Background: CDK10 is involved in cell proliferation; however, the function of CDK10 in cell development remains unclear.
Results: The knockdown of cdk10 results in the apoptosis of neural progenitor cells (NPCs) through enhanced raf1a expression.
Conclusion: Zebrafish cdk10 exhibits an important function in neurogenesis by the modulation of the raf1a level during development.
Significance: These findings emphasize the significance of cdk10 in NPC survival.
Keywords: Apoptosis, CDK (Cyclin-dependent Kinase), Development, Neurons, Zebrafish, cdk10
Abstract
In this study, we used zebrafish as an animal model to elucidate the developmental function of cdk10 in vertebrates. In situ hybridization analyses demonstrated that cdk10 is expressed throughout development with a relative enrichment in the brain in the late stages. Similar to its mammalian ortholog, cdk10 can interact with the transcription factor ETS2 and exhibit kinase activity by phosphorylating histone H1. Morpholino-based loss of cdk10 expression caused apoptosis in sox2-positive cells and decreased the expression of subsequent neuronal markers. Acetylated tubulin staining revealed a significant reduction in the number of Rohon-Beard sensory neurons in cdk10 morphants. This result is similar to that demonstrated by decreased islet2 expression in the dorsal regions. Moreover, cdk10 morphants exhibited a marked loss of huC-positive neurons in the telencephalon and throughout the spinal cord axis. The population of retinal ganglion cells was also diminished in cdk10 morphants. These phenotypes were rescued by co-injection of cdk10 mRNA. Interestingly, the knockdown of cdk10 significantly elevated raf1a mRNA expression. Meanwhile, an MEK inhibitor (U0126) recovered sox2 and ngn1 transcript levels in cdk10 morphants. Our findings provide the first functional characterization of cdk10 in vertebrate development and reveal its critical function in neurogenesis by modulation of raf1a expression.
Introduction
Protein kinase signals mediate various cellular functions such as metabolism, cell proliferation, cell growth, and apoptosis (1, 2). The cyclin-dependent kinase (CDK)2 family of proteins is a group of serine/threonine kinases that regulate cell cycle progression specifically at the G1/S and G2/M checkpoints (3, 4). Cell cycle progression requires the association of CDK with specific cyclins to form active complexes, which subsequently control the expression of downstream cell cycle genes (3, 4).
A family of cdc2-related kinases, including PISSLRE (CDK10), PCTAIRE, KKIALRE (CDK-like-1; CDKL1), KKIAMRE (CDK-like-2; CDKL2), and NKIATRE (CDK-like-3; CDKL3), was recently identified based on their biochemical and genetic structures. Their names refer to their sequence, which corresponds to the cdc2 PSTAIRE motif (5). No known cyclins are associated with cdc2-related kinases. Northern blot analyses revealed that human CDK10 is broadly expressed in normal and tumor tissues (6). In vitro kinase assays also showed that CDK10 can phosphorylate histone H1 and myelin basic protein (6). ETS2 is a CDK10-interacting protein identified by yeast two-hybridization screening (7). CDK10 is associated with the N-terminal domain of ETS2, which harbors the transactivation domain and the highly conserved point domain of ETS2. However, CDK10 is not associated with ETS1 (7). When CDK10 binds to ETS2, CDK10 can inhibit the transactivation activity of ETS2 (7). Several reports have suggested that CDK10 is involved in cell proliferation and differentiation. In human U2OS cells, the abrogation of the CDK10 signal may be achieved by using a kinase-dead form of CDK10 or antisense RNA-arrested cell cycle progression at the G2/M checkpoint (8). CDK10 was significantly down-regulated in retinoic acid-induced differentiation of retinoblastoma cells (9). Using two-dimensional gel electrophoresis and mass spectrometry analysis, Leman et al. (10) demonstrated the down-regulation of CDK10 in seminomas. CDK10 was shown to be up-regulated in follicular lymphomas compared with normal germinal center B-cells (11). CDK10 was also reportedly involved in endocrine therapy resistance in breast cancer (12). Using siRNA screening, Iorns et al. (12) found that changes in CDK10 expression can increase ETS2 transcriptional activity, elevate c-raf expression, and enhance MAPK activation, resulting in resistance to tamoxifen treatment.
Zebrafish is a widely used animal model in the study of genetic and developmental biology (13). To date, the role of CDKs in zebrafish development remains poorly understood. Zebrafish cyclin H/cdk7 is ubiquitously expressed throughout all examined stages and tissues (14). Abrogation of cyclin H expression results in defective development and apoptosis (14). Previous reports revealed that zebrafish cdk5 is ubiquitously and abundantly expressed in the brain and the eyes (15). Knockdown of zebrafish cdk5 by using siRNA decreases the number of primary sensory Rohon-Beard (RB) neurons in the trigeminal ganglia and promotes apoptosis in the brain (15, 16). Meanwhile, morpholino-based knockdown of cdk5 increases the number of supernumerary motor neurons (17). Recently, cdkl1 was reported to fulfill a function in zebrafish development. Interruption of cdkl1 expression decreases neurogenin-1 (ngn1) levels in the brain and sonic hedgehog expression in the floor plate region (18). In the present study, we used zebrafish as an animal model to investigate the developmental role of cdk10.
EXPERIMENTAL PROCEDURES
Reagents
All of the chemical reagents were purchased from Sigma or Mallinckrodt Baker. Antibodies against human CDK10, human ETS2, and GFP and HRP-conjugated goat anti-mouse IgG antibodies were purchased from Santa Cruz Biotechnology. The anti-β-actin antibody was obtained from Sigma. Anti-sox2 antibody (ab97959) was purchased from Abcam.
Maintenance of Zebrafish
The zebrafish AB strain and transgenic Tg (huC:gfp) fish were kind gifts from the Taiwan Zebrafish Core Facility (Academia Sinica, Taiwan) and Dr. Chang-Jen Huang (Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan), respectively. All of the fish were raised and maintained under standard conditions at 28.5 °C under a 14-h light/10-h dark cycle (19). The developmental stages of the embryos were determined according to methods described previously (20). All of the animal handling protocols were approved by the Chung Shan Medical University Animal Care Committee. Images of the fluorescent signals were captured using a Zeiss LSM510 confocal microscope.
RNA Extraction, Reverse Transcription-Polymerase Chain Reaction (RT-PCR), Quantitative Real Time PCR Analysis, and Plasmid Constructs
Total RNA was isolated from different tissues and stages in adult zebrafish using TRIzol (Invitrogen). First strand cDNA was synthesized from 3 μg of total RNA by Moloney murine leukemia virus reverse transcriptase (Promega) according to the manufacturer's instructions. The full coding region of cdk10 was amplified by RT-PCR using zebrafish cDNA as a template and the following primer set: forward, 5′-ATGGAGAGCACAGCAGACAC-3′; reverse, 5′-GTCATACTTTGCTCCTCTTGG-3′. The PCR was performed under the following conditions: 94 °C, 30 s; 52 °C, 60 s; 72 °C, 60 s for 30 cycles. The amplified products were then ligated into a pGEM-T-Easy vector (Promega) and transformed into competent cells. The pEGFP-cdk10 and pCS2-cdk10 were constructed by enzyme digestion and ligated into pEGFP-C1 and pCS2, respectively. Quantitative real time PCR was performed on an ABI 7000 Sequence Detection System (Applied Biosystems) with SYBR Green fluorescent label (Thermo Scientific). Primers for zebrafish raf1a (forward, 5′-AAGACATCAGCTCCTGCACA-3′; reverse, 5′-AGACGATCACTCTTTGAGGG-3′) were used. Gene expression levels were normalized to elongation factor 1α (ef1α) (forward, 5′-TGCCTTCGTCCCAATTTCAG-3′; reverse, 5′-TACCCTCCTTGCGCTCAATC-3′) and assessed using comparative CT (40 cycles) according to the manufacturer's instructions (Applied Biosystems). The expression level was normalized to ef1α and calculated using the ΔΔCT method.
TUNEL Assay
A TUNEL assay was performed according to the manufacturer's instructions (Promega). Briefly, the embryos (24 or 48 HPF) were fixed with 4% paraformaldehyde at 4 °C overnight, dehydrated by washing three times in methanol, and stored at −20 °C for at least 30 min. After rehydration, the embryos were transferred to tubes containing 100 μl of equilibration buffer and incubated at room temperature for 10 min. Next, the embryos were incubated with 100 μl of labeling solution at 37 °C for 1 h. After washing with 2× SSC buffer and phosphate-buffered saline (PBS), the TUNEL signals were examined using an upright fluorescence microscope (Zeiss AXioskop2).
Cell Culture and Transfection
Mouse neuroblastoma Neuro-2a and human embryonic kidney (HEK) 293 cells were obtained from ATCC and maintained in minimum essential medium supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 units/ml penicillin. The cells were maintained in a humidified atmosphere of 95% air and 5% CO2. Plasmid DNAs were transfected into Neuro-2a or HEK 293 cells using the Lipofectamine reagent (Invitrogen). Twenty-four hours after transfection, the cell lysates were collected.
Immunoblot Analysis and in Vitro Kinase Assay
Five hundred micrograms of total cell lysates derived from HEK 293 cells overexpressing GFP alone or the GFP-cdk10 fusion protein was subjected to immunoprecipitation using an anti-GFP antibody, and a kinase assay was performed as described previously (18, 21). After washing three times with kinase buffer (25 mm Tris, pH 7.5, 150 mm NaCl, 2 mm dithiothreitol, and 0.1 mm sodium orthovanadate), the precipitates were incubated with H1 histone in kinase buffer supplemented with 0.1 mm ATP, pH 7.0, 5 μCi of [γ-32P]ATP, and 10 mm MgCl2 at 37 °C for 10 min. After separation by electrophoresis with a 12.5% SDS-polyacrylamide gel, the phosphorylation status was determined by autoradiography of the dried gel.
Microinjection of Antisense Morpholino Oligonucleotides (MOs) and Synthetic mRNA
The following MOs were designed and synthesized by Gene Tools, LLC (Philomath, OR): cdk10 MO (5′-GGTCTGTGTCTGCTGTGCTCTCCAT-3′), which targets the start codon (from +1 to +25); 5-mismatch (5-mis) MO (5′-GGTGTCTGTCTCCTGTCCTGTCCAT-3′) where the underlines indicate the mismatched sites; and control MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′), which corresponded to the human β-globin gene and was used as a negative control. MOs were dissolved in 1× Danieau solution and injected into the embryos at the one- to four-cell stage. The pCS2-cdk10 plasmid was linearized by NotI, and the mRNA was synthesized by SP6 RNA polymerase using the mMESSAGE mMACHINE kit (Ambion) according to the manufacturer's recommendation. For the rescue experiments, morpholinos were co-injected with 11 pg of mRNA into one- to four-cell embryos.
