Abstract
To compare the pharmacokinetics of coadministered intraperitoneal ketamine and xylazine in young (8 to 10 wk; n = 6) and old rats (2 to 2.4 y; n = 6), blood samples obtained at 15 and 30 min and 1, 2, and 4 h after drug administration were analyzed by HPLC–tandem mass spectrometry. In both groups, the withdrawal reflex was absent during anesthesia and was present at 1.1 (± 0.2) and 2.6 (± 0.7) h after drug administration in young and old rats, respectively, with the first voluntary movement at 1.5 ± 0.2 and 4.9 ± 1.0 h. Drug availability of ketamine and xylazine was 6.0 and 6.7 times greater, respectively, in old than young rats. The rate constant of elimination of both drugs was greatly decreased and the elimination half-life was significantly greater in old compared with young rats. In conclusion, age and associated factors affect the availability of ketamine and xylazine when coadministered to attain clinical anesthesia, changing the pharmacokinetics of these drugs and prolonging anesthesia duration and recovery times with aging. Compared with their young counterparts, aged rats required much higher doses to attain a similar level of anesthesia. Finally, the long half-life of both ketamine and xylazine, when coadministered to old rats, may be a factor in research protocols because residual plasma concentrations could still be present for as long as 3 and 5 d, respectively, after administration.
Abbreviations: Clast, last measurable plasma concentration; Kel, terminal elimination rate constant
Ketamine, an N-methyl d-aspartate antagonist with anesthetic properties, and xylazine, an α2-adrenoreceptor agonist with sedative and antinociceptive effects, are often used in combination to anesthetize rodents. They are administrated intramuscularly, intraperitoneally, or intravenously to provide relief of pain and distress.27 Ketamine combinations are considered to be a first choice in rodents when injectable anesthetics are used.6,20 Ketamine and xylazine are metabolized mainly by liver cytochromes P450 enzymes and excreted by the kidney.11 Both drugs are rapidly absorbed and well distributed to the CNS.24 However little is known about their pharmacokinetics in aged animals. Commercial ketamine preparations are composed of 2 enantiomers (the S-enantiomer is more active and produces fewer side effects),19 and cytochrome metabolism is different across different animal species;18 therefore findings in rats may not extrapolate to other species.
The main objective of the current study was to compare the pharmacokinetics of ketamine and xylazine in young and old rats when coadministered at anesthetic doses to determine a safer, more appropriate combination of injectable drugs for anesthesia of aged rats.
Materials and Methods
Animal subjects.
SPF Sprague–Dawley (Crl:CD[SD]; Charles River Canada, St Constant, Canada) rats were used for this study. To obtain old rats, 8- to 12-wk-old rats were purchased and kept in the animal facility until experimentation. At the time of experimentation, these animals (total of 9 rats; 3 animals used for a pilot study) were 2.0 to 2.4 y of age and weighed 0.8 to 1.1 kg. Sentinel program evaluations (Standard Health monitoring: serology [rat parvovirus, Toolan H1 virus, Kilham rat virus, rat minute virus, parvovirus NS1, sialodacryoadenitis virus, Pneumocystis carinii, Sendai virus, pneumonia virus of mice, reovirus, and Mycoplasma pulmonis]; upper respiratory and gastrointestinal microbiology, parasitology [endo- and ectoparasites]; and gross necropsy; Charles River) were performed every 6 mo on 6- to 8-wk-old Sprague–Dawley rats (Crl:CD(SD); Charles River) after dirty-bedding contact for 2 mo. Results showed that these sentinels were negative for all pathogens and necropsy findings. Young rats were 8 to 10 wk old and weighed 0.35 to 0.40 kg (n = 6) at the time of the study and had 7 d of acclimation before the start of the experiments. All rats were housed in a standard laboratory animal environment (fresh filtered air, 15 changes per hour; temperature, 21 ± 2 °C; humidity, 50% ± 20%; and 12:12-h light:dark cycle). Rats were pair-housed in polycarbonate cages (Ancare, Bellmore, NY) on hardwood bedding (Teklad Certified SaniChips, Harlan Laboratories, Madison, WI) with PVC tubes for environmental enrichment. Cage dimension were 10.5 in. × 19 in. × 8 in. for young rats and 24 in. × 17 in. × 8 in. for old rats (rats were moved from small to larger cages according to their weights, according to Canadian Council on Animal Care guidelines2). Rats received tap water and a certified laboratory diet (2018 Teklad Global 18% Protein Rodent Diet, Harlan Teklad, Bartonville, IL) ad libitum. The experimental protocol was approved by the IACUC of the Faculty of Veterinary Medicine of the University of Montreal (young rats) and the Ste Justine Hospital Research Center (old rats), in accordance with the guidelines of the Canadian Council on Animal Care.2 Animals were kept in different facilities, under similar environmental conditions, and all manipulations were performed by the same experimenters.
