Abstract
NSAID administration is often chosen as a method of minimizing pain and discomfort for nonhuman primates. Of concern when using NSAID is the potential for decreased platelet aggregation due to the inhibition of cyclooxygenases 1 and 2. In both dogs and humans, the use of NSAID that are selective for cyclooxygenase 2, like meloxicam, minimizes the inhibition of platelet aggregation in comparison to nonselective NSAID, like aspirin, that inhibit both isoforms of cyclooxygenase. In this study, we measured platelet aggregation in rhesus macaques (n = 6) by using the impedance method on a multiple-electrode aggregometer at baseline, at 1 and 4 d after initiating treatment with aspirin or meloxicam, and after a washout period. There was no statistical difference between aggregation at baseline and after 1 or 4 d of meloxicam treatment, but platelet aggregation decreased after both 1 and 4 d of aspirin therapy. Our data suggest that clinically significant postoperative hemorrhage is unlikely in rhesus macaques briefly treated with meloxicam.
Abbreviations: ASPI, arachidonic acid; COX, cyclooxygenase
The privilege of using animals in biomedical research comes with the societal expectation that, whenever possible, pain and discomfort will be minimized. Many pharmaceutical options are available for the relief of pain in nonhuman primates. These options include, but are not limited to, analgesics like the µ, partial µ, and κ opioid agonists; local anesthetics; and NSAID. NSAID are used to alleviate pain secondary to tissue inflammation and are an integral component of multimodal analgesia.7 Although NSAID are potent, long-acting analgesics for postoperative pain in various species, they present various risks, including gastrointestinal irritation, hepatic toxicity, renal toxicity, and interference with hemostasis, to animals as well as research data.4,10
NSAID decrease inflammation by inhibiting cyclooxygenases (COX), of which there are 2 commonly recognized isoforms: COX1 and COX2. Although a third isoform (COX3) is known, its role in disease pathology and homeostasis is currently being investigated.14 NSAID available for clinical use are characterized by their relative effect of COX1 compared with COX2. Aspirin and etodolac, for example, block both COX1 and COX2 nonselectively, whereas meloxicam and other NSAID are COX2-selective.
Cyclooxygenase plays an important role in the arachidonic acid pathway. COX1 plays an integral role in converting arachidonic acid in cell membranes to prostaglandins and thromboxane A2, which is necessary for platelet aggregation. The production of thromboxane A2in platelets currently is thought to be exclusively mediated by COX1.5 In addition, prostaglandins function to maintain gastric mucosal integrity in the face of excessive gastric acidity, regulate gastric microcirculation, and modulate renal blood flow.19 In contrast, the COX2 isoform is increased secondary to inflammation, given that it enhances nociception and other aspects of the inflammatory process.5 These COX1-dependent adverse side effects have led to the creation of COX2-selective inhibitors for antiinflammatory and analgesic applications.10,11,17,25 Meloxicam selectively inhibited COX2 in various studies in dogs and humans in vivo.8,19
Aspirin and other nonselective COX inhibitors have been demonstrated to decrease platelet aggregation in many species.5,6,11 For some investigators, this finding prompts concerns regarding the risk of hemorrhage during intra- and postoperative periods. Aspirin blocks COX by acetlylating serine 529 within COX.26 Aspirin's inhibition of COX is not only nonselective, but its blockade of COX1 in platelets is irreversible.11 Although some studies show no significant changes in platelet aggregation in humans and dogs that received meloxicam, methods of testing varied widely, and contradictory findings have been reported for dogs.5,8,10,25 The degree of COX2-selectiveness of specific NSAID varies from species to species, for example, dogs and humans.28,32 Using a different assay, other authors have reported an innate dog-to-dog variability in responsiveness to NSAID and a decrease in platelet function in response to meloxicam, although the authors also stated that agonist choice in this assay plays a role in this finding.23 Given this variability in response to NSAID and the prevalence of meloxicam use for postoperative analgesia at our facility, we sought to determine whether the COX2-selective effects of meloxicam on platelet aggregation occur in nonhuman primates, specifically rhesus macaques, when meloxicam is used for a short time, such as the perioperative period.