Whole-mount in Situ Hybridization
Whole-mount in situ hybridization was performed as described previously (22). Digoxigenin-labeled antisense riboprobes were generated from a pGEM-T-Easy plasmid containing the full coding sequence and partial 3′-untranslated regions of cdk10. The plasmid was linearized using NotI. The RNA was transcribed in vitro by the SP6 RNA polymerase to generate antisense probes using the DIG-RNA labeling kit (Roche Applied Science).
Western Blot Analysis
Zebrafish embryos injected with the control MO, cdk10 MO, 5-mismatch MO, or cdk10 MO plus cdk10 mRNA were collected at 24 HPF. The total protein was extracted as described above. Thirty micrograms of protein was separated by SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with PBS containing 5% nonfat milk, washed with PBS buffer, and incubated with anti-human CDK10 antibody (Santa Cruz Biotechnology) overnight. Next, the membranes were incubated with an HRP-conjugated goat anti-mouse IgG antibody, and the signals were detected using an enhanced chemiluminescence kit (Millipore).
Co-immunoprecipitation
HEK 293 cells were transfected with 2 μg of pEGFP-cdk10 or pEGFP vector, and the cell lysates were collected as described above. Four hundred micrograms of proteins was subjected to immunoprecipitation using an anti-GFP antibody. The immunoprecipitated complexes were separated by electrophoresis on a 10% polyacrylamide gel followed by immunoblot analysis using anti-human ETS2 and anti-GFP antibodies. The signal was detected using an enhanced chemiluminescence kit (Millipore).
Anti-acetylated α-Tubulin Antibody Staining
Briefly, embryos at the indicated stages were collected, fixed in 4% paraformaldehyde at 4 °C overnight, and stored in methanol at −20 °C. After rehydration with PBST (PBS containing 0.1% Tween 20), the embryos were blocked with blocking solution (5% goat serum, 2% bovine serum albumin, and 1% DMSO in PBST) for 1 h and then incubated with monoclonal anti-acetylated α-tubulin at 4 °C overnight. Samples were washed with PBST and incubated with goat anti-mouse FITC-conjugated antibodies (1:500) in blocking buffer at 4 °C overnight in the dark. After washed with washing buffer (2% BSA and 1% DMSO in PBST), the images were captured by a scanning laser confocal microscope (Zeiss 510 META).
Statistical Analysis
Statistical analysis was performed by Student's t test using SPSS software (SPSS, Inc.). A p value <0.05 was considered statistically significant.
RESULTS
Identification of Zebrafish cdk10
We performed Basic Local Alignment Search Tool program analysis using the human CDK10 protein sequence to study the function of cdk10 in zebrafish. A gene (NCBI Reference Sequence accession number NM_001017622) that encodes the full-length zebrafish cdk10 was identified. Zebrafish cdk10 contains 1298 base pairs; encodes a polypeptide of 360 amino acid residues; and displays 78, 77, 77, and 76% identity to human, mouse, rat, and Xenopus orthologs, respectively. The kinase domain extended from residues 48 to 300, and the conserved PISSLRE motif was localized at amino acids 81–87 (Fig. 1A). On the basis of the phylogenetic analysis, zebrafish cdk10 was closer to Xenopus cdk10 (Fig. 1B).
FIGURE 1.
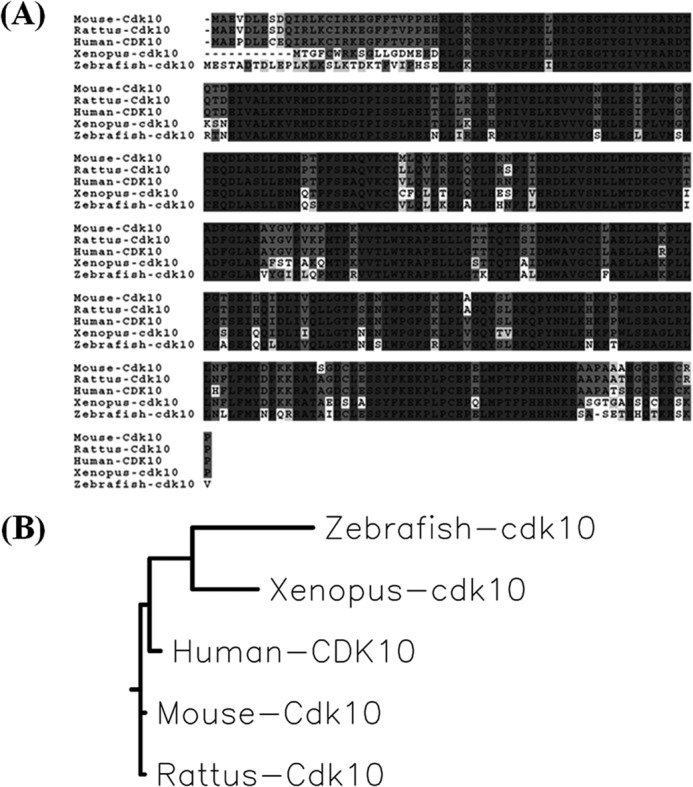
Protein sequence alignments and phylogenetic tree. A, sequence alignment of zebrafish cdk10 with that of human, rat, mouse, and Xenopus. Identical amino acids in all or three species are shaded in black and light gray, respectively. B, phylogenetic tree of zebrafish cdk10. The phylogenetic tree was created using the MegAlign program in DNASTAR using the neighbor joining algorithm.
Functional Characterization of cdk10
A previous report showed that human CDK10 interacts with the transcription factor ETS2 (7). We performed a co-immunoprecipitation assay to investigate whether cdk10 interacts with human ETS2. A strong ETS2 signal was detected in the complexes immunoprecipitated with GFP-cdk10 but not in those immunoprecipitated solely with GFP (Fig. 2A). These results demonstrated that cdk10 interacts with ETS2 and are similar to the results obtained with human and murine CDK10.
FIGURE 2.
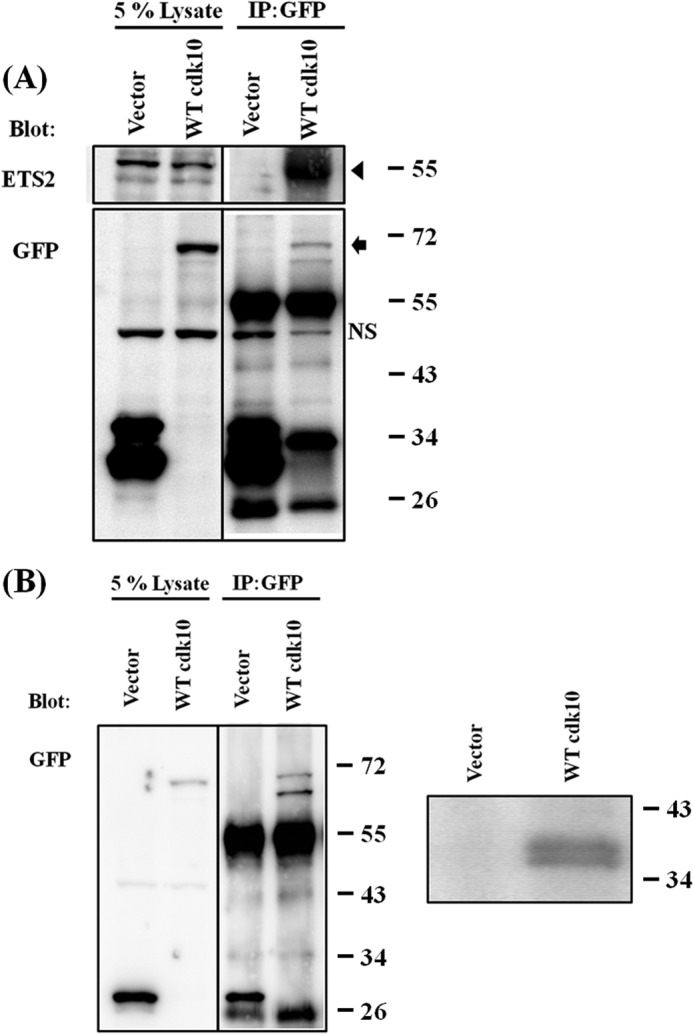
Characterization of zebrafish cdk10. A, zebrafish cdk10 interacts with ETS2. pEGFP or pEGFP-zcdk10 was transfected into HEK 293 cells. At 24 h post-transfection, cell lysates were collected and immunoprecipitated (IP) with an anti-GFP antibody. NS denotes nonspecific signal. The arrowhead indicates ETS2 signals. The arrow indicates GFP-cdk10 signals. B, in vitro kinase assay. pEGFP or pEGFP-zcdk10 was transfected into HEK 293 cells. GFP fusion proteins were immunoprecipitated with an anti-GFP antibody and subjected to an in vitro kinase assay using histone H1 as substrate. Left panel, immunocomplexes were analyzed using Western blot and anti-GFP antibody. Right panel, phosphorylated signals were detected by autoradiography.
We performed an in vitro kinase assay to confirm whether cdk10 exhibits kinase activity. The GFP-cdk10 fusion protein was overexpressed in the HEK 293 cell line and purified with an anti-GFP antibody. Histone H1 was phosphorylated by cdk10 in the presence of an isotope-labeled ATP (Fig. 2B). Similar to its mammalian ortholog, cdk10 was shown to interact with ETS2 and exhibit kinase activity during the phosphorylation of histone H1.
Temporal and Spatial Expression Patterns of cdk10
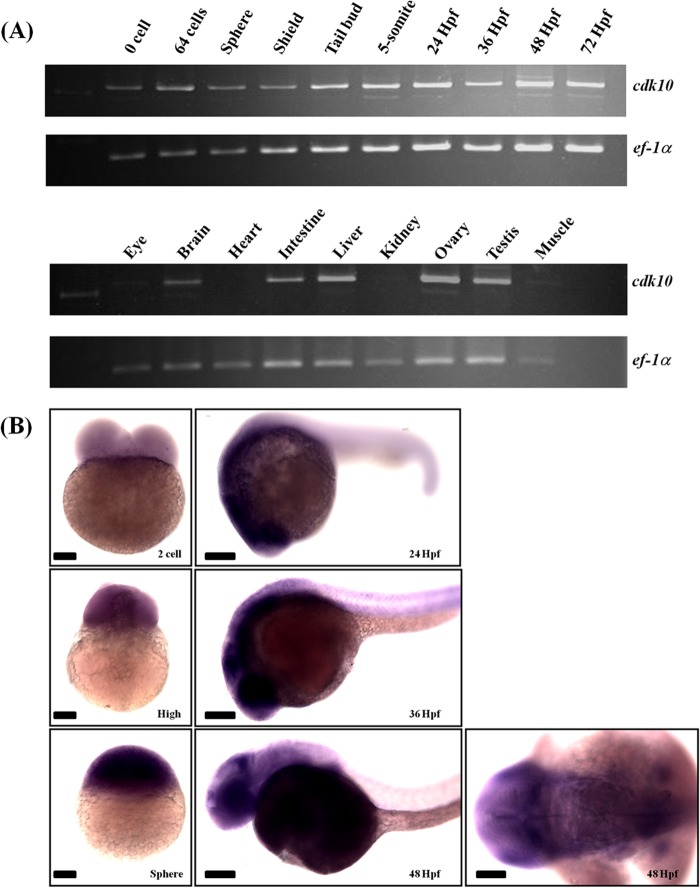
We conducted RT-PCR and whole-mount in situ hybridization to analyze the developmental expression patterns of cdk10. RT-PCR analysis showed that cdk10 was expressed in all examined zebrafish stages. Furthermore, cdk10 was predominantly expressed in the adult brain, intestine, liver, ovary, and testis. Whole-mount in situ hybridization revealed that cdk10 mRNA was ubiquitously expressed from the two-cell to sphere stages. At 24 and 36 HPF, cdk10 expression was relatively enriched in the eye and brain regions. At 48 HPF, cdk10 was predominantly expressed in the eye, tectum, hindbrain-midbrain boundary, and telencephalon (Fig. 3, A and B).