Treatments.
All rats (n = 6 per group) received ketamine (125 mg/kg IP; Ketalean, Bimeda-MTC, Cambridge, Canada) and xylazine (10 mg/kg IP; Xylamax, Bimeda-MTC). The anesthesic dose was selected from a pilot study in which 3 ketamine doses (80, 100, and 125 mg/kg; with 10 mg/kg xylazine) were evaluated in 3 old rats. The 2 lower doses of ketamine did not produce sufficiently profound anesthesia because all rats retained the withdrawal reflex at all time points. Only the 125-mg/kg dose of ketamine induced an anesthetic level where no withdrawal reflex was observed at the early time points (0.25, 0.5, and 1 h). Therefore, we used doses of 125 mg/kg ketamine and 10 mg/kg xylazine for this comparative pharmacokinetic study.
Evaluation of anesthesia depth.
After ketamine–xylazine injection, the duration of anesthesia was evaluated as the time until the first withdrawal reflex, evaluated immediately prior to each blood collection by pressing the interdigital skin of a hindpaw skin by using hemostatic forceps. In addition, recovery time was measured as the time until the first voluntary movement after ketamine–xylazine injection.
Blood sampling for the pharmacokinetic study.
The blood sampling method has previously been described.30 Briefly, jugular vein blood collections (0.3 mL per time point) were rapidly (approximately 1 min total duration) collected under isoflurane anesthesia (0.5 mL/min oxygen) when necessary (that is, when the withdrawal reflex was pinch present) by face mask. Blood was collected at 15 and 30 min and 1, 2, and 4 h after ketamine–xylazine administration. During blood collections, rats were kept on an electric heating pad; after sampling, they were placed under a heating lamp until sternally recumbent. Blood was collected in 1-mL microtainer tubes containing K2EDTA (Becton Dickenson, Franklin Lakes, NJ). Samples were maintained on ice and centrifuged (3200 × g for 10 min) within 30 min of collection. All samples were kept at −80 °C pending analysis by HPLC–tandem mass spectrometry.
Histologic preparations.
Immediately after euthanasia (isoflurane overdose) on the day after the last blood collection, the kidney and liver of each rat were collected and preserved in a buffered 10% formalin solution prior to histologic preparations (hematoxylin–eosin–saffron staining). Specimens were sent to the pathology department of the Faculty of Veterinary Medicine of the University of Montreal and evaluated by a veterinary pathologist (Dr Pierre Hélie, DMV, DACVP).
Bioanalytical methods.
Ketamine and xylazine analyses were performed by using an HPLC–tandem mass spectrometer as has previously been described.30
Pharmacokinetics.
Pharmacokinetic parameters of ketamine and xylazine in plasma were calculated using noncompartmental methods.23 The AUC from time 0 to the last measurable concentration was calculated by using the linear trapezoidal rule. The terminal rate constant of elimination (Kel) was calculated by using a minimum of 3 measured plasma concentrations, and a terminal elimination half-life was calculated as ln 2/Kel. The AUC extrapolated to infinity was calculated as AUC0-t + Clast/Kel, where Clast was the last measured plasma concentration. All pharmacokinetic parameters were calculated by using WinNonLin 5.2 (Pharsight, Mountain View, CA), and plasmatic drug profiles were modeled by using WinNonLin.
Statistical analysis.
Unpaired t tests were performed to assess differences in anesthesia duration, recovery time, and selected pharmacokinetic parameters between groups. The statistical significance level was set a priori at a P value of less than 0.05. Statistical analyses were performed by using Statistica software (version 4.3, www.statsoft.com). Data are reported as mean ± 1 SD.
