Abstract
To test the hypotheses that global decreased neuro-axonal integrity reflected by decreased N-acetylaspartate (NAA) and increased glial activation reflected by an elevation in its marker, the myo-inositol (mI), present in a CD8-depleted rhesus macaque model of HIV-associated neurocognitive disorders. To this end we performed quantitative MRI and 16×16×4 multivoxel proton MR spectroscopic imaging (TE/TR=33/1400 ms) in 5 macaques pre- and 4-6 weeks post-simian immunodeficiency virus infection. Absolute NAA, creatine, choline (Cho), and mI concentrations, gray and white matter (GM, WM) and cerebrospinal fluid fractions were obtained. Global GM and WM concentrations were estimated from 224 voxels (at 0.125 cm3 spatial resolution over ~35% of the brain) using linear regression. Pre- to post-infection global WM NAA declined 8%: 6.6±0.4 to 6.0±0.5 mM (p=0.05); GM Cho declined 20%: 1.3±0.2 to 1.0±0.1 mM (p<0.003); global mI increased 11%: 5.7±0.4 to 6.5±0.5 mM (p<0.03). Global, GM and WM brain volume fraction changes were statistically insignificant. These metabolic changes are consistent with global WM (axonal) injury and glial activation, and suggest possible GM host immune response.
Keywords: Animal Disease Models, Brain, HIV-1, Macaca mulatta, Magnetic Resonance Spectroscopy, Simian immunodeficiency virus
INTRODUCTION
HIV-associated neurocognitive disorders (HAND) are a significant problem for the million infected in the US alone (1,2). Unfortunately, incomplete understanding of its pathogenesis hinders development of effective therapies. Recent evidence has shown that despite the benefits of highly-active antiretroviral therapy, HAND incidence among advanced HIV patients has not changed and its prevalence continues to climb (3). Moreover, mounting evidence (4,5) suggests that even while the virus is maximally suppressed in cerebrospinal fluid (CSF) and blood plasma, HIV may still facilitate dementia. This is thought to be due to patients living significantly (up to 20+ years) longer with the disease (6), allowing infected monocytes, macrophages and microglia to produce a toxic cascade that ultimately results in neuronal death (7). MRI and proton MR spectroscopy (1H-MRS) have been useful in detecting brain abnormalities (8-11), and provide critical knowledge of the dynamics of cerebral injury during HIV-infection (12-15).
Due to practical challenges to retrospective HIV studies, e.g., unknown infection date and overlapping secondary conditions, animal models are often used (16). Simian immunodeficiency virus (SIV)-infected rhesus macaque shares similar pathology with HIV-infection, including development of AIDS, disease of the central nervous system and cognitive or behavioral deficits (17-20). Although several 1H-MRS studies of SIV-infected macaque models are reported, all used low spatial resolution (>3 cm3) single-voxels in few brain regions that typically suffer CSF, gray and white matter (GM, WM) partial volume (21-23). Consequently, one outstanding, therapy-relevant question remains the relative dysfunction of the global GM versus WM.
Indeed, prior histopathology of SIV-infected macaques has shown neuro-axonal dysfunction and death in both GM and WM regions (24,25), as well as glial activation in subcortical WM and cortical GM suggestive of developing encephalitis (26,27). Based on these findings, our goal in this paper was to test the hypothesis that decreases in global GM and WM N-acetylaspartate (NAA), the marker for neuronal integrity, and elevated global GM and WM myo-inositol (mI), the marker for glial activation (28,29), are already detectable in an accelerated non-human primate model of HAND. To overcome the spatial coverage restriction of single voxel 1H-MRS, we use an approach that combines the data from high, 0.125 cm3, spatial resolution 3D multivoxel 1H-MRS imaging (MRSI) with MRI tissue segmentation (30), to analyze hundreds of voxels cooperatively, thereby increasing the overall precision (30) - at the cost of sensitivity to possible regional variations. We use this approach to compare the global GM and WM levels of NAA, mI, creatine (Cr) and choline (Cho) - the markers of cellular energy/density and membrane turnover (28,29), in a large, 28 cm3 (~35%) portion of 5 rhesus macaque brains before and 4 – 6 weeks after SIV infection, when they are persistently CD8+ lymphocyte-depleted (23).
EXPERIMENTAL
Non-human primates
Five (2 females, 3 males; 5.0 – 8.6 kg weight) healthy 3 – 4 year-old adult rhesus macaques (Macaca mulatta) were scanned under constant veterinary supervision. Each was tranquilized with 15 – 20 mg/kg intramuscular ketamine hydrochloride and intubated to ensure a patent airway during the experiment (no mechanical ventilation was needed). Intravenous injection of 0.4 mg/kg atropine was used to prevent bradycardia. Continuous infusion of 0.25 mg/kg/minute propofol was maintained via a catheter in a saphenous vein. Heart and respiratory rates, oxygen saturation and end-tidal CO2 were monitored continuously and a water blanket used to prevent hypothermia. All animals were then subsequently infected by intravenous injection with SIV and their CD8+ lymphocytes persistently depleted (>28 days post-infection) to speed up progression to AIDS and SIV encephalitis, both late-stage events similar to those of HIV-infection (31,32), with an antibody targeted against CD8 (cM-T807) at 6, 8 and 12 days post-inoculation. CD8+ depletion without SIV infection has been demonstrated to not produce metabolic changes or pathological abnormalities in the rhesus macaque brain (33). The animals were rescanned 4 or 6 weeks later to assess disease activity. The protocol was approved by the Harvard Medical School and Massachusetts General Hospital Institutional Animal Care and Utilization Committees.