FIGURE 3.
Spatial and temporal expression pattern of zebrafish cdk10. A, RT-PCR analyses of cdk10 mRNA in different stages (upper panel) and different adult tissues (lower panel). Elongation factor 1α is used as internal control. B, whole-mount in situ hybridization analyses of cdk10 mRNA during zebrafish development. Zebrafish cdk10 is expressed both maternally and zygotically throughout development with relative enrichment in the brain. Stages are indicated in the lower right. Scale bars, 100 μm.
Developmental Role of cdk10
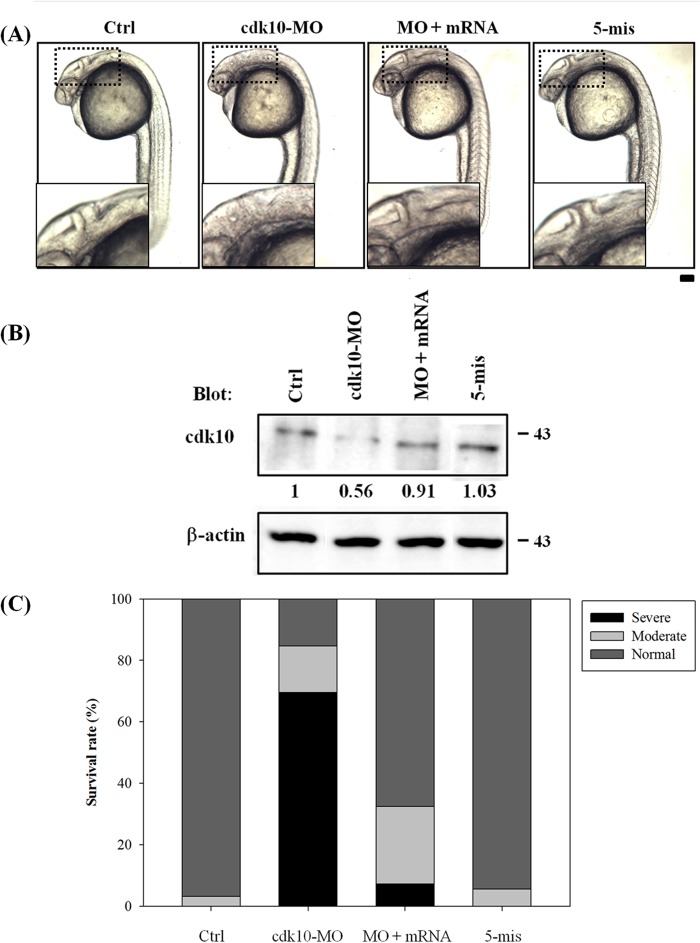
Antisense MOs that overlap with the translation start codon were designed to block the translation of the cdk10 gene and thereby explain the function of cdk10 during zebrafish development. To confirm whether cdk10 MO affects endogenous cdk10 expression, protein extracts from embryos receiving control MO, cdk10 MO, and 5-mis MO were subjected to Western blot analysis using anti-human CDK10 antibody. As shown in Fig. 4B, 1 ng of cdk10 MO reduced the amount of cdk10 protein expression by 44%. However, no clear changes in cdk10 expression were observed in embryos that received control MO or 5-mis MO. These results indicated that cdk10 MO specifically blocks the translation of the endogenous cdk10 gene. A previous report found that the microinjection of specific MOs induces off-target effects by increasing p53 activity, resulting in cell death (23). These off-target effects were avoided in this study by using specific MOs that were co-injected with 2-fold amounts of p53 MO in all MO experiments. To determine whether p53 MO contributes to the phenotype of cdk10 morphants, we injected the embryos with p53 MO alone. Indeed, no distinct phenotype was observed between the p53 morphants and the control embryos (data not shown). At 24 HPF, embryos receiving 1 ng of cdk10 MO exhibited significant developmental abnormalities such as small brain, undersized eye, and somite curvature. These embryos also lacked the isthmus region. The percentages of normal, moderate, and severe phenotypes (characterized by smaller brain size, undersized eyes, and curved somites) are presented in Fig. 4C. Developmental defects observed in cdk10 morphants as well as endogenous cdk10 protein level could be rescued by co-injection of cdk10 mRNA, demonstrating the specificity of this morpholino (Fig. 4, A and B). Dark cell masses observed in cdk10-depleted embryos indicated that these morphants underwent cell death. To verify this hypothesis, we performed a TUNEL assay to examine the embryos for apoptosis. Compared with the control MO-injected embryos, depletion of cdk10 sharply increased the number of TUNEL-positive cells in the dorsal neuronal regions at the three-somite stage (in the brain at 24 HPF as well as the forebrain and the midbrain regions at 48 HPF). Co-injection with cdk10 mRNA significantly reduced apoptosis (Fig. 5, A–C). These findings show the significance of cdk10 during development in zebrafish.
FIGURE 4.
Morphological phenotype of cdk10 morphants. A, phenotypic analyses of zebrafish embryos at 24 HPF. Embryos at one- to four-cell stages that received 1 ng of control MO (Ctrl), 1 ng of cdk10 MO, 1 ng of cdk10 MO plus 10 pg of cdk10 mRNA (MO + mRNA), and 1 ng of 5-mismatch cdk10 MO (5-mis) are depicted at 24 HPF. Compared with the normal morphology of the wild type (WT), cdk10 morphants exhibited head malformations with indistinct boundaries between the brain subdivisions as shown in the small insets. Images are shown in the lateral view with the anterior at the left. B, Western blot analysis of cdk10 knockdown efficiency. Embryos injected with the indicated MO were harvested at 24 HPF. Protein samples were prepared and probed using a human CDK10-specific antibody. β-Actin was used as the loading control. Data represent one of at least three independent experiments. C, percentages of normal, moderate, and severe phenotypes in the WT control (n = 159), cdk10 MO (n = 193), MO + mRNA (n = 279), and 5-mis MO (n = 178) embryos. Scale bar, 100 μm.
FIGURE 5.
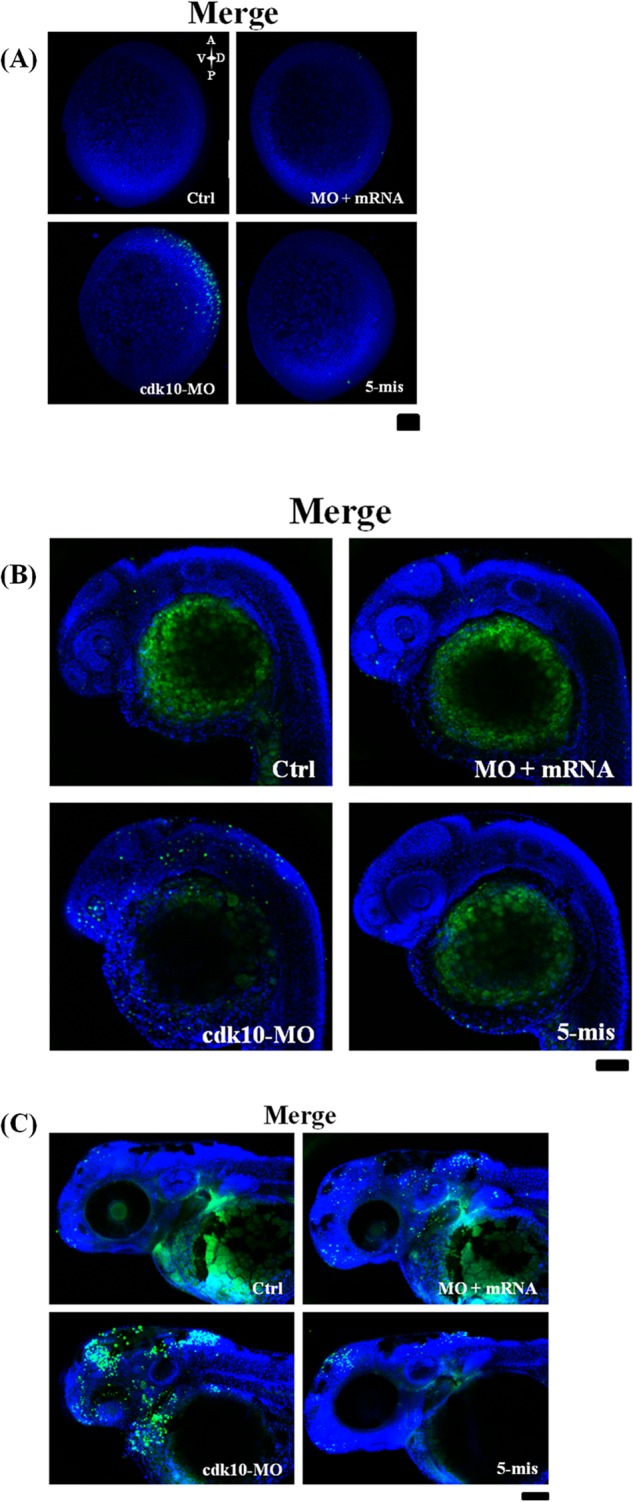
Knockdown of cdk10-induced apoptosis. A TUNEL assay was used to detect cell apoptosis in zebrafish embryos that received the indicated MO in the tail bud stage (A), at 24 HPF (B), or at 48 HPF (C). Control MO (Ctrl)-injected embryos displayed very few TUNEL-positive cells. The cdk10 MO-injected embryos that were primarily concentrated in the forebrain, midbrain, neural plate, and retina regions showed a high level of apoptosis. Apoptosis was prevented by co-injection of cdk10 mRNA. All images are shown in the lateral view with the anterior at the left (n = 10 embryos each). Scale bars, 100 μm. A, anterior; V, ventral; D, dorsal; P, posterior.
