Abstract
Rationale
Hypersignaling of corticotropin releasing factor (CRF) has been implicated in stress disorders, however many of its downstream mechanisms of action remain unclear. In vitro, CRF1 receptor activation initiates multiple cell signaling cascades, including protein kinase A (PKA), protein kinase C (PKC) and mitogen-activated protein kinase kinase MEK1/2 signaling. It is unclear however, which of these signaling cascades mediate CRF-induced behaviors during stress.
Objectives
We examined the role of PKA, PKC and MEK1/2 signaling pathways in CRF-induced anxiety as measured by startle hyperreactivity.
Methods
Mice treated with intracerbroventricular (ICV) ovine CRF (oCRF) were pretreated with the PKA inhibitor Rp-cAMPS, PKC inhibitor BIM (bisindolylmaleimide) or MEK1/2 inhibitor PD98059 (ICV) and assessed for acoustic startle reactivity.
Results
The PKC inhibitor BIM significantly attenuated CRF-induced increases in startle. BIM was also able to block startle increases induced by oCRF when both compounds were infused directly into the bed nucleus of stria terminalis (BNST). PKA and MEK1/2 inhibition had no significant effects on CRF-induced changes in startle at the dose ranges tested. CRF-induced disruption of PPI was not significantly reversed by any of the 3 pretreatments at the dose ranges tested.
Conclusions
PKC signaling is required for CRF-induced increases in startle, and this effect is mediated at least in part at the BNST. These findings suggest PKC signaling cascades: 1) may be important for the acute effects of CRF to induce startle hyperreactivity, and 2) support further research of the role of PKC signaling in startle abnormalities relevant to disorders such as posttraumatic stress disorder.
Keywords: CRF, CRH, startle, prepulse inhibition, PKC, MEK, ERK, PKA, bed nucleus of stria terminalis, signaling
Introduction
Corticotropin releasing factor (CRF) is rapidly released during emotional and physiological stress to initiate HPA secretion required for survival and coordinate the behavioral and physiological coping with the stressors. Most animal studies showed the anxiogenic effects of enhanced CRF neurotransmission in the central nervous system (dominantly via type-1 CRF receptor signaling; Takahashi 2001; Muller et al. 2003), however, it can also exert stress coping actions and long-term stress adaptation (via type-2 CRF receptor signaling; Bale et al. 2000). The startle response is an acute defensive response, and is increased during stress or threat in humans and animals (Davis et al. 1993; Grillon et al. 1993). In rodents, CRF treatment mimics stress effects on startle, and both CRF and stressinduced increases in startle can be blocked by CRF1 receptor antagonist treatment or deletion of the CRF1 receptor gene (Swerdlow et al. 1989; Risbrough et al. 2004, 2009; Walker et al. 2009b). Elevated CRF levels or acute stress exposure both reduce habituation and inhibition of startle in animals (Conti et al. 2002; (Risbrough 2003, 2004); (Bakshi et al. 2012). In humans, exaggerated startle reactivity and abnormally high cerebrospinal fluid levels of CRF are reported in individuals with posttraumatic stress disorder (PTSD) (Morgan et al. 1995; Grillon et al. 1996; Bremner et al. 1997; Baker et al. 1999; Sautter et al. 2003; for review see Risbrough and Stein 2006). Therefore, it has been hypothesized that brain CRF hypersecretion may contribute to increased arousal and dysregulation of the startle response in PTSD. This hypothesis is supported by data showing that central CRF administration increases startle reaction and decreases PPI in several experimental paradigms, such as unconditioned startle, light and fear potentiated startle (Swerdlow et al. 1989; Risbrough et al. 2009; Walker et al. 2009b; Bijlsma et al. 2011). Conversely, pharmacological antagonism of the CRF1 receptor before or immediately after predator stress blocks the development of enduring startle hyperreactivity in mice (Adamec et al. 2010), suggesting that CRF hypersignaling during and after stress triggers a cascade of molecular events contributing to the persistent effects of trauma on startle hyperreactivity. One of the remaining questions is to identify downstream signaling pathways mediating CRF receptor activation effects on acute defensive behaviors such as startle reaction.
CRF acts on the two cloned G-protein-coupled CRF receptor subtypes (CRF1 and CRF2) to generate signals via the second messenger protein kinases A (PKA) and C (PKC) pathways as well as the extracellular regulated kinase-mitogen activated protein kinase (MEK-ERK/MAPK) cascade. To activate the PKA pathway, CRF receptors couple to Gsα thereby stimulating adenylate cyclase generation of cAMP; cAMP then activates PKA which phosphorylates cellular targets, modulates gene transcription, and influences neuronal excitability (Dautzenberg et al. 2004; Hillhouse and Grammatopoulos 2006; Hauger et al. 2009). To activate the PKC pathway, CRF receptors couple to the Gq protein, stimulating phospholipase C activity and intracellular calcium mobilization, in turn, leading to phosphorylation of PKC which also can phosphorylate cytosolic proteins, modulates gene transcription, and influence cell excitability (Ungless et al. 2003; Hauger et al. 2003; Dautzenberg et al. 2004; Hillhouse and Grammatopoulos 2006; Gutknecht et al. 2009). Various cellular mechanisms regulate CRF receptor signaling via the MEK-ERK cascade which modulates intracellular and nuclear events including gene transcription and neuronal plasticity (Sweatt 2001; Punn et al. 2006; Hauger et al. 2009).
Involvement of the PKA pathway in startle reactivity is supported by evidence that cAMP increases startle response when administered into the spinal cord or the nucleus reticularis pontis (Boulis and Davis 1990; de Lima and Davis 1995). Additionally, increased PKA activity results in disrupted PPI (Kelly et al. 2007), and PKA mediates the startle hyperactivity phenotype in phosphodiesterase-4B deficient mice (Siuciak et al. 2008). The inhibition of PKC in the spinal cord, however, increases startle reactivity (Boulis and Davis 1990). Although these data suggest that PKA and PKC play a role in the execution of startle response and sensorimotor gating, their role in startle hyperreactivity induced by stress, CRF or threat stimuli are unknown. Activation of the MEK-ERK pathway is required for longterm potentiation in the amygdala, and for Pavlovian fear conditioning and fear-potentiated startle (Schafe et al. 2000; (Di Benedetto et al. 2009). Central CRF administration and stress also activate the MEK-ERK signaling in several neuronal circuits that modulate startle behavior including the BNST, amygdala, and hippocampus (Schafe et al. 2000; (Di Benedetto et al. 2009; Todorovic et al. 2009), suggesting the MEK-ERK signaling could also play a role in stress or CRF-induced startle hyperreactivity.
Here we tested the hypothesis that PKA, PKC and/or MEK-ERK signaling mediate the effects of CRF on startle behaviors. To test this hypothesis we examined the effects of pretreatment with PKA, PKC and/or MEK inhibitors on CRF-induced increases in startle and reductions in PPI. We also examined if this mechanism was found directly in the BNST, a critical region for CRF receptor activation effects on startle behavior (Waddell et al. 2008; Walker et al. 2009a; Lee and Davis, 1997).