Platelet aggregation can be measured based on the principles of light transmittance, impedance, or luminescence, or a combination of these methods by a variety of instruments currently available.24 In the current study, we measured aggregation by using electrode impedance aggregometry, a type of whole-blood aggregometry. This method is based on how the attachment of platelets to 2 electrodes modulates electrical resistance between the electrodes. The change of resistance is proportional to the number of platelets adhering to the electrodes and is recorded continuously by the device.30 This method has been used to study the effect of various medications on platelet aggregation in humans.18
The primary goal of the current study was to test our null hypothesis: that meloxicam has no measurable effect on platelet aggregation, as determined by using the aggregometer, when compared with the premedication baseline value. The second null hypothesis we tested was that aspirin would also have no measureable effect on platelet aggregation when compared with premedication baseline as measured by the same assay. The study was designed to address whether a single dose of meloxicam affected platelet aggregation as determined by the aggregometer; whether 4 d of daily meloxicam administration affected platelet function; whether a single dose of aspirin affect platelet aggregation; whether 4 consecutive days of aspirin administration affected platelet function; and whether either medication affected platelet aggregation after cessation of treatment.
Materials and Methods
Animals.
Six intact adult male rhesus macaques (Macaca mulatta; weight 10.8 to 17.0 kg; age, 8.5 to 10.5 y) were used in a crossover design, in which each animal received 4 d of treatment with each of 2 NSAID (aspirin and meloxicam) and platelet aggregation was measured. All animals were housed at the University of California, San Francisco, an AAALAC-accredited institution. All research and animal care at this facility were performed in accordance with the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.1,16 All research performed was IACUC-approved. The colony is enzootic for Macacine herpesvirus 1. Macaques were singly housed indoors on a 12:12-h light:dark cycle and were fed primate biscuits (no. 5050, Lab Fiber Plus, PMI Nutrition International, Brentwood, MO) twice daily and assorted produce. All animals had ad libitum access to reverse-osmosis, deionized, UV-sterilized water throughout the study. All macaques had access to edible, destructible, or indestructible enrichment items daily on an alternating schedule. All subjects were transferred from a neurophysiology project, and all cranial and orbital implants had been removed 3 or more months prior to enrollment in this study.
Drug treatments.
The 6 macaques were randomized by lottery into 2 treatment groups, an aspirin-first group and a meloxicam-first group, each consisting of 3 animals. All macaques received either aspirin (20 mg/kg PO twice daily; of 81 mg/tablet chewable low-dose orange flavored-aspirin, Bayer, Pittsburgh, PA) first and then meloxicam (0.3 mg/kg SC or PO daily; 1.5 mg/mL oral suspension or 5 mg/mL injectable, Boehinger Ingelheim, Metacam, St Joseph, MO) or the reverse. The personnel collecting and analyzing samples were blinded in regard to treatment group. The first dose of meloxicam was given subcutaneously, to best assure consistent uptake for testing the effects of a single-dose treatment and to best model perioperative dosing at our facility. Follow-up oral medication administration for 3 d at the doses we commonly use at our facility allowed examination of short-term cumulative dosing. Aspirin is only available as an oral formulation in the United States, and all aspirin doses were administered orally. Due to equipment constraints for the platelet assay, animals were assigned randomly by lottery into 2 testing groups (Monday or Tuesday), with sample collection on Monday and Thursday or Tuesday and Friday, respectively. All macaques underwent initial blood work 25 d before treatment. All animals received 4 d of each NSAID, with 18 d from the last day of the first medication to the first day of the other. Twenty days after administration of the second drug, blood was collected and analyzed for the final time. Platelet function was assayed before NSAID treatments, on days 1 and 4 of each NSAID, and at 18 d after the last NSAID administration.
Sample collection and analysis.