Knockdown of cdk10 Abrogates Several Central Nervous System (CNS) Marker Expression
We examined the expression of sox2 (a known neural stem cell marker) and ngn1 (a marker of differentiating neurons at the three-somite stage) to evaluate the effect of cdk10 on neural development. Depletion of cdk10 resulted in expansion and reduction of sox2 expression compared with wild-type control embryos (Fig. 6A). Loss of cdk10 reduced ngn1 expression in dorsal neuron precursor cells (Fig. 6B). At 24 HPF, morphant embryos showed a significant disruption of ngn1 expression in the forebrain and the cranial ganglia (Fig. 6C). Significant reductions in zath3 (a late proneural gene) and nes (a marker for stem cells) were observed in cdk10 morphants (Fig. 6D). In addition, wnt1 (alar plate midbrain region) expression was significantly reduced in the dorsal midline neurons of cdk10-depleted morphants at 24 HPF (Fig. 6E). We subsequently determined whether forebrain patterning events were disrupted in cdk10 mutants. The expression of emx1 (a marker involved in telencephalic patterning) was markedly reduced in cdk10 morphants (Fig. 6F). The expression of islet2 (a dorsal root ganglion marker) was significantly reduced in RB neurons, whereas motor neurons were not affected in cdk10 morphants (Fig. 6G). These findings suggest the significance of cdk10 in the formation of forebrain and midbrain as well as RB neurons.
FIGURE 6.
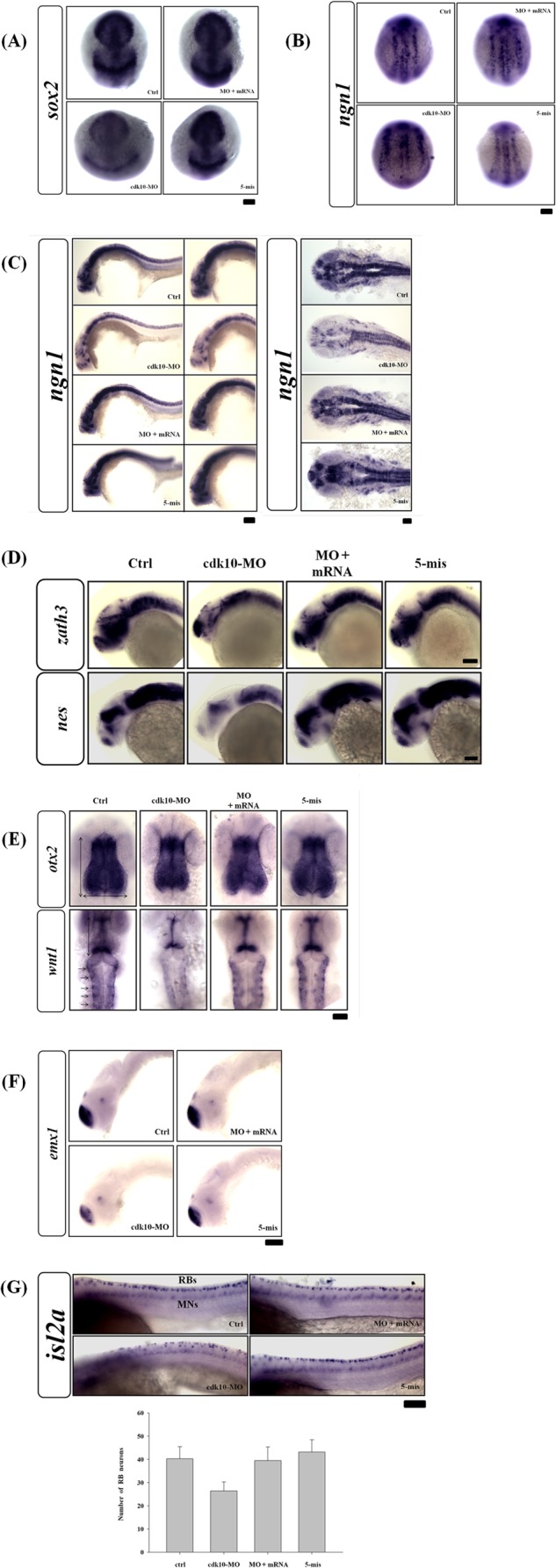
Knockdown of cdk10-impaired zebrafish neuronal development and affected RB sensory neuronal population. In preparation for whole-mount in situ hybridization with specific neuronal markers, embryos that received the indicated MO with or without corresponding RNA were collected at the tail bud stage (A and B) and at 24 HPF (C–F). A and B, depletion of cdk10 induced a local decrease and expansion of the neural plate markers sox2 and ngn1. C, the cdk10 morphants significantly disrupted the ngn1 expression in the CNS (right and middle panels, lateral view; left panel, dorsal view). D, lateral views of whole-mount in situ hybridization detecting zath3 and nes in embryos at 24 HPF. Expression of zath3 and nes was altered in cdk10 morphants. These defects can be complemented by co-injection of cdk10 mRNA. E, loss of wnt1 expression in dorsal midline neurons (denoted with an arrow) was found in cdk10 morphants, whereas no apparent change in otx2 expression was observed (dorsal view). F, the expression of the telencephalic marker emx1 was reduced in cdk10 morphants (lateral view). G, the number of RB neurons (RBs) was decreased in cdk10 morphants as shown by the decrease in islet2 expression. Conversely, the number of motor neuron cells (MNs) was not affected in cdk10 morphants. Lower panel, quantitative analyses of islet2-positive RB neurons. Data were obtained from at least three independent experiments. Scale bars, 100 μm. Ctrl, control. Error bars represent S.D.
To determine the role of cdk10 during neurodevelopment, we conducted acetylated α-tubulin (AcTub) staining to determine whether disruption of cdk10 affects neuronal differentiation. At 24 HPF, AcTub staining revealed the presence of anterior commissure, postoptic commissure, supraoptic tract, nucleus of the tract of anterior commissure, dorsal-ventral diencephalic tract, epiphyseal cluster, and tract of the posterior commissure in the forebrain and the midbrain in the embryos injected with control and 5-mis MOs (Fig. 7A). Axonal scaffolds in cdk10 morphants visibly deteriorated. Reduced arborization was evident in several neurons, including those in the spinal cord (Fig. 7B). Embryos co-injected with cdk10 mRNA resembled those of the control group. Similar results were also observed at 48 HPF at which time the staining patterns of the tectal neurons, hindbrain neurons, and tubulin-labeled axons were severely reduced in cdk10 morphants. Co-injection with specific cdk10 mRNA restored these defects (Fig. 7C). Therefore, depletion of cdk10 caused abnormalities in the number of neurons and in axonal pathfinding, indicating that cdk10 is involved in the proper maintenance of neurons.
FIGURE 7.
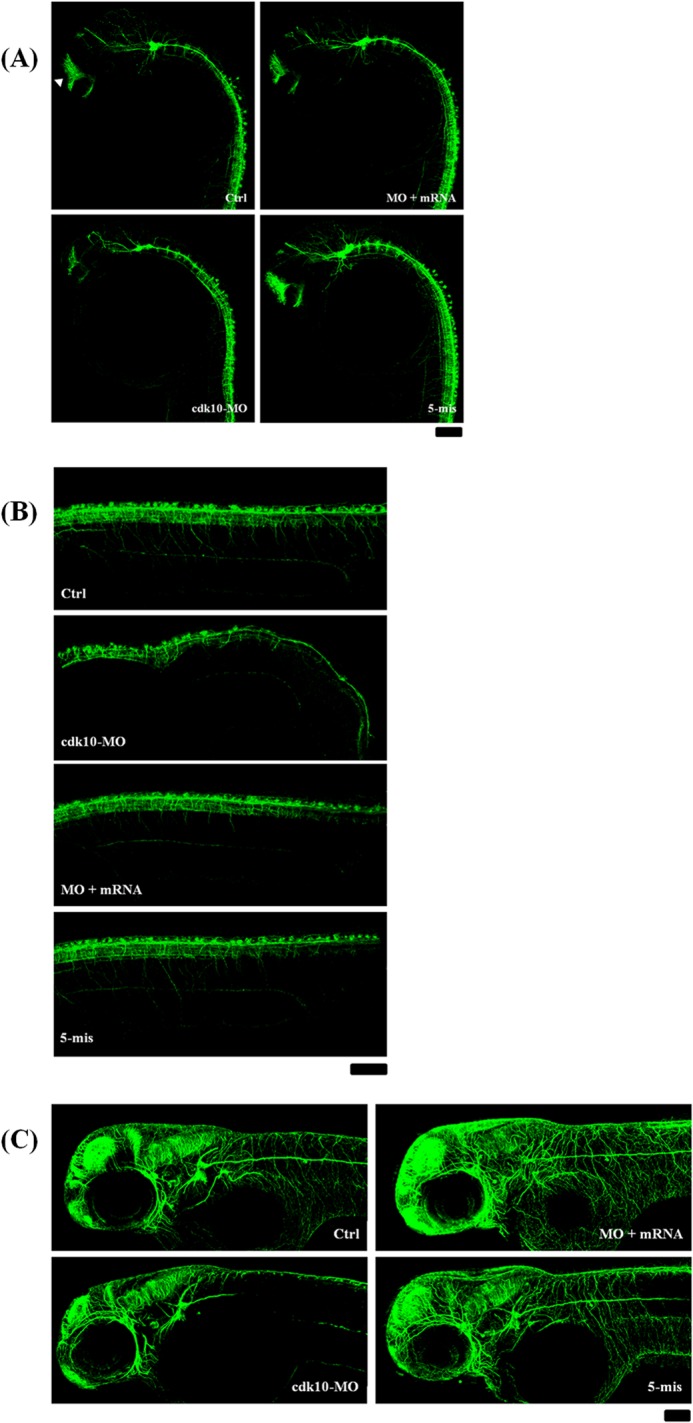
Effects of cdk10 depletion on neurogenesis as visualized by AcTub staining. AcTub expression (an axonal marker) was examined using confocal microscopy at 24 (A and B) and 48 HPF (C). A, axonal scaffolds in cdk10 morphants were visibly deteriorated. The arrowhead indicates anterior commissure. B, a decrease in the number of dorsal neurons, including RB neurons, was observed in cdk10 morphants. C, the staining patterns of the tectal neurons, hindbrain neurons, and tubulin-labeled motor axons were severely reduced in cdk10 morphants at 48 HPF. All images are shown in the lateral view. Scale bars, 100 μm. Ctrl, control.
We also used the Tg (huC:gfp) model to track the generation of terminally differentiated neurons. A lateral view of the embryos at 24 HPF revealed that the control, 5-mis MO, and cdk10 MO plus cdk10 mRNA groups exhibited high levels of huC expression in the telencephalic cluster and neural tube. We observed that cdk10 morphants exhibited abrogated huC expression in the forebrain region and a significantly decreased huC population in the neural tube throughout the spinal cord axis (Fig. 8, A and B). At 48 HPF, only a small number of huC-positive neurons were present in the tectum and hindbrain regions of cdk10 morphants (Fig. 8C). A dorsal view of the embryos revealed that cdk10 morphants also exhibited severe defects of neuronal organization in the olfactory bulb, telencephalic cluster, and retinal ganglion (Fig. 8D). Thus, cdk10 knockdown in zebrafish embryos affects neuronal development in the brain.