Methods
Subjects
Male C57BL/6 mice (2 months of age) from Jackson Laboratories (Bar Harbor, Maine) were used for these studies. All mice were housed in a temperature and humidity-controlled room with a 12:12 h reverse light/dark cycle (lights off at 08:00) and food and water available ad libitum. All startle testing occurred between 10:00 and 18:00. All experiments were conducted in accordance with the statement revised and approved by the Society for Neuroscience in January 1995 and in accordance with “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003), as approved by the University of California, San Diego.
Surgery and histological verification
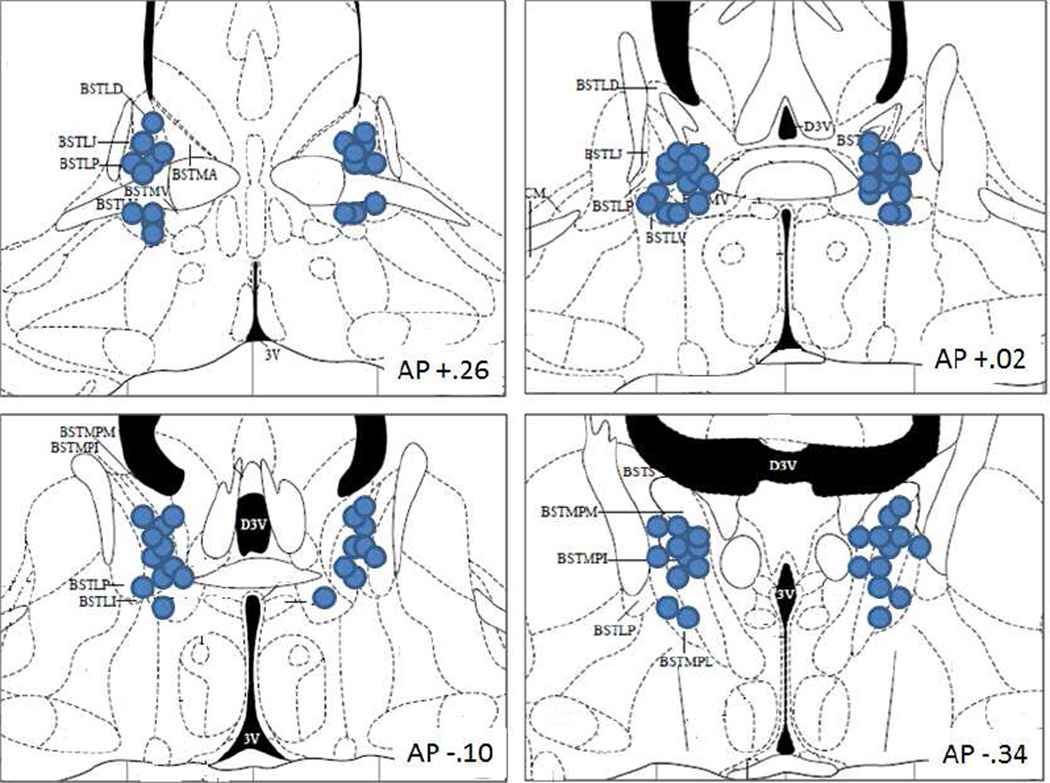
All mice were anesthetized using a ketamine/acepromazine cocktail (4.2/.835 mg/kg respectively). For the ICV cannulation surgery, a 23 gauge 7 mm unilateral guide cannulae was inserted into the lateral ventricle (flat skull; anteroposterior, −0.1 mm; mediolateral, ±1.1 mm; dorsoventral, −1.5 mm below dura). A 30 gauge 8 mm injector was used for ICV infusions (1 mm below the cannula tip). For the BNST surgeries, guide cannula (23 gauge, 7 mm) were inserted bilaterally above the ventricles and 2.6 mm above the BNST, to minimize damage or blockage of the ventricles (flat skull; anteroposterior, −0.22 mm; mediolateral, ±0.8 mm; dorsoventral, −1.25 mm below dura). A beveled 30 gauge 9.6 mm injector was used to pass through the ventricle to pierce the BNST below (2.6 mm below the cannula tip). All cannula were secured with one skull screw and dental cement (Den-Mat Corp., Santa Maria, CA) and closed with a removable stylet. Mice recovered for at least 1 week prior to testing. Within 1 week after study completion, mice were anesthetized and 1.5 µl of dye was infused to check for cannulae placement. Mice were killed immediately and as the brain was removed, the presence of dye in the fourth ventricle was noted. The presence of dye in the lateral and fourth ventricle was also noted by making a coronal cut along the guide tract. All brains were digitally scanned with the cut side on the slides. For BNST injections, brains were fixed with 4% paraformaldehyde, cut to 25 micron sections and Nissl stained. Mice with evidence of needle tracks outside the BNST were removed from analysis. See Figure 1 for schematic of injection sites of mice included in the analysis. Ten mice were removed from data analysis due to incorrect cannula placement. A total of 3 outliers in startle or prepulse inhibition measures (>3 standard deviations from the mean) were removed (1 mouse from the 0.3 nmol BIM/aCSF group, peak startle=366; 1 mouse from the 1.0 nmol BIM/oCRF group, peak startle=831; 1 mouse from the 3.7 nmol RpCAMPS/oCRF group, PPI=−27).
Fig. 1.
Injector tip placements in BNST of mice included in behavioral analyses. The approximate distance posterior to bregma in millimeters is indicated in the lower right of each panel. Coronal sections are from the atlas of (Paxinos and Franklin 2001). bed nucleus of the stria terminalis (BST); lateral division, dorsal part (BSTLD); juxtacapsular part (BSTLJ); lateral division, posterior part (BSTLP); lateral division, ventral part (BSTLV); medial division, posterior part (BSTMP); medial division, posterolateral part (BSTMPL); medial division, posteromedial part (BSTMPM); medial division, ventral part (BSTMV).
Drugs
oCRF (Bachem, Torrance, CA) was infused (0.24 nmol in 5 ul, ICV) 45 minutes before test. Doses and timing were based on previous studies (Risbrough et al. 2003, 2004; Gresack and Risbrough 2011). The PKC inhibitor BIM (Calbiochem, San Diego, CA) was infused (0.01–3.0 nmol in 5 ul, ICV) 60 minutes before oCRF. Both oCRF and BIM were dissolved in aCSF vehicle. The PKA inhibitor Rp-cAMPS (Biolog Lifescience Institute, Bremen, Germany) was infused (30–200 nmol dissolved in sterile water, 5ul ICV) 60 minutes before oCRF. The MEK inhibitor PD98059 (Calbiochem, San Diego, CA) was similarly infused (1.1–11 nmol in 2 ul, ICV) 60 min prior to oCRF. The vehicle for the MEK inhibitors was 30% DMSO/water. Doses and time course for all inhibitors were based on observations from pilot experiments and previous reports (Bourtchouladze et al. 1998; Schafe et al. 1999, 2000; Lu et al. 2001; Sananbenesi et al. 2003; Selvamani et al. 2007). ICV infusions were by gravity after which the injector remained in place for 1 min before removal. In all studies except the oCRF dose response in the BNST (see below), a between subject design was used.
