Abstract
Background:
Women with anovulatory polycystic ovary syndrome (PCOS) are generally insulin-resistant and as a consequence are often treated with the biguanide metformin. Results with metformin have, however, been variable with some studies demonstrating induction of regular cycles and an increase in ovulation, whereas others do not. Hence more understanding is needed regarding the mechanism of metformin's actions in ovarian granulosa cells especially in light of previous demonstrations of direct actions.
Objective:
The aim of this study was to investigate metformin's interaction with the FSH/cAMP/protein kinase A pathway, which is the primary signaling pathway controlling CYP19A1 (aromatase) expression in the ovary.
Methods:
The effect of metformin on FSH and forskolin-stimulated aromatase expression in human granulosa cells was measured by quantitative real-time PCR. Activity was assessed after transfection with a promoter II-luciferase construct, and by an RIA measuring conversion of androgen to estrogens. The effect on FSH receptor (FSHR) mRNA was assessed by quantitative PCR. Levels of phosphorylated cAMP response element binding protein (CREB) and CREB-regulated transcription coactivator 2 (CRTC2) were measured by Western blotting and cAMP by a bioluminescent assay.
Results:
Metformin markedly reduced FSH but not forskolin-stimulated aromatase expression and activity. This effect was exerted by inhibition of basal and ligand-induced up-regulation of FSHR expression. Metformin also reduced FSH-induced phosphorylation of CREB and hence CRE activity, which could potentially disrupt the CREB–CREB-binding protein–CRTC2 coactivator complex that binds to CRE in promoter II of the aromatase gene. This is mediated in an AMP-activated protein kinase-independent manner, and does not involve alteration of cAMP levels.
Conclusion:
These finding have implications for the use of metformin in the treatment of anovulation in women with PCOS.
Women with anovulatory polycystic ovary syndrome (PCOS) are generally insulin-resistant and as a consequence are often treated with the biguanide metformin. Results with metformin have, however, been variable with some studies demonstrating induction of regular menstrual cycles and increases in both ovulation and live birth rates (in women with PCOS) (1, 2), whereas others have shown no effect on ovulation (3, 4). The patients who will most benefit remain to be identified, and this is due in part to a lack of understanding of the mechanism by which metformin exerts its effects. The systemic antihyperglycemic and insulin-sensitizing effects of metformin are well documented (5), but metformin also exerts direct effects on insulin-mediated steroidogenesis and glucose uptake in ovarian cells. Metformin exerted its effects on the expression and activity of aromatase (encoded by CYP19A1) via the ERK pathway (6, 7) and was also able to directly enhance the insulin-mediated translocation of glucose transporters to the granulosa cell plasma membrane (8). However, many questions regarding the mechanisms by which metformin exerts its effects on the ovary remain to be elucidated, for example, how metformin interacts with the FSH-stimulated cAMP/protein kinase A (PKA)/cAMP response element binding protein (CREB) pathway, the primary signaling pathway for the regulation of CYP19A1 in the ovary (9). Interestingly, treatment of women with PCOS with metformin was associated with a reduction in aromatase activity in response to FSH, supporting a direct effect (10, 11).
FSH binding activates cAMP and PKA to affect aromatase gene expression via the activation of cAMP-responsive transcriptional regulatory proteins. The best known is CREB, which when phosphorylated binds to a cAMP response element (CRE) in the gonad-specific promoter II (PII) of CYP19A1 (12, 13). Downstream of CRE is another important regulatory binding site for the orphan nuclear receptor steroidogenic factor 1 (SF-1, official symbol NR5A1). Activation of both leads to recruitment of a cohort of multiple coregulators and transcription factors such as CREB-binding protein (CBP), which then activate PII-specific expression to induce aromatase expression (12).
In 2003, a new family of CREB coactivators were identified, known either as CREB-regulated transcription coactivators (CRTCs) or as transducers of regulated CREB activity (TORC) (14, 15). The 3 members of the CRTC/TORC family have a common N-terminal domain that associates with CREB DNA binding/dimerization domain (bZIP) to mediate transcriptional activity of CREB in response to both cAMP and calcium influx (16). Studies have demonstrated the involvement of CRTCs (principally CRTC2) in the regulation of cAMP-responsive genes, primarily CYP19A1 promoter activity in MCF-7 cells (17) and StAR, P450scc and 3β-HSD in rat granulosa cells (18). Under basal conditions, CRTC2 is sequestered in the cytoplasm via phosphorylation, and nuclear entry is brought about by dephosphorylation by calcium and cAMP (19).
Regulation of FSHR (FSH receptor) transcription is not well understood, but an E-box (element that binds transcription factors) in the proximal promoter, along with SF-1 and the upstream proteins stimulatory factor-1 and -2, appear important (20). In addition, transgenic mice studies have indicated that FSHR transcription is also highly dependent on regulatory elements that lie distal to the promoter region (21). The aim of this study was to investigate the interaction of metformin with the FSH-stimulated cAMP/PKA pathway in human granulosa cells to further elucidate the mechanism of action of metformin in women with PCOS. We hypothesized that metformin is able to directly regulate FSHR in addition to CYP19A1 expression, thereby influencing follicle development in the ovary.
Materials and Methods
KGN cell culture, real-time PCR, and activity assays
All experiments used the KGN human granulosa-like tumor cell line. Cells were cultured overnight at 3 × 105 cells per well in DMEM-F12 (Life Technologies) plus 1% fetal calf serum before addition of medium containing aromatase substrate testosterone (5 × 10−7M) and 10−7M metformin (16 ng/mL); FSH at 1, 5, and 10 ng/mL with or without metformin; or forskolin (Fsk) at 10μM and 25μM with or without metformin (all drugs from Sigma-Aldrich Co Ltd except hFSH from Endocrine Services). After another 48 hours in culture, the medium was removed and stored to measure estradiol (E2) as an indicator of aromatase activity using an RIA (22). The cells were lysed, and RNA was extracted and reverse transcribed to cDNA (6). In additional experiments, cells were treated as above, with the addition of 10 and 100 ng/mL activin to up-regulate FSHR mRNA expression.
In vivo luteinization is associated with a cAMP-driven up-regulation of LHR and corresponding decrease in expression of many FSH-driven genes. To determine whether Fsk treatment was able to induce mRNA expression of LH receptor (LHR), cells were treated with 25μM Fsk with or without metformin for 24 to 72 hours before lysis for RNA extraction.
Real-time quantitative PCR (qPCR) was performed using the Bio-Rad CFX real-time cycler (Bio-Rad Laboratories Ltd). Duplicate PCRs were performed with SYBR Green I master mix (Agilent Technologies) (22) with L19 (mitochondrial ribosomal protein L19) as the reference gene. Primer sequences are listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) (Sigma-Aldrich). All primer pairs annealed at 60°C except FSHR primers, which annealed at 50°C. Data are the mean of gene expression ratio ± SEM.