FIGURE 8.
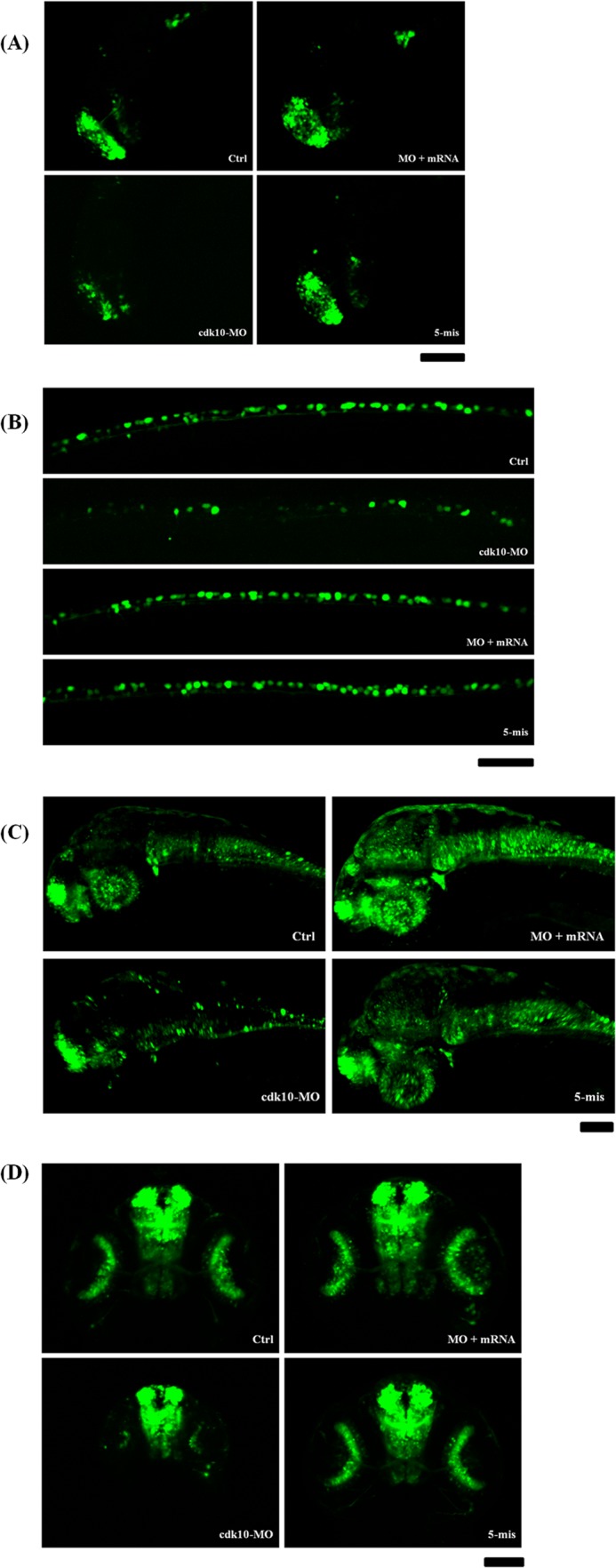
Effects of cdk10 depletion on neurogenesis on the basis of the Tg (huC:gfp) model. Embryos that received the indicated MO were collected at 24 (A and B) and 48 HPF (C and D). A, the huC expression in the telencephalon decreased in cdk10 morphants. Reduced numbers of RB neurons were also observed in cdk10 morphants. C and D, the cdk10 morphants exhibited abrogated huC expression in the forebrain region and a significantly decreased huC population of neurons in the retina. A–C, lateral view. Scale bars, 100 μm. Ctrl, control.
Zebrafish cdk10 Did Not Directly Contribute to the Cell Cycle
The results showed that cdk10 knockdown induced cell apoptosis (Fig. 5) and hindered CNS development (Figs. 7 and 8). This phenomenon of cell death may result in compensatory proliferation, thereby influencing cell cycle distribution and abrogation of differentiation. This finding is consistent with the results of previous studies on Drosophila (24), chicken (25), and zebrafish embryos (26). We then performed in vitro and in vivo assays to assess cell cycle progression using gain and loss of cdk10 function. HEK 293 cells with or without cdk10 overexpression were collected and then analyzed for populations of cell cycle by flow cytometry. Our findings revealed no apparent change in cell cycle distribution in the cells transfected with the control or cdk10 (Fig. 9A). Despite the cdk10 knockdown, the transcript levels of cdk inhibitors such as cdkn1b, cdkn1c, ccnd1, and PCNA (a proliferation marker), were similar to the control in the CNS region (Fig. 9B). This result indicates that zebrafish cdk10 did not elicit apoptosis-associated compensatory proliferation.
FIGURE 9.
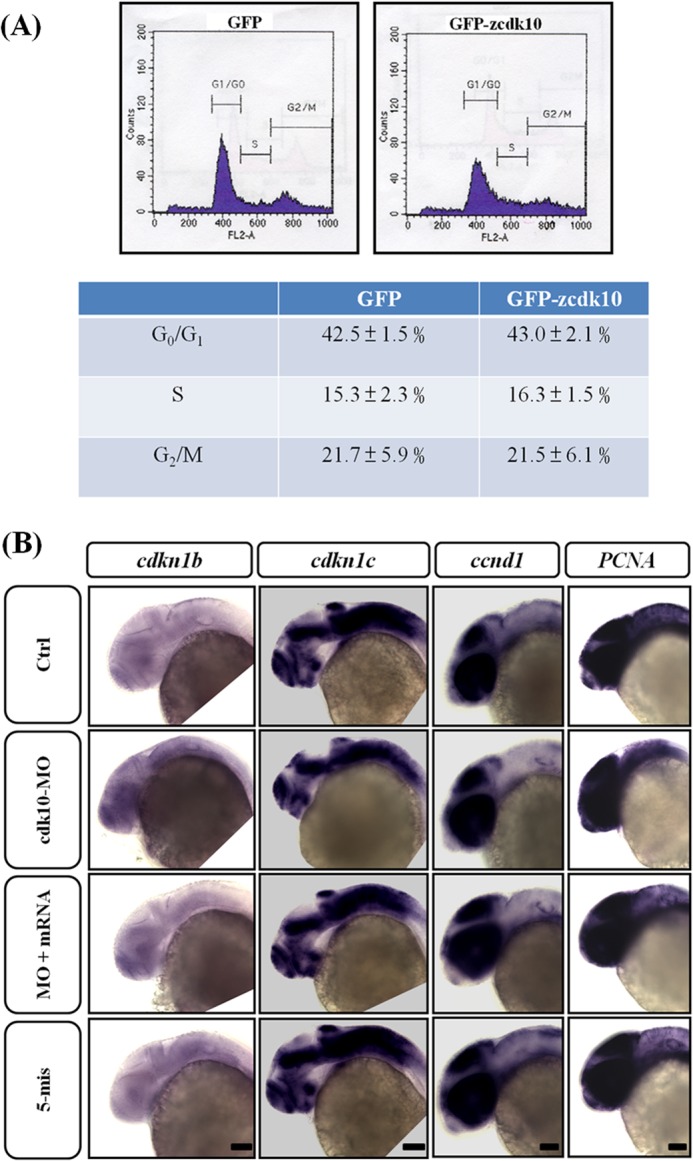
Zebrafish cdk10 did not participate in cell cycle progression in vitro and in vivo. A, HEK 293 cell were transfected with pEGFP or pEGFP-cdk10. At 24 h post-transfection, cells were collected and subjected to flow cytometric analysis. The lower panel indicates the percentages of cells in the G0/G1, S, and G2/M phases of the cell cycle. B, lateral views of cdkn1b, cdkn1c, ccnd1, and PCNA expression in embryos injected with control (Ctrl), cdk10 MO, MO + mRNA, and 5-mis MO at 24 HPF. Knockdown of cdk10 showed no apparent difference in cdkn1b, cdkn1c, ccnd1, and PCNA mRNA transcription, suggesting that cdk10 deficiency exhibited no influence on the progression of the cell cycle. Scale bars, 100 μm.
Decrease in Differentiation of Retinal Cells in cdk10 Morphants
Using the Tg (huC:gfp) model, we observed fewer retinal ganglion cells (RGCs) in cdk10 MO-injected embryos. We analyzed the expression of zath5, which is expressed in a wavelike fashion in the inner retina before the retinal ganglion cell differentiation (27). The expression of zath5 in the control retina was found around the retina to the ventrotemporal region at 36 HPF, whereas the zath5 signal was observed in differentiated RGCs near the ciliary region in normal retinas at 48 HPF. However, the spread of zath5 expression from the ventral to the dorsal region was significantly repressed at 36 HPF. Although retinas of cdk10 morphants seemed to have high levels of zath5 expression, most zath5-expressing cells were located adjacent to the optic stalk even at 48 HPF. Embryos co-injected with cdk10 mRNA resembled those of the control group. In addition, brn3, which is the downstream factor of zath5 (28), exhibited severely diminished expression in the ganglion cell layer (Fig. 10, A and B). This result indicates that the onset of zath5 expression was not delayed. However, fewer neurons were subsequently generated. Thus, cdk10 is required during RGC production.
FIGURE 10.