For the dose response of oCRF in the BNST, oCRF (0.02 and 0.04 nmol in 0.25 ul/side) were infused directly into the BNST and testing was 60 min after injection (doses chosen based on Davis et al. 1997 in rats). In this study, we used a cross over design with a 1 week washout between injections and counterbalancing for dose order. In the second BNST experiment, we used a between subject design to reduce confounds of damage to the ventricle by repeated injections. BIM (0.06 nmol in 0.25 ul/side) was infused directly into the BNST 60 min prior to oCRF (0.02 nmol in 0.25 ul/side) which was infused 45 minutes prior to test. BNST infusions were at a 0.1 ul/min rate after which injectors remained in place for 3 min before removal. This 3 min period was to ensure adequate diffusion into tissue and to minimize fluid loss to the ventricles upon injector removal.
Apparatus
Startle and PPI testing were performed in ventilated, sound-attenuating commercial startle chambers (39 × 38 × 58 cm, SR-LAB system, San Diego Instruments, San Diego, CA) consisting of a nonrestrictive Plexiglas cylinder (5 cm in diameter) into which the animal was placed. The cylinder was attached to a Plexiglas platform. Movements by the mouse were detected by a piezoelectric accelerometer attached below the platform. The mouse’s movements were then digitized and stored by an interface and computer assembly. A loudspeaker provided continuous broadband 65 dB background noise and all acoustic stimuli (see below). Beginning at startle stimulus onset, 65 consecutive 1-ms readings were recorded to obtain the average amplitude of the mouse’s startle response. The chamber house-light was off during all testing. Apparatus was calibrated and stimuli were verified as previously described (Risbrough and Geyer 2005).
Behavioral Testing
The entire experimental session consisted of a 2 min acclimation period, followed by a block of four 120 dB pulse trials (Block 1) and a variable prepulse intensity block of trials (Block 2). The variable prepulse intensity block consisted of 10 startle pulse-alone trials (each at 120 dB, 40 ms duration) and 30 prepulse + pulse trials. The prepulse + pulse trials were evenly divided among the following trial types (10 each): a 69, 73, or 77 dB prepulse preceding a 120 dB pulse stimulus. All prepulses were 20 ms in duration and all startle pulses were 40 ms. The interstimulus interval between prepulse and pulse onset was 100 ms. The average intertrial interval for all trial types throughout the session was 15 sec (range of 6–21 sec). All trial types within the prepulse intensity block were presented in a pseudorandom order.
The startle testing session for the within-subject BNST dose response study was slightly different. It also began with four 120-dB trials (Block 1). Unlike the studies above, Block 1 was followed by a block of different pulse intensities to examine if CRF effects in the BNST were dependent on pulse intensity (8 each of 90, 105 and 120 dB, 40 ms in duration). CRF showed a similar pattern of drug action in both Block 1 and across the different pulse intensity block, thus for consistency to compare to the ICV studies we will show Block 1 data. The next block was the same prepulse trial block as described for the other studies (“Block 2”).
Data Analysis
The average startle magnitude over the record window (65 ms) was used for all data analysis. As in previous studies, CRF has the strongest effect on startle in the initial phase of the session (Block 1) before prepulse trials are introduced (Gresack and Risbrough 2011), this block was used for all startle analyses. To test the effects of the inhibitors on CRF-induced increases in startle a two-way analysis of variance (ANOVA) was used with BIM, PD98059 or Rp-cAMPs pretreatment and oCRF treatment as the between subject factors. For PPI assessment, percentage of PPI was first calculated using the formula: 100-((average startle of the prepulse + pulse trials/average startle in the pulse alone trial) *100). A three-way ANOVA, with BIM, PD98059, or Rp-cAMPs pretreatment and oCRF treatment as the between subject factors and prepulse intensity as the within subject factor was used for %PPI statistical analysis. Post hoc analyses followed significant main or interaction effects as appropriate using Tukey’s or Dunnet’s post hoc tests.
Results
Contribution of PKC to CRF-induced alterations in startle
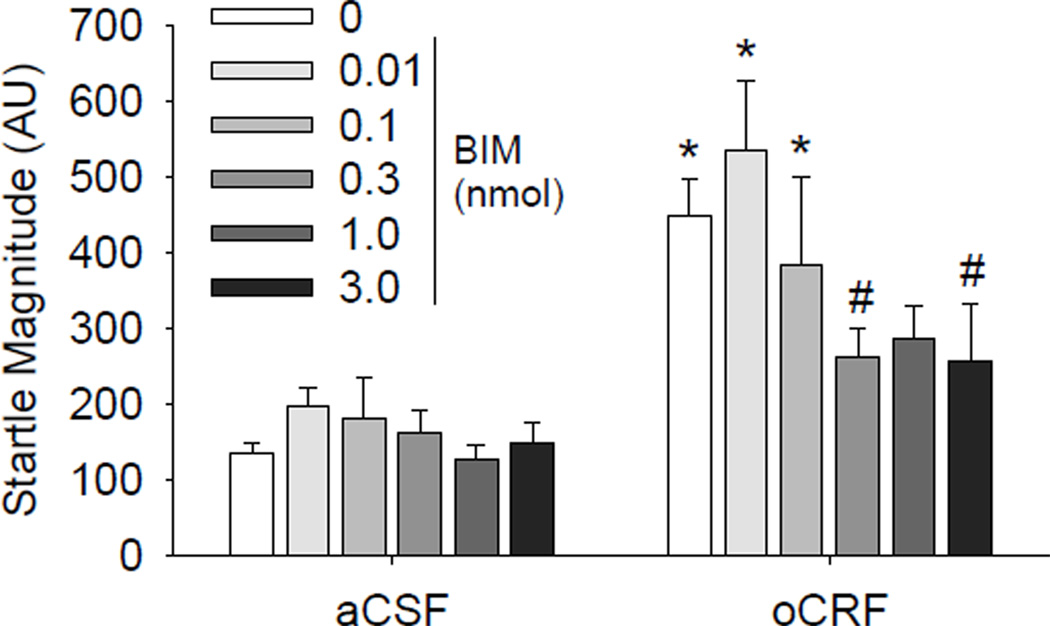
To investigate potential regulation of startle behavior by Gq-coupled CRF receptor signaling, we pretreated mice with BIM, which is reported to have the best selectivity for competitively inhibiting the ATP binding site of protein kinase C (especially the α-, β-, δ-, ε- and ζ-isoforms of PKC) with less effects on other kinases (Hauger et al. 2003). oCRF increased startle magnitude however this effect was dependent upon treatment with the PKC inhibitor BIM (CRF × BIM interaction: F(5,83) = 3.00, p <0.05). Both the 0.3 and 3 nmol doses of BIM significantly attenuated oCRF-induced increases in startle (Fig.2; p<0.01 vs. veh/oCRF treated group, Dunnet’s post hoc test). BIM treatment had no significant effect on startle when given alone (Fig.2).