KGN cell culture, transfection, and reporter gene assays
KGN cells were plated at 2 × 104 cells per well and incubated overnight. To investigate PII activity, the cells were transfected with a CYP19A1 PII-516 reporter construct expressing firefly luciferase (kindly donated by Dr Brown and Professor Simpson, Prince Henry's Institute, Melbourne, Australia) (23). To investigate CRE activity, cells were transfected with a CRE-pADneo2 C6-BL reporter construct also expressing firefly luciferase (courtesy of Dr Axel Themmen) (24). In addition, 5 ng/μL of a control plasmid expressing Renilla luciferase from a constitutive promoter, and 6 ng/μL of a transcription enhancing element, PVAi, was added to each well (courtesy of Dr Axel Nohturrft, St George's University of London) and incubated for another 24 hours (25). Cells were serum-starved for 2 hours, and then triplicate wells were treated as described in the PCR assay. After another 24 hours in culture, luciferase reporter assays were carried out using the Dual-Glo Luciferase Assay System (Promega) (25). Data are presented as the mean of the ratio of luciferase to renilla luminescence normalized to the control ± SEM.
KGN cell culture and cAMP assay
KGN cells were plated at 2 × 104 cells per well and incubated overnight in DMEM plus1% fetal calf serum before treatment with metformin from 10−7M to 10−4M for 24 hours followed by exposure to serum-free media containing FSH (5 and 10 ng/mL) or Fsk (25μM) with or without metformin for 1 hour, after which cAMP was measured using the cAMP-Glo Max assay (Promega). This is a bioluminescent assay that measures cAMP levels in cells using a coupled luciferase reaction: as cAMP increases, it binds to PKA and the regulatory subunits undergo conformational change releasing the catalytic subunits. These then catalyze transfer of the terminal phosphate of ATP to a PKA substrate, consuming ATP in the process. Remaining ATP is measured by luciferase-based Kinase-Glo reagent where luminescence is inversely proportional to cAMP levels. Data in the table are mean of cAMP (nanomolar) ± SEM. The results are presented graphically after normalization to the control (no treatment).
KGN cell culture, protein fractionation, and Western blot analysis
KGN cells were plated at 1 × 106 cells per well, incubated overnight, treated with 10−7M metformin (16 ng/mL) for 24 hours and subsequently exposed to 5 ng/mL FSH with or without metformin; 25μM Fsk with or without metformin and 2mM 5′-aminoimidazole-4-carboxamide ribonucleoside (AICAR) with or without FSH/Fsk. AICAR activates the AMP-activated protein kinase (AMPK) pathway and was added to determine the involvement of this pathway in the mechanism. After 1 hour, cells were scraped into ice-cold PBS and centrifuged, and the pellets were resuspended into RIPA buffer (Cell Signaling Technologies, New England Biolabs [UK] Ltd) with protease and phosphatase inhibitors and stored at −80°C. For the time-course experiments, cells were exposed to FSH or Fsk for 30 or 60 minutes or 3 or 24 hours before lysing for protein extraction.
To determine the effect of treatment on expression of CRTC2 (TORC2) and phosphorylated CREB (pCREB), Western blotting was performed using the Odyssey Imaging System (Li-Cor Biosciences) (8). Primary antibodies were used at the following concentrations: anti-CRTC2 at 1:500 (sc-46272; Santa Cruz Biotechnology), anti-pCREB at 1:500 (no. 9198) and anti-CREB at 1:1000 (no. 9104) (both from Cell Signaling), and anti–α-tubulin at 1:2000 (Abcam). The former 2 were detected with goat antirabbit IR680 (1:5000) and the latter two with goat antimouse IR800 (1:5000) (from Li-Cor Biosciences).
Statistical evaluation
All data represent the mean ± SEM of triplicate observations from a minimum of 3 or more independent experiments. Statistical significance was determined by ANOVA followed by post hoc tests: unpaired Student's or paired t test when 2 groups were compared (depending on the design of the experiment) or a one-sample t test when comparing with normalized control values. If the data were not normally distributed, a Wilcoxon matched-pairs signed rank test was used to compare 2 groups. Significance was set at P ≤ .05. Quantitative real-time PCR data were analyzed using the ΔΔCt method if the amplification efficiencies of the reactions were 100% ± 5%. For reactions (usually the case with the FSHR qPCR) where the amplification efficiency fell outside this range, then the Pfaffl method was used to calculate the fold change in expression ratios between the means of the 2 treatment groups (26). Data from the Western blots represent the mean densitometry measurements taken from all individual experiments and normalized to α-tubulin and then the control, except for pCREB, which was normalized to total CREB first. For the transfection experiments, the ratio of luciferase to renilla luminescence was calculated for each well and the mean ratio for each treatment group then determined and normalized to the untreated control within each experiment to give the fold change in CRE activity.
Results
The effect of metformin on FSH- and Fsk-stimulated aromatase expression and activity
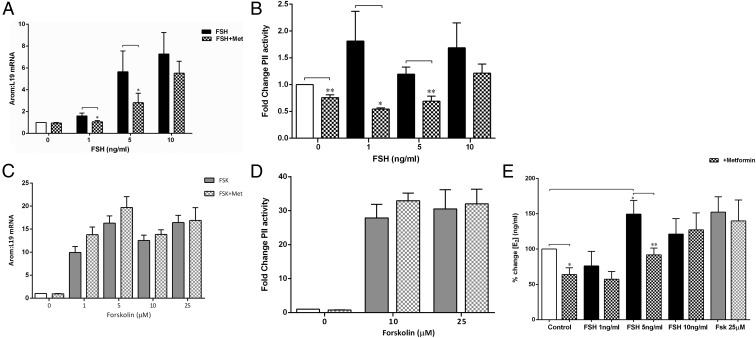
FSH had a dose-related stimulatory effect on aromatase mRNA expression (ANOVA P < .0001), which was significantly attenuated by addition of metformin at 1 and 5 ng/mL (P < .05), with no inhibition at 10 ng/mL FSH (Figure 1A). This was via a direct significant inhibition of both basal (P = .006) and FSH-stimulated (P < .05) PII expression and activity (Figure 1B). The substantial increase in PII activity at 1 ng/mL FSH (Figure 1B) was not reflected in total aromatase mRNA (Figure 1A) or E2 levels (Figure 1E).
Figure 1.