For each blood sample, macaques were fasted overnight and sedated with ketamine (10 to 15 mg/kg IM; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA), except for one subject that received ketamine (10 to 15 mg/kg IM, Fort Dodge Animal Health) and midazolam (0.05 mg/kg IM; Midazolam, Hospira, Lake Forest IL). Blood was collected via the saphenous vein, with the cephalic vein as an alternate method of collection. For each sample, 10 to 20 mL blood (approximately 0.1% to 0.2% of body weight in grams for a 10-kg animal at any single blood draw) was taken. Blood was taken 2 to 3 h after drug administration to coincide with expected peak blood levels according to the manufacturer of injectable meloxicam. Blood was collected on day 0 (baseline data: CBC, clinical chemistry, PTT, PT, platelet count, and aggregometry assay) and days 18 (first drug, day 1), 21 (first drug, day 4), 39 (second drug, day 1), 42 (second drug, day 4), and 63 (final sample) for analysis of CBC, platelet count, and platelet aggregation.
All samples for platelet aggregation, PT, or PTT were collected into citrate tubes; those for analysis of CBC and platelet count were collected into EDTA tubes; and those for serum biochemistry (Idexx Laboratories, Sacramento, CA) were collected into serum separator tubes to evaluate for underlying disease or indications of clotting disorders.
Multiplate platelet aggregometer.
Platelet function was assessed by using the Multiplate multiple-electrode aggregometer (Verum Diagnostica, Munich, Germany) within 2 h of sample collection. Briefly, 0.3 mL of whole blood in citrate was diluted in warmed normal saline containing 3 mM CaCl2 and incubated for 3 min at 37 °C with continuous stirring in a Multiplate test cell. Each test cell contains 2 sets of 3-mm silver-coated copper wires, across which electrical resistance is measured at 0.57-s intervals. Platelet activation was induced by using ADP (0.2 mM), arachidonic acid (ASPI; 15 mM), or collagen (100 μg/mL). Platelet adhesion to the electrodes was detected as increasing electrical impedance, measured by duplicate sets of sensor wires in each test cell. Agonist responses are reported as area under the aggregation curve (U) over a 6-min measurement period.
Statistical analysis.
A factorial ANOVA tested the main effects of treatment (aspirin or meloxicam), sequence (aspirin or meloxicam first), and time within sequence (day 1 or day 4) and an interaction effect of time and treatment on change from baseline platelet aggregation.
Because we found no treatment-order effect, paired t test compared baseline and posttreatment platelet aggregation data for both drugs at all time points and at the final sample 21 d after the last NSAID treatment. Stata 10.1 (College Station, TX) was used for all analyses.
Results
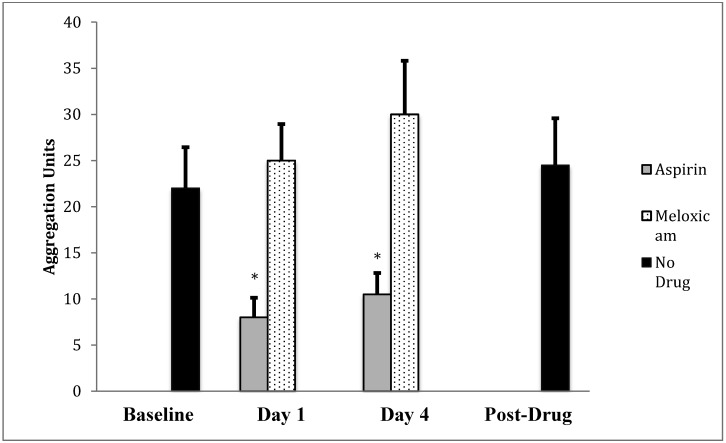
Platelet aggregation using the ASPI agonist did not differ after 1-d or 4-d meloxicam administration compared with baseline levels. Platelet aggregation was significantly (P < 0.05 for both comparisons) decreased with aspirin administration at both the 1-d and 4-d time points when compared with baseline using the ASPI agonist (Figure 1). For all macaques in both treatment orders, platelet aggregation using the ASPI agonist did not differ between the premedication baseline sample and the day 63 sample, which was collected 21 d after the last NSAID administration. There were no residual effects of aspirin on day 18 or 21.