Results
In young and old rats, the withdrawal reflex was absent during ketamine–xylazine anesthesia and returned at 1.1 ± 0.2 and 2.6 ± 0.7 h (mean ± 1 SD; P < 0.0001), respectively, after the drug administration. The first voluntary movement after ketamine–xylazine anesthesia was observed at 1.5 ± 0.2 and 4.9 ± 1.0 h (P < 0.0001) in young and old rats respectively. After recovery, no signs of toxicity (salivation, vocalization, erratic recovery, dyspnea, spastic jerking movements, convulsions, muscular tremors) were observed in either group.
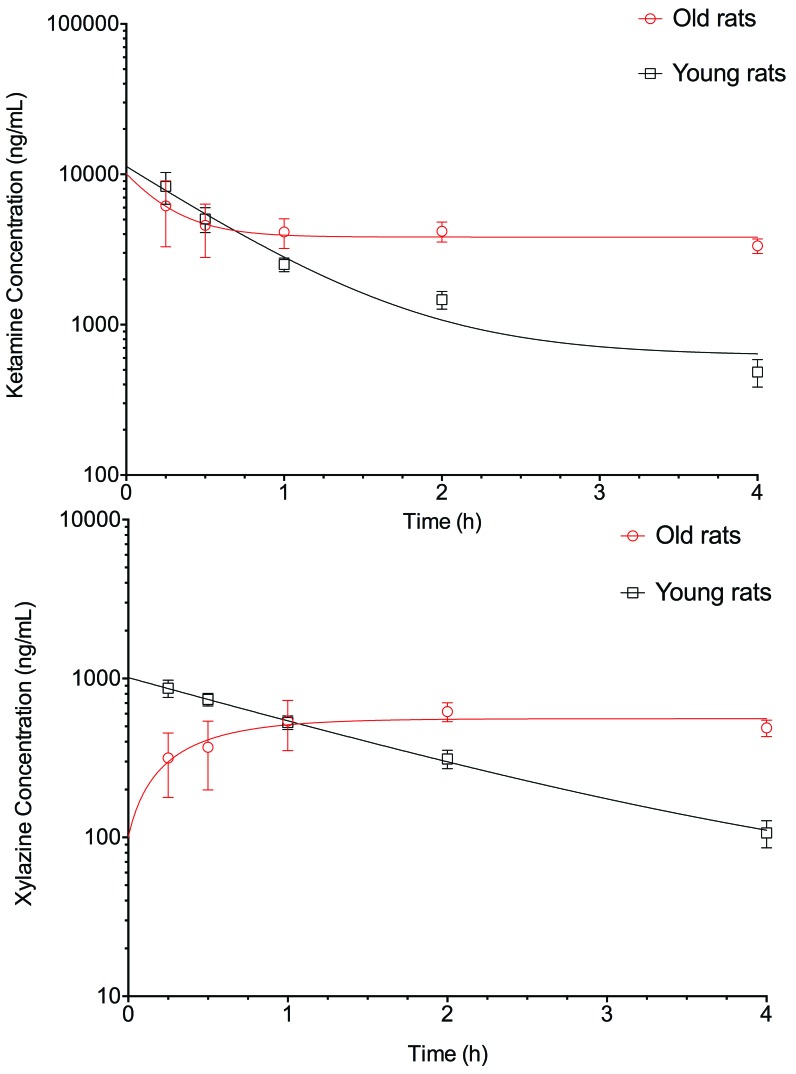
Semilogarithmic graphs of the plasmatic concentrations of ketamine and xylazine are presented in Figure 1. Pharmacokinetics parameters are presented in Table 1. Drug availability (AUC) was 6.0 and 6.7 times greater for ketamine and xylazine, respectively, in old rats compared with young rats. Kel was greatly decreased for both drugs and the elimination half-life was significantly greater in old rats than in young rats.
Figure 1.
Concentration (mean ± SD)–time profiles of ketamine (top) and xylazine (bottom) in young and old rats (n = 6 per group) after a single intraperitoneal injection of ketamine (125 mg/kg) and xylazine (10 mg/kg).
Table 1.
