Abstract
Although mosquitoes are well known vectors of human and animal diseases, pathogens are only minor components of their total endogenous microbial communities. The midguts of many insects, including mosquitoes, contain diverse microbial communities. In this study, we used denaturing gradient gel electrophoresis to identify the diversity of bacteria in field-collected adult female Culiseta melanura (Diptera: Culicidae) (Coquillett) and Coquillettidia perturbans (Diptera: Culicidae) (Walker). Few significant differences in bacterial fauna between the two mosquito species were found, but the results suggest that host life history may be a determinant of the endogenous bacterial communities in mosquitoes. The dominant bacteria in the current study are frequently identified as major components of other mosquito species’ microbial flora, suggesting the establishment of a stable association between the mosquitoes and the microbes after initial acquisition from the environment.
Keywords: Culiseta melanura, Coquillettidia perturbans, DGGE, 16S rDNA, microbial community
Introduction
Studies of environmental microbiomes provide unprecedented insights to how microbial communities function and the roles of individual species. Like the gut of vertebrate species (including humans), the insect gut can be thought of as an environment with a thriving microbial community. Determining microbial contents of insect microbiomes can facilitate a heightened understanding of the ecology of vector-borne diseases and lead to new control methods for the insects that spread plant and human pathogens (Douglas, 2007).
Although some of the non-pathogenic organisms that dominate the mosquito microbiome have important roles in digestion and fecundity (Fouda et al., 2001) and mediating the transmission of human pathogens, comparatively little is known about the overall composition and dynamics of mosquito microbiomes: factors that likely affect the dynamics of the diseases that mosquitoes transmit (Cirimotich et al., 2011). The microbial communities that reside within mosquitoes may be influenced by behavior, genetics, and mosquito life histories. Hence, the microbiomes may be regarded as proxies for certain aspects of mosquito biology.
Previous studies identified many new types of bacteria associated with mosquitoes and extended our knowledge of mosquito-associated microbial communities, especially with respect to differences before and after blood meals (Demaio et al., 1996) and between lab-reared and field-collected insects (Rani et al., 2009). Bacteria may be found in reproductive tissues and several somatic tissues besides the midgut (Zouache et al., 2009; Saridaki & Bourtzis, 2010) and some bacteria may play important roles in the specific tissues in which they reside. Therefore, the aim of the present study is to examine the overall microbial communities within whole mosquitoes in a series of comparative studies.
Most previous mosquito-microbiome studies to date have focused on Anopheles spp., Culex spp., or Stegomyia (formerly Aedes) spp., which are the most common disease vectors in the developing world. In this study polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) was used to describe, characterize, and compare the microbial communities associated with adult females of two mosquito species: Culiseta melanura (Diptera: Culicidae) (Coquillet) and Coquillettidia perturbans (Diptera: Culicidae) (Walker). Both species are important vectors of North American arboviruses including Eastern Equine Encephalitis virus (EEEV) and West Nile virus. In addition, Cs. melanura and Cq. perturbans were chosen because of their seasonal and spatial proximity to each other and the differences between their hosts and larval habitats. Culiseta melanura feeds primarily on birds and resides in swamps, where its larvae are well protected in crypts formed by the roots of trees (Molaei & Andreadis, 2006). Coquillettidia perturbans opportunistically feeds on both birds and mammals and lays its eggs in marshes dominated by dense aquatic plants (Apperson et al., 2004). The body segments of the mosquito compartmentalize organs; reproductive tissues reside solely in the abdomen, the midgut is present in both thorax and abdomen and the salivary glands are present in the thorax (Clements, 1992). Thus, the bacterial communities were examined within the thorax and abdomen separately to determine whether tissue-specific differences could be detected. The goals of this study were to identify and compare the bacteria associated within these lesser-studied mosquitoes. It is anticipated that the findings of the current study will be useful in future efforts to determine the specific ecological roles of different bacteria and how these bacteria compositions may reveal life histories of these important vectors.
Materials and Methods
Sampling
A total of 102 Cs. melanura and 147 Cq. perturbans adult female mosquitoes were collected using a New Jersey light trap (model 1112, John W. Hock Company, Gainesville, FL) in Raynham, Massachusetts, Bristol County, USA on seven dates between June and September 2006. Mosquitoes were transferred into 0.5-ml microcentrifuge tubes and stored at −20°C.
DNA Extraction
Individually mosquitoes were surface sterilized by rinsing them twice with 70% ethanol and once with distilled water. Each mosquito was placed on a sterilized microscope slide and separated the head, thorax, and abdomen using a single-use 20-gauge needle. The abdomen and thorax were placed into separate 1.5-ml microcentrifuge tubes and stored at −80°C. The DNA was extracted from the individual mosquito parts using Epicentre Master Complete DNA and RNA purification kits (Epicentre Technologies, Madison, WI) following the manufacturer’s protocols. Total DNA from each insect was dissolved in 30 µl water.