A, Effect of metformin and FSH on aromatase mRNA. Cells were treated for 48 hours with testosterone (as an aromatase substrate) and increasing doses of FSH (black solid bars) with or without metformin (Met) (10−7M) (checked bars). Aromatase mRNA was measured by real-time qPCR and expressed as a fold change in expression relative to control values (no treatment) and normalized to L19 mRNA expression. Metformin reduced the FSH-mediated increase in CYP19 mRNA levels at 1 and 5 ng/mL FSH (mean ± SEM, n = 5–7; *, P < .05, paired t test). B, Effect of metformin on FSH-stimulated PII activity. Cells were transfected with the CYP19-PII-516 reporter construct and incubated for 24 hours before serum starvation for 2 hours. The cells were then treated with increasing doses of FSH (black solid bars) with or without metformin (10−7M) (checked bars). Luciferase reporter assays demonstrated that metformin reduced basal and FSH-stimulated PII activity at 1 and 5 ng/mL FSH (data expressed as the fold change in the ratio of luciferase: renilla activity and normalized to the control) (n = 5–7, mean ± SEM; **, P < .005, paired t test). C, Effect of metformin and Fsk on aromatase mRNA. Cells were treated for 48 hours with increasing doses of Fsk (gray solid bars) (doses shown on graph) with or without metformin (checked bars) (10−7M) and testosterone as an aromatase substrate. Aromatase mRNA was measured as described in A. Metformin had no effect on the Fsk-mediated increase in CYP19 mRNA levels (mean ± SEM, n = 5–7). D, Effect of metformin on Fsk-stimulated PII activity. Cells were transfected with the CYP19A1 reporter construct as described for B. Metformin had no effect on Fsk-stimulated increase in PII activity (data expressed as fold change activity of luciferase compared with renilla) (n = 5–7, mean ± SEM). E, Effect of metformin on FSH- and Fsk-stimulated E2 levels. The medium from cells treated as described in A was removed, and the conversion of testosterone to E2 was measured as an indicator of aromatase activity using an RIA. Metformin (checked bars) significantly reduced basal (white bar) E2 levels (*, P < .05, 1-sample t test) as well as FSH-stimulated E2 (black bars) at 5 ng/mL (mean ± SEM, n = 5–8; *, P < .05; **, P < .005, unpaired t test). It had no effect on the Fsk-stimulated (gray bars) increase in E2. E2 levels are expressed as a percent change normalized to basal.
Consistent with the mRNA and transfection data, metformin significantly reduced both basal (P = .002) and 5 ng/mL FSH-stimulated E2 levels (P = .009), as measured by testosterone conversion to E2 (Figure 1E). Fsk increased aromatase mRNA dose-dependently (ANOVA P < .0001) and also PII activity (Figure 1, C and D). Fsk treatment resulted in a 28-fold higher PII activity than FSH (Figure 1D), and metformin was unable to reduce this increase in aromatase (Figure 1C), PII activity (Figure 1D), or E2 levels (Figure 1E).
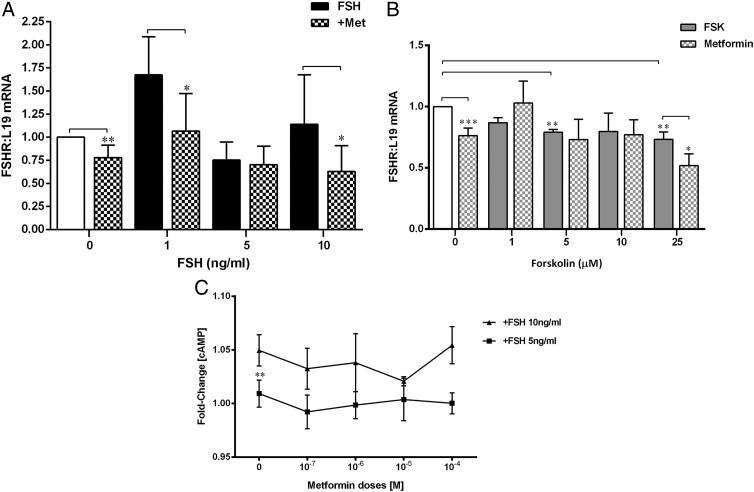
Metformin attenuates FSHR expression without altering cAMP levels
Because metformin reduced FSH- but not Fsk-mediated increases in aromatase expression and activity, we conjectured that the effect might be upstream of cAMP, ie, on the FSH receptor itself. Interestingly, metformin reduced basal FSHR mRNA expression (P < .005, 1-sample t test) and the ligand-mediated increase in FSHR induced by 1 and 10 ng/mL FSH (P < .05, Wilcoxon matched-pairs t test) (Figure 2A). Fsk reduced basal FSHR mRNA levels (ANOVA P = .05), particularly at 5μM (P = .01, 1-sample t test) and 25μM (P = .005, 1-sample t test), and unexpectedly, the addition of metformin to the latter dose of Fsk further attenuated FSHR mRNA expression (P = .003, paired t test) (Figure 2B).
Figure 2.
A, Effect of metformin (Met) and FSH on FSHR mRNA. Cells were treated as described in Figure 1A, and FSHR mRNA was measured by real-time qPCR and expressed as described previously. Metformin (checked bars) reduced basal mRNA levels of FSHR (mean ± SEM, n = 5–7; **, P < .005, 1-sample t test) as well as FSH (black bars) stimulation of its own receptor at 1 and 10 ng/mL FSH (*, P < .05, Wilcoxon matched-pairs signed rank t test). B, Effect of metformin and Fsk on FSHR mRNA. Cells were treated with Fsk (gray solid bars) as described in Figure 1C, which significantly decreased basal levels of FSHR mRNA with 5μM and 25μM (**, P < .005, ***, P < .0005, 1-sample t test). This decrease in FSHR mRNA was further attenuated by metformin (checked gray bars) (*, P < .05, paired t test) (mean ± SEM, n = 5–7). C, Effect of metformin on intracellular cAMP levels. Cells were treated with metformin at a range of doses from 10−7M to 10−4M for 24 hours and then exposed to FSH at 5 or 10 ng/mL for 1 hour. Intracellular cAMP levels were measured using a bioluminescent assay and normalized to the control (no treatment). Increasing FSH concentration from 5 to 10 ng/mL produced a significant increase in the fold change of cAMP levels (**, P = .003, paired t test), and metformin did not alter this.
To further up-regulate FSHR expression, cells were cultured with activin (10 and 100 ng/mL), and qPCR analysis for aromatase, FSHR, and SF-1 mRNA was performed. Although activin did not alter the expression of any of these genes with or without FSH, the addition of metformin to activin and FSH (both at 10 ng/mL) significantly down-regulated both aromatase and FSHR mRNA levels (data not shown).
FSH significantly increased intracellular cAMP levels as the dose increased from 5 to 10 ng/mL (Figure 2C). However, metformin had no effect on FSH- or Fsk-induced cAMP levels (Supplemental Table 2), confirming that metformin's attenuation of FSH-induced aromatase is via a reduction in FSHR expression and not a decrease in cAMP levels.