Pharmacokinetic parameters after a single intraperitoneal injection of ketamine (125 mg/kg) and xylazine (10 mg/kg)
| Young rats | Old rats | |
| Ketamine | ||
| AUC0-t (ng × h/mL) | 8,539.5 | 15,970.7a |
| AUCinf (ng × h/mL) | 9,422.7 | 56,927.3a |
| Kel (/h) | 0.5491 | 0.0817a |
| T1/2 (h) | 1.26 | 8.48a |
| Xylazine | ||
| AUC0-t (ng × h/mL) | 1,465.8 | 2,040.3a |
| AUCinf (ng × h/mL) | 1,666.0 | 11,168.1a |
| Kel (/h) | 0.53337 | 0.0536a |
| T1/2 (h) | 1.30 | 12.93a |
AUC0-t, AUC from time zero to the last measured concentration; AUC0-inf, AUC extrapolated to infinity; Kel, terminal elimination rate constant; T1/2, terminal elimination half-life.
Value significantly (P < 0.0001) different from that for young rats.
No abnormal histopathologic findings were present in livers or kidneys from either young or old rats.
Discussion
Many injectable anesthetic drug combinations have been used in rats, including fentanyl– droperidol and ketamine with either medetomidine or xylazine.9,10,32 Ketamine–xylazine is one of the most common anesthetic combinations in mice, rats, hamsters, and guinea pigs.1,21,28 Ketamine also is used for its sedative and analgesic properties in many species.8 In addition to its analgesic and anesthetic properties, ketamine has many advantages including simple administration, wide margin of safety, and its ability to be combined with other drugs.7 Xylazine is mainly used for its sedative and analgesic properties.13,14
Xylazine and ketamine are both eliminated mainly via urine. Ketamine is metabolized primarily into norketamine.16 Xylazine is extensively metabolized into many metabolites,18 however as much as 70% of the xylazine dose is eliminated in urine.20,23 The half-life of ketamine is variable in different species.14 In rats, the reported half-life for ketamine is 2 h and for xylazine is 1 h.5,30 Our findings show that the half-life of both drugs was approximately 1.3 h in young Sprague–Dawley rats but 8.5 and 13 h, respectively, in old rats. These results suggest that drug clearance is hampered markedly in aged rats. Together with reduced drug clearance, these changes explain in part the prolonged duration of anesthesia and slow recovery seen in old rats. In addition, the low clearance is reflected in greatly increased drug availability, measured as the AUC. AUC (exposure) is the most important pharmacokinetic parameter in terms of clinical efficacy and toxicity. Concerning efficacy, reflexes in our rats returned after drug administration with no apparent decrease in blood concentration. We currently have no clear answer for this apparent discrepancy. Important differences in drug concentrations between nervous tissue and plasma may occur due to the activation of drug efflux pumps. Additional studies are required to understand the relationship between anesthetic depth and the concentrations of drugs in nervous tissue and plasma. The significant increase in the AUC for both drugs suggests that toxicity will occur with repeated administrations, given that the clearance of a drug usually is calculated as 7 to 9 times the half-life (that is, 2.5 to 3 d for ketamine and 4 to 5 d for xylazine).23 Furthermore, considering that old rats usually have more degenerative renal lesions than do young rats, we expect that aged rats will have greater difficulty in eliminating the drugs, thus increasing the AUC and consequently toxicity to tissues. Given the biochemical, physiologic, and anesthetic differences between our experimental groups, our results strongly suggest that the ketamine–xylazine injectable drug combination is a poor anesthetic choice in aged rats.
Many factors can affect the pharmacokinetics of drugs, including age, sex, nutrition, environmental conditions, and disease.12,15,27,29 Various age-associated changes might affect the metabolism of drugs, such as chronic subclinical inflammation, obesity (for example, storage of lipid-soluble drugs in fat), and diminished exercise.17 Our results show a clear effect of age and associated factors on the pharmacokinetics of ketamine and xylazine. Because both ketamine and xylazine are metabolized by the liver cytochrome P450 enzymes and excreted by the kidney,16 age-associated changes in liver metabolism could very well explain our findings.31 Significant ultrastructural changes of hepatocellular organelles (for example, endoplasmic reticulum) occur with aging and can be correlated with biochemical changes.26 The hepatic metabolism of various substrates was altered in rats that varied in age from 3 to 24 mo, due to qualitative changes in cytochromes P450.22 Although drug metabolism might also occur in kidney and brain tissue, no previous publication has addressed this question in rats. In addition, plasma concentrations of the drugs may vary due to physiologic changes associated with aging; for example, glomerular filtration rates decrease with aging, although the total number of nephrons does not seem to be altered.3,4 Therefore pharmacokinetic changes associated with aging may reflect both metabolic and physiologic alterations. More studies are required to assess the pharmacokinetics of ketamine and xylazine in aged animals and should consider the metabolism of these drugs in liver, kidney, and brain tissue as well as the effects of other age-induced physiologic changes. We did not find any pathologic changes in the kidneys of the old rats; therefore the expected aged-associated degenerative are not a concern in the present study.