MRI
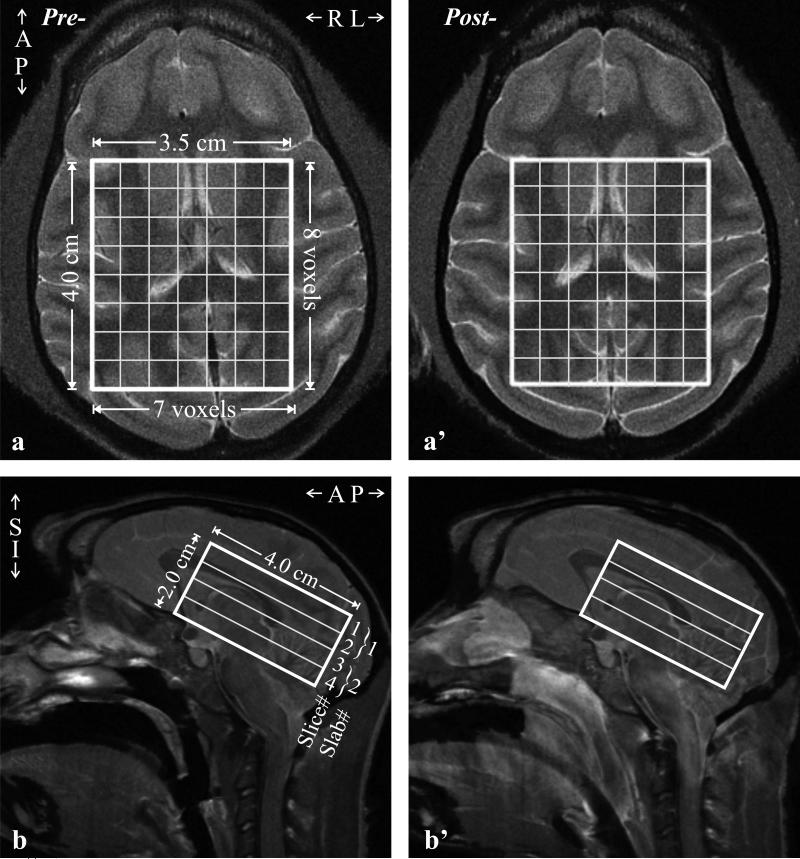
All experiments were done in a 3 T whole-body MR imager (Magnetom TIM Trio, Siemens AG, Erlangen, Germany), running software version VB13P. It was equipped with the manufacturer's circularly-polarized transmit-receive knee-coil capable of delivering a peak 2 KHz (45.2 μT) radio-frequency (RF) B1 field with ~1 kW of power. To guide the 1H-MRS volume-of-interest (VOI) and for tissue segmentation, sagittal and axial T2-weighted turbo spin echo (TSE) MRI: TE/TR=16/7430 ms, 140×140 mm2 field-of-view (FOV), 512×512 matrix, were acquired. To cover the entire macaque head, 24 sagittal images 2.0 mm thick each and 40 axial 1.2 mm thick slices were obtained, as shown in Fig. 1.
Fig. 1.
Left (pre-infection): Axial (a) and sagittal (b) T2-weighted turbo spin echo (TSE) MRI of a female macaque head showing the location and size of the 4.0×3.5×2.0 cm3 volume-of-interest (VOI) and localization grid (solid white frame) and two second-order Hadamard slabs, }1 and }2, in the IS direction (b).
Right (a’, b’): Same as (a, b) six weeks post-SIV infection in that animal. Note similar VOI placement and brain coverage.
Multivoxel 3D 1H-MRSI
A 4.0 cm anterior-posterior (AP) ×3.5 cm left-right (LR) ×2.0 cm inferior-superior (IS) =28 cm3 VOI was image-guided, onto the corpus callosum and angled along the genu-splenium line of each animal to maximize the number of brain voxels within it while avoiding air-filled sinuses and skull lipids, as shown in Fig. 1. The manufacturer's automatic procedure adjusted the first- and second-order shims to 26±1 Hz full-width-at-half-maximum (FWHM) VOI water line. The VOI was excited using TE=33 ms PRESS with two, 1 cm-thick, second-order Hadamard-encoded slabs (two 0.5 cm thick slices each), interleaved along the IS direction every 720 ms for an effective TR of 1440 ms for each slab (and slice), as shown in Fig. 1b. This results in optimal signal-to-noise-ratio (SNR) and spatial coverage (34), as well as allows a strong, 9 mT/m Hadamard slice-selection gradient to keep the maximal 1.6 ppm chemical shift displacement between NAA and mI to 0.5 mm, 10% of each slice's thickness (35). The four slices’ planes were encoded with 16×16 2D-Chemical shift imaging over an 8×8 cm2 (LR×AP) FOV to yield nominal (0.5 cm)3=0.125 cm3 voxels [1.12×1.12×0.75≈0.15 cm3 given the FWHM of the 2D point spread function (36)]. The VOI was defined in their planes by the two 9 ms 4.9 kHz bandwidth 180° RF pulses, under 3.3 and 2.9 mT/m. The localization grid produced 8×7 voxels per slice, for a total of 224 in the VOI, as shown in Fig. 1. The MRS signal was acquired for 256 ms at ±1 kHz bandwidth. Each 16×16×2 1H-MRSI scan took ~12.5 minutes and the 4 averages ~50 minutes.