Mechanism of action of metformin via pCREB, CRTC2, and CRE
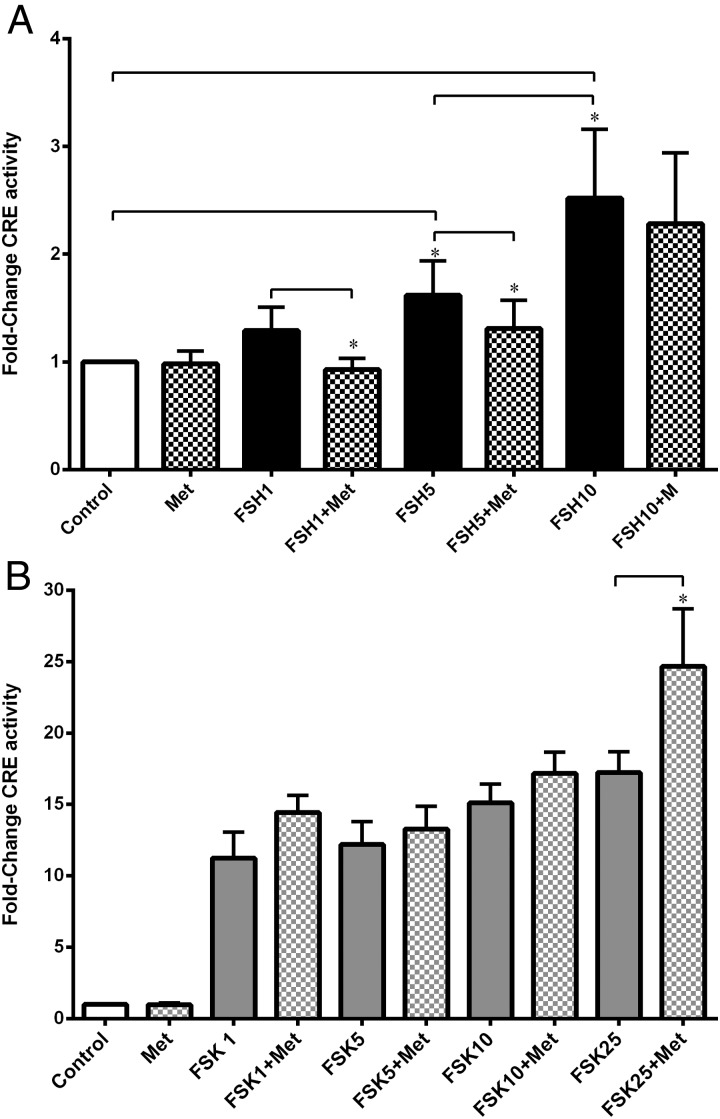
FSH dose-dependently increased CRE activity, but metformin attenuated this at 1 and 5 ng/mL FSH (P < .05) but had no effect on 10 ng/mL (Figure 3A) or on Fsk-mediated up-regulation of CRE (Figure 3B).
Figure 3.
Effect of metformin on FSH- and Fsk-stimulated CRE activity. Cells were transfected with the CRE-C6-BL reporter construct and treated as described previously. Luciferase reporter assays demonstrated that metformin (checked bars) significantly reduced CRE activity in the presence of 1 and 5 ng/mL FSH (black bars) (*, P < .05, ratio of paired t test) (A) but had no effect on Fsk-stimulated CRE (gray solid bars), apart from in the presence of 25μM Fsk where metformin significantly up-regulated CRE activity (B) (data expressed as fold change in ratio of luciferase to renilla luminescence, normalized to the control) (n = 5–7, mean ± SEM; *, P = .039, ratio of paired t test).
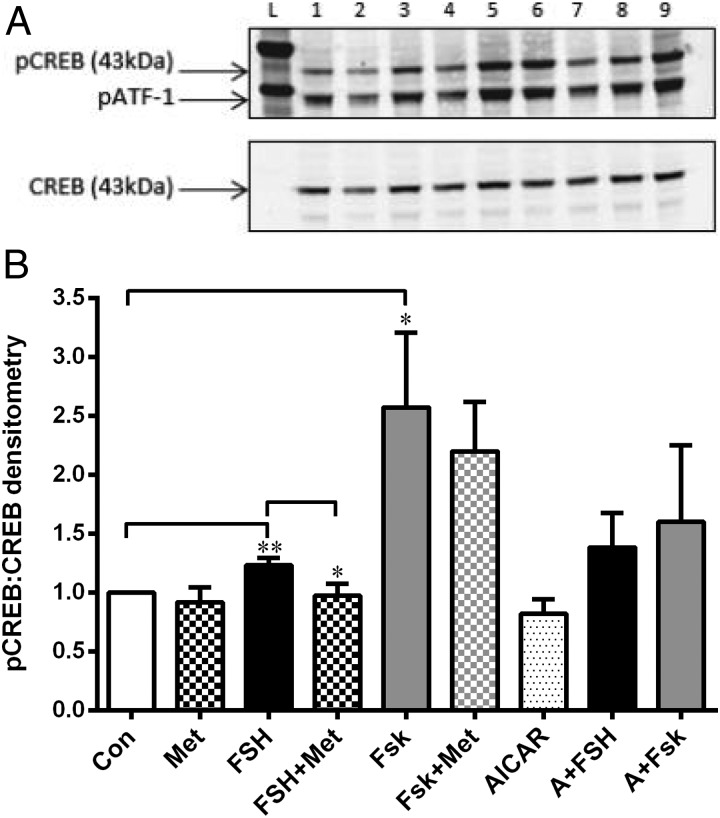
We determined that the optimal time for detection of pCREB after stimulation with either FSH or Fsk was 1 hour. Densitometric analysis of the immunoblots for pCREB/CREB showed that, as expected, FSH (5 ng/mL) increased pCREB compared with control (P = .016), and this was reduced to basal levels by metformin (P = .03) (Figure 4, A and B). Fsk (25μM) significantly increased pCREB, and this was not attenuated by metformin (Figure 4, A and B). AICAR had no effect on basal or FSH/Fsk-up-regulated pCREB levels (Figure 4C).
Figure 4.
Effect of metformin on pCREB. A, Representative Western blot using total protein lysates from cells treated with metformin (10−7M) for 24 hours and then exposed to either FSH (5 ng/mL) or Fsk (25μM) with or without metformin for 1 hour. Cells were also treated with AICAR (2 mM) with or without FSH/Fsk for 1 hour before lysis and then blotted with anti–α-tubulin (1:2500), anti-pCREB (1:500), and anti-CREB (1:1000). The anti-pCREB antibody also detects the phosphorylated form of the CREB-related protein, ATF-1 (activating transcription factor). The effect of all treatments on pCREB levels is described in B. Lanes L, ladder; lane 1, control; lane 2, metformin; lane 3, FSH; lane 4, FSH plus metformin; lane 5, Fsk; lane 6, Fsk with or without metformin; lane 7, AICAR; lane 8, AICAR plus FSH; lane 9, AICAR plus Fsk. B, Graph of densitometry analysis of Western blots (mean ± SEM, n = 4). FSH (black bar) significantly increased levels of pCREB (**, P = .016, 1-sample t test), which was significantly reduced by metformin (checked bar) (*, P = .03, unpaired t test, n = 4–7 mean ± SEM). Fsk (gray bar) up-regulated pCREB (*, P = .048, 1-sample t test), and this was not affected by the addition of metformin. AICAR (spotted bar) did not alter basal levels of pCREB, but neither did it reduce the FSH- or Fsk-stimulated increase in pCREB. Abbreviations: Con, control; Met, metformin.