PCR-DGGE
For PCR-DGGE analyses, bacterial 16S-rRNA fragments (Muyzer et al., 1993; Schabereiter-Gurtner et al., 2003) were amplified using a GC-clamped forward primer, 341f (5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG G CCT ACG GGA GGC AGC AG-3′), and reverse primer 518r (5′-ATT ACC GCG GCT GCT GG-3′). The primers targeted fragments ranging from 150 to 200 bp, which are ideal for DGGE. All reactions were carried out in 40-µl volumes containing 1 µl DNA, 1× buffer, 2.5 µM MgCl2, 0.2 mM dNTP, 0.2 µM of each primer and 2 U of Taq polymerase (Promega, Madison, WI). All master mixes were treated with ultraviolet light for 10 min prior to the addition of Taq polymerase and DNA. DNase/RNAse free molecular-grade water was used as a negative control. PCRs were performed in an Eppendorf ep mastercycler (Eppendorf, Westbury, NY) using the following program: 94°C for 1 min; 3 cycles of 94°C for 15 s, 61°C for 15 s, and 72°C for 1 min; followed by 3 cycles of 94°C for 15 s, 58°C for 15 s, and 72°C for 1 min; then 28 cycles of 94°C for 15 s, 55°C for 15 s, and 72°C for 1 min; and finally, an extension step at 72°C for 6 min. To confirm that the PCR had resulted in the correct-sized fragments, 2 µl of each of the PCR products on a 1% (w/v) agarose gel were separated and stained them with ethidium bromide for visualization. PCR products that did not yield visible bands were not separated using DGGE and the corresponding mosquito samples were removed from the dataset.
The PCR products were separated by DGGE using a D-Code System (Bio-Rad, Hercules, CA) in 10% (v/v) polyacrylamide (37.5:1 acrylamide:bisacrylamide) gels containing a linear denaturing gradient ranging from 40–70% of urea and formamide (100% denaturant contains 7 M urea and 40% v/v formamide). Electrophoresis of the PCR products (300 ng/µl) was performed in 1.25× Tris-acetate-EDTA running buffer at a constant temperature of 56°C at 70 V for 17 h. To facilitate comparisons between gels, a marker was generated using a subsample of bands isolated from the mosquitoes and was run on each gel. After completion of electrophoresis, the gels were stained with 0.1 µl/ml SYBR gold (Invitrogen Corporation, Carlsbad, CA) for 40 min.
Amplicon bands of corresponding electrophoretic mobility were excised and placed them into individual microcentrifuge tubes containing 100 µl water and then eluted the DNA at 4°C overnight. The eluted amplicons via PCR were re-amplified using the same primers and parameters as described above, with 2 µl eluted DNA as template. PCR products were purified and sequenced using a modified 341f oligonucleotide (5′-CCT ACG GGA GGC AGC AG-3′), without the GC-clamp, on an ABI 3130XL genetic analyzer (Applied Biosystems, Foster City, CA). For identification purposes, bands were assigned an arbitrary number corresponding to their electrophoretic mobility, with the smaller numbers corresponding to the bands of slower mobility. To determine the identity of putatively unique bands, multiple bands of identical mobility were sequenced when present.
Sequence and Statistical Analysis
Sequencher (Gene Codes, Ann Arbor, MI) was used to edit and assemble the nucleotide sequences. The finished nucleotide sequences for matches were compared against known species using the BLASTn algorithm (http://www.ncbi.nlm.nih.gov/BLAST) and assigned identities to the bacterial genera (Table 1). Two bands corresponding to Cq. perturbans and Cs. melanura 18S rRNA were excluded from the dataset. The presence/absence of the 57 bands was recorded in each mosquito sample. Chi-squared tests were performed to analyze the differences between the microbiomes of Cs. melanura and Cq. perturbans, and between the thorax and abdomen subsamples within each species.
Table 1.
Taxonomic identities of bands separated by denaturing gradient gel electrophoresis based on 16S-rRNA gene sequences
| Class and family | Band no. |
BLASTn nearest similarity (accession no.) | Query length (bp) |
% similarity |
|---|---|---|---|---|
| α-Proteobacteria | ||||
| Anaplasmataceae | 25 | Wolbachia endosymbiont (GU592789) | 128 | 100 |
| Bartonellaceae | 51 | Bartonella washoensis (AB519066) | 133 | 100 |
| Acetobacteraceae | 54 | Asaia sp. (FN821397) | 135 | 100 |
| Acetobacteraceae | 55 | Asaia sp. (FN821397) | 128 | 100 |
| Bacilli | ||||