Brain Volumetry
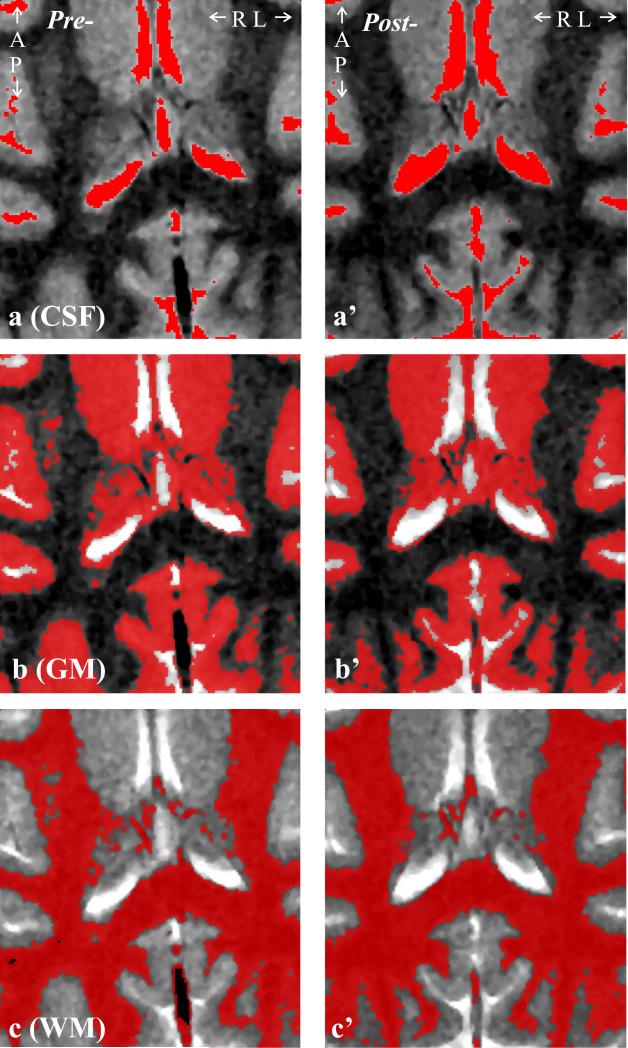
The TSE images were segmented with our FireVoxel package (37). It first corrects all images for non-uniform intensities due to the coil's RF inhomogeneities, using the common histogram devolution technique of Sled et al. (38). Next, a WM signal intensity, IWM, is selected in a periventricular “seed” region. Following automatic detection of all pixels at or above 50% (but below 172.5% to exclude the CSF) of IWM, a tissue-mask is constructed per slice in three steps: morphological erosion, recursive region growth retaining pixels connected to the seed; and morphological inflation to reverse the effect of erosion. Pixels of intensity above 172.5% of IWM are classified as CSF, as shown in Fig. 2a. Following a similar process, all pixels above 120% (but below 172.5%) of IWM, are classified as part of the GM mask; and pixels under 120% (but over 50% to exclude air cavities) classified as WM, as shown in Fig. 2b and 2c. The masks were co-registered with the 1H-MRSI grid using in-house software (MATLAB, The Mathworks Inc., Natick, MA) estimating their volume in every j-th voxel in the k-th animal (VjkGM, VjkWM, VjkCSF).
Fig. 2.
Left: Axial T2-weighted TSE image shows the anatomical coverage of the 4.0×3.5 cm2 VOI, overlaid with the: a: CSF, b: GM and c: WM masks generated by the FireVoxel package. Note the tissue type differentiation performance.
Right: a’-c’: Same as a-c, post-infection of the same female macaque.
Metabolic quantification
The 1H-MRSI data was processed offline using in-house software written in IDL (Research Systems Inc., Boulder, CO). The data was voxel-shifted to align the NAA grid with the VOI, then Fourier transformed in the time, AP and LR dimensions and Hadamard reconstructed along the IS direction with no spectral or spatial filters. The spectra were each automatically frequency aligned and phased in reference to the NAA peak. Relative levels of the i=NAA, Cr, Cho (choline + phosphorylethanolamine), mI metabolite in the j=1...224 VOI voxel of the k=1..5 animal were estimated from their peak areas, Sijk, using the SITools-FITT parametric spectral modeling software using Cho, Cr, mI, NAA, glutamate and glutamine full model functions (39). The Sijk-s were scaled into absolute amounts, Qijk, relative to a 0.5 L sphere of Civitro=12.5, 10.0, 3.0 and 7.5 mM NAA, Cr, Cho and mI in water at physiological ionic strength to load the coil and similar VOI size and position in order to sample the B1 profile as closely as possible:
| [1] |
where V is the voxel volume, the SR is the sphere's voxels’ metabolites’ signal, Pj180° and PR180° the RF power for a non-selective 1 ms 180° inversion pulse on the animal and reference.
To account for different relaxation times in vivo (T1vivo, T2vivo) and in the reference phantom (T1vitro, T2vitro), the Qijk in Eq. [1] were corrected with a factor for each metabolite, i:
| [2] |
with 316, 177 and 264 ms macaque 3 T T2vivo values for NAA, Cr and Cho used (40), that represent a 60:40 GM:WM brain fractions in the VOI. For mI, we used the human T2vivo=200 ms value reported by Posse et al. (41). We also used the respective macaque 3 T T1vivo values of 1232, 1238, 1107 and 1170 ms (41,42). No age-related T1/T2s differences were anticipated in this cohort of animals of similar ages as those in a previous report (40,42). The corresponding values measured in the phantom were T2vitro=483, 288, 200, 233 ms and T1vitro=605, 336, 235, 280 ms.
The global tissue concentration of each metabolite in the VOI, Cik, was obtained as:
| [3] |
This Cik has the advantage of (number of voxels)½≈15 fold lower variance than the individual voxels’ and, hence, expected to yield proportionally better precision, as described by Kreis (43).
Global WM and GM concentrations
Since the CSF does not contribute to the 1H-MRS signal, the i-th metabolite amount in the j-th voxel in the k-th animal can be modeled as the sum of two compartments’ (GM, WM) amounts:
| [4] |
where CikWM, CikGM are the (unknown) global WM and GM metabolites’ concentrations and the GM and WM fi-s are given by Eq. [2]. The T2vivos used were 325, 178, 274 and 200 ms for NAA, Cr, Cho and mI in GM; 311, 181, 255 and 200 ms in WM (40,41). Since no significant GM and WM T1vivo differences are reported (42), we used the values following Eq. [2]. Although CikWM and CikGM cannot both be derived from Eq. [4], since the macaque brain's GM and WM spatial heterogeneity is on a scale smaller than the voxels, each has different, independent VjkWM and VjkGM coefficients. The resulting over-determined system of j=224 equations in CikWM and CikGM was solved with linear regression. The regression fitting error per voxel was calculated for each metabolite for every animal pre- and post-infection and averaged. The intra-animal coefficient of variation (CV= standard deviation/mean) of this approach was shown to be under 5% for all metabolites (30).