Figure 1.
ASPI-induced platelet aggregation (AUC; mean ± SEM) before drug administration (baseline), at 2 time points for aspirin and for meloxicam, and at 21 d after the final drug administration. *, Change from baseline was statistically significant (P < 0.05, 2-tailed t test) for aspirin after both 1 and 4 d of administration but not for meloxicam at either time point.
Treatment order did not have an effect when the ASPI agonist was used. There were no residual effects of aspirin detectable by day 18 or 21 by using the ASPI agonist. Other main effects were nonsignificant (sequence, F1, 23 = 1.8, P > 0.05; time within sequence, F1, 23 = 0.9, P > 0.05). In addition, the interaction effect of day×treatment failed to reach statistical significance (F1,23 = 0.1, P > 0.05), suggesting that the change in baseline platelet aggregation did not differ by day within treatment. Results indicated that pretreatment (mean ± 1 SD, 4.4 ± 10.9) and posttreatment (5.1 ± 12.5) platelet aggregation values were similar (t5, P > 0.05).
Table 1 reflects the baseline reading and how aggregation changed with medication administration throughout the study for each macaque. All preliminary blood work was within normal reference ranges. CBC and platelet counts remained within normal parameters throughout the study for all macaques. No animals became ill during the study. Collagen and ADP agonists did not reveal any statistically significant differences from baseline for either aspirin or meloxicam.
Table 1.
Changes in platelet aggregation (U) by using the ASPI agonist relative to baseline value
| Aspirin | Meloxicam | |||||
| Macaque no. | Baseline | Day 1 | Day 4 | Day 1 | Day 4 | After treatment |
| 1 | 25 | −17 | −6 | −3 | −8 | 0 |
| 2 | 22 | −18 | −15 | 0 | 14 | −9 |
| 3 | 22 | −19 | −18 | 14 | 8 | 3 |
| 4 | 44 | −28 | −28 | 2 | 16 | 2 |
| 5 | 20 | −12 | −8 | 4 | 10 | 4 |
| 6 | 11 | 3 | −2 | 15 | 19 | 0 |
| Overall (mean ± 1 SD) | 24 ± 10 | −15 ± 9 | −13 ± 9 | 5 ± 7 | 10 ± 9 | 0 ± 4 |
Discussion
There was no statistically significant deviation from baseline in platelet aggregation for rhesus macaques after either a single dose or 4 d of consecutive doses of meloxicam when the ASPI agonist was used. This finding contrasts with that of aspirin, which caused a marked change at both time points. This result strengthens the argument for meloxicam's potential use as a preemptive analgesic administered before surgery or intraoperatively.
Aspirin was chosen as the positive control in this study because of its well-known antithrombotic effects that are due to irreversibly inhibiting COX, to ensure that the aggregometer could differentiate changes in platelet aggregation in macaques as it does in humans when the ASPI agonist is used.21 A statistical difference was detected in the ASPI agonist aggregation values when data obtained after aspirin administration were compared with baseline values. The use of ASPI as the agonist of choice reflects the fact that it is responsive to cyclooxygenase-dependent aggregation, making it more sensitive to the COX-inhibitory effects of NSAID, in contrast to the other agonists available.22
In the current study, impedance aggregometry was selected as the method of determining platelet aggregation. Although light-transmission aggregometry, which evaluates luminosity as aggregation occurs in platelet-rich plasma after stimulation with a platelet agonist, is considered to be the ‘gold standard,’ its cost, poor standardization, and the need for a skilled technician to operate the machine make its use challenging in clinical veterinary use.20,27 In addition, the centrifugation used in light-transmission aggregometry can destroy platelets.31 Furthermore, whole-blood aggregometry has been noted to have a higher sensitivity than that of light-transmission aggregometry for accurately measuring platelet aggregation in some studies in humans.9
An 18- to 21-d interval between medications was chosen because it is considered an adequate time frame to encompass the lifespan of platelets in many species.3,12,29 Minimal data are available on platelet lifespan in rhesus macaques, which has been found to be as short as 5 to 6 d.13 Although the lack of a sample between the administrations of the first and second drug is a weakness of the present study, we found no residual effect of aspirin at 18 to 21 d after its last administration, and we found no evidence of a carryover effect of either NSAID. There was no statistical difference in platelet aggregation 21 d after the last NSAID compared with preNSAID baseline, regardless of which NSAID was administered most recently. Likewise, day 1 of meloxicam in ‘aspirin first’ macaques that had received aspirin 18 d earlier did not differ from predrug baseline values. The return to baseline values after aspirin treatment is consistent with what would be predicted by using time periods that are 2 to 3 times longer than the average lifespan of a rhesus platelet.