| Enterococcaceae | 12 | Enterococcus silesiacus (HM209744) | 160 | 100 |
| Enterococcaceae | 13 | Enterococcus silesiacus (HM209744) | 152 | 99 |
| Carnobacteriaceae | 20a | Carnobacterium sp. (HM141122) | 160 | 100 |
| Streptococcaceae | 21 | Lactococcus lactis (HM218820) | 161 | 100 |
| Enterococcaceae | 26 | Enterococcus sp. (GU905013) | 160 | 99 |
| Bacillaceae | 31 | Bacillus sp. (HQ113214) | 160 | 99 |
| Leuconostocaceae | 32a | Fructobacillus ficulneus (NR025153) | 159 | 97 |
| Mollicutes | ||||
| Spiroplasmataceae | 2 | Spiroplasma (FJ57213) | 160 | 97 |
| Spiroplasmataceae | 6 | Spiroplasma culicicola (NR025701) | 160 | 100 |
| Flavobacteria | ||||
| Flavobacteriaceae | 14 | Chryseobacterium sp. (EF442766) | 155 | 100 |
| Spirochaetes | ||||
| Spirochaetaceae | 9 | Spironema culicis (AF166259) | 161 | 99 |
| Spirochaetaceae | 10 | uncultured spirochete (AY605143) | 161 | 88 |
| γ-Proteobacteria | ||||
| Enterobacteriaceae | 15 | Erwinia rhapontici (HM008943) | 160 | 95 |
| Enterobacteriaceae | 27 | Enterobacter sp. (HQ014913) | 153 | 99 |
| Enterobacteriaceae | 29 | Enterobacter sp. (FN555398) | 153 | 100 |
| Enterobacteriaceae | 30 | Enterobacter sp. (AM748792) | 157 | 100 |
| Enterobacteriaceae | 32a | Ewingella americana (FN811904) | 158 | 99 |
| Enterobacteriaceae | 33 | Enterobacter sp. (HQ014913) | 153 | 100 |
| Enterobacteriaceae | 34 | Erwinia sp. (EF016525) | 159 | 99 |
| Enterobacteriaceae | 35 | Pantoea sp. (AY659872) | 157 | 99 |
| Enterobacteriaceae | 36 | Brenneria quercina (HM196337) | 158 | 100 |
| Enterobacteriaceae | 37 | Hafnia sp. (HM489947) | 153 | 99 |
| Enterobacteriaceae | 38 | Erwinia rhapontici (AB087715) | 159 | 100 |
| Enterobacteriaceae | 39 | Pantoea sp. (AY659872) | 157 | 100 |
| Enterobacteriaceae | 40 | Ewingella americana (FN811903) | 158 | 100 |
| Enterobacteriaceae | 41 | Erwinia persicina (EF693792) | 158 | 99 |
| Enterobacteriaceae | 42 | Serratia sp. (HM099664) | 158 | 99 |
| Enterobacteriaceae | 44 | Yersinia mollaretii (HM031473) | 157 | 99 |
| Enterobacteriaceae | 46a | Erwinia rhapontici (AB087715) | 158 | 99 |
| Enterobacteriaceae | 46a | Erwinia sp. (HM462346) | 158 | 99 |
| Enterobacteriaceae | 47a | Ewingella americana (DQ383802) | 153 | 100 |
| Enterobacteriaceae | 47a | Serratia sp. (HM242285) | 160 | 100 |
| Enterobacteriaceae | 48 | Enterobacter sp. (GU086387) | 160 | 99 |
| Enterobacteriaceae | 49 | Rahnella sp. (GU068647) | 157 | 98 |
| Halomonadaceae | 28 | Zymobacter palmae (AF211871) | 160 | 95 |
| Moraxellaceae | 18 | Acinetobacter (DQ314740) | 160 | 98 |
| Moraxellaceae | 19 | Acinetobacter sp. (AJ633639) | 152 | 95 |
| Moraxellaceae | 22 | Acinetobacter sp. (GQ464378) | 152 | 96 |
| Moraxellaceae | 23 | Acinetobacter sp. (DQ314740) | 155 | 99 |
| Pseudomonadaceae | 16 | Pseudomonas sp. (AB548846) | 154 | 100 |
| Pseudomonadaceae | 17 | Pseudomonas sp. (HQ166064) | 153 | 100 |
| Pseudomonadaceae | 20a | Pseudomonas sp. (AB548838) | 153 | 100 |
| Pseudomonadaceae | 24 | Pseudomonas sp. (AB548840) | 153 | 100 |
| Uncultured bacteria | ||||
| 3 | uncultured bacterium (DQ980728) | 158 | 96 | |
| 5 | uncultured bacterium (DQ343058) | 158 | 96 | |
| 8 | uncultured bacterium (DQ343058) | 159 | 97 | |
| 50 | uncultured bacterium (AY627574) | 160 | 99 |
Bands migrate to identical positions on the gel.
Results
A total of 57 unique bands separated by DGGE were identified based on relative mobility. Two of the bands corresponded to Cq. perturbans and Cs. melanura 18S rRNAs. Although mosquito rRNA bands were from subsequent analyses, their consistent amplification in all mosquito samples served as a positive indicator of the presence of extracted DNA.
Of the 57 unique bands, 47 contained single fragments that could be directly sequenced. All of the sequenced bands yielded strong BLAST hits to known bacterial species. The nucleotide-sequence identities ranged from 88–100%, but most matches had identities greater than 99% (Table 1). Ten of the extracted gel bands contained multiple nucleotide-sequence fragments, as revealed in the chromatograms from the sequencing runs. Bands containing two or more sequence fragments could not be directly sequenced. In four instances, bands with apparently identical DGGE motilities from different mosquitoes yielded unique sequences with substantially different BLAST results (Table 1).