Statistical Analyses
The metabolite change for each metabolite and the brain volume change in each compartment (i.e., global VOI tissue, WM, and GM) were computed for each animal by taking their pre-minus the post-infection level so that a positive change reflects a decline over time. The lower and upper limits of a 95% confidence interval for the true mean change from the “pre” to “post”-infection scans were estimated for each metabolite in each tissue compartment. The 5-animal sample size was insufficient to permit a nonparametric test of whether there was a change in any metabolite for any tissue compartment. As a result, paired sample t tests were used to assess MRS as well as brain volume changes from the “pre” to “post”-infection scans. Significance was tested at the p<0.05 level and SAS version 9.0 (SAS Institute, Cary, NC) was used for all calculations.
RESULTS
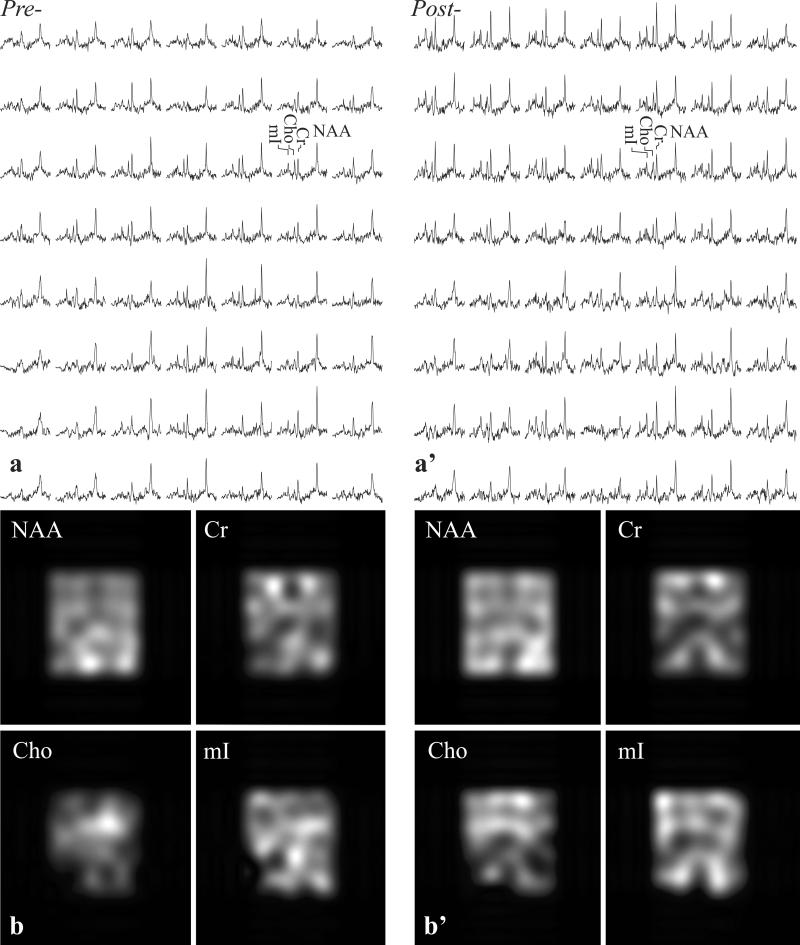
Example of the VOI position and size, pre- and post-infection, is shown in Fig. 1. The corresponding GM, WM and CSF VOI masks, segmented from the TSE images for VGM and VWM, are shown in Fig. 2. Spectra from the VOI and metabolic maps generated from them, pre- and post-infection, are shown in Fig. 3. Note that the lateral ventricles in the MRI in Fig. 1a, a’, can be detected in the metabolic map for each of the metabolites, reflecting SNR and localization performance. Shimming yielded voxel linewidth of 5.9±0.9 Hz FWHM (mean±standard deviation) in the 2240 voxels (224 voxels/scan ×2 scans/animal ×5 animals) and SNRs of NAA: 25±8, Cr: 16±6, Cho: 10±3 and mI: 10±4, as shown in Fig. 3. Excellent fit reliability with mean voxel Cramer-Rao lower bounds (CRLBs) below 15% were obtained for NAA, Cr, Cho, and mI. To optimize the analyses reliability, VOI voxels were included only if their CRLBs were <20% for all metabolites. The global regression fitting errors per voxel for GM/WM were: NAA=0.04±0.02, Cr=0.03±0.01, Cho=0.002±0.001, mI=0.04±0.04 mM amongst the five animals and two timepoints.
Fig. 3.
Top: Real part of the 7×8 (LR×AP) 1H spectra matrices from the VOI in Fig. 1a and a’, on common chemical shift and intensity scales pre- (a) and six weeks post- (a’) SIV infection. Note the SNR in those (0.5)3=0.125 cm3 voxels: NAA: 25±8, Cr: 16±6, Cho: 10±3 and mI: 10±4 and spectral resolution, leading to excellent fit reliability reflected by voxel CRLBs below 15% for these four metabolites.
Bottom (b, b’): NAA, Cr, Cho, and mI metabolic maps from the spectra in a, a’. Note the gross anatomical features reflecting spatial localization.
GM and WM tissue volumes in the VOI were 16.0±0.7 cm3 and 10.1±0.6 cm3 pre-infection , and 15.0±1.1 cm3 and 10.6±0.9 cm3 post- infection,.None of these changes were significant (p>0.2 for both). Since the % WM and GM volumes changes seem to be offsetting, i.e., the total tissue volume in the VOI appears to remain unchanged, these variations probably reflect different tissue sampling from slight VOI misregistration in the follow-up scan of each animal.
