimulus complex including the illumination of a white stimulus light above the active lever and a tone (2 kHz, 15 dB above ambient noise), followed by a 20-s timeout period, during which additional responses on the active lever resulted in no programmed consequences. Responses on the inactive lever were recorded but had no consequence. For each session, rats had to self-administer at least 10 infusions to fulfill the maintenance criterion. In a separate experiment, rats were trained on the same 2-hr/FR1 daily cocaine or yoked saline self-administration schedule for 2 days followed by 9 extended sessions that allowed rats to earn i.v. infusions over the course of a 6-hr/FR1 session (LgA group). Yoked-saline control rats were attached to infusion lines identical to those used for cocaine self-administering rats in the operant chambers. Yoked saline subjects received 50 μl of 0.9% saline during daily sessions whenever the paired self-administering rat received a cocaine infusion.
Immunoblotting
Two hr after the end of the last self-administration session, rats in both experiments were rapidly decapitated and brains were removed and placed in a brain matrix (Braintree Scientific, Inc., Warren, MI). Two mm coronal slices at AP 4.7-2.7 from Bregma were cut and the dmPFC was bilaterally punched and frozen at -80°C. Protein was extracted by sonication in ice-cold RIPA lysis buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 2mM EDTA, 10% Glycerol, 1% Triton X-100, 1% Igepal CA-630, 1% sodium deoxycholic acid) containing complete mini protease inhibitor (Roche Diagnostics, Indianapolis, IN), Halt phosphatase inhibitor (Pierce Chemical, Rockford, IL), and 1 mM PMSF. After a 30 min incubation, homogenates were centrifuged at 10000g for 20 min at 4°C. The resulting supernatants were collected and subjected to protein analysis with the Micro-Bicinchoninic Acid assay kit (Pierce, Rockford, IL). Equal amounts of protein extracts (15μg) were boiled in Lammeli buffer containing 1% β-mercaptoethanol for 5 min and run on 7.5% or 10% SDS-PAGE gels, then transferred to PVDF membranes. Membranes were then blocked with 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST; pH=7.6) for 1 hr at room temperature and incubated with primary antibody against p-ERK1/2 (1:2000; Cell Signaling, Beverly, MA), p-PP2Ac Tyr307 (1:5000; Abcam, Cambridge, MA), p-GluN2B Tyr1472 (1:1000; Sigma-Aldrich, St. Louis, MO), p-GluA1 Ser845 (1:2000), GluA2 (1:4000), p-CREB Ser133 (1:2000)(Millipore, Bedford, MA) or GluN1 (1:1000; BD Biosciences, San Jose, CA) overnight at 4°C. After three washes with blocking solution, membranes were incubated with the appropriate secondary antibodies for 1 hr at room temperature followed by three more washes with TBST. Antibody binding was detected by using an enhanced chemiluminescence kit (ECL Plus; GE Healthcare Bio-Sciences, Piscataway, NJ). Membranes were then stripped and re-probed with total protein by antisera against ERK (1:4000; Cell Signaling), GluA1 (1:5000; Abcam), GluN2B (1:1000; BD Biosciences), PP2Ac (1:5000; Millipore), or CREB (1:500; Millipore) for quantitation. Equal loading proteins were further confirmed by probing with anti-calnexin antiserum (CNXN; 1:10000; Enzo Life Sciences, Farmingdale, NY). All Western blot analyses were performed a minimum of two times to confirm the data. The integrated density of each phosphoprotein and total protein band was measured using ImageJ software (National Institute of Health, Bethesda, MD).
Immunoprecipitation
Immunoprecipitation of STEP was used followed by immunoblotting with a phospho-STEP antiserum because the phospho-specific antiserum was raised against the PKA site in the KIM domain of STEP that is common to several other kinases (Paul et al. 2003). The procedure of immunoprecipitation was described previously (Paul et al. 2003; Sun et al. 2009). Briefly, equal amount of protein extracts (250 μg) were incubated in RIPA lysis buffer containing a protease inhibitor mixture (as described above) with a monoclonal anti-STEP antibody (3.6 μg; Novus Biologicals, Littleton, CO) overnight, followed by the addition of 20 μl of protein A/G agarose beads (Santa Cruz Technologies, Santa Cruz, CA) for 4 hr at 4°C. Beads were then washed 3 times in the RIPA lysis buffer (1 ml). After final centrifugation, Lammeli buffer containing 1% β-mercaptoethanol (50μl) was added, boiled for 5min, loaded to 10% SDS-PAGE gels and analyzed by immunoblotting procedures as described above. The primary antibody against p-STEP Ser221 (1:4000; a gift from Dr. Paul Lombroso, Yale University) was used to detect the phosphorylation of STEP. The same membranes were stripped and re-probed with the monoclonal anti-STEP (1:5000) for quantification. Equal loading proteins were further confirmed by the intensity of IgG bands (~50 KDa).