Fig. 2.
Effect of PKC inhibition on CRF-induced startle hyperreactivity. Mice were treated with vehicle or BIM (ICV) 105 min before testing and treated with aCSF or oCRF (0.24 nmol) 45 min before testing. N=4–5 for BIM 0.01–0.1 and N=6–13 for the remaining groups. *p<0.05 vs. respective vehicle group, Tukey’s post hoc test; #p<0.05 vs. vehicle/oCRF, Dunnet’s post hoc test
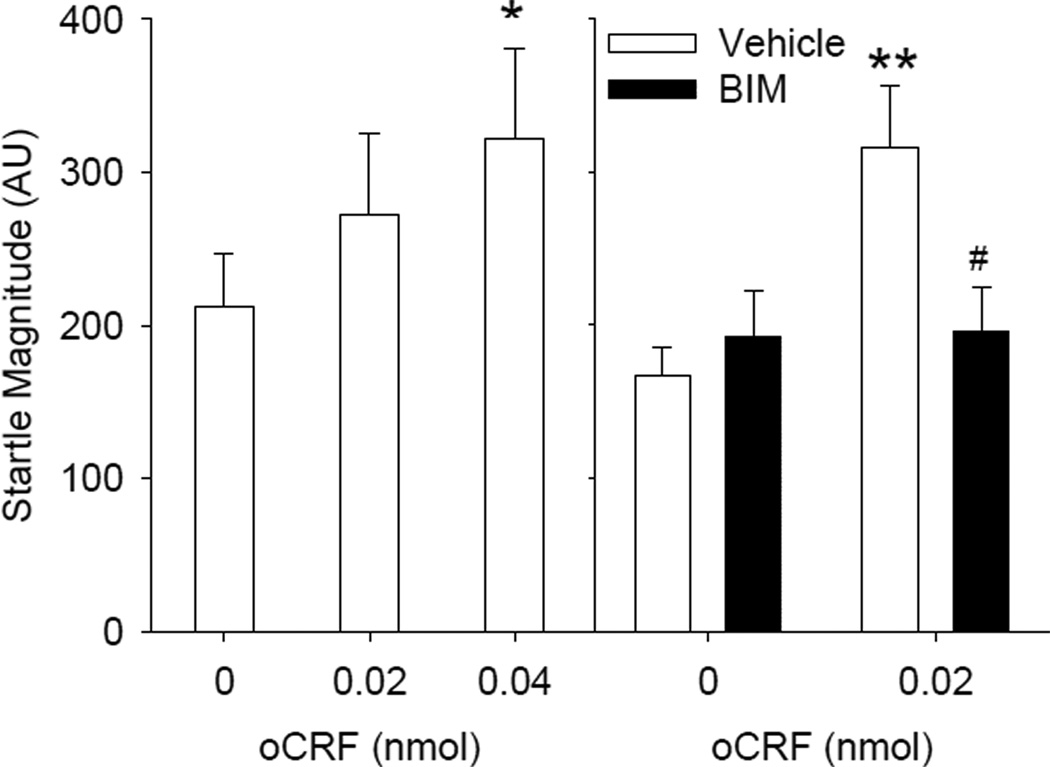
To determine if the BNST is the neural substrate for CRF effects on startle we first examined the effects of oCRF infusions in the BNST in mice. Using a within subject design we found that oCRF induced significant increases in startle reactivity at the 0.04 nmol dose (Fig.3) (p<0.05 simple comparison following a main effect of dose: F(2,22)=3.78, p<0.05). In the block presenting different pulse intensities (90, 105, 120 dB), oCRF effects were most predominant at the 120-dB trials (CRF × intensity: F(4,44)=2.82, p<0.05; data not shown). oCRF infused into the BNST showed a trend to reduce prepulse inhibition (main effect of oCRF: F(2,22)=2.44, p=0.11; data not shown).
Fig. 3.
Effect of PKC inhibition of CRF signaling in the BNST. Mice were treated with vehicle or BIM (0.06 nmol) 105 min before testing and treated with aCSF or oCRF (0.02 nmol) 45 min before testing (N=8–9). *p<0.05 vs. vehicle/aCSF; #p<0.05 vs. vehicle/oCRF, Tukey’s post hoc test
To determine effects of BIM on oCRF-induced alteration in startle, we chose the 0.02 and 0.06 nmol dose of oCRF and BIM respectively, which is 10 and 5 times lower than the effective dose using the ICV route (Fig.2; Risbrough et al. 2003, 2004). oCRF-induced increases in startle magnitude were reversed by pretreatment with BIM into the BNST (Fig.3; oCRF × BIM: F(1,31)=5.64, p <0.05). Tukey’s post hoc analyses indicated that startle magnitude in the oCRF/Veh group was significantly greater than all other groups (ps < 0.05).
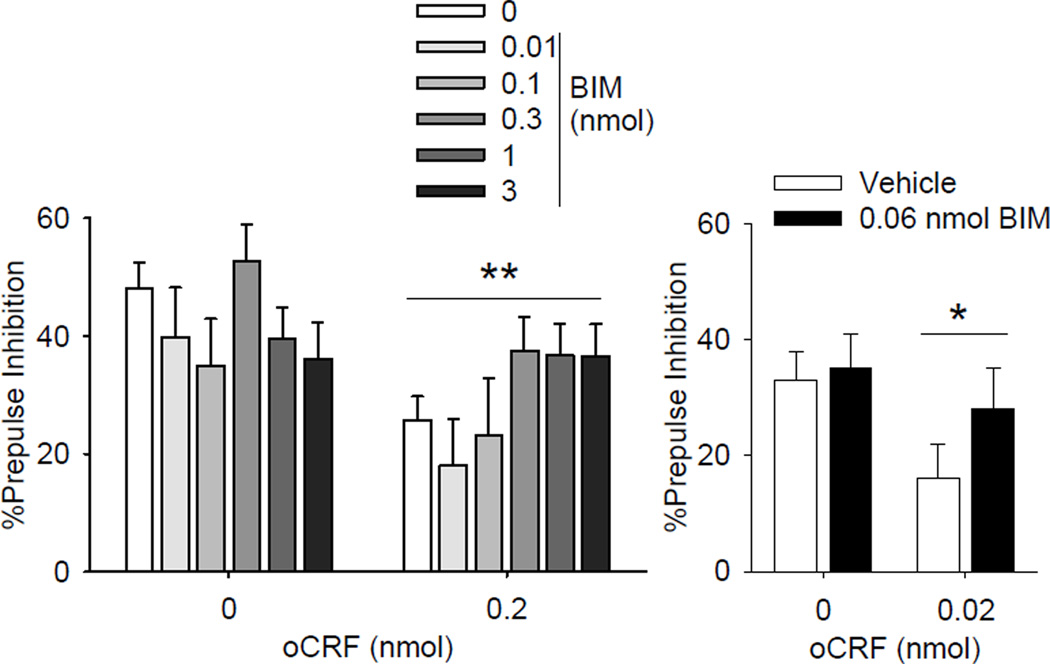
ICV infusions of oCRF also decreased %PPI but this effect was not significantly altered by pretreatment with BIM (Figure 4 left; main effect of CRF: F(1,83) = 10.78, p < 0.01; oCRF × BIM F(5,83)=1.79, p=.12). When infused into the BNST, oCRF-induced decreases in PPI were not significantly affected by BIM pre-treatment (Figure 4; main effect of oCRF: F(1,31) = 4.41, p <0.05;.oCRF × BIM: F(1,31)<1, p=0.44).