The microbial sequences associated with adult female Cs. melanura and Cq. perturbans revealed the presence of both gram-negative and gram-positive bacteria representing six classes and 15 families (Table 2). The most ubiquitous bacterial sequences corresponded to the Enterobacteriaceae family, which comprised the majority of the unique bands (35%). The genera present in the greatest numbers of individual mosquitoes of both species were Erwinia (49.4%), Acinetobacter (33.1%), Asaia (26.4%), Pseudomonas (25.1%), Enterobacter (29.7%), and Enterococcus (25.9%) (Table 2). Wolbachia was the dominant genus in Cq. perturbans, having been found in 66% of individuals. Wolbachia was only present in Cq. perturbans and was confined solely to the abdomen of that species. Pseudomonas sp. was only present in Cs. melanura, with a similar distribution in the thorax and abdomen. Chi-squared comparisons of frequencies of the remaining bacterial taxa present in both Cs. melanura and Cq. perturbans revealed no significant differences. Likewise the frequency distribution for these taxa were the same for thorax and abdomen subsamples within each species.
Table 2.
Distribution of bacteria genera among Cs. melanura and Cq. perturbans based on the presence/absence of denaturing gradient gel electrophoresis bands.
| Class and family | Genus | Both mosq. (%) n=239 |
Cs. melanura (%) 95 |
Thorax (%) 90 |
Abdomen (%) 89 |
Cq. perturbans (%) 144 |
Thorax (%) 132 |
Abdomen (%) 141 |
|---|---|---|---|---|---|---|---|---|
| α-Proteobacteria | ||||||||
| Anaplasmataceae | Wolbachia | 95 (39.7) | 0 | 0 | 0 | 95 (66) | 0 | 95 (67.3) |
| Bartonellaceae | Bartonella | 10 (4.2) | 4 (4.2) | 1 (1.1) | 4 (4.5) | 6 (4.2) | 3 (2.3) | 5 (3.5) |
| Acetobacteraceae | Asaia | 63 (26.4) | 22 (23.2) | 19 (21.3) | 13 (14.8) | 41 (28.5) | 40 (30.3) | 26 (18.4) |
| Bacilli | ||||||||
| Bacillaceae | Bacillus | 29 (12.1) | 13 (13.7) | 11 (12.4) | 8 (9.1) | 16 (11.1) | 14 (10.6) | 5 (3.5) |
| Carnobacteriaceae | Carnobacterium | 20 (8.4) | 14 (14.7) | 3 (3.4) | 13 (14.8) | 6 (4.2) | 4 (3.0) | 5 (3.5) |
| Enterococcaceae | Enterococcus | 62 (25.9) | 29 (30.5) | 14 (15.7) | 22 (25.0) | 33 (22.9) | 21 (15.9) | 18 (12.8) |
| Leuconostocaceae | Fructobacillus | 24 (10) | 8 (8.4) | 5 (5.6) | 8 (9.1) | 15 (10.4) | 10 (7.6) | 12 (8.5) |
| Streptococcaceae | Lactococcus | 16 (6.7) | 9 (9.5) | 6 (6.7) | 7 (8.0) | 7 (4.9) | 3 (2.3) | 5 (3.5) |
| Mollicutes | ||||||||
| Spiroplasmataceae | Spiroplasma | 5 (2.1) | 2 (2.1) | 1 (1.1) | 1 (1.1) | 3 (2.1) | 1 (0.8) | 3 (2.1) |
| Flavobacteria | ||||||||
| Flavobacteriaceae | Chryseobacterium | 21 (8.8) | 11 (11.6) | 8 (9.0) | 6 (6.8) | 11 (7.6) | 8 (6.1) | 6 (4.3) |
| Spirochaetes | ||||||||
| Spirochaetaceae | Spironema | 1 (0.4) | 1 (1.1) | 0 | 1 (1.1) | 0 | 0 | 0 |
| Spirochaetaceae | uncultured spirochete | 4 (1.7) | 2 (2.1) | 0 | 2 (2.3) | 2 (1.4) | 2 (1.5) | 1 (0.71) |
| γ-Proteobacteria | ||||||||
| Enterobacteriaceae | Brenneria | 8 (3.3) | 4 (4.2) | 2 (2.2) | 4 (4.5) | 4 (2.8) | 2 (1.5) | 3 (2.1) |
| Enterobacteriaceae | Enterobacter | 71 (29.7) | 36 (37.9) | 17 (19.1) | 29 (33) | 42 (29.1) | 31 (23.5) | 30 (21.3) |
| Enterobacteriaceae | Yersinia mollaretii | 22 (9.2) | 11 (11.6) | 7 (7.9) | 8 (9.1) | 11 (7.6) | 8 (6.1) | 3 (2.1) |
| Enterobacteriaceae | Erwinia | 118 (49.4) | 57 (60.0) | 28 (31.5) | 52 (59.1) | 61 (42.4) | 50 (37.9) | 46 (32.6) |
| Enterobacteriaceae | Ewingella americana | 40 (16.7) | 17 (17.9) | 7 (7.9) | 16 (18.2) | 22 (15.3) | 15 (11.4) | 16 (11.3) |
| Enterobacteriaceae | Hafnia | 8 (3.3) | 4 (4.2) | 4 (4.5) | 4 (4.5) | 4 (2.8) | 4 (3.0) | 4 (2.8) |
| Enterobacteriaceae | Pantoea | 24 (10.0) | 12 (12.6) | 6 (6.7) | 9 (10.2) | 12 (8.3) | 7 (5.3) | 10 (7.1) |
| Enterobacteriaceae | Rahnella | 17 (7.1) | 6 (6.3) | 3 (3.4) | 6 (6.8) | 11 (7.6) | 7 (5.3) | 10 (7.1) |
| Enterobacteriaceae | Serratia | 35 (14.6) | 22 (23.2) | 8 (9.0) | 31 (35.2) | 13 (9.0) | 10 (7.6) | 8 (5.7) |
| Halomonadaceae | Zymobacter palmae | 8 (3.3) | 2 (2.1) | 0 | 2 (2.3) | 6 (4.2) | 4 (3.0) | 4 (2.8) |
| Moraxellaceae | Acinetobacter | 79 (33.1) | 37 (38.9) | 24 (27) | 26 (29.5) | 42 (29.1) | 36 (27.3) | 23 (16.3) |
| Pseudomonadaceae | Pseudomonas | 60 (25.1) | 38 (40.0) | 10 (11.2) | 35 (39.8) | 22 (15.3) | 11 (8.3) | 16 (11.3) |
| Uncultured bacteria | ||||||||
| uncultured bacterium | 33 (13.8) | 13 (13.7) | 7 (7.9) | 11 (12.5) | 20 (13.9) | 13 (9.8) | 9 (6.4) |
Discussion