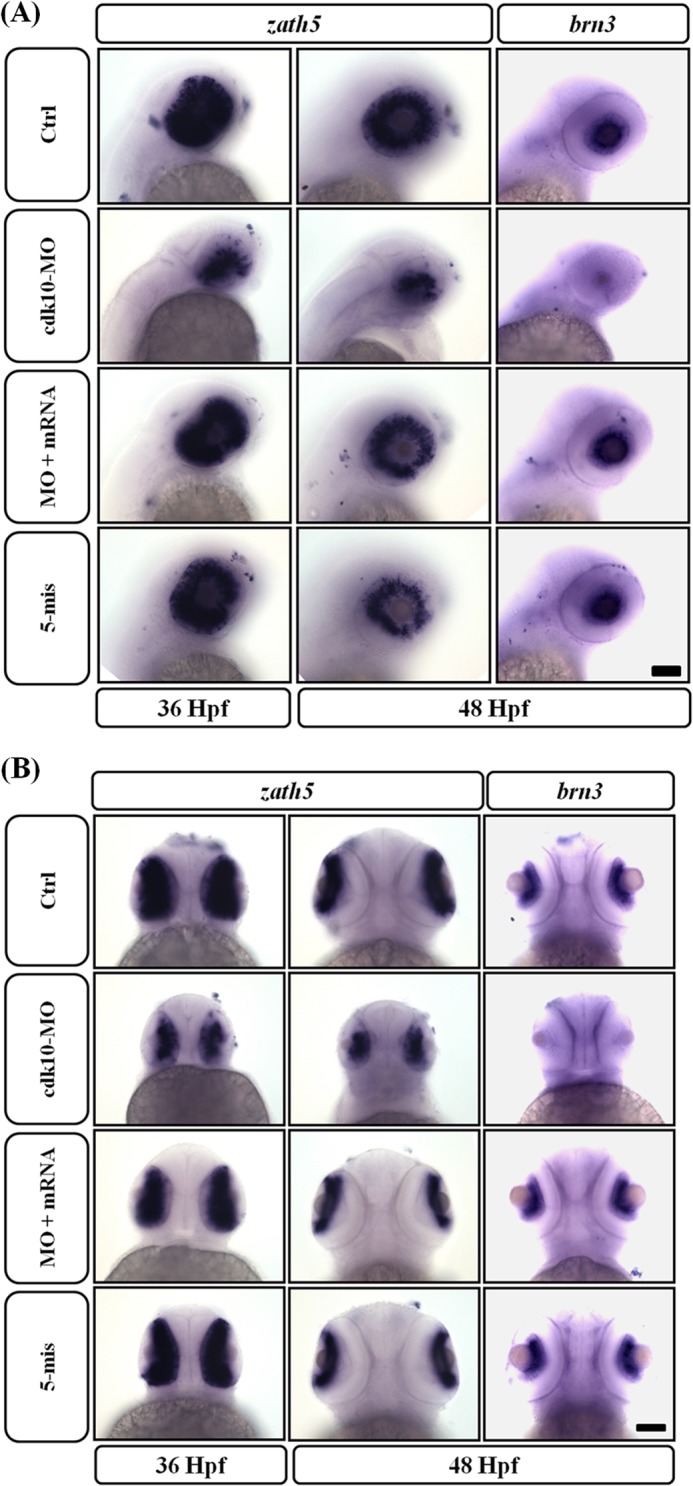
Changes in cdk10 levels impaired RGC differentiation. A and B, expression of zath5 (36 and 48 HPF) and brn3 (48 HPF) mRNAs was examined by whole-mount in situ hybridization. At 36 HPF, zath5 is undetected except in a small number of cells in cdk10-deficit embryos. At 48 HPF, the ciliary margin strongly expressed the zath5 signal in the control retina, whereas the zath5 signal is expressed near the optic stalk in the cdk10 morphant retina. The expression of the downstream effector brn3 is reduced severely at 48 HPF. Scale bars, 100 μm. Ctrl, control.
Zebrafish cdk10 Protects Neural Progenitor Survival
We evaluated whether the apoptotic cells observed in cdk10 morphants were NPCs. We collected embryos at 80% epiboly and conducted immunostaining with fluorescent sox2 antibody prior to TUNEL assay. In both early and late stages, most apoptotic cells were co-localized with sox2-positive cells in the anterior neural plate and throughout the axis of CNS in cdk10 morphants. Co-injection with specific cdk10 mRNA evidently reduced apoptosis, indicating that the apoptotic cells in cdk10 morphants are NPCs (Fig. 11, A and B). By contrast, p53 morphants presented no obvious TUNEL-positive signals in the early stages. These results show that the abrogation of cdk10 may cause severe damage during NPC maintenance and subsequently disrupted neurogenesis.
FIGURE 11.
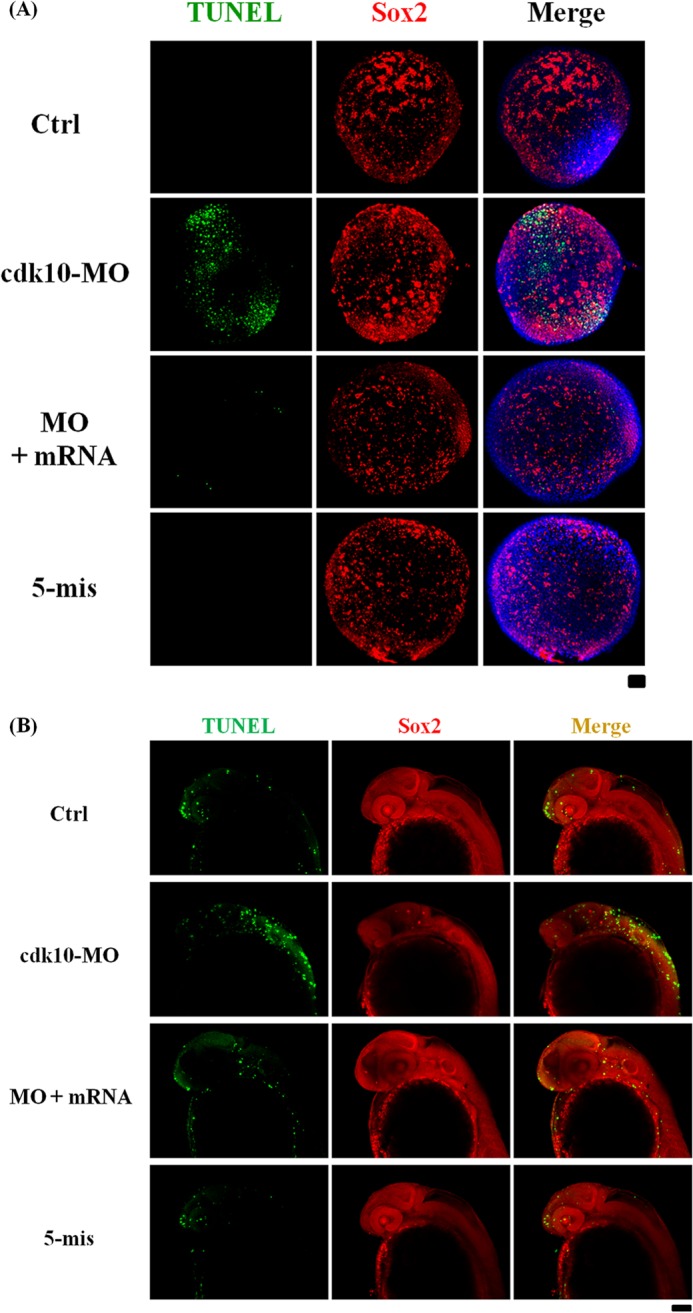
Depletion of cdk10 induced NPC apoptosis. Fluorescent images of TUNEL staining (in green) and sox2 expression (in red) in control (Ctrl), cdk10 MO, MO + mRNA, and 5-mis MO in embryos at 80% epiboly (A) and 24 HPF (B). TUNEL- and sox2-positive cells were co-localized (in yellow), representing apoptotic NPCs (n = 20 embryos each). Scale bars, 100 μm.
raf1a Is Involved in cdk10-dependent Neuron Progenitor Survival
Previous studies reported that loss of CDK10 induces ETS2-dependent RAF1 gene expression, thereby activating the mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)-extracellular signal-regulated kinase 1/2 (ERK1/2) pathway (12). This finding suggests that the zebrafish raf1a ortholog is also regulated by cdk10. To verify this hypothesis, we analyzed the transcriptional level of raf1a in cdk10-deficient embryos. The depletion of cdk10 markedly elevated the transcription of raf1a (Fig. 12A). Co-injection with cdk10 mRNA reduced raf1a expression to the basal level. No apparent alternation of raf1a expression was found in the embryos that received 5-mis MO. Embryos were co-treated with the MEK inhibitor U0126 in the presence of cdk10 MO, and the expression of sox2 and ngn1 were determined to assess whether the raf1a involved in cdk10 depletion induced NPC loss and aberrant neural differentiation. The knockdown of cdk10 sharply decreased sox2 expression at 80% epiboly. In addition, ngn1 expression was significantly lower compared with the control at the tail bud stage and 24 HPF. Co-injecting cdk10 mRNA restored sox2 and ngn1 expression. Interestingly, treatment with U0126 in cdk10 morphants recovered sox2 and ngn1 expression to the same level as that of the control groups. By contrast, treatment with U0126 alone reduced sox2 and ngn1 expression, suggesting that basal levels of ERK1/2 activities were still required for normal neurogenesis (Fig. 12B). Our collective findings revealed that raf1a rescued the impaired NPC development caused by cdk10 deficiency.
FIGURE 12.
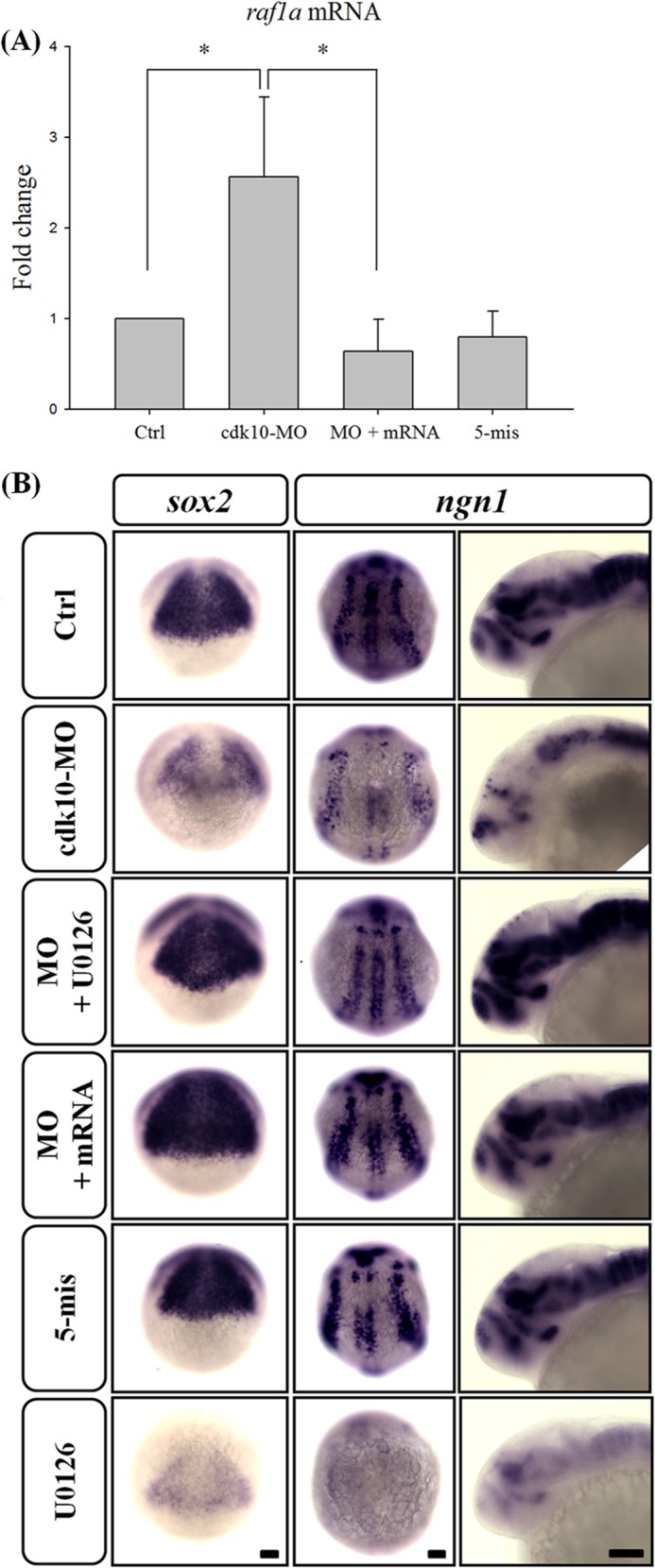
Knockdown of cdk10 increased the mRNA level of raf1a, contributing to the impairment of NPC development. A, quantitative RT-PCR of the raf1a gene in control (Ctrl), cdk10 MO, MO + mRNA, and 5-mis MO in embryos at 24 HPF. Expression was normalized to ef1α. Knockdown of cdk10 significantly enhanced the transcription of raf1a. *, p < 0.05. Error bars represent S.D. B, embryos received the indicated MO with or without the MEK inhibitor U0126 and were collected at 80% epiboly, tail bud stage, or 24 HPF. Embryos were then subjected to whole-mount in situ hybridization using sox2 or ngn1 riboprobe. The severe diminution of sox2 and ngn1 expression observed in the cdk10 morphant was effectively eliminated by adding 10 μm U0126. Scale bars, 100 μm.