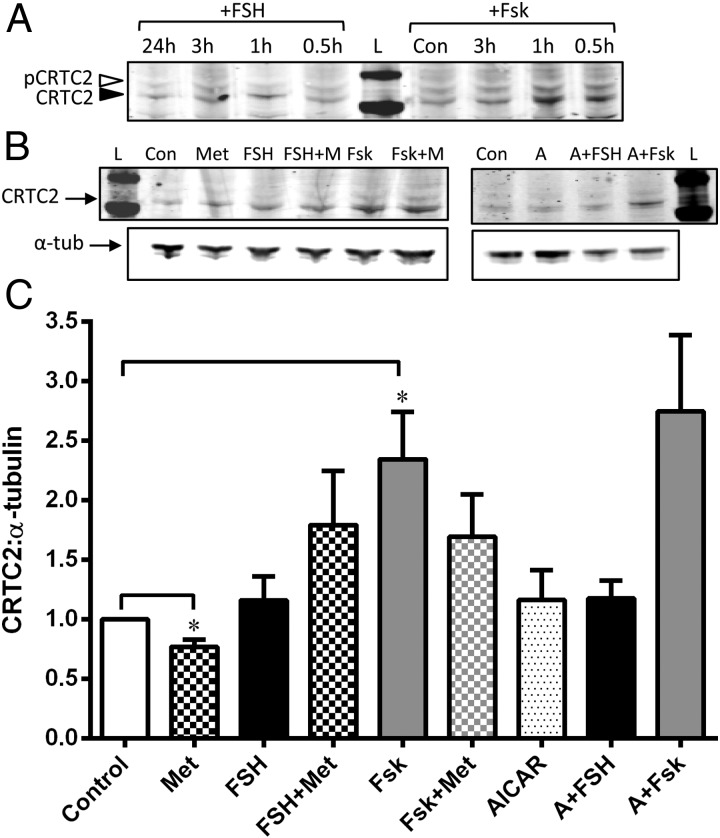
Anti-CRTC2 antibody detected both the phosphorylated (cytosolic, open arrowhead) and nonphosphorylated (nuclear, filled arrowhead) forms (Figure 5A), revealing a clear increase in the nonphosphorylated form (nuclear) at 1 hour with both Fsk and FSH treatment, the time used in subsequent experiments. Densitometric analysis of the nonphosphorylated form (which would be translocated into the nucleus to bind to the CREB-CBP complex) showed that metformin keeps the basal levels low (P = .02), ie, retains CRTC2 in the cytosol, but is unable to prevent the FSH- or Fsk-stimulated dephosphorylation and nuclear translocation of CRTC2 (Figure 5, B and C). Interestingly, AICAR had no effect on CRTC2 either basally or in the presence of FSH/Fsk (Figure 5, B and C).
Figure 5.
Effect of metformin on CRTC2. Panel A, Representative Western blot showing the effect of FSH or Fsk with time on phosphorylated (cytosolic) and nonphosphorylated (nuclear) CRTC2 levels. Cells were treated with 5 ng/mL FSH for 24 hours, 3 hours, 1 hour, and 30 minutes or 25μM Fsk for 3 hours, 1 hour, and 30 minutes before extraction of total protein lysates. Western blotting was performed with anti-CRTC2 antibody (1:2500), which detects both the phosphorylated (pCRTC2) (open arrowhead) and nonphosphorylated (CRTC2) (solid arrowhead) forms. Exposure to Fsk for 30 minutes and 1 hour significantly increased the expression of nonphosphorylated CRTC2 compared with pCRTC2 and after 1 hour FSH also increased CRTC2 levels. Panel B, Cells were treated as described for Figure 4A and lysates blotted with anti-CRTC2 and α-tubulin. The levels of CRTC2 (nuclear) were related to α-tubulin, and the effect of all treatments is analyzed and described in panel C. Panel C, Graph of densitometry analysis of Western blot experiments described in panel B. Metformin (checked bars) decreased levels of CRTC2 from basal (*, P = .02, 1-sample t test), indicating increased retention of pCRTC2 in the cytoplasm. Fsk (gray bars) significantly increased levels of CRTC2 (*, P = .03, 1-sample t test) as did FSH (black bars), indicating increased movement of dephosphorylated CRTC2 into the nucleus. Addition of metformin (checked bars) to either FSH/Fsk was unable to alter this. Unlike metformin, AICAR (spotted bars) did not alter basal CRTC2 levels, nor was it able to affect the FSH/Fsk-induced increase in nuclear localization of CRTC2. Abbreviations: A, AICAR; Con, control; L, ladder; Met or M, metformin; α-tub, α-tubulin.
LHR expression induction
There was no induction of LHR mRNA expression by Fsk at 24, 48, or 72 hours and hence no additional effect of metformin (data not shown).
Discussion
This is the first demonstration that metformin reduces FSH-stimulated aromatase expression and activity in granulosa cells. It does so by reducing FSHR mRNA and consequently the activity of FSH as measured by aromatase expression and E2, without altering cAMP levels. This involves blocking activation of CRE on PII of CYP19 via inhibition of pCREB and hence possible disruption of the formation of the CREB-CRTC2 coactivator complex. This is via an AMPK-independent mechanism. This is especially relevant for our understanding of the mechanism of action of metformin in the ovaries of women with PCOS.
It is well established that FSH is the major inducer of aromatase activity in granulosa cells and that this occurs primarily via activation of the cAMP/PKA signaling pathway (9) as demonstrated in our study. Interestingly, metformin down-regulated aromatase mRNA via an abrogation of PII activity, which translated into reduced FSH-stimulated E2. It is noteworthy that this was seen only at 1 and 5 ng/mL FSH. Previous dose-finding experiments in cultured primary granulosa cells from follicles <12 mm diameter from normal and polycystic ovaries revealed that the peak E2 response to FSH was elicited by a dose of between 2.5 and 5 ng/mL (27), which is the range in which metformin exerted its inhibitory effect on FSH action in this study.