Bacteria that associate with insects are involved in key biological processes including generating nutrients to augment poor food resources, detoxifying plant allelochemicals, producing digestive enzymes, and aiding in the prevention of enteric infections (Dillon & Dillon, 2004). Bacteria can also protect insects from parasitoids, parasitic nematodes, and fungal pathogens, and may contribute to overcoming environmental stresses such as heat and suboptimal nutrition (Kikuchi, 2009; Oliver et al., 2010). The recognition of the importance of arthropod symbionts and the increased availability of culture-independent molecular tools has led to many studies investigating the microbial communities associated with insects (Shi et al., 2010). Such studies helped to distinguish obligate symbionts from those that play more transient roles.
Intra- and interspecies bacterial diversity
Using PCR-DGGE, the bacterial diversity was characterized among female Cs. melanura and Cq. perturbans mosquitoes. The most abundant bacterial genera identified by our study were Pseudomonas (25.1%), Acinetobacter (33.1%), Enterobacter (29.7%), Enterococcus (25.9%), and Asaia (26.4%). Although reportedly common among other mosquito species, Bacillus, Serratia, and Pantoea were found in only 12.1%, 14.6%, and 10%, respectively, of mosquitoes in our study (Table 2). All of the highly abundant genera identified by our study were previously isolated from mosquitoes using both culture-dependent and culture-independent techniques; and are known to infect a diverse array of mosquito species with vastly different life histories from around the globe (Demaio et al., 1996; Straif et al., 1998; Crotti et al., 2009).
Significant interspecific occurrence was observed for only two bacterial taxa among the set of all detected bacteria. One Pseudomonas isolate was more common in Cs. melanura, while Wolbachia was more frequent in Cq. perturbans. Intraspecific comparisons between the thorax and abdomen showed that Wolbachia resides exclusively in the abdomens of Cq. perturbans. However, most of the bacteria that observed appear in similar abundances in the two mosquito hosts, suggesting that mosquitoes acquire the majority of their bacteria from the same environmental sources.
There was considerable intraspecific variation in the microbiota of both mosquito species, i.e. between individuals within a species. This may be to due to natural variation in microbial flora or variation in the number or timing of blood meals. A protein-rich blood bolus is an excellent nutrient source for bacteria that usually maintain low population densities in the mosquito midgut. Bacterial population sizes can increases 70 to 16,000-fold in adult mosquitoes 48 h after a blood meal (Demaio et al., 1996). The bacterial populations decrease and return to pre-blood meal levels within a 3-day period (Demaio et al., 1996). The variation in the microbiota within each mosquito species could be due to spikes in the growth of certain species following a recent blood meal. Future studies should measure mosquito age and feeding status to look for correlations between bacterial species and number of blood meals.
Bacteria originating from plant or environmental sources
Our results suggest that many of the bacteria found in mosquitoes are acquired from the environment while feeding on something other than blood. The literature supports the observation that mosquitoes may acquire bacteria from their environment. For example, the bacterium, Thorsellia anopheles is the dominant bacterium in the midgut of adult Anopheles gambiae captured in Kenya (Briones et al., 2008). This bacterium also resides in an aquatic rice paddy environment nearby to where the mosquitoes were captured, indicating that mosquitoes can acquire bacteria from the environment. In addition, field-collected mosquitoes, as compared to lab-reared mosquitoes, had a higher abundance of the same bacteria types that were observed in our study (Rani et al., 2009). Hence, the microbial community may serve as a record of an individual’s feeding habits. Mosquitoes exploit different nutritional sources and habitats throughout their development (Clements, 1992). As larvae, they consume microbes in their aquatic environment. They move to terrestrial habitats and feed upon plant nectar and/or vertebrate blood as adults. Based on previous studies with mosquitoes (Lindh et al., 2005; Rani et al., 2009), the majority of bacteria observed appear to have an environmental or plant origin, indicating that they were most likely acquired directly from the mosquito’s habitat.