Anesthetic agents such as ketamine frequently are coadministered with xylazine; both of these drugs are metabolized by CYP3A. An in vitro study25 has shown that ketamine has an inhibitory effect on xylazine metabolism, perhaps explaining our finding that the plasma concentration of ketamine at 1 h after administration was greatly increased in old rats. The marked difference in substrate concentration and a reduction in CYP expression may amplify the effect of CYP3A substrate competition and may explain the significant reduction in xylazine clearance that we noted in our old rats. It is important to note that pharmacokinetic analyses usually are done with a single drug at a time, whereas we here calculated the various pharmacokinetic parameters after the coadministration of ketamine and xylazine, which reflects the clinical situation. Because both drugs are metabolized by similar hepatic enzymes, the importance of this approach is revealed in our aged rats.
In conclusion, age and associated factors significantly affect ketamine and xylazine availability when the drugs are coadministered to attain clinical anesthesia, subsequently changing the pharmacokinetics of these drugs and leading to prolonged anesthesia duration and recovery times in aged rats. We also found that, compared with young rats, old rats required much greater anesthetic doses to attain a similar level of anesthesia. The long half-life of both ketamine and xylazine when coadministered to old rats may be a factor in research protocols, because residual plasma concentrations may be present for as long as 3 and 4 d, respectively, after their administration.
Acknowledgments
We thank Dr Elie Haddad (Centre Hospitalier Ste-Justine) for the donation of the old rats.
References
- 1.Branson KR. 2001. Injectables anesthetics, p 213–267. In: Adams HR Veterinary pharmacology and therapeutics, 8th ed. Ames (IA): Iowa State Press. [Google Scholar]
- 2.Canadian Council on Animal Care 1993. Guide to the care and use of experimental animals, vol 1, 2nd ed. Ottawa (Canada): Canadian Council on Animal Care. [Google Scholar]
- 3.Corman B, Pratz J, Poujeol P. 1985. Changes in anatomy, glomerular filtation, and solute excretion in aging rat kidney. Am J Physiol 248:R282–R287 [DOI] [PubMed] [Google Scholar]
- 4.Corman B, Michel JB. 1987. Glomerular filtration, renal blood flow, and solute excretion in conscious aging rats. Am J Physiol 253:R555–R560 [DOI] [PubMed] [Google Scholar]
- 5. European Agency for the Evaluation of Medicinal Products. 2002. Committee for veterinary medicinal products. Xylazine hydrochloride: a summary report. London (UK): European Agency for the Evaluation of Medicinal Products.
- 6.Flecknell PA. 1996. Laboratory animal anesthesia. London (UK): Academic Press. [Google Scholar]
- 7.Gaertner DJ, Hallman TM, Hankenson FC, Batchelder MA. 2008. Anesthesia and analgesia for laboratory rodents, p 240–282. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals. San Diego (CA): Academic Press. [Google Scholar]
- 8.Green CJ. 1981. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10-year experience. Lab Anim 15:163–170 [DOI] [PubMed] [Google Scholar]
- 9.Hayton SM, Kriss A, Muller DP. 1999. Comparison of the effects of 4 anaesthetic agents on somatosensory evoked potentials in the rat. Lab Anim 33:243–251 [DOI] [PubMed] [Google Scholar]
- 10.Hedenqvist P, Roughan JV, Flecknell PA. 2000. Sufentanil and medetomidine anaesthesia in the rat and its reversal with atipamezole and butorphanol. Lab Anim 34:244–251 [DOI] [PubMed] [Google Scholar]
- 11.Hijazi Y, Boulieu R. 2002. Contribution of CYP3A4, CYP2C9, and CYP2B6 in N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 30:853–858 [DOI] [PubMed] [Google Scholar]