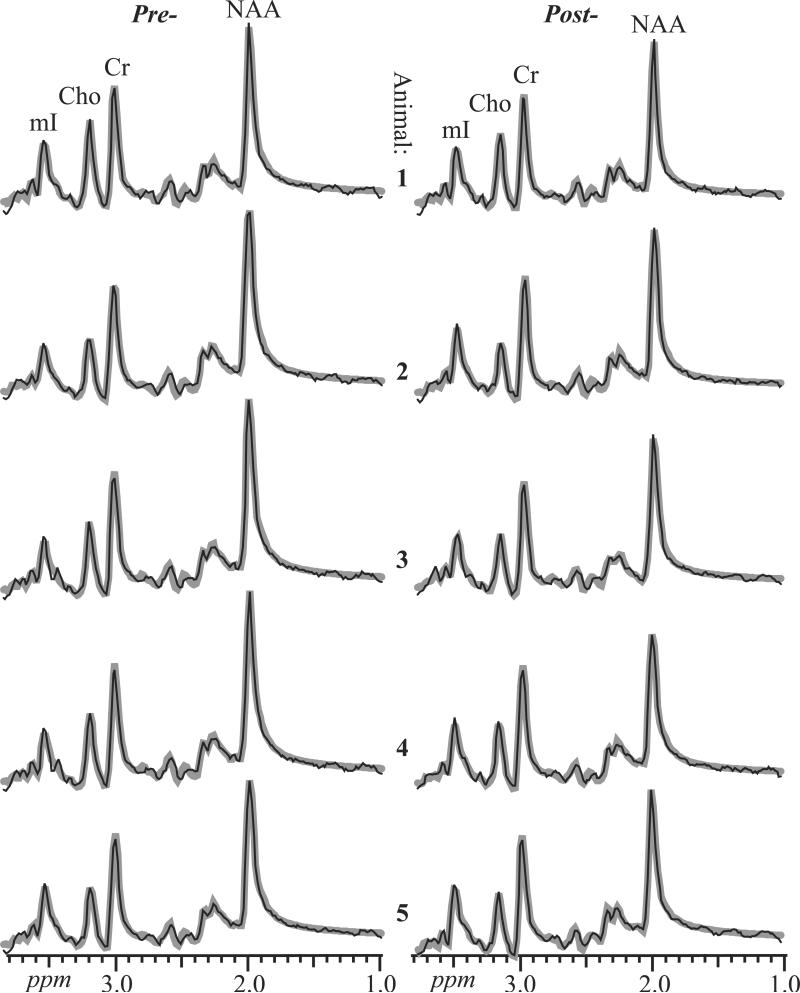
The sums of all 224 spectra in the VOI (equivalent to the numerator of Eq. [3]) for each animal pre- and post-infection, overlaid with their fits, are shown in Fig. 4. They exhibit SNRs of 390±30, 223±12, 151±17 and 144±17 for NAA, Cr, Cho and mI, a dramatic ~224½ ≈ 15 fold gain over the source 0.125 cm3 voxels (compare Fig. 4 with Fig. 3). The single-voxels’ spectral resolution is also maintained in the sums, as reflected by their 8.2±0.8 Hz FWHM; a result of pre-alignment, this further contributes to their SNR increase.
Fig. 4.
Real part of the aligned and summed 1H spectra from all VOI voxels (thin black lines) representing Eq. [3], for each of the animals pre- (left) and 4 (animals 4 and 5) or 6 (animals 1-3) weeks post-SIV-infection (right), superimposed with their fitted model functions (thick gray lines), on common intensity and chemical shift scales. Animals 1 and 2 are female; 3 – 5 male. Note the excellent SNRs (reflected by CRLBs of less than 2% for the four metabolites) and spectral resolution compared with individual voxels .
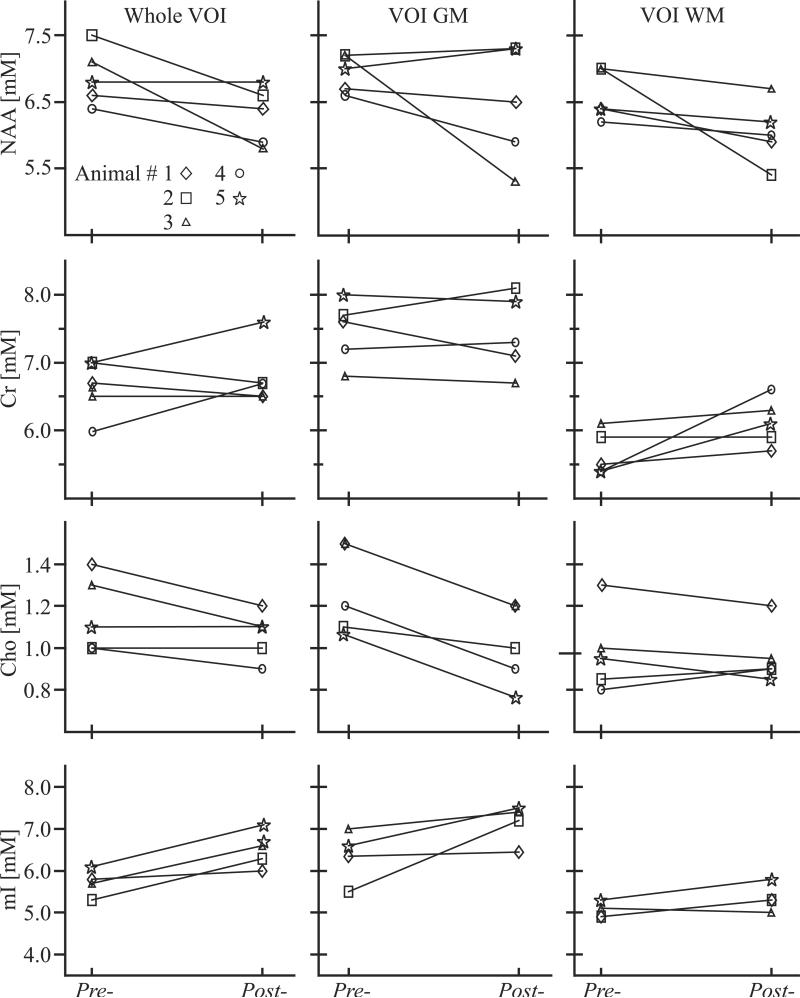
The resultant global (Eq. [3]) as well as GM and WM (Eq. [4]) concentrations for each metabolite in each animal, pre- and post-SIV-infection, are shown in Fig. 5. They reveal a significant global VOI Cho decline (-13%, p<0.03), with a 95% confidence interval (CI) of [-0.25, -0.08 mM] and mI increase (11%, p<0.03), CI=[0.18, 1.38 mM]. A significant Cho decline (-20%, p<0.003), CI=[-0.39, -0.16 mM] was found only in the GM. Slight increases at the “trend” level (p~0.1) were also observed in the WM Cr and mI, perhaps reflecting the small sample size and smaller VOI WM volume (see above; the WM volume was on average ⅔ of the GM's). Since NAA is known to always decline in all adult neuro-pathologies, we tested at the single-sided level and found a significant global decline in the whole VOI (-9%, p<0.04), CI=[-1.31, 0.07 mM] and in the WM (-8%, p=0.05) CI=[-1.23, 0.13 mM], but no significant change in GM. No metabolite differences greater than two standard deviations from the mean of all animals were observed between males and frmales when the group was divided according to gender.