Statistical analysis
Results are expressed as mean ± standard error of the mean (S.E.M.). For behavioral data, the number of active lever presses and infusions was plotted over the course of self-administration sessions. Paired t-tests were used to determine difference in the number of active lever presses and cocaine infusions and the amount of cocaine intake (mg/kg) during the 1st and last cocaine self-administration sessions. The intensity of protein bands was normalized by either the respective total protein or CNXN followed by Student’s t-tests. Determination of significant differences was considered at a 0.05 level (P<0.05).
Results
The number of active lever presses and cocaine infusions during ShA, the first 2hr of LgA, or the entire LgA6-hr sessions throughout self-administration are shown in Fig. 1a-c. The ShA group pressed significantly more on the active lever, received more cocaine infusions, and consumed more cocaine on the last day than on the first day (t (10) = 3.12, p<0.01, t (10) = 4.16, p<0.01, and t (10) = 3.56, p<0.01, respectively; Fig 1d-e left). Similarly, the number of active lever presses, cocaine infusions and the amount of cocaine intake of the LgA group were significantly greater during the first 2 hr (t (15) = 2.93, p< 0.01, t (15) = 3.35, p< 0.01, and t (15) = 2.66, p<0.01, respectively; Fig 1d-e middle bars), and during the entire 6 hr of the last session (t (15) = 2.87, p< 0.01, t (15) = 3.53, p< 0.01, and t (15) = 2.91, p<0.01, respectively; Fig 1d-e right bars) than during the first session.
Fig. 1.
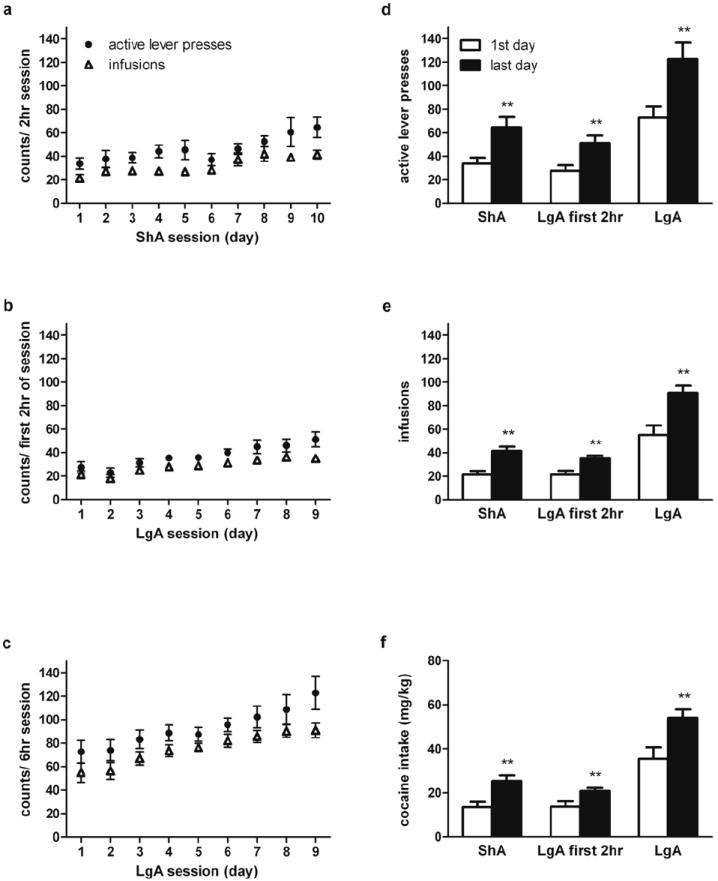
The Number of active (drug-paired) lever presses, and cocaine infusions across cocaine self-administration sessions in ShA (a), first 2 hr of LgA (b), and the entire 6 hr sessions of LgA groups (c). The number of active lever presses (d), cocaine infusions (e), and amount of cocaine intake (mg/kg, f) during the first and the last day of ShA, first 2 hr of LgA, and the entire LgA 6-hr sessions. Significant differences between the first and last day within each group are indicated (**p<0.01; N=6-9 per group).