DISCUSSION
CDK10 is a serine/threonine protein kinase from the CDK family (5). Recent studies suggested that human CDK10 functions as a tumor suppressor gene, preventing cell proliferation in breast cancer (12), biliary tract cancer cells (29), and hepatoma (30). However, the developmental function of CDK10 remains unclear. We present evidence that zebrafish cdk10 is involved in NPC survival. The disruption of cdk10 expression by morpholinos caused defects in the brain by triggering apoptosis in the brain and the dorsal neurons at 24 and 48 HPF, respectively. Whole-mount in situ hybridization revealed the down-regulation of several neuronal markers such as ngn1, emx1, wnt1, and islet2 in morphants. Co-injection with cdk10 mRNA rescued these phenotypes. Acetylated tubulin staining and Tg (huC:gfp) zebrafish demonstrated that the disruption of cdk10 expression alters telencephalic patterns and decreases RB neurons and RGCs. The results of this study show that cdk10 is an essential gene for zebrafish during normal embryogenesis.
The cdk10 mRNA transcripts were diffusedly expressed in the early stages (prior to the sphere stage) and more abundant in the brain at 24 and 48 HPF. The progressively restricted pattern of cdk10 expression was similar to that of other cdk and cdk-related genes in zebrafish. The cdk7/cyclin H and cdkl1 genes are ubiquitously distributed before gastrulation (14, 18). At 24 HPF and thereafter, cdk7 and cyclin H are primarily expressed in the brain, eye, and heart (14). The expression of cdkl1, which is another cdc2-related protein kinase, is restricted to the floor plate, pronephric ducts, and hypochord at 12 HPF (18). These cdks may exhibit redundant functions during the early developmental stage and perform specific functions during late developmental stages.
Specific morpholinos were designed to knock down cdk10 to assess the developmental role of cdk10 in zebrafish. A previous report showed that morpholinos may cause off-target effects that result in neuronal apoptosis because of p53 activation (23). To prevent off-target effects, p53 MO was co-injected with cdk10 MO into zebrafish embryos. The knockdown of cdk10 caused no obvious abnormalities. However, small brains and eyes as well as slight pericardial edema were observed in the morphants. Interestingly, impaired cdk10 expression resulted in apoptosis in the dorsal regions at the tail bud stage as well as the dorsal neurons and anterior brain regions where cdk10 is abundantly expressed. These findings were consistent with the whole-mount in situ hybridization data. The expression of sox2, which is a neural progenitor marker, was found to have expanded and decreased in cdk10 morphants. Reduced expression of ngn1, emx1, and wnt1 in cdk10 morphants was evident. Expression of a dominant negative form of cyclin H, which decreases cdk7 activities, results in apoptosis in the early stage of zebrafish embryos (14). Previous reports demonstrated the down-regulation of zebrafish cdk5 by using siRNA, which leads to apoptosis in the brain and a subsequent reduction in the number of primary sensory neurons in the trigeminal ganglion (15, 31). The present study shows that cdk10 is essential for cell survival in zebrafish.
At 24 HPF, in situ hybridization of embryos by using an RNA probe against islet2 indicated that reduction in the number of RB neurons was more significant in embryos injected with cdk10 MO than in those injected with control MO. AcTub staining revealed a large reduction in the number of RB neurons along the body axis in cdk10 morphants. RB neurons are primary sensory neurons that gradually die within the first 2 days of development (32). A previous report showed that cdk5 may mediate RB neuronal survival. The knockdown of zebrafish cdk5 by specific siRNAs decreased the number of RB neurons in the spinal cord (15). Phosphorylation of dihydropyrimidinase-like proteins 2 and 3 (DPYSL2 and DPYSL3) by cdk5 and the dual specificity tyrosine-phosphorylated and -regulated kinase (DYRK2) were essential in the proper localization of RB and neural crest cells during neurulation in zebrafish development (33). Conversely, cdk5 knockdown using specific MOs induced motor neuron development (17). Exposure to perfluorooctane sulfonate resulted in motor neuron defects in zebrafish that may be attributed to increased cdk5 expression (34). However, no apparent changes in motor neuron expression were observed in cdk10 morphants. Our findings show that cdk10 exhibits a significant function in the development of the spinal sensory but not the motor nervous system. However, further investigation must be conducted to determine whether DPYSL2 and DPYSL3 are also downstream targets of cdk10.
Human CDK10 significantly affects the progression from the G2/M phase of the cell cycle (8). Overexpression of human CDK10 inhibits the proliferation of human biliary tract cancer (29) and hepatocellular carcinoma (30). By contrast, zebrafish cdk10 is not involved in G2/M phase progression. No apparent change in G2/M phase population was observed in cells overexpressing rodent cdk10 (35). An RNA probe of cdkn1b, cdkn1c, and ccnd1 revealed no considerable effects in cdk10-deficient embryos. These observations show that cdk10 exhibits various functional activities in cell cycle progression that differ between human and zebrafish.
RGCs, the output neurons of the retina, contribute to a topographical spatial code that corresponds with their position within the retinal compartments and in higher visual centers of the brain (36). A recent study suggested that elevation of phospho-NR2A(Ser-1232) by Cdk5/p35 contributes to the apoptotic death of RGCs in experimental glaucoma rats (37). In an in vivo optic nerve crush model, the combined treatment with the cdk5 inhibitor indolinone A and the Rho-associated protein kinase inhibitor Y-27632 produced a strong stabilizing effect on neuronal survival (38). Administration of the CDK4 inhibitor reduced ischemia-reperfusion injury-induced neural apoptosis (39). By contrast, cdk5 was found to support neuronal survival by Bcl-2 phosphorylation (40) and significantly influence the extension and maintenance of axons as well as the stability and steering of RGCs (41). Administration of the pan-CDK inhibitor AG-012986 also induced apoptosis in retina cells (42).
A previous report showed that depletion of CDK10 expression led to elevated Raf1 levels, consequently activating the MEK-ERK1/2 pathway (12). According to its duration and magnitude, Raf-MEK-ERK1/2 signaling controls diverse cell responses such as proliferation, differentiation, and cell death (29). In the nervous system, the Raf-MEK-ERK1/2 pathway affects the genesis of neural progenitors (29). Treatment with the ERK1/2 inhibitor PD98059 prevented traumatic brain injury in the hippocampus by suppressing ERK1/2 activation (30). Similarly, treatment with U0126 rescued the glutamate-induced neuronal injury by suppressing ERK1/2 activation (43). Death of striatal neurons by dopamine induction has been attributed to ERK1/2 activation (44). Yang et al. (45) recently demonstrated that Raf-MEK-ERK1/2 enhancement causes neurogenesis defects in cultured cortical neurons. Similarly, the present study demonstrated that the knockdown of cdk10 expression triggered raf1a expression and decreased expression of neuron progenitor markers such as sox2 and ngn1. Nevertheless, the exposure of embryos to U0126 alone under a non-lethal dosage severely diminished the transcriptional level of sox2 and ngn1, indicating the significance of raf1a in maintaining normal neurogenesis. Moreover, Raf1 exhibited a neuroprotective ability during serum withdrawal (46). Inhibition of Raf activities led the otic epithelia to undergo apoptosis, thereby reducing the neurogenesis of the acoustic-vestibular ganglion (47). The results of the present study collectively suggest that fine-tuning the modulation between cdk10 and the Raf-MEK-ERK1/2 axis is necessary for NPC survival.
Thus, the results of ours study emphasize that cdk10 may positively regulate NPC development. Changes in cdk10 expression led to NPC apoptosis in the early stages and subsequent impairment in differentiating neurons. Whole-mount in situ hybridization revealed a significant reduction in the number of RB neurons in cdk10 morphants. Tg (huC:gfp) zebrafish revealed a decrease in the number of RB neurons and RGCs at 24 and 48 HPF. The blocked cdk10 expression elicited an increase in the raf1a level, consequently decreasing NPC survival, whereas the MEK inhibitor reversed these phenomena. In summary, our findings show that cdk10 significantly affects NPC survival by Raf-MEK-ERK1/2 pathway modulation.
Acknowledgments
We thank the Zebrafish Core in Academia Sinica, Institute of Cellular and Organismic Biology, which is supported by National Science Council (NSC) Grant NSC-99-2321-B-001-027, the Taiwan Zebrafish Core Facility for providing the zebrafish AB strain and Tg (huc:gfp), and the National Health Research Institute, which is supported by NSC Grant 100-2321-B-400-003. Upright fluorescence microscopy was performed in the Instrument Center of Chung Shan Medical University, which is supported by the National Science Council, Ministry of Education, and Chung Shan Medical University.
This work was supported by National Science Council of Taiwan Grant NSC-101-2311-B-040-001.
- CDK
- cyclin-dependent kinase
- MO
- morpholino oligonucleotide
- RB
- Rohon-Beard
- RGC
- retinal ganglion cell
- nes
- nestin
- CDKL
- CDK-like
- ngn1
- neurogenin-1
- ef1α
- elongation factor 1α
- HPF
- h postfertilization
- AcTub
- acetylated α-tubulin
- DPYSL
- dihydropyrimidinase-like protein
- 5-mis
- 5-mismatch
- PCNA
- proliferating cell nuclear antigen.