Fig. 5.
NAA, Cr, Cho and mI concentrations’ line-plots for all animals, pre- and 4 (animals 4 - 5) or 6 (animals 1-3) weeks post-SIV-infection, for the whole VOI and its GM and WM. Note significant >10% declines in VOI Cho and NAA, elevated mI, as well as GM Cho and WM NAA declines.
DISCUSSION
Although several SIV-infected macaque models 1H-MRS studies have been reported to date, these have been limited by low, >3 cm3, spatial resolution single-voxels that excluded >95% of the brain and suffered CSF, GM, and WM partial volume (21-23). To overcome such limitations, we combined the data from high spatial resolution (0.125 cm3) 3D multi-voxel MRSI with tissue segmentation from the MRI. This enabled us to analyze the metabolic and structural data from hundreds of voxels cooperatively, increasing the overall precision, at the cost of sensitivity to possible regional or specific structure variations (30,43).
Due to the sporadic nature and low incidence of HAND and its consequent paucity of postmortem studies, non-human primate models provide an alternative to histopathology for studying its neuropathogenesis. In this context, 1H-MRS provides a nondestructive tool for detecting brain metabolite abnormalities in intact animals, facilitating longitudinal studies that histopathology does not. Towards this end, this study aims to quantify the global GM and WM changes associated with SIV-infection in order to test the hypothesis that global neuro-axonal injury and glial cell activation develop in an accelerated nonhuman primate model of HAND.
WM neuronal dysfunction
Indeed, we find significant NAA decline in WM that appears earlier or is more profound than in GM. Although evidence of focal neuronal damage in frontal cortex was seen with single-voxel 1H-MRS (22), it is possible that global GM dysfunction does not occur until later stages of infection, as suggested previously (27). Nonetheless, a finding of lower WM NAA is consistent with ex vivo studies showing NAA/Cr decline in centrum semiovale and frontal WM in acute- and chronically-infected animal and human samples (44). Several in vivo animal studies also found 5-10% WM NAA/Cr decreases 4 weeks post-infection (23,45,46), consistent with us, and some even showed WM NAA decline 27 days post-infection was proportional to synaptic and dendritic integrity deterioration and neuronal loss in quantitative immunohistochemistry (23,46).
Diffuse GM and WM glial activation
Increased global mI levels support our hypothesis of diffuse glial activation (28,29,47). Previous HIV studies have also suggested a role for gliosis, reflected by elevated mI in both WM and GM of patients with mild HIV-dementia and clinically asymptomatic patients (12,48); these fit well with immunohistochemical studies showing astrocytic and microglial hypertrophy in both the WM and GM of HIV patients with, or without, dementia (49). In SIV-infected macaque models, elevated mI has been reported in several GM and WM regions using single voxel 1H-MRS (22). Evidence seems to suggest a correspondence with increased levels of glial fibrillary acidic protein and ionized calcium binding adaptor molecule-1, immunohistochemistry markers of astrogliosis and microglial activation, at 4 (or 8) weeks post-infection (46).
GM host immune response
The observed 20% global GM Cho decline (see Fig. 5) is consistent with a host immunological response to developing SIV encephalitis, reported previously in the basal ganglia (50). That is, immunopathological evidence has shown that macaques with basal ganglia Cho/Cr near uninfected level or below at 4 weeks post infection (after an initial increase at 2 weeks post infection) revealed no later signs of encephalitis, whereas those whose levels remained elevated did (50). Moreover, studies of both the traditional (21) as well as accelerated (46) SIV macaque models of HAND have also demonstrated large reductions in Cho and Cho/Cr (after initial elevation) 2 to 4 weeks post-infection, consistent with our findings of decreased Cho at 4 weeks.
Although it has not been established what role this return to pre-infection levels plays in the context of disease progression, it is reasonable to assume that an immune response is involved given the similar Cho/Cr changes reported after neuroprotective treatment in SIV-infected macaques (22,45), and following antiretroviral treatment in HIV-infected patients (51). Furthermore, both treatments have been shown to support host immune defense through: (i) downregulation of microglial activation in the former (52); and (ii) reduction of infected monocyte trafficking into the central nervous system in the latter (23).
Finally, increased energy demand may also occur due to glial activation, as reflected by slight increases in WM Cr. Evidence for this can be found in a previous study (46) that showed significant Cr elevation at 8-weeks post- compared with pre-infection and a correspondence of this change with greater histopathological evidence of gliosis.
Caveats
This study is also subject to several limitations. First, the decision to maximize the sensitivity to diffuse, global changes, especially for the weaker mI signal, comes at the cost of localization. Although the original MRSI data is available, regional analyses may suffer 10× lower sensitivity to metabolic changes due to 22-45% intra-voxel CVs (53). Consequently, a ~25% decline in parietal cortex neuron number 4 weeks after SIV-infection (46) would go undetected. Second, due to the air-tissue interfaces (i.e., paranasal sinuses) in the frontal region that cause severe B0 field inhomogeneity (54)—leading to signal dropout-- our VOI placement was limited to exclude most of the frontal brain - an area known to be affected in HAND. However, since our method assesses global GM and WM, exclusion or inclusion of specific region(s) should not affect the findings in diffuse diseases significantly. For example, given their relatively modest, 2.2 cm3, 14% (55) fraction of the VOI's 16 cm3 GM volume and the fact that changes there are of the order of % (see Fig. 3), areas vulnerable to high viral burden, e.g., basal ganglia, cannot in of themselves explain the global GM changes observed. Third, although we account for T1 and T2 in healthy macaques, possible pathology effects on either remain unknown. Determining these effects was too costly and time-consuming, especially since pathology-related changes may vary over the 4-6 week infection period. However, use of short, TE=33 ms and TR≈T1 has been shown to reduce the metabolic quantification variations from T1 and T2 variations of up to 10% to below the ~5% voxel SNR (42,56). Fourth, because viral and host immune function can vary over a 2-week period (23), use of two post-infection scan times could have obscured some effects. Due to cost constraints and lack of preliminary differences between the two groups, we combined their data to improve statistical power. Logistical constraints notwithstanding, future studies would benefit from more animals scanned at multiple times, including closer to infection, when alterations in cerebral metabolism due to initial viral activity may be more evident.