The current study documents the first report of Erwinia in mosquitoes, with half of the sampled mosquitoes being infected (Table 2). Erwinia includes phytopathogens that cause soft rot and fire blight in many plants (Permbelon & Kelman, 1980). Some Erwinia species can be vectored by Drosophila directly feeding on infected fruit, or by pollinators collecting nectar from flowers (Permbelon & Kelman, 1980; Hildebrand et al., 2012). Erwinia has been isolated from several plant feeding insects, including thrips and aphids (Harada et al., 1997; de Vries et al., 2001). Erwinia is most likely acquired while feeding on plants, and consequently, its prevalence among our mosquitoes may reflect the mosquitoes’ degree of nectar-feeding behavior. Some Erwinia are also obligate endosymbionts, such as “Ca. Erwinia dacicola” in the olive fly (Tephritidae: Diptera) (Capuzzo et al., 2005). The exact nature and mode of Erwinia infection in Cs. melanura and Cq. perturbans will require further investigation.
An Asaia species with 99% nucleotide identity to flower-dwelling Asaia bogorensis and Asaia siamensis (Katsura et al., 2001) was in 23.2% of Cs. melanura and 28.5% of Cq. perturbans (Table 2). This acetic acid bacterium was originally isolated from plants but has since been found to infect a variety of insects that feed on plant tissue, phloem, or nectar (Chouaia et al., 2010). Asaia was isolated from several mosquito species; including An. stephensi, An. maculipennis, An. gambiae, St. aegypti, and St. albopicta; and stably associates with the salivary glands, midgut, and reproductive tissues of adults (Favia et al., 2007; Crotti et al., 2009). The ability of Asaia to be transferred both horizontally and vertically makes this bacterium a possible candidate for novel mosquito control strategies, wherein the bacterium could be modified to express molecules that directly target human pathogens within the mosquito (Favia et al., 2008).
Vertebrate host-associated bacteria
Although many mosquito-associated bacteria are likely obtained from environmental sources (e.g., standing water and flower nectar), some bacteria were probably acquired while feeding on blood or tissue of associated vertebrate hosts. One of those bacterial species is Ewingella americana, a bacterium originally cultured from Eastern Bluebird feathers (Shawkey et al., 2005). It was present in Cs. melanura, an ornithophilic mosquito known to feed on bluebirds (Molaei & Andreadis, 2006). It was also found in the more opportunistic bird ectoparasite Cq. perturbans, consistent with the catholic host choice of this species and its role as a bridge vector for avian-borne pathogens. The occurrence of Ewingella americana could prove useful as a proxy for determining host feeding behavior.
Also present was Bartonella spp., a gram-negative bacteria that infects mammalian blood cells. Mammals can act as reservoirs of Bartonella, and some insects can serve as vectors for members of this genus (Breitschwerdt & Kordick, 2000). Bartonella washoensis, previously isolated from California ground squirrels (Kosoy et al., 2003), was found in both Cs. melanura and Cq. perturbans. Although Cq. perturbans is known to feed regularly on mammals and is therefore likely to have acquired B. washoensis while doing so, Cs. melanura feeds primarily on birds and would seem less likely to have acquired the bacterium by feeding on an infected mammal. B. washoensis may have been transmitted from mammals to birds by Cq. perturbans and then acquired from the birds by Cs. Melanura, thus demonstrating a bridge vector pattern opposite to that regarded as most important in arbovirus epizootiology.
Wolbachia pipientis, a known mosquito endosymbiont, was present in 66% of Cq. perturbans (Table 2). In contrast, there is no evidence of Wolbachia in Cs. melanura. This is consistent with previous reports that have observed Wolbachia within other Coquillettidia species, but not Culiseta (Ricci et al., 2002). Wolbachia is a maternally inherited rickettsia-like α-Proteobacteria that has been associated with various arthropods. It causes reproductive abnormalities such as male killing, cytoplasmic incompatibility, parthenogenesis, and feminization (Werren et al., 2008). To our knowledge, the current study is the first documentation of Wolbachia in Cq. perturbans. Some Wolbachia strains are under consideration as control agents for mosquito-borne diseases such as Dengue fever and malaria. Wolbachia are known to reduce mosquito population sizes (Brelsfoard & Dobson, 2012). Wolbachia also has potential utility to introduce transgenic traits into susceptible mosquito populations and perhaps to interfere with pathogen development (Hoffmann et al., 2011). It has been shown that Wolbachia infections can provide a protective effect against West Nile Virus infection within the mosquito (Glaser & Meola, 2010).
Whole microbiome characterization is powerful tool to identify important aspects of the ecology and life history of arthropod vectors. Cs. melanura and Cq. perturbans are both important arbovirus vectors, and cataloging their microbial communities will help us to better understand the roles that they play in enzootic and epizootic transmission of diseases such as WNV, EEEV and Dengue fever. Cs. melanura feeds primarily on birds and is therefore an important maintenance vector of EEEV; while Cq. perturbans has a broader host range and plays a fundamental role as a bridge vector from those enzootic cycles into humans and companion animals. Because the microbial communities within insects are registers of the past activities and habitats of the insects, they can serve as markers for assessing the dynamics of disease transmission and the risk of human exposure to disease. In some cases, the microbiome may reflect past host feeding choices. Other bacteria are possibly environmentally acquired and among these, there may be several for which the association with mosquitoes is merely coincidental. But some of the species detected in our survey, especially a first report of Wolbachia, are clearly stable symbionts. How these species interact with each other, the roles they play in the physiology of their hosts, and how they affect the transmission of known infectious agents are subjects for further investigation.