As with the highest FSH dose, metformin had no effect on Fsk-induced aromatase expression or activity, possibly due to induction of high cAMP levels. Our findings differ from those of Brown et al (28) who showed a reduction in Fsk-mediated PII activity by metformin in primary human breast adipose stromal cells. However, they used significantly higher doses of metformin (10μM and 50μM) compared with 100nM used in this study and a different cell type. High metformin doses are known to produce different effects (29). Because metformin did not alter intracellular cAMP in the presence of 5 or 10 ng/mL FSH, an alternative explanation for our results is that it acts upstream of cAMP on the FSHR itself. Indeed, to our surprise, metformin significantly reduced basal levels of FSHR mRNA. In addition, it also reduced ligand-induced FHSR up-regulation. FSH itself was unable to stimulate the expression of its receptor further, and it is known that there is considerable species variation and also dose and time dependence (reviewed in Ref. 30). Unfortunately, we were also unable to increase FSHR by treating cells with activin. The activin signaling pathway is present in KGN cells, but there is extremely low expression of type 1B activin receptor, which is the main activin receptor for these cells (31). Despite this, metformin still reduced aromatase and FSHR mRNA expression in the presence of both activin and FSH. This is an extremely important finding because FSHR mRNA levels have been shown to parallel those of FSHR protein in the human ovary (32). Moreover, genetic models of FSH and FSHR mutations have indicated that FSH signaling is quite sensitive to the quantity of functional FSHR (33). This also explains our previous observation of a reduction in basal aromatase expression by metformin that was not via activation of the ERK pathway (6). Interestingly a number of clinical studies showed that addition of metformin to FSH during induction of ovulation lowered serum androgens and E2 and also produced significantly more monofollicular cycles (11, 34). Our results may explain these findings.
Fsk treatment reduced FSHR mRNA, which is consistent with observations that high doses of cAMP analog and Fsk attenuate both the binding and mRNA steady-state levels of FSHR in the ovary and testis (35, 36). Unexpectedly, this attenuation was enhanced further in the presence of metformin. In vivo, luteinization is associated with an E2- and cAMP-driven increase in LHR mRNA and a rapid and dramatic decrease in mRNA levels of many FSH-stimulated genes including aromatase (37). However, in our experiments, the decrease in FSHR mRNA induced by Fsk was not accompanied by an induction in LHR mRNA, even after 72 hours exposure. Moreover, there was no accompanying reduction in aromatase, although this may be accounted for by the fact that CRE activity remains high in the presence of Fsk, and in fact, the addition of metformin to the highest dose of Fsk used further enhanced CRE activity. The KGN cells' physiology is closer to that of immature granulosa cells than preovulatory cells, and so LH receptors are not present, but presumably the pathway for their induction would be; hence, this requires further investigation.
The mechanism of FSHR transcriptional regulation is not well known, particularly in the human ovary. SF-1 has been shown to be involved in both FSHR and aromatase regulation (38, 39), but none of our treatment conditions altered SF-1 expression. As stated earlier, it has been shown that FSH-induced transcription occurs via phosphorylation and activation of CREB, which then binds to CRE to promote transcription of aromatase via PII (12, 13). Interestingly metformin was able to significantly reduce CRE activity in the presence of FSH, but not the Fsk-mediated effect. Investigation of CREB phosphorylation, demonstrated a rapid (1 hour) increase in phosphorylation by both FSH and Fsk, with the effect of Fsk being double that of FSH. Although metformin was able to reduce the FSH-stimulated increase in pCREB, AICAR had no effect on the FSH- or Fsk-mediated increases in pCREB, suggesting that, in the ovary at least (17), inhibition of cAMP-driven aromatase by metformin may be AMPK-independent (Figure 6).
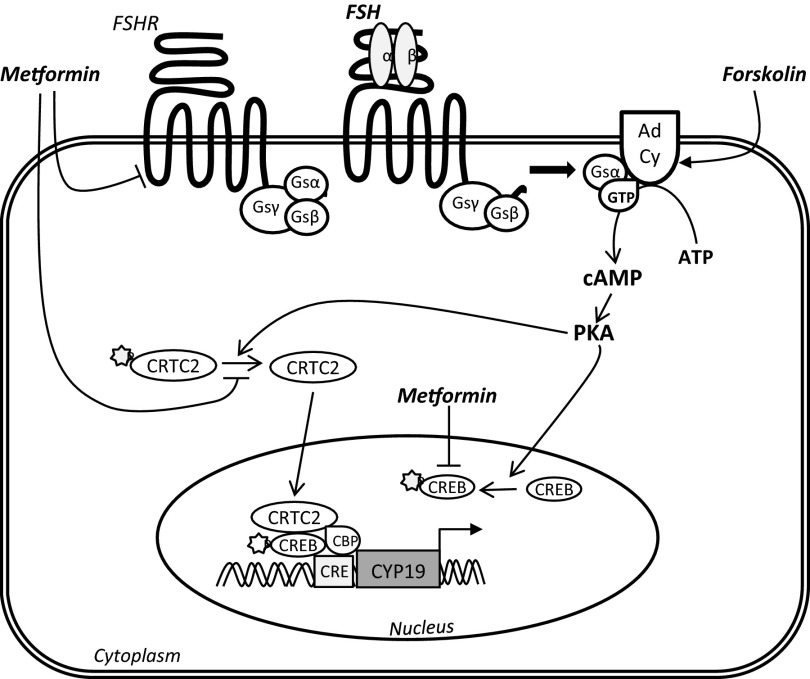
Figure 6.
Hypothetical mechanism of metformin's action on FSH activity in granulosa cells. The FSHR is a G protein-coupled receptor, which upon binding by FSH activates adenylyl cyclase (Ad Cy) to produce cAMP from ATP, which then acts to activate PKA downstream. Fsk directly stimulates adenylyl cyclase activity to greatly increase intracellular cAMP levels. Activation of PKA results in phosphorylation of CREB (pCREB), which allows it to bind to a CRE on PII of the aromatase gene (CYP19). In addition, activated PKA dephosphorylates CRTC2, allowing it to enter into the nucleus and bind to CRE in a coactivator complex with CBP and pCREB, resulting in CYP19 transcription. We have shown that metformin reduces the levels of FSHR, which then reduces FSH activity as measured by aromatase expression and E2 production. It does this without altering intracellular cAMP levels. Metformin decreases levels of pCREB, which may inhibit CYP19 transcription by disrupting the pCREB-CRTC2-CBP coactivator complex that stimulates CRE activation on PII. This is supported by the reduction in FSH-stimulated CRE activity brought about by metformin. This occurs independently of AMPK activation. Metformin is also able to prevent the dephosphorylation of basal CRTC2, thus retaining it in the cytosol. It is, however, unable to prevent the cAMP-driven dephosphorylation and nuclear translocation of CRTC2 induced by exposure to either FSH or Fsk.
In addition to CREB phosphorylation, what is of importance is the corecruitment of other transcriptional regulators to the promoter (40). In rat granulosa cells, FSH was able to stimulate progesterone secretion via recruitment of the CRTC2, CREB, and CBP complex to up-regulate key steroidogenic genes (Star, CYP11a1, and HSD3b) (18). Metformin enhanced the basal retention of CRTC2 in the cytoplasm, but because metformin (at physiologically relevant doses) does not alter cAMP levels, it was ineffective in preventing the FSH/Fsk-mediated dephosphorylation and translocation of CRTC2 into the nucleus, as was AICAR. Despite this, metformin still reduced FSH-mediated increases in pCREB and CRE activity and hence aromatase expression (Figure 6).