Fig. 4.
Effect of PKC inhibition on CRF-induced disruption of PPI. Left: Mice were treated with vehicle or BIM (ICV) 105 min before testing and treated with aCSF or oCRF (0.24 nmol) 45 min before testing. N=4–5 for BIM 0.01–0.1 and N=6–13 for the remaining groups. **p<0.01 Main effect of CRF infusion, see results for details. Right: Effect of PKC inhibition of CRF signaling in the BNST. Mice were treated with vehicle or BIM (0.06 nmol) 105 min before testing and treated with aCSF or oCRF (0.02 nmol) 45 min before testing (N=8–9). *p<0.05 Main effect of CRF infusion, see results for details.
Contribution of PKA to CRF-induced alterations in startle
Here we determined if pretreatment with the highly selective, potent PKA inhibitor Rp-cAMPS, which has no effect on Gq-coupled CRF receptor signaling (Dautzenberg et al. 2004), would alter startle responses to oCRF. oCRF produced similar increases in startle and reductions in PPI across both vehicle and Rp-cAMPS-pretreated mice (Table 1; main effect of CRF: Startle: F(1,102)=16. 25, p<0.001; PPI: F(1,102)=4.59, p<0.05, no significant interactions with Rp-cAMPS: Startle and PPI: F(3,102)<1, p>0.6). Rp-cAMPS pretreatment alone had no significant main effects on startle or PPI.
Table 1.
MEK1/2 (PD98059) and PKA (Rp-cAMPS) inhibitors do not affect CRF-induced alterations in startle and PPI.
| PD98059 (nmol) | Rp-cAMPS (nmol) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1.1 | 3.7 | 11 | 0 | 30 | 100 | 200 | |
| Startle | ||||||||
| aCSF | 80±20 | 144±23 | 121±15 | 159±30 | 212±30 | 209±18 | 185±18 | 221±56 |
| oCRF* | 396±84 | 306±83 | 364±51 | 271±26 | 331±70 | 377±65 | 294±54 | 355±40 |
| %PPI | ||||||||
| aCSF | 64±7 | 58±3 | 49±7 | 63±5 | 49±4 | 40±3 | 35±7 | 42±6 |
| oCRF* | 39±5 | 39±7 | 43±8 | 44±5 | 38±6 | 32±5 | 34±4 | 31±4 |
Main effect of oCRF treatment with no significant main effects or interactions with PD98059 (N=8–11) or Rp-cAMPS (N=11–15) pretreatment. All treatments were ICV, please see results for statistics.
Contribution of MEK1/2-ERK to CRF-induced alterations in startle
Finally, we examined the role of CRF receptor signaling via the MEK1/2-ERK cascade in CRFinduced startle behavior. oCRF increased startle magnitude and reduced PPI independently of MEK1/2 inhibitor PD98059 treatment (Table 1; main effect of CRF: Startle: F(1,68) = 42.88, p < 0.001; PPI: F(1,68)=19.18, p<0.001, no significant interactions with PD98059: Startle : F(3,68)=2.1, p=0.11; PPI: F(3,68)<1, p=0.4). PD98059 treatment alone had no significant main effects on startle or PPI.
Discussion
Here we showed that ICV administration of oCRF induced a marked increase in startle magnitude which was attenuated by the PKC inhibitor BIM. ICV oCRF-induced startle hyperreactivity was reproduced by targeted administration of oCRF into the BNST, an effect that also could be attenuated by intra-BNST pretreatment with BIM. BIM treatment alone had no effect on startle magnitude, suggesting BIM’s effects were specific to blocking CRF receptor-induced PKC signaling. In contrast, inhibition of PKA and MEK1/2-ERK pathways by ICV injection of selective inhibitors did not affect CRF-induced increases in startle significantly. Prepulse inhibition was consistently decreased by oCRF treatment but none of the inhibitors were able to significantly attenuate this effect at the dose ranges tested. We have previously established an important role for brain CRF1 receptor hypersignaling in the marked increase in startle and decrease in PPI by demonstrating that pretreatment with a selective CRF1 receptor antagonist or targeting deletion of the CRF1 receptor gene can block CRF effects on startle behavior (Risbrough et al. 2003, 2004). The present findings broaden our knowledge of the downstream mechanisms of CRF receptor effects on startle behavior, indicating a central role of CRF receptor signaling via the PKC pathway in CRF-induced startle hyperreactivity but not in PPI deficits. These findings extend previous findings in rats (Lee and Davis 1997), that the BNST is critically involved in CRF-induced hyperreactivity, by showing that CRF receptor PKC signaling in the BNST can mediate CRF effects on startle. CRF infusions into the BNST also reduce PPI, suggesting CRFinduced activation in this region is also sufficient to induce PPI deficits. Neither ICV nor BNST BIM pretreatment significantly reversed CRF-induced PPI deficits. However, there did appear to be a nonsignificant increase in PPI in BIM/CRF treated groups compared to VEH/CRF treated groups, while groups treated with BIM alone showed slightly reduced PPI. Thus PKC inhibition may play a weak role in PPI with differential consequences dependent upon CRF tone, however the present studies were not powered to detect this potentially weaker effect size.
The PKC pathway is involved in several behavioral effects of CRF and stress. CRF facilitation of contextual fear conditioning and long-term potentiation in the hippocampus is blocked by pretreatment with the PKC inhibitor BIM (Blank et al. 2002, 2003). PKCγ and ε deficient mice show decreased anxiety in avoidance paradigms and reduced CRF in the amygdala (Bowers et al. 2000; Lesscher et al. 2008). Although the causal relationship between CRF and PKC activity is not clear in the latter study, the findings do support a potential coupling between the CRF and PKC pathways. PKC activation is also required for acquisition of other learned avoidance responses, such as taste aversion (Yasoshima and Yamamoto 1997), inhibitory avoidance (Walker and Gold 1994), or in stressful spatial memory tasks (Wehner et al. 1990). Blockade of the constitutively active isoform of PKC, PKMζ, reduces fear potentiated startle (Parsons and Davis 2011) or predator scent-induced startle hyperreaactivity (Cohen et al. 2010). These data support the role of different PKC isoforms in defensive responses, anxiety and stress-related learning and conditioning paradigms. Here, we showed that PKC pathway mediates the effect of CRF on defensive startle, suggesting that PKC plays a role in stress-induced startle reactivity and supports the importance of this CRF receptor signaling pathway in stress responses.