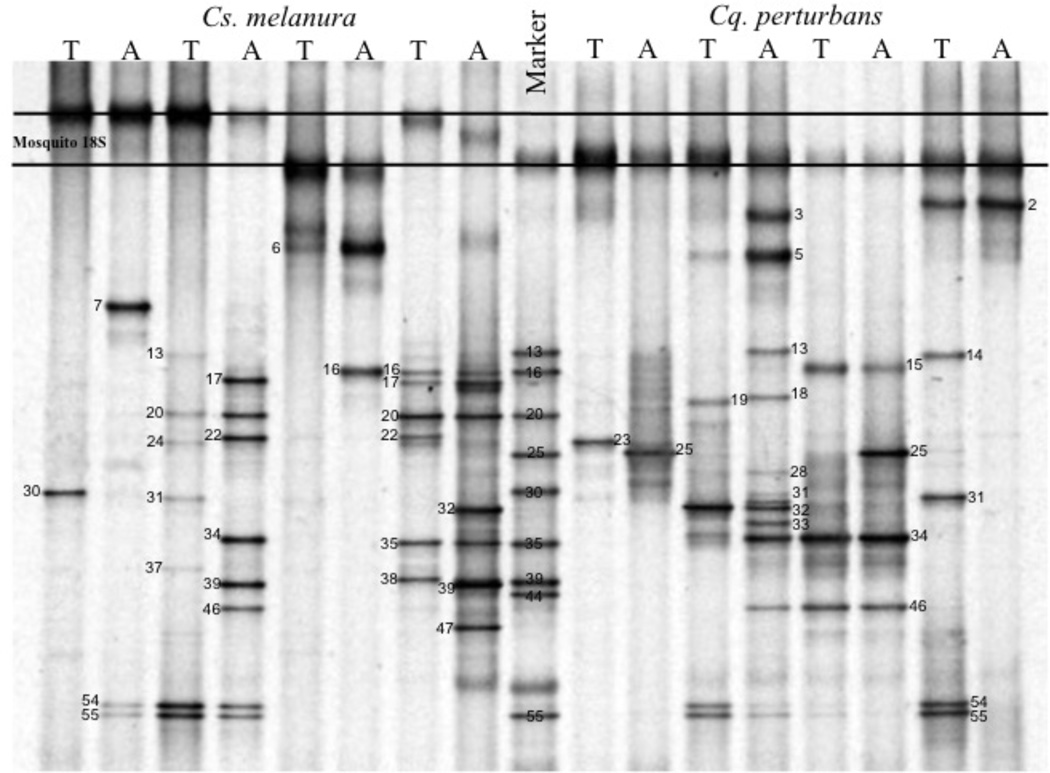
Fig 1.
Denaturing gradient gel electrophoretic profile of 16S rDNA gene fragments amplified by polymerase chain reaction from four representatives of each mosquito species, Cs. melanura and Cq. perturbans. All bands recovered from the mosquitoes in this study are not represented. The concentration of denaturing agents in the gradient gel range from 40% to 70%, top to bottom. Numbers were arbitrarily assigned to bands based on electrophoretic mobility, and each corresponds to a different bacterial identity (Table 1). T: thorax. A: abdomen
Acknowledgements
We thank F. Kopliku, J. Faraj, and D. Jaworski for experimental assistance and the Bristol County Mosquito Control Project for aid in mosquito collection. This research was supported by National Institutes of Health grant RO1-GM07077 (SMR) and the Massachusetts Agricultural Experiment Station (MAS 00983).