In women with PCOS, the response to metformin treatment could depend on the underlying disturbance; if it is primarily related to insulin resistance and defective insulin signaling, then metformin could help systemically (41) and locally in the ovary (6), but if it is related to a defect in steroidogenesis, then the effectiveness of metformin treatment may be more variable. Metformin has been shown to have the beneficial effect of reducing androgen levels, but it is clear that it also reduces aromatase and hence E2. We can speculate that if there is initial accelerated growth/proliferation of granulosa cells due to augmentation of insulin and FSH in women with PCOS (42, 43), leading to hyperresponsiveness and premature luteinization, then metformin could slow this down by decreasing both the FSH- and insulin-mediated increase in aromatase directly and indirectly via a reduction in FSHR expression, which could be beneficial. It has been shown that granulosa cells from women with PCOS have higher levels of FSHR expression compared with those from normal ovaries (44, 45). In effect, metformin could be acting to reset the follicular growth system, and because it takes at least 3 months to grow a cohort of antral follicles that can enter the menstrual cycle, this would take time. This hypothesis is supported by the recent findings that 3 months pretreatment of obese (2) and nonobese (1) women with metformin improved pregnancy and live-birth rates. In addition, a recent Cochrane review on metformin concluded that ovulation and pregnancy rates were higher in women with PCOS taking metformin (46). However, the effect of metformin on potentiating the down-regulation of FSHR expression brought about by sustained cAMP exposure could be detrimental to hyperresponsive follicles from women with PCOS, likewise its reduction of aromatase activity (47). Although as a single treatment metformin has failed to fulfill its promise in women with PCOS, it has proven to have utility when given in conjunction with ovulation-inducing agents where, interestingly, it appears to significantly reduce the incidence of ovarian hyperstimulation syndrome (48, 49). Our results may point to a possible mechanism for this beneficial action.
To summarize, metformin was able to reduce FSH-stimulated aromatase expression and activity by reducing FSHR expression. Metformin also reduced FSH-induced phosphorylation of CREB and hence CRE activity, which could result in disruption of the CREB-CBP-CRTC2 coactivator complex that binds to CRE in PII of the aromatase gene. This is mediated in an AMPK-independent manner. This has implications for the use of metformin in treating women with anovulatory PCOS.
Supplementary Material
Acknowledgments
We thank Michael Lacey for assistance with the RIA. We acknowledge the Biomics Units, St George's University of London, for access to the real-time cycler. We are indebted to Professor Ilpo Huhtaniemi for his generous advice.
This work was supported by the Wellcome Trust (WT073572MA, 081420/Z/06/Z to S.R. and H.D.M) and St George's University of London.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AICAR
- 5′-aminoimidazole-4-carboxamide ribonucleoside
- AMPK
- AMP-activated protein kinase
- CRE
- cAMP response element
- CREB
- cAMP response element binding protein
- CRTC
- CREB-regulated transcription coactivator
- E2
- estradiol
- FSHR
- FSH receptor
- Fsk
- forskolin
- PCOS
- polycystic ovary syndrome
- pCREB
- phosphorylated CREB
- PII
- promoter II
- PKA
- protein kinase A
- qPCR
- quantitative PCR
- SF-1
- steroidogenic factor 1
- TORC
- transducers of regulated CREB activity.
References
- 1. Kjøtrød SB, Carlsen SM, Rasmussen PE, et al. Use of metformin before and during assisted reproductive technology in non-obese young infertile women with polycystic ovary syndrome: a prospective, randomized, double-blind, multi-centre study. Hum Reprod. 2011;26(8):2045–2053 [DOI] [PubMed] [Google Scholar]
- 2. Morin-Papunen L, Rantala AS, Unkila-Kallio L, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97(5):1492–500 [DOI] [PubMed] [Google Scholar]
- 3. Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21(1):80–89 [DOI] [PubMed] [Google Scholar]
- 4. Trolle B, Flyvbjerg A, Kesmodel U, Lauszus FF. Efficacy of metformin in obese and non-obese women with polycystic ovary syndrome: a randomized, double-blinded, placebo-controlled cross-over trial. Hum Reprod. 2007;22(11):2967–2973 [DOI] [PubMed] [Google Scholar]
- 5. Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755–772 [DOI] [PubMed] [Google Scholar]
- 6. Rice S, Pellatt L, Ramanathan K, Whitehead SA, Mason HD. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology. 2009;150(10):4794–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003;79(4):956–962 [DOI] [PubMed] [Google Scholar]
- 8. Rice S, Pellatt LJ, Bryan SJ, Whitehead SA, Mason HD. Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J Clin Endocrinol Metab. 2011;96(3):E427–E435 [DOI] [PubMed] [Google Scholar]
- 9. Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18(9):1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. la Marca A, Morgante G, Palumbo M, Cianci A, Petraglia F, De Leo V. Insulin-lowering treatment reduces aromatase activity in response to follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil Steril. 2002;78(6):1234–1239 [DOI] [PubMed] [Google Scholar]
- 11. van Santbrink EJ, Hohmann FP, Eijkemans MJ, Laven JS, Fauser BC. Does metformin modify ovarian responsiveness during exogenous FSH ovulation induction in normogonadotrophic anovulation? A placebo-controlled double-blind assessment. Eur J Endocrinol. 2005;152(4):611–617 [DOI] [PubMed] [Google Scholar]
- 12. Michael MD, Michael LF, Simpson ER. A CRE-like sequence that binds CREB and contributes to cAMP-dependent regulation of the proximal promoter of the human aromatase P450 (CYP19) gene. Mol Cell Endocrinol. 1997;134(2):147–156 [DOI] [PubMed] [Google Scholar]
- 13. Hinshelwood MM, Michael MD, Simpson ER. The 5′-flanking region of the ovarian promoter of the bovine CYP19 gene contains a deletion in a cyclic adenosine 3′,5′-monophosphate-like responsive sequence. Endocrinology. 1997;138(9):3704–3710 [DOI] [PubMed] [Google Scholar]
- 14. Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12(2):413–423 [DOI] [PubMed] [Google Scholar]
- 15. Iourgenko V, Zhang W, Mickanin C, et al. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci U S A. 2003;100(21):12147–12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252(5011):1427–1430 [DOI] [PubMed] [Google Scholar]
- 17. Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69(13):5392–5399 [DOI] [PubMed] [Google Scholar]