Our results reveal global tissue injury and recovery while also distinguishing GM- from WM-related dysfunction during the later stages of SIV infection in an accelerated macaque model of HAND. Although host immunological response appears a likely explanation to some of the changes observed during viral infection, its disparate effects in GM and WM suggest distinct pathological pathways that need additional study. These, for example, might examine how neuroprotective intervention in conjunction with anti-inflammatory agents affect the GM and WM and whether alone, or taken together, might be better strategies for treatment of the disease.
ACKNOWLEDGEMENTS
We thank Drs. Andrew A. Maudsley and Brian J. Soher for their SITools-FITT software and Dr. Joanne Morris and Ms. Shannon Luboyeski for animal veterinary care.
Supported by NIH Grants EB01015, NS050520, NS050041, NS051129, NS059331, NS040237 and NS059331. The Athinoula A. Martinos Center for Biomedical Imaging is supported by National Center for Research Resources grant number P41RR14075. Assaf Tal acknowledges support from the Human Frontiers Science Project's Cross-Disciplinary Fellowship.
Abbreviations Used
- HIV
human immunodeficiency virus
- HAND
HIV-associated neurocognitive disorders
- SIV
simian immunodeficiency virus
- GM
gray matter
- WM
white matter
- CSF
cerebrospinal fluid
- MRSI
magnetic resonance spectroscopic imaging
- VOI
volume-of-interest
- FWHM
full-width at half-maximum
- NAA
N-acetylaspartate
- Cho
choline
- Cr
creatine
- mI
myo-inositol
- CD8
cluster of differentiation 8
REFERENCES
- 1.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 2.Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4(2):163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 3.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 4.Pialoux G, Fournier S, Moulignier A, Poveda JD, Clavel F, Dupont B. Central nervous system as a sanctuary for HIV-1 infection despite treatment with zidovudine, lamivudine and indinavir. AIDS. 1997;11(10):1302–1303. doi: 10.1097/00002030-199710001-00009. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham PH, Smith DG, Satchell C, Cooper DA, Brew B. Evidence for independent development of resistance to HIV-1 reverse transcriptase inhibitors in the cerebrospinal fluid. AIDS. 2000;14(13):1949–1954. doi: 10.1097/00002030-200009080-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, Phillips A, Porter K. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63(2):213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 7.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 8.Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43(10):2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- 9.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61(3):369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- 10.Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Eckert MA, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, Toga AW, Reiss AL. Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J Neurosci. 2005;25(16):4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castelo JM, Courtney MG, Melrose RJ, Stern CE. Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Arch Neurol. 2007;64(9):1275–1280. doi: 10.1001/archneur.64.9.1275. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52(1):100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhoff DJ, MacKay S, Bachman L, Poole N, Dillon WP, Weiner MW, Fein G. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993;43(3 Pt 1):509–515. doi: 10.1212/wnl.43.3_part_1.509. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed MA, Lentz MR, Lee V, Halpern EF, Sacktor N, Selnes O, Barker PB, Pomper MG. Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology. 2010;254(2):577–586. doi: 10.1148/radiol.09081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, Venna N, Williams K, Rosenberg ES, Gonzalez RG. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72(17):1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campsmith ML RP, Hall HI, Green T. MMWR Weekly. Volume 2008: Div of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. CDC; 2008. [Google Scholar]
- 17.Desrosiers RC. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 18.Murray EA, Rausch DM, Lendvay J, Sharer LR, Eiden LE. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science. 1992;255(5049):1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- 19.Burudi EM, Fox HS. Simian immunodeficiency virus model of HIV-induced central nervous system dysfunction. Adv Virus Res. 2001;56:435–468. doi: 10.1016/s0065-3527(01)56035-2. [DOI] [PubMed] [Google Scholar]
- 20.Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151(3):793–803. [PMC free article] [PubMed] [Google Scholar]
- 21.Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51(6):1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- 22.Ratai EM, Pilkenton SJ, Greco JB, Lentz MR, Bombardier JP, Turk KW, He J, Joo CG, Lee V, Westmoreland S, Halpern E, Lackner AA, Gonzalez RG. In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci. 2009;10:63. doi: 10.1186/1471-2202-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115(9):2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, Lee PL, Masliah E, Lackner AA. Early brain injury in the SIV-macaque model of AIDS. AIDS. 2000;14(18):2841–2849. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- 25.Mankowski JL, Queen SE, Tarwater PM, Fox KJ, Perry VH. Accumulation of beta-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. Journal of neuropathology and experimental neurology. 2002;61(1):85–90. doi: 10.1093/jnen/61.1.85. [DOI] [PubMed] [Google Scholar]
- 26.Berman NE, Marcario JK, Yong C, Raghavan R, Raymond LA, Joag SV, Narayan O, Cheney PD. Microglial activation and neurological symptoms in the SIV model of NeuroAIDS: association of MHC-II and MMP-9 expression with behavioral deficits and evoked potential changes. Neurobiology of Disease. 1999;6(6):486–498. doi: 10.1006/nbdi.1999.0261. [DOI] [PubMed] [Google Scholar]
- 27.Xing HQ, Moritoyo T, Mori K, Tadakuma K, Sugimoto C, Ono F, Hayakawa H, Izumo S. Simian immunodeficiency virus encephalitis in the white matter and degeneration of the cerebral cortex occur independently in simian immunodeficiency virus-infected monkey. Journal of neurovirology. 2003;9(4):508–518. doi: 10.1080/13550280390218904. [DOI] [PubMed] [Google Scholar]