References
- Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, et al. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–438. doi: 10.1128/cmr.13.3.428-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsfoard CL, Dobson SL. Population genetic structure of Aedes polynesiensis in the Society Islands of French Polynesia: implications for control using a Wolbachia-based autocidal strategy. Parasit Vectors. 2012;5:80. doi: 10.1186/1756-3305-5-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Shililu J, Githure J, Novak R, Raskin L. Thorsellia anophelis is the dominant bacterium in a Kenyan population of adult Anopheles gambiae mosquitoes. Isme Journal. 2008;2:74–82. doi: 10.1038/ismej.2007.95. [DOI] [PubMed] [Google Scholar]
- Capuzzo C, Firrao G, Mazzon L, Squartini A, Girolami V. 'Candidatus Erwinia dacicola', a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin) Int J Syst Evol Microbiol. 2005;55:1641–1647. doi: 10.1099/ijs.0.63653-0. [DOI] [PubMed] [Google Scholar]
- Chouaia B, Rossi P, Montagna M, Ricci I, Crotti E, Damiani C, et al. Molecular evidence for multiple infections as revealed by typing of Asaia bacterial symbionts of four mosquito species. Appl Environ Microbiol. 2010;76:7444–7450. doi: 10.1128/AEM.01747-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich CM, Ramirez JL, Dimopoulos G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe. 2011;10:307–310. doi: 10.1016/j.chom.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. The biology of mosquitoes: Volume 1: Development, Nutrition and Reproduction. New York: Chapman and Hall; 1992. [Google Scholar]
- Crotti E, Damiani C, Pajoro M, Gonella E, Rizzi A, Ricci I, et al. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ Microbiol. 2009;11:3252–3264. doi: 10.1111/j.1462-2920.2009.02048.x. [DOI] [PubMed] [Google Scholar]
- de Vries EJ, Breeuwer JA, Jacobs G, Mollema C. The association of Western flower thrips, Frankliniella occidentalis, with a near Erwinia species gut bacteria: transient or permanent? J Invertebr Pathol. 2001;77:120–128. doi: 10.1006/jipa.2001.5009. [DOI] [PubMed] [Google Scholar]
- Demaio J, Pumpuni CB, Kent M, Beier JC. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am J Trop Med Hyg. 1996;54:219–223. doi: 10.4269/ajtmh.1996.54.219. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Douglas AE. Symbiotic microorganisms: untapped resources for insect pest control. Trends Biotechnol. 2007;25:338–342. doi: 10.1016/j.tibtech.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci U S A. 2007;104:9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favia G, Ricci I, Marzorati M, Negri I, Alma A, Sacchi L, et al. Bacteria of the genus Asaia: a potential paratransgenic weapon against malaria. Adv Exp Med Biol. 2008;627:49–59. doi: 10.1007/978-0-387-78225-6_4. [DOI] [PubMed] [Google Scholar]
- Fouda MA, Hassan MI, Al-Daly AG, Hammad KM. Effect of midgut bacteria of Culex pipiens L. on digestion and reproduction. J Egypt Soc Parasitol. 2001;31:767–780. [PubMed] [Google Scholar]
- Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Oyaizu H, Kosako Y, Ishikawa H. Erwinia aphidicola, a new species isolated from pea aphid, Acyrthosiphon pisum. J. Gen. Appl. Microbiol. 1997;43:349–354. doi: 10.2323/jgam.43.349. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Dickler E, Geider K. Occurrence of Erwinia amylovora on insects in a fire blight orchard. J. Phytopathology. 2012;148:251–256. [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Katsura K, Kawasaki H, Potacharoen W, Saono S, Seki T, Yamada Y, et al. Asaia siamensis sp. nov., an acetic acid bacterium in the alpha-proteobacteria. Int J Syst Evol Microbiol. 2001;51:559–563. doi: 10.1099/00207713-51-2-559. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y. Endosymbiotic bacteria in insects: their diversity and culturability. Microbes Environ. 2009;24:195–204. doi: 10.1264/jsme2.me09140s. [DOI] [PubMed] [Google Scholar]
- Kosoy M, Murray M, Gilmore RD, Jr, Bai Y, Gage KL. Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. J Clin Microbiol. 2003;41:645–650. doi: 10.1128/JCM.41.2.645-650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh JM, Terenius O, Faye I. 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 2005;71:7217–7223. doi: 10.1128/AEM.71.11.7217-7223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG. Identification of avian- and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, U.S.A. J Med Entomol. 2006;43:1088–1093. doi: 10.1603/0022-2585(2006)43[1088:IOAAMB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- Permbelon MC, Kelman A. Ecology of the Soft Rot Erwinias. Annu Rev Phytopathol. 1980;18:361–387. [Google Scholar]
- Rani A, Sharma A, Rajagopal R, Adak T, Bhatnagar RK. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 2009;9:96. doi: 10.1186/1471-2180-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci I, Cancrini G, Gabrielli S, D'Amelio S, Favi G. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J Med Entomol. 2002;39:562–567. doi: 10.1603/0022-2585-39.4.562. [DOI] [PubMed] [Google Scholar]
- Saridaki A, Bourtzis K. Wolbachia: more than just a bug in insects genitals. Curr Opin Microbiol. 2010;13:67–72. doi: 10.1016/j.mib.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Schabereiter-Gurtner C, Lubitz W, Rolleke S. Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. J Microbiol Methods. 2003;52:251–260. doi: 10.1016/s0167-7012(02)00186-0. [DOI] [PubMed] [Google Scholar]
- Shawkey MD, Mills KL, Dale C, Hill GE. Microbial diversity of wild bird feathers revealed through culture-based and culture-independent techniques. Microb Ecol. 2005;50:40–47. doi: 10.1007/s00248-004-0089-4. [DOI] [PubMed] [Google Scholar]
- Shi WB, Syrenne R, Sun JZ, Yuan JS. Molecular approaches to study the insect gut symbiotic microbiota at the 'omics' age. Insect Science. 2010;17:199–219. [Google Scholar]
- Straif SC, Mbogo CN, Toure AM, Walker ED, Kaufman M, Toure YT, et al. Midgut bacteria in Anopheles gambiae and An. funestus (Diptera: Culicidae) from Kenya and Mali. J Med Entomol. 1998;35:222–226. doi: 10.1093/jmedent/35.3.222. [DOI] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Zouache K, Voronin D, Tran-Van V, Mousson L, Failloux AB, Mavingui P. Persistent Wolbachia and cultivable bacteria infection in the reproductive and somatic tissues of the mosquito vector Aedes albopictus. PLoS One. 2009;4:e6388. doi: 10.1371/journal.pone.0006388. [DOI] [PMC free article] [PubMed] [Google Scholar]