The downstream events that mediate CRF-PKC signaling effects on startle are unknown, however there are several neurotransmitter systems and neural substrate candidates. For instance, CRFinduced PKC activation was shown to increase serotonin-enhanced GABA release in the prefrontal cortex (Tan et al. 2004), increase field potential amplitudes in the basolateral amygdala (Ugolini et al. 2008), facilitate LTP and GABA release in the central amygdala (Pollandt et al. 2006; Bajo et al. 2008), induce NMDA current depression in hippocampal neurons (Sheng et al. 2008), and increase excitability and firing rates of dopaminergic neurons of the ventral tegmental area (Ungless et al. 2003; Wanat et al. 2008). The altered excitability of these regions may contribute to CRF-induced startle hyperreactivity as these region are known to modulate startle (Lee and Davis 1997; Walker et al. 2009a). For instance, increased dopamine transmission has been shown to increase startle responses (Davis and Aghajanian 1976; Davis 1980; Davis et al. 1986) and dopamine D1 receptor activation mediates CRF effects on startle in rats (Meloni et al. 2006). However, in mice CRF-induced startle is unaffected by the genetic disruption and pharmacological blockade of D1 or D2 receptors suggesting species differences in the signaling transduction pathways mediating CRF effects on startle (Vinkers et al. 2007). In mice, we have found that noradrenergic transmission via α1 receptor activation plays a permissive role in CRFinduced startle hyperreactivity (Gresack and Risbrough 2011). Agonist-activated α1 receptors couple to Gq and can play a role in BNST-mediated anxiety behaviors via alteration in BNST neuronal excitability (Cecchi et al. 2002; (McElligot et al. 2010). Accordingly BIM-induced inhibition of PKC pathways in the BNST may be disrupting NE signaling required for CRF effects on startle. Thus it is possible that disruption of PKC is blocking CRF1 activation effects or possible downstream α1 activation effects on startle.
Based on our present findings, CRF-induced startle hyperreactivity is linked to the PKC signaling cascade in the BNST, a key regulator of CRF-induced startle and its potentiation by stressful stimuli (Lee and Davis 1997; Koob 1999; Waddell et al. 2008; Walker et al. 2009a; Sink et al. 2012). These findings in mice are in line with the known mediation of the BNST in CRF effects on startle in rats (Lee and Davis, 1997). The fact that both the BIM (0.06 nmol) and oCRF (0.02 nmol) doses that were effective in the BNST are lower than doses that are ineffective when given ICV suggests that the effects of BIM to block oCRF induced increases in startle are unlikely to be due to diffusion in to the ventricles (highest ineffective ICV dose of BIM 0.1 nmol Figure 2, highest ineffective ICV dose of oCRF 0.06 nmol, Gresack and Risbrough, 2011). Although it is possible that CRF/BIM diffused to neighboring regions surrounding the BNST, the BNST either has (1) much higher CRF receptor expression and peptide levels compared to immediately surrounding nuclei (preoptic area, substantia innominata) or (2) CRF receptor activation in neighboring nuclei (globus pallidus) is associated with anxiolytic effects, opposite to what we see in the present studies (van Pett et al. 2000, Sztainberg et al 2011). Additionally, BNST controls other stress-related behaviors, such as inhibitory avoidance, social and non-social anxiety and defensive behavior (Liang et al. 2001; Cooper and Huhman 2005; Sahuque et al. 2006; Lee et al. 2008). Confirming the role of BNST in stress responses, we showed that acute CRF receptor activation in the BNST induces startle hyperreactivity which is mediated (at least partly) by PKC pathway activation. Interestingly, unlike acute applications of CRF or acute stress induction, chronic CRF overexpression in the BNST does not affect startle reactivity (Regev et al. 2011; Sink et al. 2012). Chronic CRF overexpression may result in (1) differential receptor desensitization, internalization, and downregulation, (2) shift receptor (CRF1 or CRF2) expression and activity ratios or (3) induce other compensatory changes resulting in an altered behavioral outcome such that startle behavior is no longer affected (Price et al. 2002; Greetfeld et al. 2009; Waselus et al. 2009). CRF modulates GABAergic and glutamatergic transmission in the BNST, thus determining BNST excitability to different threatening stimuli (Kash and Winder 2006; Nobis et al. 2011; Oberlander and Henderson 2012). Hence, long-term CRF overexpression may significantly alter the neuronal excitability of this region.
In contrast to PKC blockade, the PKA inhibitor Rp-cAMPS did not block the effect of CRF on startle and PPI at any doses used in this study. It is possible that higher doses of Rp-cAMPS might be more effective, however in pilot studies we found doses higher than 200 nmol were toxic, precluding their use. Another possible reason Rp-cAMPS was ineffective might be the treatment window used in the present studies (1.75 hrs before testing). However it has been previously reported that PKA inhibition up to 3 hrs before testing blocks memory formation (Bourtchouladze et al. 1998) suggesting that Rp-cAMPS has long lasting effects. These negative findings are in contrast to cAMP, adenylate cyclase activators or phosphodiesterase inhibitor treatments which increase startle if administered intrathecally or into the nucleus reticularis pontis, a central regulator of the startle response (Kehne et al. 1986; Boulis and Davis 1990; de Lima and Davis 1995). However none of these treatments alter startle when administrated into the lateral ventricles (Kehne et al. 1986; Boulis and Davis 1990; de Lima and Davis 1995). Taken together with the present studies, PKA signaling may play a significant role in the execution of the startle response at the startle circuit but play less of a role in forebrain regions that modulate startle. This explanation is based on the assumption that ICV administered treatments have more access to forebrain regions compared to brain stem and spinal cord.
Similar to PKA inhibition, MEK1/2 inhibition had no significant effects on startle plasticity either alone or in conjunction with CRF treatment. Pilot studies using an alternative MEK1/2 inhibitor, U0126 (3 ug ICV, data not shown) also showed no effects on CRF-induced increases in startle or reductions in PPI. The MEK-ERK/MAPK pathway induces long-term behavioral effects by regulating gene transcription and neuronal plasticity, processes that occur over longer time periods than examined in our study (Sweatt 2001; Punn et al. 2006; Hauger et al. 2009). Therefore, a plausible interpretation of the lack of MEK1/2 inhibitor efficacy on startle reactivity is the short-term nature of our paradigm. CRF1 receptors could activate the MEK-ERK pathway within 1h, but protein translation and subsequent steps may require additional time. Supporting this interpretation, CRF-induced MEK-ERK pathway activation was able to increase the stress-induced enhancement of fear conditioning 24h after stress (Radulovic et al. 1999; Sananbenesi et al. 2003). In accordance, Schafe et al. (1999) showed that the inhibition of MAPK (and PKA) reduced contextual and auditory fear memories on a long-term (24h) but left short-term (1h) fear memory intact. Similarly, MEK inhibition immediately post training blocks recall of inhibitory avoidance 24h later (Walz et al. 2000). These data support the importance of de novo protein synthesis in the mediation of MEK-ERK pathway effects on behavior. However, the blockade of MEK-ERK pathway reduces depressive symptoms in the forced swim and tail suspension test in a 6–30 min time frame (Todorovic et al. 2009) showing that this pathway is also capable of having short-term effects on stress-related behaviors. Based on this later finding, treatment time course is less likely a factor in the present negative results.