REFERENCES
- 1. Aouadi M., Binetruy B., Caron L., Le Marchand-Brustel Y., Bost F. (2006) Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie 88, 1091–1098 [DOI] [PubMed] [Google Scholar]
- 2. Deacon E. M., Pettitt T. R., Webb P., Cross T., Chahal H., Wakelam M. J., Lord J. M. (2002) Generation of diacylglycerol molecular species through the cell cycle: a role for 1-stearoyl,2-arachidonyl glycerol in the activation of nuclear protein kinase C-βII at G2/M. J. Cell Sci. 115, 983–989 [DOI] [PubMed] [Google Scholar]
- 3. Morgan D. O. (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13, 261–291 [DOI] [PubMed] [Google Scholar]
- 4. Morgan D. O. (1995) Principles of CDK regulation. Nature 374, 131–134 [DOI] [PubMed] [Google Scholar]
- 5. Meyerson M., Enders G. H., Wu C. L., Su L. K., Gorka C., Nelson C., Harlow E., Tsai L. H. (1992) A family of human cdc2-related protein kinases. EMBO J. 11, 2909–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullrich F., MacLachlan T. K., Sang N., Druck T., Veronese M. L., Allen S. L., Chiorazzi N., Koff A., Heubner K., Croce C. M., Giordano A. (1995) Chromosomal mapping of members of the cdc2 family of protein kinases, cdk3, cdk6, PISSLRE, and PITALRE, and a cdk inhibitor, p27Kip1, to regions involved in human cancer. Cancer Res. 55, 1199–1205 [PubMed] [Google Scholar]
- 7. Kasten M., Giordano A. (2001) Cdk10, a Cdc2-related kinase, associates with the Ets2 transcription factor and modulates its transactivation activity. Oncogene 20, 1832–1838 [DOI] [PubMed] [Google Scholar]
- 8. Li S., MacLachlan T. K., De Luca A., Claudio P. P., Condorelli G., Giordano A. (1995) The cdc-2-related kinase, PISSLRE, is essential for cell growth and acts in G2 phase of the cell cycle. Cancer Res. 55, 3992–3995 [PubMed] [Google Scholar]
- 9. Li A., Zhu X., Brown B., Craft C. M. (2003) Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest. Ophthalmol. Vis. Sci. 44, 996–1007 [DOI] [PubMed] [Google Scholar]
- 10. Leman E. S., Magheli A., Yong K. M., Netto G., Hinz S., Getzenberg R. H. (2009) Identification of nuclear structural protein alterations associated with seminomas. J. Cell. Biochem. 108, 1274–1279 [DOI] [PubMed] [Google Scholar]
- 11. Husson H., Carideo E. G., Neuberg D., Schultze J., Munoz O., Marks P. W., Donovan J. W., Chillemi A. C., O'Connell P., Freedman A. S. (2002) Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood 99, 282–289 [DOI] [PubMed] [Google Scholar]
- 12. Iorns E., Turner N. C., Elliott R., Syed N., Garrone O., Gasco M., Tutt A. N., Crook T., Lord C. J., Ashworth A. (2008) Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell 13, 91–104 [DOI] [PubMed] [Google Scholar]
- 13. Chen E., Ekker S. C. (2004) Zebrafish as a genomics research model. Curr. Pharm. Biotechnol. 5, 409–413 [DOI] [PubMed] [Google Scholar]
- 14. Liu Q. Y., Wu Z. L., Lv W. J., Yan Y. C., Li Y. P. (2007) Developmental expression of cyclin H and Cdk7 in zebrafish: the essential role of cyclin H during early embryo development. Cell Res. 17, 163–173 [DOI] [PubMed] [Google Scholar]
- 15. Kanungo J., Li B. S., Zheng Y., Pant H. C. (2006) Cyclin-dependent kinase 5 influences Rohon-Beard neuron survival in zebrafish. J. Neurochem. 99, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grant P., Sharma P., Pant H. C. (2001) Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism. Eur. J. Biochem. 268, 1534–1546 [PubMed] [Google Scholar]
- 17. Kanungo J., Zheng Y. L., Amin N. D., Kaur S., Ramchandran R., Pant H. C. (2009) Specific inhibition of cyclin-dependent kinase 5 activity induces motor neuron development in vivo. Biochem. Biophys. Res. Commun. 386, 263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu L. S., Liang C. J., Tseng C. Y., Yeh C. W., Tsai J. N. (2011) Zebrafish Cyclin-dependent protein kinase-like 1 (zcdkl1): identification and functional characterization. Int. J. Mol. Sci. 12, 3606–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westerfield M., Doerry E., Kirkpatrick A. E., Driever W., Douglas S. A. (1997) An on-line database for zebrafish development and genetics research. Semin. Cell Dev. Biol. 8, 477–488 [DOI] [PubMed] [Google Scholar]
- 20. Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 21. Hsu L. S., Tseng C. Y. (2010) Zebrafish calcium/calmodulin-dependent protein kinase II (cam-kii) inhibitors: expression patterns and their roles in zebrafish brain development. Dev. Dyn. 239, 3098–3105 [DOI] [PubMed] [Google Scholar]
- 22. Thisse C., Thisse B. (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 [DOI] [PubMed] [Google Scholar]
- 23. Robu M. E., Larson J. D., Nasevicius A., Beiraghi S., Brenner C., Farber S. A., Ekker S. C. (2007) p53 activation by knockdown technologies. PLoS Genet. 3, e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan Y., Bergmann A. (2008) Apoptosis-induced compensatory proliferation. The cell is dead. Long live the cell! Trends Cell Biol. 18, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Graham V., Khudyakov J., Ellis P., Pevny L. (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39, 749–765 [DOI] [PubMed] [Google Scholar]
- 26. Ko C. Y., Tsai M. Y., Tseng W. F., Cheng C. H., Huang C. R., Wu J. S., Chung H. Y., Hsieh C. S., Sun C. K., Hwang S. P., Yuh C. H., Huang C. J., Pai T. W., Tzou W. S., Hu C. H. (2011) Integration of CNS survival and differentiation by HIF2α. Cell Death Differ. 18, 1757–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kay J. N., Link B. A., Baier H. (2005) Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development 132, 2573–2585 [DOI] [PubMed] [Google Scholar]
- 28. Liu W., Mo Z., Xiang M. (2001) The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc. Natl. Acad. Sci. U.S.A. 98, 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu J. H., Zhong X. Y., Zhang W. G., Wang Z. D., Dong Q., Tai S., Li H., Cui Y. F. (2012) CDK10 functions as a tumor suppressor gene and regulates survivability of biliary tract cancer cells. Oncol. Rep. 27, 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhong X. Y., Xu X. X., Yu J. H., Jiang G. X., Yu Y., Tai S., Wang Z. D., Cui Y. F. (2012) Clinical and biological significance of Cdk10 in hepatocellular carcinoma. Gene 498, 68–74 [DOI] [PubMed] [Google Scholar]
- 31. Kanungo J., Li B. S., Goswami M., Zheng Y. L., Ramchandran R., Pant H. C. (2007) Cloning and characterization of zebrafish (Danio rerio) cyclin-dependent kinase 5. Neurosci. Lett. 412, 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cole L. K., Ross L. S. (2001) Apoptosis in the developing zebrafish embryo. Dev. Biol. 240, 123–142 [DOI] [PubMed] [Google Scholar]
- 33. Tanaka H., Morimura R., Ohshima T. (2012) Dpysl2 (CRMP2) and Dpysl3 (CRMP4) phosphorylation by Cdk5 and DYRK2 is required for proper positioning of Rohon-Beard neurons and neural crest cells during neurulation in zebrafish. Dev. Biol. 370, 223–236 [DOI] [PubMed] [Google Scholar]
- 34. Zhang L., Li Y. Y., Chen T., Xia W., Zhou Y., Wan Y. J., Lv Z. Q., Li G. Q., Xu S. Q. (2011) Abnormal development of motor neurons in perfluorooctane sulphonate exposed zebrafish embryos. Ecotoxicology 20, 643–652 [DOI] [PubMed] [Google Scholar]
- 35. Bagella L., Giacinti C., Simone C., Giordano A. (2006) Identification of murine cdk10: association with Ets2 transcription factor and effects on the cell cycle. J. Cell. Biochem. 99, 978–985 [DOI] [PubMed] [Google Scholar]
- 36. McLaughlin T., Torborg C. L., Feller M. B., O'Leary D. D. (2003) Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160 [DOI] [PubMed] [Google Scholar]
- 37. Chen J., Miao Y., Wang X. H., Wang Z. (2011) Elevation of p-NR2A(S1232) by Cdk5/p35 contributes to retinal ganglion cell apoptosis in a rat experimental glaucoma model. Neurobiol. Dis. 43, 455–464 [DOI] [PubMed] [Google Scholar]
- 38. Bermel C., Tönges L., Planchamp V., Gillardon F., Weishaupt J. H., Dietz G. P., Bähr M., Lingor P. (2009) Combined inhibition of Cdk5 and ROCK additively increase cell survival, but not the regenerative response in regenerating retinal ganglion cells. Mol. Cell. Neurosci. 42, 427–437 [DOI] [PubMed] [Google Scholar]
- 39. Sakamoto K., Ohki K., Saito M., Nakahara T., Ishii K. (2011) Small molecule cyclin-dependent kinase inhibitors protect against neuronal cell death in the ischemic-reperfused rat retina. J. Ocul. Pharmacol. Ther. 27, 419–425 [DOI] [PubMed] [Google Scholar]
- 40. Cheung Z. H., Gong K., Ip N. Y. (2008) Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2. J. Neurosci. 28, 4872–4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hahn C. M., Kleinholz H., Koester M. P., Grieser S., Thelen K., Pollerberg G. E. (2005) Role of cyclin-dependent kinase 5 and its activator P35 in local axon and growth cone stabilization. Neuroscience 134, 449–465 [DOI] [PubMed] [Google Scholar]
- 42. Illanes O., Anderson S., Niesman M., Zwick L., Jessen B. A. (2006) Retinal and peripheral nerve toxicity induced by the administration of a pan-cyclin dependent kinase (cdk) inhibitor in mice. Toxicol. Pathol. 34, 243–248 [DOI] [PubMed] [Google Scholar]
- 43. Lesuisse C., Martin L. J. (2002) Immature and mature cortical neurons engage different apoptotic mechanisms involving caspase-3 and the mitogen-activated protein kinase pathway. J. Cereb. Blood Flow Metab. 22, 935–950 [DOI] [PubMed] [Google Scholar]
- 44. Chen J., Rusnak M., Lombroso P. J., Sidhu A. (2009) Dopamine promotes striatal neuronal apoptotic death via ERK signaling cascades. Eur. J. Neurosci. 29, 287–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang K., Cao F., Sheikh A. M., Malik M., Wen G., Wei H., Ted Brown W., Li X. (2013) Up-regulation of Ras/Raf/ERK1/2 signaling impairs cultured neuronal cell migration, neurogenesis, synapse formation, and dendritic spine development. Brain Struct. Funct. 218, 669–682 [DOI] [PubMed] [Google Scholar]
- 46. Rössler O. G., Giehl K. M., Thiel G. (2004) Neuroprotection of immortalized hippocampal neurones by brain-derived neurotrophic factor and Raf-1 protein kinase: role of extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase. J. Neurochem. 88, 1240–1252 [DOI] [PubMed] [Google Scholar]
- 47. Magariños M., Aburto M. R., Sánchez-Calderón H., Muñoz-Agudo C., Rapp U. R., Varela-Nieto I. (2010) RAF kinase activity regulates neuroepithelial cell proliferation and neuronal progenitor cell differentiation during early inner ear development. PLoS One 5, e14435. [DOI] [PMC free article] [PubMed] [Google Scholar]