Due to the low p-ERK1 signal, only the intensity of the p-ERK2 signal was quantified. In the LgA group, cocaine self-administration decreased the protein expression of p-ERK2 and p-CREB in the dmPFC when compared to yoked saline-treated rats (p-ERK: t (13) = -3.85, p<0.01; p-CREB: t (13) = -3.63, p<0.01; Fig.2a and 2b, respectively), findings that are similar to those in our previous study in ShA rats (Whitfield et al. 2011). As shown in Fig.2c, the protein level of p-STEP in the dmPFC of both ShA and LgA cocaine groups was also significantly reduced (ShA: t (9) = -2.37, p< 0.05; LgA: t (12) = -2.49, p< 0.05). In contrast, the level of p-PP2Ac Tyr307 was significantly increased in ShA and LgA rats after cocaine self-administration (ShA: t (10) = 3.26, p< 0.01; LgA: t (13) = 3.73, p<0.01; Fig.2d). There were no significant changes in total protein levels between cocaine- and saline-treated animals when normalized to CNXN or IgG after immunoprecipitation (Supplementary Table 1).
Fig. 2.
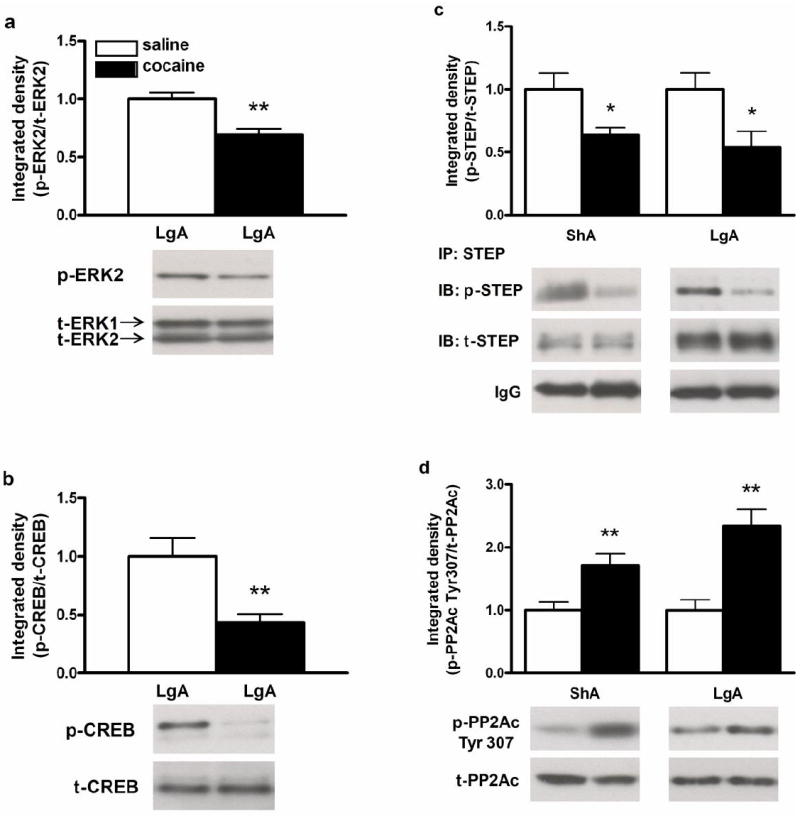
Phospho-protein levels in the dmPFC 2 hr after the last ShA and/or LgA cocaine self-administration session. p-ERK (a), p-CREB (b), p-STEP (c), and p-PP2Ac Tyr307 (d). Lower panels represent typical Western blot images for each protein. Significant differences from respective yoked-saline controls are indicated (*p<0.05 and **p<0.01; N=5-9 per group).
GluN2B phosphorylation levels were significantly reduced in the dmPFC of rats with a ShA or LgA cocaine self-administration history when compared to their respective yoked saline-treated counterparts (ShA: t (10) = -2.53, p< 0.05; LgA: t (13) = -3.68, p< 0.01; Fig 3a). Similarly, total GluN2B protein expression was decreased after cocaine self-administration in both ShA and LgA rats (ShA: t (10) = -2.38, p< 0.05; LgA: t (13) = -2.92, p< 0.05; Fig. 3b). Because both p-GluN2B and total GluN2B were reduced to a similar extent after ShA and LgA cocaine self-administration, their ratio (p-GluN2B/total GluN2B) did not differ between groups (Fig 3c). In addition, total GluN1 protein expression was significantly decreased in both ShA and LgA cocaine groups (ShA: t (10) = -2.47, p< 0.05; LgA: t (13) = -2.16, p< 0.05; Fig. 3d). In contrast, there were significant reductions of p-GluA1 Ser845 and total GluA1 in LgA, but not in ShA, cocaine groups (p-GluA1 Ser845: t (13) = -2.80, p< 0.05; GluA1: t (13) = -2.49, p< 0.05; Fig. 4a and 4b, respectively). Further analysis demonstrated a significant reduction in the ratio of p-GluA1 Ser845 to total GluA1 only in the LgA cocaine group (t (13) = -2.42, p< 0.05; Fig. 4c). Similarly, total GluA2 protein expression was not changed in the ShA group but there was a significant reduction of GluA2 in LgA rats (t (13) = -2.70, p< 0.05; Fig. 4d).