PTSD subjects exhibit increased fear learning and startle hyperreactivity (Grillon and Baas 2003; Grillon et al. 2009; Jovanovic et al. 2009; Glover et al. 2011) as well as elevated CRF levels in cerebrospinal fluid (Bremner et al. 1997; Baker et al. 1999; Sautter et al. 2003). Thus it is possible that CRF hypersignaling plays a role in PTSD risk or symptoms (Steckler and Risbrough 2012). CRF receptor activation enhances fear learning and anxiety via a PKC mechanism (Blank et al. 2002, 2003; Walker et al. 2009a) in addition to the present findings that PKC signaling pathway mediates CRFinduced startle hyperreactivity. Interestingly, PKC signaling can mediate apical dendritic spine loss in hippocampal pyramidal neurons during chronic stress while PKC inhibition reverses this stress effect (Hains et al. 2009). These data support the CRF-coupled PKC pathway as a target for future PTSD pharmacotherapies. Initial negative results with CRF1 antagonists in depression and anxiety clinical trials have shifted CRF1 drug development towards seeking alternative mechanisms, such as utilizing biased agonism or signal transduction selectivity (Zorrilla and Koob 2010; Hauger et al. 2012). In accordance, there is growing body of evidence for the signal transduction selective nature of existing CRF1 antagonists, showing their selectivity on Gq, Gi or Gs proteins or their competitive and non– competitive actions on different G protein subtypes (Berger et al. 2006; Beyermann et al. 2007; Zorrilla and Koob 2010). This specificity could be utilized to develop drugs with more specific actions and improved side effect profile in the future. There are several different PKC isoforms that show specific distributions in the brain and at the subcellular level, e.g. location at pre- and postsynaptic compartments (Casabona 1997), with most isoforms mediating stress-induced neurobiological and behavioral alterations in some form (see above). Thus, there is a need for further studies to clarify the functional role of PKC isoforms underlying stress and CRF-induced behavioral and neurobiological alterations, e.g. following traumatic experiences.
Acknowledgments
This study was funded by a Veterans Affairs Merit Grant to RLH, Veterans Affairs Center of Excellence for Stress and Mental Health to VBR and RLH and NIMH 074697 to VBR. The authors declare no conflict of interest.
References
- Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. 2010;13(6):747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105(24):8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156(4):585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Alsene KM, Roseboom PH, Connors EE. Enduring sensorimotor gating abnormalities following predator exposure or corticotropin- releasing factor in rats: a model for PTSD-like information-processing deficits? Neuropharmacology. 2012;62(2):737–748. doi: 10.1016/j.neuropharm.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24(4):410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Berger H, Heinrich N, Wietfeld D, Bienert M, Beyermann M. Evidence that corticotropin-releasing factor receptor type 1 couples to Gs- and Gi-proteins through different conformations of its Jdomain. Br J Pharmacol. 2006;149(7):942–947. doi: 10.1038/sj.bjp.0706926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyermann M, Heinrich N, Fechner K, Furkert J, Zhang W, Kraetke O, Bienert M, Berger H. Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain. Br J Pharmacol. 2007;151(6):851–859. doi: 10.1038/sj.bjp.0707293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma EY, van Leeuwen ML, Westphal KG, Olivier B, Groenink L. Local repeated corticotropin-releasing factor infusion exacerbates anxiety- and fear-related behavior: differential involvement of the basolateral amygdala and medial prefrontal cortex. Neuroscience. 2011;173:82–92. doi: 10.1016/j.neuroscience.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22(9):3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23(2):700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulis NM, Davis M. Blockade of the spinal excitatory effect of cAMP on the startle reflex by intrathecal administration of the isoquinoline sulfonamide H-8: comparison to the protein kinase C inhibitor H-7. Brain Res. 1990;525(2):198–204. doi: 10.1016/0006-8993(90)90864-8. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5(4–5):365–374. [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet. 2000;30(2):111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154(5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casabona G. Intracellular signal modulation: a pivotal role for protein kinase C. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(3):407–425. doi: 10.1016/s0278-5846(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112(1):13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Matar MA, Kaplan Z, Zohar J. Mapping the brain pathways of traumatic memory: inactivation of protein kinase M zeta in different brain regions disrupts traumatic memory processes and attenuates traumatic stress responses in rats. Eur Neuropsychopharmacol. 2010;20(4):253–271. doi: 10.1016/j.euroneuro.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Conti LH, Murry JD, Ruiz MA, Printz MP. Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology (Berl) 2002;161(3):296–303. doi: 10.1007/s00213-002-1025-2. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF-sub-2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2005;119(4):1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Gutknecht E, Van der Linden I, Olivares-Reyes JA, Dürrenberger F, Hauger RL. Cell-type specific calcium signaling by corticotropin-releasing factor type 1 (CRF1) and 2a (CRF2(a)) receptors: phospholipase C-mediated responses in human embryonic kidney 293 but not SKN-MC neuroblastoma cells. Biochem Pharmacol. 2004;68(9):1833–1844. doi: 10.1016/j.bcp.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurochemical modulation of sensory-motor reactivity: acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980;4(2):241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Aghajanian GK. Effects of apomorphine and haloperidol on the acoustic startle response in rats. Psychopharmacology (Berl) 1976;47(3):217–223. doi: 10.1007/BF00427605. [DOI] [PubMed] [Google Scholar]
- Davis M, Commissaris RL, Cassella JV, Yang S, Dember L, Harty TP. Differential effects of dopamine agonists on acoustically and electrically elicited startle responses: comparison to effects of strychnine. Brain Res. 1986;371(1):58–69. doi: 10.1016/0006-8993(86)90810-3. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58(1–2):175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352(1362):1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima TC, Davis M. Involvement of cyclic AMP at the level of the nucleus reticularis pontis caudalis in the acoustic startle response. Brain Res. 1995;700(1–2):59–69. doi: 10.1016/0006-8993(95)00837-g. [DOI] [PubMed] [Google Scholar]
- Di Benedetto B, Kallnik M, Weisenhorn DM, Falls WA, Wurst W, Hölter SM. Activation of ERK/MAPK in the lateral amygdala of the mouse is required for acquisition of a fear-potentiated startle response. Neuropsychopharmacology. 2009;34(2):356–366. doi: 10.1038/npp.2008.57. [DOI] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress Anxiety. 2011;28(12):1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greetfeld M, Schmidt MV, Ganea K, Sterlemann V, Liebl C, Müller MB. A single episode of restraint stress regulates central corticotrophin- releasing hormone receptor expression and binding in specific areas of the mouse brain. J Neuroendocrinol. 2009;21(5):473–480. doi: 10.1111/j.1365-2826.2009.01865.x. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Risbrough VB. Corticotropin-releasing factor and noradrenergic signalling exert reciprocal control over startle reactivity. Int J Neuropsychopharmacol. 2011;14(9):1179–1194. doi: 10.1017/S1461145710001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Foot M, Davis M. Fear-potentiated startle: relationship to the level of state/trait anxiety in healthy subjects. Biol Psychiatry. 1993;33(8–9):566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114(9):1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996;64(3):169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66(1):47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht E, Van der Linden I, Van Kolen K, Verhoeven KF, Vauquelin G, Dautzenberg FM. Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling. Mol Pharmacol. 