- 12.Jenkins AJ. 2007. Toxicokinetics and factors affecting pharmacokinetic parameters, p 21–24. In: Karch SB. Pharmacokinetics and pharmacodynamics of abused drugs. Boca Raton (FL): CRC Press. [Google Scholar]
- 13.Kästner SB. 2006. α2 agonists in sheep: a review. Vet Anaesth Analg 33:79–96 [DOI] [PubMed] [Google Scholar]
- 14.Lamont LA, Tranquilli WJ, Grimm KA. 2000. Physiology of pain. Vet Clin North Am Small Anim Pract 30:703–728 [DOI] [PubMed] [Google Scholar]
- 15.Majewski-Tiedeken CR, Rabin CR, Siegel SJ. 2008. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2, and FVB inbred mouse strains. Drug Alcohol Depend 92:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer RE, Fish RE. 2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers, p 27–82. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals. San Diego (CA): Academic Press. [Google Scholar]
- 17.Mézière A, Paillaud E, Plaud B. 2013. Anesthesia in the elderly. Presse Med 42:197–201 [DOI] [PubMed] [Google Scholar]
- 18.Mössner LD, Schmitz A, Thorman W, Mevissen M. 2011. Inhibition of cytochrome P450 enzymes involved in ketamine metabolism by use of liver microsomes and specific cytochrome P450 enzymes from horses, dogs, and humans. Am J Vet Res 72:1505–1513 [DOI] [PubMed] [Google Scholar]
- 19.Pai A, Hening M. 2007. Ketamine. Contin Educ Anaesth Crit Care Pain 7:59–63 [Google Scholar]
- 20.Park Choo HY, Choi SO. 1991. The metabolism of xylazine in rats. Arch Pharm Res 14:346–351 [Google Scholar]
- 21.Richardson CA, Flecknell PA. 2005. Anaesthesia and postoperative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim 33:119–127 [DOI] [PubMed] [Google Scholar]
- 22.Rikans LE, Notley BA. 1982. Age-related changes in rat hepatic microsomal drug metabolism are substrate selective. J Pharmacol Exp Ther 220:574–578 [PubMed] [Google Scholar]
- 23.Rowland M, Towzer TN. 1995. Clinical pharmacokinetics: concepts and application, p 367–389. Philadelphia (PA): Lippincott Williams and Wilkins. [Google Scholar]
- 24.Salonen JS. 1992. Chemistry and pharmacokinetics of the α2-adrenoreceptor agonist, p 191–200. In: Short CE, Van Poznak A. Animal pain. New York (NY): Churchill Livingstone. [Google Scholar]
- 25.St-Germain-Lavoie D, Pailleux F, Vachon P, Beaudry F. 2013. Characterization of xylazine in vitro metabolism in rat liver microsomes using liquid chromatography–hybrid triple quadrupole linear ion trap mass spectrometry. Biomed Chromatogr 27:882–888 [DOI] [PubMed] [Google Scholar]
- 26.Schmucker DL. 1990. Hepatocyte fine structure during maturation and senescence. J Electron Microsc Tech 14:106–125 [DOI] [PubMed] [Google Scholar]
- 27.Song G, Wu H, Yoshino K, Zamboni WC. 2012. Factors affecting the pharmacokinetics and pharmacodynamics of liposomal drugs. J Liposome Res 22:177–192 [DOI] [PubMed] [Google Scholar]
- 28.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154 [DOI] [PubMed] [Google Scholar]
- 29.Struck MB, Andrutis KA, Ramirez HE, Battles AH. 2011. Effect of a short-term fast on ketamine–xylazine anesthesia in rats. J Am Assoc Lab Anim Sci 50:344–348 [PMC free article] [PubMed] [Google Scholar]
- 30.Veilleux-Lemieux D, Beaudry F, Hélie P, Vachon P. 2012. Effects of endotoxemia on the pharmacodynamics and pharmacokinetics of ketamine and xylazine anesthesia in Sprague–Dawley rats. Vet Med Res Reports 3:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wauthier V, Verbeeck RK, Buc Calderon P. 2004. Age-related changes in the protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as in their hepatic activities in Wistar rats. What role for oxidative stress? Arch Toxicol 78:131–138 [DOI] [PubMed] [Google Scholar]
- 32.Wixson SK, White WJ, Hughes HC, Lang CM, Marshall WK. 1987. A comparison of pentobarbital, fentanyl–droperidol, ketamine–xylazine, and ketamine–diazepam anesthesia in adult male rats. Lab Anim Sci 37:726–730 [PubMed] [Google Scholar]