Fig. 3.
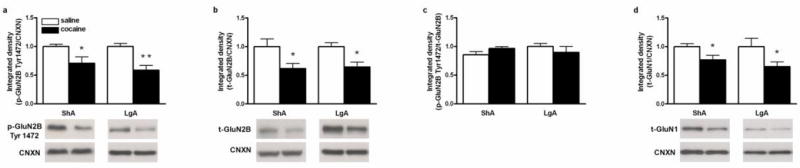
Effects of ShA and LgA cocaine self-administration on protein levels of p-GluN2B Tyr1472 (a), t-GluN2B (b), p-GluN2B Tyr1472/t-GluN2B ratio (c), t-GluA1 (d) in the dmPFC. Lower panels represent typical Western blot images for each protein. Significant differences from respective yoked-saline controls are indicated (*p<0.05 and **p<0.01; N=6-9 per group).
Fig. 4.
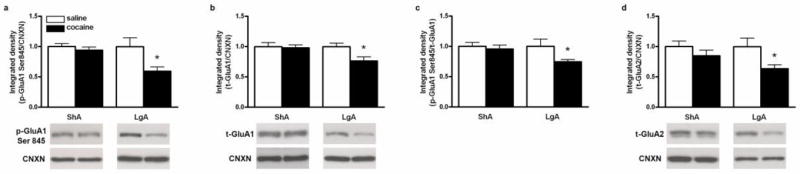
Effects of ShA and LgA cocaine self-administration on protein levels of p-GluA1 (a), t-GluA1 (b), p-GluA1 Ser845/t-GluA1 ratio, and t-GluA2 (d) in the dmPFC. Lower panels represent typical Western blot images for each protein. Significant differences from respective yoked-saline controls are indicated (*p<0.05; N=6-9 per group).
Discussion
Similar to our findings in ShA cocaine self-administering rats (Whitfield et al. 2011), this study established that p-ERK and p-CREB levels in the dmPFC were decreased 2hr after LgA cocaine self-administration. Further, both ShA and LgA cocaine elevated p-PP2Ac, consistent with deactivation, and blunted p-STEP, consistent with activation, making STEP a more likely candidate than PP2A to dephosphorylate ERK during early withdrawal. The decrease in STEP-regulated p-GluN2B Tyr1472 in the dmPFC 2 hr after ShA or LgA cocaine strengthens this conclusion and is likely to have led to internalization and degradation of GluN complexes. In contrast, a significant reduction of p-GluA1 Ser845, GluA1, and GluA2 was found only in LgA rats, suggesting that AMPA receptor expression is a more specific marker of LgA neuroadaptations in the dmPFC than GluN2B. Finally, the decreases in total GluN1, GluN2B, and/or total GluA1/2 in the dmPFC support the idea that the PFC is hyporesponsive during early withdrawal from both ShA and LgA cocaine self-administration.
Both active lever pressing and cocaine intake were escalated in LgA rats after cocaine self-administration, as previously reported (Ahmed and Koob 1998, 1999; Ben-Shahar et al. 2007, 2009; Briand et al. 2008). Similarly, lever pressing and total cocaine intake were increased between the first to last session in the ShA rats. The latter finding differs from previous studies that have reported a lack of escalation in cocaine intake in ShA rats but the duration of daily self-administration sessions in those studies was most commonly 1 hr. Among the few studies that have analyzed the time course of drug intake in a 2-hr psychostimulant self-administration paradigm, escalation was reported after 2 hr, but not 1 hr, access to methamphetamine (Rogers et al. 2008). Although escalation from the first to last day was detected in the 2 hr and 6 hr data of LgA rats in the present study, the number of active lever presses and the amount of cocaine intake in the LgA rats during the first 2 hr did not differ from those of the ShA rats. This finding also differs from 1 hr ShA reports in the literature (Ahmed and Koob 1998, 1999; Ben-Shahar et al. 2007, 2009).