- 18. Fang WL, Lee MT, Wu LS, et al. CREB coactivator CRTC2/TORC2 and its regulator calcineurin crucially mediate follicle-stimulating hormone and transforming growth factor β1 upregulation of steroidogenesis. J Cell Physiol. 2012;227(6):2430–2440 [DOI] [PubMed] [Google Scholar]
- 19. Screaton RA, Conkright MD, Katoh Y, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119(1):61–74 [DOI] [PubMed] [Google Scholar]
- 20. Hermann BP, Hornbaker K, Rice DA, Sawadogo M, Heckert LL. In vivo regulation of follicle-stimulating hormone receptor by the transcription factors upstream stimulatory factor 1 and upstream stimulatory factor 2 is cell specific. Endocrinology. 2008;149(10):5297–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hermann BP, Hornbaker KI, Maran RR, Heckert LL. Distal regulatory elements are required for Fshr expression, in vivo. Mol Cell Endocrinol. 2007;260–262:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rice S, Mason HD, Whitehead SA. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. 2006;101(4–5):216–225 [DOI] [PubMed] [Google Scholar]
- 23. Michael MD, Kilgore MW, Morohashi K, Simpson ER. Ad4BP/SF-1 regulates cyclic AMP-induced transcription from the proximal promoter (PII) of the human aromatase P450 (CYP19) gene in the ovary. J Biol Chem. 1995;270(22):13561–13566 [DOI] [PubMed] [Google Scholar]
- 24. Piersmaa D, Verhoef-Posta M, Look MP, et al. 2007 Polymorphic variations in exon 10 of the luteinizing hormone receptor: Functional consequences and associations with breast cancer. Mol Cell Endocrinol. 2007;276(1–2):63–70 [DOI] [PubMed] [Google Scholar]
- 25. Zhang S, Nohturfft A. Studying membrane biogenesis with a luciferase-based reporter gene assay. J Vis Exp. 2008;pii:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab. 1994;79(5):1355–1360 [DOI] [PubMed] [Google Scholar]
- 28. Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat. 2010;123(2):591–596 [DOI] [PubMed] [Google Scholar]
- 29. Wiernsperger NF. Membrane physiology as a basis for the cellular effects of metformin in insulin resistance and diabetes. Diabetes Metab. 1999;25(2):110–127 [PubMed] [Google Scholar]
- 30. Findlay JK, Drummond AE. Regulation of the FSH receptor in the ovary. Trends Endocrinol Metab. 1999;10(5):183–188 [DOI] [PubMed] [Google Scholar]
- 31. Mukasa C, Nomura M, Tanaka T, et al. Activin signaling through type IB activin receptor stimulates aromatase activity in the ovarian granulosa cell-like human granulosa (KGN) cells. Endocrinology. 2003;144(4):1603–1611 [DOI] [PubMed] [Google Scholar]
- 32. Minegishi T, Tano M, Igarashi M, et al. Expression of follicle-stimulating hormone receptor in human ovary. Eur J Clin Invest. 1997;27(6):469–474 [DOI] [PubMed] [Google Scholar]
- 33. Burns KH, Matzuk MM. Genetic models for the study of gonadotropin actions. Endocrinology. 2002;143(8):2823–2835 [DOI] [PubMed] [Google Scholar]
- 34. De Leo V, la Marca A, Ditto A, Morgante G, Cianci A. Effects of metformin on gonadotropin-induced ovulation in women with polycystic ovary syndrome. Fertil Steril. 1999;72(2):282–285 [DOI] [PubMed] [Google Scholar]
- 35. Nakamura K, Minegishi T, Takakura Y, et al. Hormonal regulation of gonadotropin receptor mRNA in rat ovary during follicular growth and luteinization. Mol Cell Endocrinol. 1991;82(2–3):259–263 [DOI] [PubMed] [Google Scholar]
- 36. Maguire SM, Tribley WA, Griswold MD. Follicle-stimulating hormone (FSH) regulates the expression of FSH receptor messenger ribonucleic acid in cultured Sertoli cells and in hypophysectomized rat testis. Biol Reprod. 1997;56(5):1106–1111 [DOI] [PubMed] [Google Scholar]
- 37. Richards JS, Haddox M, Tash JS, Walter U, Lohmann S. Adenosine 3′,5′-monophosphate-dependent protein kinase and granulosa cell responsiveness to gonadotropins. Endocrinology. 1984;114(6):2190–2198 [DOI] [PubMed] [Google Scholar]
- 38. Levallet J, Koskimies P, Rahman N, Huhtaniemi I. The promoter of murine follicle-stimulating hormone receptor: functional characterization and regulation by transcription factor steroidogenic factor 1. Mol Endocrinol. 2001;15(1):80–92 [DOI] [PubMed] [Google Scholar]
- 39. Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol. 2003;207:39–45 [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Odom DT, Koo SH, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102(12):4459–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Romualdi D, De Cicco S, Tagliaferri V, Proto C, Lanzone A, Guido M. The metabolic status modulates the effect of metformin on the antimullerian hormone-androgens-insulin interplay in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(5):E821–E824 [DOI] [PubMed] [Google Scholar]
- 42. Mason HD, Willis DS, Holly JM, Franks S. Insulin preincubation enhances insulin-like growth factor-II (IGF-II) action on steroidogenesis in human granulosa cells. J Clin Endocrinol Metab. 1994;78(5):1265–1267 [DOI] [PubMed] [Google Scholar]
- 43. Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83(11):3984–3991 [DOI] [PubMed] [Google Scholar]
- 44. Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(11):4456–4461 [DOI] [PubMed] [Google Scholar]
- 45. González-Fernández R, Peña Ó, Hernández J, Martín-Vasallo P, Palumbo A, Ávila J. Patients with endometriosis and patients with poor ovarian reserve have abnormal follicle-stimulating hormone receptor signaling pathways. Fertil Steril. 2011;95(7):2373–2378 [DOI] [PubMed] [Google Scholar]
- 46. Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, d-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012;5:CD003053. [DOI] [PubMed] [Google Scholar]
- 47. Palomba S, Falbo A, Di Cello A, Cappiello F, Tolino A, Zullo F. Does metformin affect the ovarian response to gonadotropins for in vitro fertilization treatment in patients with polycystic ovary syndrome and reduced ovarian reserve? A randomized controlled trial. Fertil Steril. 2011;96(5):1128–1133 [DOI] [PubMed] [Google Scholar]
- 48. Palomba S, Falbo A, Carrillo L, et al. Metformin reduces risk of ovarian hyperstimulation syndrome in patients with polycystic ovary syndrome during gonadotropin-stimulated in vitro fertilization cycles: a randomized, controlled trial. Fertil Steril. 2011;96(6):1384–1390 [DOI] [PubMed] [Google Scholar]
- 49. Palomba S, Falbo A, La Sala GB. Effects of metformin in women with polycystic ovary syndrome treated with gonadotrophins for in vitro fertilisation and intracytoplasmic sperm injection cycles: a systematic review and meta-analysis of randomised controlled trials. BJOG. 2013;120(3):267–276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.