Limitations of the current study relate mostly to the small sample size available for the project. To minimize the risk of residual carryover effect from one NSAID affecting the findings during the second NSAID treatment, a crossover design was used to assign the macaques to an aspirin-first or meloxicam-first group. Because of equipment limitations, within each treatment group, animals were further divided into groups to be tested on either Monday and Thursday or Tuesday and Friday. The population was not large enough to rigorously test for the effect of treatment group or day of the week, but the dramatic change in platelet function in response to aspirin treatment and the return to baseline at the end of the study suggest that group assignment did not induce significant artifacts.
A further unavoidable complication was the need to sedate the macaques for blood collection; thus the no-NSAID samples at the start and end of the study are in fact sedation-only controls. One animal required a ketamine–midazolam mixture because of a prior history of poor sedation with ketamine only. Ketamine has been shown to affect platelet function, and midazolam alone can have effects on platelet aggregation.2,14 Although suboptimal, sedation of nonhuman primates, especially for short-term studies, is often clinically required to ensure both human and animal safety.
Finally, normal values have not been established for rhesus macaques for the platelet aggregometer; in fact, this report is the first published use of this equipment for this species. The dramatic response to aspirin treatment in the current study verifies that the aggregometer is capable of detecting COX1-induced changes in platelet function in rhesus macaques by using the ASPI agonist, supporting our interpretation that the lack of meloxicam effect is a true finding and not due to an inability to detect changes in platelet function. It is noteworthy that the other 2 agonists did not reveal any drug-associated change in platelet function. Because the ADP agonist does not activate platelets via thromboxane, neither drug we tested had an effect on aggregation.31 The collagen agonist, like the ASPI agonist, activates platelets primarily through thromboxane formation and has demonstrated a decrease in platelet aggregation similar to that of the ASPI agonist in humans.31 Perhaps the collagen agonist is not as sensitive as is ASPI to COX1-associated effects in macaques. Additional study with a larger sample size would be necessary to evaluate this issue.
Short courses of meloxicam given to rhesus macaques should be considered as a component of multimodal postoperative analgesia. Concern regarding a decrease in platelet aggregation does not appear to be a justifiable reason to eliminate this medication as a choice.
Acknowledgments
We thank Mitchell Cohen and Michael Kutcher for kindly allowing access to the Multiplate aggregometer. Thanks also to the veterinary technicians, in particular Salomon Martinez and Michael Sheets, and the husbandry staff in LARC at UCSF for keeping the animals so healthy and happy and for seeing to their retirement to sanctuary and to Dr John Kornak for statistical advice. In addition, we thank all of the veterinarians in the UCSF LARC department—Diana Bauer, Kristina Cataline, Krista Lindstrom, John Parker, Clifford Roberts, and James Wilkerson— for aiding in the editing process.
References
- 1.Animal Welfare Act as Amended 2008. 7 USC §2131–2159.