Previously, using the 2-hr ShA model, we have shown that cocaine resulted in a reduction of p-ERK and p-CREB in the dmPFC 2 hr after the cessation of the last self-administration session (Whitfield et al. 2011). In this paradigm, the cocaine-induced decrease in p-CREB in dmPFC depended on p-ERK deactivation because local infusion of the selective MAP kinase kinase inhibitor, U-0126, blocked the ability of cocaine to cause the p-ERK and p-CREB shut-off. Attenuation of p-ERK and p-CREB in the dmPFC of LgA animals in the present study suggests that an ERK/CREB shut-off is a fundamental neuroadaptation that occurs during early withdrawal even after intense cocaine exposure. This ERK/CREB shutoff contrasts with reports that p-ERK and p-CREB increase after chronic cocaine in response to dopamine D1 and NMDA receptor stimulation (Lu et al. 2006; Girault et al. 2007; Pascoli. et al 2011). However, those data were derived from the striatum of rats non-contingently treated with cocaine or in response to cocaine-paired cues (Miller and Marshall 2005; Valjent et al. 2000). One exception is a study by Edwards et al. (2007) that demonstrated an increase in p-ERK, but not p-CREB, in the mPFC immediately following the last of 18 daily 4-hr cocaine SA sessions. The overall data from these different studies suggest that there is a bidirectional regulation of ERK and CREB activity in vivo that is similar to that documented in response to glutamate or NMDA receptor activation in vitro (Chandler et al. 2001; Hardingham et al. 2002; Jiang et al. 2000; Paul and Connor 2010). For example, in cortical cultures, stimulation of NMDA receptors induced early, brief Ca2+ influx and p-ERK induction followed by prolonged Ca2+ accumulation resulting in p-ERK suppression mediated by calcineurin-induced dephosphorylation and activation of STEP (Paul and Connor 2010). Thus, in the current experiment, the reduction of p-ERK and p-CREB in the dmPFC of cocaine self-administering rats may result from elevated STEP activity (dephosphorylation) during early withdrawal. In contrast, in both ShA and LgA rats, p-PP2Ac Tyr307 was increased after cocaine self-administration, indicating a reduction of PP2A activity. Therefore, although PP2A activation dephosphorylates ERK and MEK, the upstream activator of ERK, in vitro (Alessi et al. 1995; Junttila et al. 2008), it is not likely that PP2A directly dephosphorylates ERK in the dmPFC during cocaine withdrawal.
To further examine the activation of STEP, we measured the effect of cocaine self-administration on GluN2B in the dmPFC. Similar to the p-ERK and p-CREB shut-off, cocaine decreased p-GluN2B and total GluN2B protein expression in both ShA and LgA groups during early withdrawal. In cell and neuronal cultures, by binding to and dephosphoryating p-GluN2B Tyr1472, STEP leads to the internalization of the GluN1/GluN2B receptor complex with inhibitory effects on NMDA receptor-mediated long-term potentiation (LTP) and NMDA-induced p-ERK expression (Braithwaite et al. 2006; Kurup et al. 2010; Pelkey et al. 2002). In addition, STEP may also indirectly modulate GluN2B function by preventing the autophosphoryation of Fyn (Nguyen et al. 2002), a member of the Src-family kinases. Fyn has been demonstrated to play an important role in p-GluN2B Tyr1472 induction (Nakazawa et al. 2001). Taken together, it is plausible that, through direct interaction with NMDA receptors and/or indirect modulation of Fyn activity, increased STEP activity is responsible for p-GluN2B reduction after both LgA and ShA cocaine self-administration.
In addition to the reduction of p-GluN2B Tyr1472, a decrease of total GluN2B was found in both ShA and LgA cocaine animals. It is possible that after STEP-mediated dephosphorylation/internalization, GluN2B is subjected to degradation. For example, a recent study demonstrated that the downregulation of GluN2B in both surface membrane and total protein homogenates in the striatum after repeated amphetamine treatment was caused by ubiquitination and proteasomal degradation of GluN2B-anchoring proteins (Mao et al. 2009). Alternatively, in cultured hippocampal neurons, calpain-mediated GluN2B subunit cleavage has been documented without influencing its surface membrane expression (Simpkins et al. 2003). Since the ratio of p-GluN2B Tyr1472/t-GluN2B was not altered after either ShA or LgA cocaine self-administration (Fig. 3c), the decreased total GluN2B protein expression likely underlies the reduction in Tyr1472 phosphorylated GluN2B. Therefore, further experimental clarification is necessary to demonstrate a causal role of STEP in GluN2B trafficking, membrane surface expression and protein cleavage/degradation in response to cocaine self-administration during early withdrawal.