2009;75(3):648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106(42):17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Olivares-Reyes JA, Braun S, Catt KJ, Dautzenberg FM. Mediation of corticotropin releasing factor type 1 receptor phosphorylation and desensitization by protein kinase C: a possible role in stress adaptation. J Pharmacol Exp Ther. 2003;306(2):794–803. doi: 10.1124/jpet.103.050088. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Olivares-Reyes JA, Dautzenberg FM, Lohr JB, Braun S, Oakley RH. Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology. 2012;62(2):705–714. doi: 10.1016/j.neuropharm.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27(3):260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167(1–2):151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51(5):1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Astrachan DI, Astrachan E, Tallman JF, Davis M. The role of spinal cord cyclic AMP in the acoustic startle response in rats. J Neurosci. 1986;6(11):3250–3257. doi: 10.1523/JNEUROSCI.06-11-03250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Isiegas C, Cheung YF, Tokarczyk J, Yang X, Esposito MF, Rapoport DA, Fabian SA, Siegel SJ, Wand G, Houslay MD, Kanes SJ, Abel T. Constitutive activation of Galphas within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2007;32(3):577–588. doi: 10.1038/sj.npp.1301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46(9):1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17(16):6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33(11):2586–2594. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, McMahon T, Lasek AW, Chou WH, Connolly J, Kharazia V, Messing RO. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 2008;7(3):323–333. doi: 10.1111/j.1601-183X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- Liang KC, Chen HC, Chen DY. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol. 2001;44(1):33–43. [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21(16):RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, Winder DG. Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc Natl Acad Sci U S A. 2010;107(5):2271–2276. doi: 10.1073/pnas.0905568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26(14):3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA3rd, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry. 1995;38(6):378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kühn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6(10):1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Henderson LP. Corticotropin-releasing factor modulation of forebrain GABAergic transmission has a pivotal role in the expression of anabolic steroid-induced anxiety in the female mouse. Neuropsychopharmacology. 2012;37(6):1483–1499. doi: 10.1038/npp.2011.334. doi: 10.1038/npp.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. β-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptordependent and cocaine-regulated mechanism. Biol Psychiatry. 2011;69(11):1083–1090. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMζ inhibition in the amygdala. Nat Neurosci. 2011;14(3):295–296. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, California: Academic Press; 2001. [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24(6):1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 2002;162(4):406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- Punn A, Levine MA, Grammatopoulos DK. Identification of signaling molecules mediating corticotropin-releasing hormone-R1alpha-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol Endocrinol. 2006;20(12):3179–3195. doi: 10.1210/me.2006-0255. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Rühmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19(12):5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16(7):714–728. doi: 10.1038/mp.2010.64. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA. Anxiogenic treatments do not increase fear-potentiated startle in mice. Biol Psychiatry. 2005;57(1):33–43. doi: 10.1016/j.biopsych.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34(6):1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 2003;170(2):178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24(29):6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50(4):550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl) 2006;186(1):122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23(36):11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54(12):1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20(21):8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6(2):197–110. [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Lincoln C, Uphouse L. The PKC inhibitor, bisindolymaleimide, blocks DOI's attenuation of the effects of 8-OH-DPAT on female rat lordosis behavior. Behav Brain Res. 2007;179(1):99–106. doi: 10.1016/j.bbr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Zhang Y, Sun J, Gao L, Ma B, Lu J, Ni X. Corticotropin-releasing hormone (CRH) depresses n-methyl-D-aspartate receptor-mediated current in cultured rat hippocampal neurons via CRH receptor type 1. Endocrinology. 2008;149(3):1389–1398. doi: 10.1210/en.2007-1378. [DOI] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2012 doi: 10.1038/mp.2011.188. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2008;197(1):115–126. doi: 10.1007/s00213-007-1014-6. [DOI] [PubMed] [Google Scholar]
- Sztainberg Y, Kuperman Y, Justice N, Chen A. An anxiolytic role for CRF receptor type 1 in the globus pallidus. J Neurosci. 2011;31:17416–17424. doi: 10.1523/JNEUROSCI.3087-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62(2):617–627. doi: 10.1016/j.neuropharm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76(1):1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9–41) Neuropsychopharmacology. 1989;2(4):285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25(7–8):627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Tan H, Zhong P, Yan Z. Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci. 2004;24(21):5000–5008. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic C, Sherrin T, Pitts M, Hippel C, Rayner M, Spiess J. Suppression of the MEK/ERK signaling pathway reverses depression-like behaviors of CRF2-deficient mice. Neuropsychopharmacology. 2009;34(6):1416–1426. doi: 10.1038/npp.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini A, Sokal DM, Arban R, Large CH. CRF1 receptor activation increases the response of neurons in the basolateral nucleus of the amygdala to afferent stimulation. Front Behav Neurosci. 2008;2:2. doi: 10.3389/neuro.08.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39(3):401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Risbrough VB, Geyer MA, Caldwell S, Low MJ, Hauger RL. Role of dopamine D1 and D2 receptors in CRF-induced disruption of sensorimotor gating. Pharmacol Biochem Behav. 2007;86(3):550–558. doi: 10.1016/j.pbb.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Bouton ME, Falls WA. Central CRF receptor antagonist a-helical CRF9–41 blocks reinstatement of extinguished fear: the role of the bed nucleus of the stria terminalis. Behav Neurosci. 2008;122(5):1061–1069. doi: 10.1037/a0013136. [DOI] [PubMed] [Google Scholar]
- Walker DL, Gold PE. Intra-amygdala kinase inhibitors disrupt retention of a learned avoidance response in rats. Neurosci Lett. 1994;176(2):255–258. doi: 10.1016/0304-3940(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34(6):1533–1542. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz R, Roesler R, Quevedo J, Sant'Anna MK, Madruga M, Rodrigues C, Gottfried C, Medina JH, Izquierdo I. Time-dependent impairment of inhibitory avoidance retention in rats by posttraining infusion of a mitogen-activated protein kinase kinase inhibitor into cortical and limbic structures. Neurobiol Learn Mem. 2000;73(1):11–20. doi: 10.1006/nlme.1999.3913. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586(8):2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66(1):76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Sleight S, Upchurch M. Hippocampal protein kinase C activity is reduced in poor spatial learners. Brain Res. 1990;523(2):181–187. doi: 10.1016/0006-8993(90)91485-y. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Yamamoto T. Rat gustatory memory requires protein kinase C activity in the amygdala and cortical gustatory area. Neuroreport. 1997;8(6):1363–1367. doi: 10.1097/00001756-199704140-00009. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15(9–10):371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]