- 2.Atkinson PM, Taylor DI, Chetty N. 1985. Inhibition of platelet aggregation by ketamine hydrochloride. Thromb Res 40:227–234 [DOI] [PubMed] [Google Scholar]
- 3.Becker RC, Smyth S. 2009. The evolution of platelet-directed pharmacotherapy. J Thromb Haemost 7 Suppl 1:266–271 [DOI] [PubMed] [Google Scholar]
- 4.Bergh MS, Budsberg SC. 2005. The coxib NSAIDs: potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med 19:633–643 [DOI] [PubMed] [Google Scholar]
- 5.Brainard BM, Meredith CP, Callan MB, Budsberg SC, Shofer FS, Driessen B, Otto CM. 2007. Changes in platelet function, hemostasis, and prostaglandin expression after treatment with nonsteroidal antiinflammatory drugs with various cyclooxygenase selectivities in dogs. Am J Vet Res 68:251–257 [DOI] [PubMed] [Google Scholar]
- 6.Bush HL, Jr, Jakubowski JA, Sentissi JM. 1988. Early healing after carotid endarterectomy: effect of high- and low-dose aspirin on thrombosis and early neointimal hyperplasia in a nonhuman primate model. J Vasc Surg 7:275–283 [PubMed] [Google Scholar]
- 7.Comittee on Recognition and Alleviation of Pain in Laboratory Animals 2009. Recognition and alleviation of pain in laboratory animals. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 8.de Meijer A, Vollaard H, de Metz M, Verbruggen B, Thomas C, Novakova I. 1999. Meloxicam, 15 mg/day, spares platelet function in healthy volunteers. Clin Pharmacol Ther 66:425–430 [DOI] [PubMed] [Google Scholar]
- 9.Dyszkiewicz-Korpanty AM, Frenkel EP, Sarode R. 2005. Approach to the assessment of platelet function: comparison between optical-based platelet-rich plasma and impedance-based whole-blood platelet aggregation methods. Clin Appl Thromb Hemost 11: 25–35 [DOI] [PubMed] [Google Scholar]
- 10.Fresno L, Moll J, Penalba B, Espada Y, Andaluz A, Prandi D, Ruiz de Gopegui R, Garcia F. 2005. Effects of preoperative administration of meloxicam on whole blood-platelet aggregation, buccal mucosal bleeding time, and haematological indices in dogs undergoing elective ovariohysterectomy. Vet J 170:138–140 [DOI] [PubMed] [Google Scholar]
- 11.Gurbel PA, Bliden KP, DiChiara J, Newcomer J, Weng W, Neerchal NK, Gesheff T, Chaganti SK, Etherington A, Tantry US. 2007. Evaluation of dose-related effects of aspirin on platelet function: results from the aspirin-induced platelet effect (ASPECT) study. Circulation 115:3156–3164 [DOI] [PubMed] [Google Scholar]
- 12.Harker LA, Hunt P, Marzec UM, Kelly AB, Tomer A, Hanson SR, Stead RB. 1996. Regulation of platelet production and function by megakaryocyte growth and development factor in nonhuman primates. Blood 87:1833–1844 [PubMed] [Google Scholar]
- 13.Harker LA, Marzec UM, Kelly AB, Cheung E, Tomer A, Nichol JL, Hanson SR, Stead RB. 1997. Prevention of thrombocytopenia and neutropenia in a nonhuman primate model of marrow suppressive chemotherapy by combining PEGylated recombinant human megakaryocyte growth and development factor and recombinant human granulocyte colony-stimulating factor. Blood 89:155–165 [PubMed] [Google Scholar]
- 14.Henrotin Y, Sanchez C, Balligand M. 2005. Pharmaceutical and nutraceutical management of canine osteoarthritis: present and future perspectives. Vet J 170:113–123 [DOI] [PubMed] [Google Scholar]
- 15.Hsiao G, Shen MY, Chou DS, Chang Y, Lee LW, Lin CH, Sheu JR. 2004. Mechanisms of antiplatelet and antithrombotic activity of midazolam in in vitro and in vivo studies. Eur J Pharmacol 487:159–166 [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Jakubowski JA, Stampfer MJ, Vaillancourt R, Deykin D. 1985. Cumulative antiplatelet effect of low-dose enteric-coated aspirin. Br J Haematol 60:635–642 [DOI] [PubMed] [Google Scholar]