- 28.Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clinical radiology. 2009;64(1):12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Mountford CE, Stanwell P, Lin A, Ramadan S, Ross B. Neurospectroscopy: the past, present and future. Chem Rev. 2010;110(5):3060–3086. doi: 10.1021/cr900250y. [DOI] [PubMed] [Google Scholar]
- 30.Tal A, Kirov I, Gonen O. The Role of Gray and White Matter Segmentation in Quantitative Proton MR Spectroscopic Imaging. NMR in Biomedicine. 2012 doi: 10.1002/nbm.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36(2):156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- 32.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 33.Ratai EM, Pilkenton S, He J, Fell R, Bombardier JP, Joo CG, Lentz MR, Kim WK, Burdo TH, Autissier P, Annamalai L, Curran E, O'Neil SP, Westmoreland SV, Williams KC, Masliah E, Gilberto Gonzalez R. CD8+ lymphocyte depletion without SIV infection does not produce metabolic changes or pathological abnormalities in the rhesus macaque brain. J Med Primatol. 2011;40(5):300–309. doi: 10.1111/j.1600-0684.2011.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goelman G, Liu S, Hess D, Gonen O. Optimizing the efficiency of high-field multivoxel spectroscopic imaging by multiplexing in space and time. Magn Reson Med. 2006;56(1):34–40. doi: 10.1002/mrm.20942. [DOI] [PubMed] [Google Scholar]
- 35.Goelman G, Liu S, Fleysher R, Fleysher L, Grossman RI, Gonen O. Chemical-shift artifact reduction in Hadamard-encoded MR spectroscopic imaging at high (3T and 7T) magnetic fields. Magn Reson Med. 2007;58(1):167–173. doi: 10.1002/mrm.21251. [DOI] [PubMed] [Google Scholar]
- 36.Mareci T, Brooker H. Essential considerations for spectral localization using indirect gradient encoding of spatial information. J Magn Reson. 1991;92:229–246. [Google Scholar]
- 37.Mikheev A, Nevsky G, Govindan S, Grossman R, Rusinek H. Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J Magn Reson Imaging. 2008;27(6):1235–1241. doi: 10.1002/jmri.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 39.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40(6):822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Gonen O, Fleysher R, Fleysher L, Babb JS, Soher BJ, Joo CG, Ratai EM, Gonzalez RG. Metabolite proton T(2) mapping in the healthy rhesus macaque brain at 3 T. Magn Reson Med. 2009;62(5):1292–1299. doi: 10.1002/mrm.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Ugurbil K, Lim KO, Alger JR. Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med. 2007 doi: 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Fleysher R, Fleysher L, Joo CG, Ratai EM, Gonzalez RG, Gonen O. Brain metabolites B1-corrected proton T1 mapping in the rhesus macaque at 3 T. Magn Reson Med. 2010;63(4):865–871. doi: 10.1002/mrm.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreis R, Slotboom J, Hofmann L, Boesch C. Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magn Reson Med. 2005;54(4):761–768. doi: 10.1002/mrm.20673. [DOI] [PubMed] [Google Scholar]
- 44.Cheng LL, Ma MJ, Becerra L, Ptak T, Tracey I, Lackner A, Gonzalez RG. Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997;94(12):6408–6413. doi: 10.1073/pnas.94.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratai EM, Bombardier JP, Joo CG, Annamalai L, Burdo TH, Campbell J, Fell R, Hakimelahi R, He J, Autissier P, Lentz MR, Halpern EF, Masliah E, Williams KC, Westmoreland SV, Gonzalez RG. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS One. 2010;5(5):e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratai EM, Annamalai L, Burdo T, Joo CG, Bombardier JP, Fell R, Hakimelahi R, He J, Lentz MR, Campbell J, Curran E, Halpern EF, Masliah E, Westmoreland SV, Williams KC, Gonzalez RG. Brain creatine elevation and N-Acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of neuroAIDS. Magn Reson Med. 2011;66(3):625–634. doi: 10.1002/mrm.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3-5):289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 48.Laubenberger J, Haussinger D, Bayer S, Thielemann S, Schneider B, Mundinger A, Hennig J, Langer M. HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology. 1996;199(3):805–810. doi: 10.1148/radiology.199.3.8638009. [DOI] [PubMed] [Google Scholar]
- 49.Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, McArthur JC, Trapp BD. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993;34(3):339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- 50.Fuller RA, Westmoreland SV, Ratai E, Greco JB, Kim JP, Lentz MR, He J, Sehgal PK, Masliah E, Halpern E, Lackner AA, Gonzalez RG. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004;5:10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, Miller EN. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999;53(4):782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- 52.Zemke D, Majid A. The potential of minocycline for neuroprotection in human neurologic disease. Clin Neuropharmacol. 2004;27(6):293–298. doi: 10.1097/01.wnf.0000150867.98887.3e. [DOI] [PubMed] [Google Scholar]
- 53.Wu WE, Kirov II, Zhang K, Babb JS, Joo CG, Ratai EM, Gonzalez RG, Gonen O. Cross-sectional and longitudinal reproducibility of rhesus macaque brain metabolites: A proton MR spectroscopy study at 3 T. Magn Reson Med. 2011 doi: 10.1002/mrm.22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duyn JH, Gillen J, Sobering G, van Zijl PC, Moonen CT. Multisection proton MR spectroscopic imaging of the brain. Radiology. 1993;188(1):277–282. doi: 10.1148/radiology.188.1.8511313. [DOI] [PubMed] [Google Scholar]
- 55.Yin D, Valles FE, Fiandaca MS, Forsayeth J, Larson P, Starr P, Bankiewicz KS. Striatal volume differences between non-human and human primates. J Neurosci Methods. 2009;176(2):200–205. doi: 10.1016/j.jneumeth.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaaraoui W, Fleysher L, Fleysher R, Liu S, Soher BJ, Gonen O. Human brain-structure resolved T(2) relaxation times of proton metabolites at 3 Tesla. Magn Reson Med. 2007;57(6):983–989. doi: 10.1002/mrm.21250. [DOI] [PubMed] [Google Scholar]