STEP activity is regulated by a balance between PKA and protein phosphatase 1 (PP1), Stimulation of PKA induces p-STEP and decreases its capacity to dephosphorylate p-ERK (Nika et al. 2004; Paul et al. 2000). PKA activation also indirectly modulates STEP activity by phosphorylating DARPP-32 Thr34, leading to inhibition of PP1, thereby decreasing PP1-STEP binding affinity and increasing p-STEP expression (Valjent et al. 2005). In contrast, phosphorylation of DARPP-32 Thr75 inhibits PKA activity and subsequent p-DARPP-32 Thr34 activation (Bibb et al. 1999; Nairn et al. 2004; Nishi et al. 1997). Evidence for a reduction in PKA-mediated signaling was reported after chronic treatment with cocaine or methamphetamine that decreased Thr34, but increased Thr75, phosphorylation of DARPP-32 in the striatum (Bibb et al. 2001; Chen and Chen 2005). Altogether, these data suggest that a decrease in PKA activity may mediate the activation of STEP, leading to ERK and p-GluN2B dephosphorylation by (1) potentiating the inhibitory action of PP1 on STEP and/or (2) increasing p-DARPP-32 Thr75. The inhibitory action of PP1 is augmented by calcineurin-induced dephosphorylation of DARPP-32 or inhibitor-1 (Mulkey et al 1994; Braithwaite et al 2006). Thus, cocaine may initially stimulate D1R-mediated phosphorylation of GluN2B Tyr 1472 via Fyn (Pascoli et al 2011), which when prolonged, will increase calcineurin–mediated STEP activation and ERK/GluN2B dephosphorylation followed by GluN degradation. Another possible mechanism underlying STEP activation may involve metabotropic GluR stimulation. Stimulation of mGluR5 receptors in striatal and hippocampal cultures has been reported to elevate STEP activity, STEP-regulated GluA1/GluA2 internalization, and p-PP2Ac Tyr307 (Mao et al. 2005; Zhang et al. 2008). Moreover, mGluR5 receptor dimer expression and its functional activity are increased in the mPFC of ShA animals 22 hr after the last 1hr/daily cocaine session (Hao et al. 2010). Hence, future experiments will examine whether changes in PKA/calcineurin activity and/or mGluR5-dependent molecular cascades contribute to STEP-mediated signaling in ShA and LgA animals.
Consistent with a previous study demonstrating a reduction of NMDA receptor expression in the PFC after 20 min of withdrawal from a ShA paradigm (Ben-Shahar et al. 2007), the GluN1 subunit was decreased in our current ShA cocaine groups. In contrast to a similar decrease in our LgA group, an increase or no alteration of the GluN1 subunit in the PFC was reported after LgA (8hr/daily for 15 days) or binge cocaine access (8hr/daily for 15 days + 24hr/daily for additional 6 days) followed by 16 hr or 2 weeks of withdrawal (Hemby et al. 2005; Tang et al. 2004). These data suggest that dynamic GluN1 changes depend heavily on the duration of withdrawal from cocaine self-administration. GluN1 subunits are normally retained in the endoplasmic reticulum. After binding to GluN2 subunits, the GluN1/2 complex is delivered to the cell surface via the secretory pathway (Lau and Zukin 2007). A decrease in the GluN1/GluN2B complex on the membrane surface after ShA and LgA cocaine self-administration may lead to a reduction in neuronal activity in the PFC during early cocaine withdrawal.
In addition to GluN1 and GluN2B alterations, cocaine reduced p-GluA1 Ser845, GluA1, and GluA2 exclusively in the dmPFC of LgA rats. The greater cocaine intake in LgA rats than in ShA rats likely caused the differential alterations in GluA receptor subunit expression in dmPFC, raising the possibility that PKA activity was diminished during early withdrawal from LgA, but not ShA, cocaine self-administration. In addition, in response to presynaptic overactivation, the postsynaptic level of AMPA receptors is known to be downregulated by receptor internalization and proteasomal degradation (Hou et al. 2011; Wang et al. 2012). Thus, the excessive cocaine intake in LgA animals may lead to prolonged elevation in presynaptic dopamine transmission in the dmPFC that promotes GluA1/2 degradation during early withdrawal. This idea is supported by a recent report demonstrating augmented extracellular dopamine levels and diminished glutamate levels in the mPFC in response to LgA cocaine SA (Ben-Shahar et al. 2012).