- 18.Jambor C, Weber CF, Gerhardt K, Dietrich W, Spannagl M, Heindl B, Zwissler B. 2009. Whole-blood multiple electrode aggregometry is a reliable point-of-care test of aspirin-induced platelet dysfunction. Anesth Analg 109:25–31 [DOI] [PubMed] [Google Scholar]
- 19.Jones CJ, Streppa HK, Harmon BG, Budsberg SC. 2002. In vivo effects of meloxicam and aspirin on blood, gastric mucosal, and synovial fluid prostanoid synthesis in dogs. Am J Vet Res 63:1527–1531 [DOI] [PubMed] [Google Scholar]
- 20.Lordkipanidze M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. 2007. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J 28:1702–1708 [DOI] [PubMed] [Google Scholar]
- 21.Malinin AI, Atar D, Callahan KP, McKenzie ME, Serebruany VL. 2003. Effect of a single-dose aspirin on platelets in humans with multiple risk factors for coronary artery disease. Eur J Pharmacol 462:139–143 [DOI] [PubMed] [Google Scholar]
- 22.Marschner CB, Kristensen AT, Spodsberg EH, Wiinberg B. 2012. Evaluation of platelet aggregometry in dogs using the Multiplate platelet analyzer: impact of anticoagulant choice and assay duration. J Vet Emerg Crit Care (San Antonio) 22:107–115 [DOI] [PubMed] [Google Scholar]
- 23.Mullins KB, Thomason JM, Lunsford KV, Pinchuk LM, Langston VC, Wills RW, McLaughlin RM, Mackin AJ. 2012. Effects of carprofen, meloxicam, and deracoxib on platelet function in dogs. Vet Anaesth Analg 39:206–217 [DOI] [PubMed] [Google Scholar]
- 24.Pakala R, Waksman R. 2011. Currently available methods for platelet function analysis: advantages and disadvantages. Cardiovasc Revasc Med 12:312–322 [DOI] [PubMed] [Google Scholar]
- 25.Rinder HM, Tracey JB, Souhrada M, Wang C, Gagnier RP, Wood CC. 2002. Effects of meloxicam on platelet function in healthy adults: a randomized, double-blind, placebo-controlled trial. J Clin Pharmacol 42:881–886 [DOI] [PubMed] [Google Scholar]
- 26.Roth GJ, Calverley DC. 1994. Aspirin, platelets, and thrombosis: theory and practice. Blood 83:885–898 [PubMed] [Google Scholar]
- 27.Sibbing D, Braun S, Jawansky S, Vogt W, Mehilli J, Schomig A, Kastrati A, von Beckerath N. 2008. Assessment of ADP-induced platelet aggregation with light-transmission aggregometry and multiple-electrode platelet aggregometry before and after clopidogrel treatment. Thromb Haemost 99:121–126 [DOI] [PubMed] [Google Scholar]
- 28.Streppa HK, Jones CJ, Budsberg SC. 2002. Cyclooxygenase selectivity of nonsteroidal antiinflammatory drugs in canine blood. Am J Vet Res 63:91–94 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka R, Murota A, Nagashima Y, Yamane Y. 2002. Changes in platelet lifespan in dogs with mitral valve regurgitation. J Vet Intern Med 16:446–451 [DOI] [PubMed] [Google Scholar]
- 30.Toth O, Calatzis A, Penz S, Losonczy H, Siess W. 2006. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost 96:781–788 [PubMed] [Google Scholar]
- 31.Velik-Salchner C, Maier S, Innerhofer P, Streif W, Klingler A, Kolbitsch C, Fries D. 2008. Point-of-care whole-blood impedance aggregometry versus classical light-transmission aggregometry for detecting aspirin and clopidogrel: the results of a pilot study. Anesth Analg 107:1798–1806 [DOI] [PubMed] [Google Scholar]
- 32.Wilson JE, Chandrasekharan NV, Westover KD, Eager KB, Simmons DL. 2004. Determination of expression of cyclooxygenase-1 and -2 isozymes in canine tissues and their differential sensitivity to nonsteroidal antiinflammatory drugs. Am J Vet Res 65:810–818 [DOI] [PubMed] [Google Scholar]