Together with attenuated GluN1 and GluN2B, decreased GluA1/2 and the ERK/CREB shut-off in LgA rats suggests that hypoactivity in the dmPFC during early withdrawal is accentuated after prolonged cocaine self-administration. Numerous studies have demonstrated hypoactivity in the PFC of human cocaine addicts (Franklin et al. 2002; Lim et al. 2002; Matochik et al. 2003; Volkow and Fowler 2000). Further, during cocaine self-administration, PFC hyperexcitability has been detected during repeated cocaine infusions and may be augmented by basal hypoactivity between infusions (Sun and Rebec 2006). On the other hand, with longer abstinence from or extinction of cocaine self-administration, elevated GluN2B, GluA1 and p-GluNA1 Ser845 have been detected in the mPFC (Ben-Shahar et al. 2009; Ghasemzadeh et al. 2011; Sun et al. 2013), suggesting a biphasic response during withdrawal. The underlying mechanism of biphasic glutamate receptor expression during different phases of withdrawal is unknown. However, it may indicate a homeostatic synaptic plasticity in PFC activation: an early reduction of GluN2B and/or GluA1 in response to cocaine-induced overactivation followed by a compensatory augmentation due to activity deprivation during later withdrawal (Sun et al 2013).
In conclusion, STEP is a potential phosphatase that mediates the ERK/CREB shut-off and attenuates GluN2B phosphorylation during early withdrawal from ShA and LgA cocaine self-administration. Activation of mGluRs may contribute to STEP activation after both ShA and LgA cocaine whereas a reduction of PKA-mediated signaling combined with internalization and degradation may underlie decreased p-GluA1 and t-GluA1 expression after LgA cocaine, representing molecular mechanisms that produce hypoactivity in the dmPFC. Understanding the signaling events early in withdrawal may provide a potential intervention strategy in human cocaine abusers that delays or prevents relapse after abstinence. This notion is supported by our previous studies indicating that a single intra-dmPFC BDNF infusion immediately after the cessation of cocaine self-administration normalized the ERK/CREB dephosphorylation and resulted in a long-term inhibitory effect on context-, cue- and cocaine primed-induced relapse in rats (Berglind et al. 2007; Whitfield et al. 2011). Therefore, in future studies, we will evaluate whether the manipulation of STEP and/or GluN2B activation in the dmPFC mimics the suppressive effect of intra-dmPFC BDNF on subsequent drug seeking.
Supplementary Material
Acknowledgments
The authors thank Phong Do, Andrew Nowak, and John Yang for excellent technical assistance and Dr. Paul Lombroso for the phospho-STEP antiserum. This research was supported by P50 DA015369.
Footnotes
The authors have no conflicts of interest.
References
- Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings BA, Dilworth SM, Mischak H, Kolch W, Baccarini M. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J Biol Chem. 2000;275:22300–4. doi: 10.1074/jbc.M003259200. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–12. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–95. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci. 2000;20:7571–8. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–8. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res. 2006;1095:148–53. doi: 10.1016/j.brainres.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar OM, Szumlinski KK, Lomniac KD, Cohen A, Gordon E, Ploense KL, Demartini J, Bernstein N, Rudy NM, Nabhan AN, Sacramento A, Pagano K, Carosso GA, Woodward N. Extended access to cocaine self administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol. 2012;17:746–757. doi: 10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, Szumlinski KK. Deficits in ventromedial prefrontal cortex Group I metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33:495–506. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–66. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–9. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–80. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–71. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–44. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, Oksenberg D, Nikolich K. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006;23:2847–56. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276:2627–36. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Chen PC, Chen JC. Enhanced Cdk5 activity and p35 translocation in the ventral striatum of acute and chronic methamphetamine-treated rats. Neuropsychopharmacology. 2005;30:538–49. doi: 10.1038/sj.npp.1300604. [DOI] [PubMed] [Google Scholar]
- Chung H, Nairn AC, Murata K, Brautigan DL. Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic subunit favors association with the alpha 4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry. 1999;38:10371–6. doi: 10.1021/bi990902g. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Oliva JM, Ghasemzadeh MB, kalivas PW, Ambrosio E. Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-asminitration. Ann NY Acad Sci. 2002;965:7–91. doi: 10.1111/j.1749-6632.2002.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Bachtell RK, Self DW. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci. 2007;25:2201–13. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res. 2011;1413:60–71. doi: 10.1016/j.brainres.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2010;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–8. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Gilbert J, Man HY. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72:806–18. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Mizuno N, Masu M. Molecular characterization of the family of N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Jiang Q, Gu Z, Zhang G, Jing G. Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res. 2000;857:71–7. doi: 10.1016/s0006-8993(99)02364-1. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–21. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–57. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:412–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lee H-K, Barbarosle M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–5. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–74. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–68. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Yoshimatsu T, Hamabe W, Fukazawa Y, Kumamoto K, Ozaki M, Kishioka S. Involvement of serine/threonine protein phosphatases sensitive to okadaic acid in restraint stress-induced hyperlocomotion in cocaine-sensitized mice. Br J Pharmacol. 2006;148:405–12. doi: 10.1038/sj.bjp.0706769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, Fibuch EE, Wang JQ. Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons. J Biol Chem. 2005;280:12602–10. doi: 10.1074/jbc.M411709200. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–10. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–20. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–93. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. N Engl J Med. 1996;334:965–72. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–84. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka R. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–9. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–77. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–9. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Nika K, Hyunh H, Williams S, Paul S, Bottini N, Tasken K, Lombroso PJ, Mustelin T. Haematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase in T-cells: dynamics and subcellular location. Biochem J. 2004;378:335–42. doi: 10.1042/BJ20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–55. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–8. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Herve D, Heck N, Girault J-A, Caboche J, Vanhoutte P. Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellualr signal-regulated kinase activation. Biol Psychiatr. 2011;69:218–227. doi: 10.1016/j.biopsych.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. NeuroReport. 2003;14:2229–32. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Paul S, Connor JA. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem. 2010;114:1107–18. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nature Neuroscience. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–8. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–38. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–88. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–24. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins KL, Guttmann RP, Dong Y, Chen Z, Sokol S, Neumar RW, Lynch DR. Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci. 2003;23:11322–31. doi: 10.1523/JNEUROSCI.23-36-11322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Coleman NT, Zelek-Molik A, Barry SM, Whitfield TW, Jr, McGinty JF. Relapse to cocaine-seeking after abstinence is regulated by cAMP-dependent protein kinase A in the prefrontal cortex. Addiction Biol. 2013 doi: 10.1111/adb.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of acute cocaine on ERK and DARPP-32 phosphorylation pathways in the caudate-putamen of Fischer rats. Brain Res. 2007;1178:12–9. doi: 10.1016/j.brainres.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Quinones-Jenab V, Jenab S. Cocaine effects on dopamine and NMDA receptors interactions in the striatum of Fischer rats. Brain Res Bull. 2009;80:377–81. doi: 10.1016/j.brainresbull.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–33. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Carreira MB, Smith LN, Zirlin BC, Neve RL, Cowan CW. Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron. 2012;73:108–20. doi: 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashev R, Moura PJ, Venkitaramani DV, Prosperetti C, Centonze D, Paul S, Lombroso PJ. A substrate trapping mutant form of striatal-enriched protein tyrosine phosphatase prevents amphetamine-induced stereotypies and long-term potentiation in the striatum. Biol Psychiatry. 2009;65:637–45. doi: 10.1016/j.biopsych.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–9. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–6. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Ding YS, Wang GJ, Gatley SJ. Positron emission tomography radioligands for dopamine transporters and studies in human and nonhuman primates. Adv Pharmacol. 1998;42:211–4. doi: 10.1016/s1054-3589(08)60730-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Wang G, Gilbert J, Man HY. AMPA receptor trafficking in homeostatic synaptic plasticity: functional molecules and signaling cascades. Neural Plast. 2012;2012:825364. doi: 10.1155/2012/825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Glutamate-dopamine interactions mediate the effects of psychostimulant drugs. Addict Biol. 1999;4:141–50. doi: 10.1080/13556219971641. [DOI] [PubMed] [Google Scholar]
- Wassarman DA, Solomon NM, Chang HC, Karim FD, Therrien M, Rubin GM. Protein phosphatase 2A positively and negatively regulates Ras1-mediated photoreceptor development in Drosophila. Genes Dev. 1996;10:272–8. doi: 10.1101/gad.10.3.272. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–53. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31:834–42. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–6. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga A, Torres J, Ubeda J, Pulido R. Interaction of mitogen-activated protein kinases with the kinase interaction motif of the tyrosine phosphatase PTP-SL provides substrate specificity and retains ERK2 in the cytoplasm. J Biol Chem. 1999;274:21900–7. doi: 10.1074/jbc.274.31.21900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


