Background: Phagocytic mechanisms are important for understanding the host-pathogen interactions of intracellular parasitic pathogens.
Results: The entry and early intracellular trafficking of B. abortus are dependent on clathrin cooperation with Rab5.
Conclusion: Clathrin influences phagocytic pathways of B. abortus associating with Rab5 at a host subcellular site allowing replication.
Significance: This study promotes the early control of B. abortus infection.
Keywords: Bacteria, Bacterial Pathogenesis, Host-Pathogen Interactions, Microbial Pathogenesis, Microbiology
Abstract
Lipid raft-associated clathrin is essential for host-pathogen interactions during infection. Brucella abortus is an intracellular pathogen that circumvents host defenses, but little is known about the precise infection mechanisms that involve interaction with lipid raft-associated mediators. The aim of this study was to elucidate the clathrin-mediated phagocytic mechanisms of B. abortus. The clathrin dependence of B. abortus infection in HeLa cells was investigated using an infection assay and immunofluorescence microscopy. The redistribution of clathrin in the membrane and in phagosomes was investigated using sucrose gradient fractionation of lipid rafts and the isolation of B. abortus-containing vacuoles, respectively. Clathrin and dynamin were concentrated into lipid rafts during B. abortus infection, and the entry and intracellular survival of B. abortus within HeLa cells were abrogated by clathrin inhibition. Clathrin disruption decreased actin polymerization and the colocalization of B. abortus-containing vacuoles with clathrin and Rab5 but not lysosome-associated membrane protein 1 (LAMP-1). Thus, our data demonstrate that clathrin plays a fundamental role in the entry and intracellular survival of B. abortus via interaction with lipid rafts and actin rearrangement. This process facilitates the early intracellular trafficking of B. abortus to safe replicative vacuoles.
Introduction
Brucella species are Gram-negative, facultative intracellular bacteria and the etiological agent of brucellosis in many animals and humans (1, 2). The ability of these bacteria to escape killing within phagocytes is hypothesized to be involved in their virulence by promoting invasion and chronic infections (3, 4), but the exact molecular mechanisms are unknown.
Previous studies explored the mechanisms that underlie intracellular survival by investigating the trafficking of Brucella-containing vacuoles (BCVs)2. These studies revealed that BCVs interact with early endocytic compartments before acquiring the lysosome-associated membrane protein 1 (LAMP-1) and progressively exclude themselves from endocytic compartments to circumvent fusion with terminal lysosomes (5, 6). However, a recent study that used live cell imaging to investigate BCV trafficking confirmed that fusion with lysosomes occurs in the intermediate stages of BCV trafficking and is required for the maturation of BCVs into endoplasmic reticulum-derived replicative organelles (7).
Lipid rafts are specialized membrane microdomains enriched in cholesterol, glycosylphosphatidylinositol-anchored proteins and GM1 gangliosides (8). There is growing evidence concerning the potential role of lipid rafts in host-pathogen interactions, and lipid rafts have been implicated as portals of entry for intracellular pathogens (9, 10). Several studies have implicated lipid rafts in the entry and endocytic pathway of Brucella abortus in host cells. These studies indicated that lipid raft-associated molecules, such as glycosylphosphatidylinositol-anchored proteins, GM1 gangliosides, and cholesterol, are selectively integrated into Brucella-containing macropinosomes following the internalization of Brucella into macrophages and continuously sustain a dynamic state of the phagosomal membrane (11–13). In addition, the route by which Brucella is internalized into phagocytic cells determines the intracellular fate of this bacterium, and this event is modulated by lipid rafts (11, 14).
Clathrin is an endocytic coat protein that mediates the internalization of a variety of transmembrane receptors and their ligands, and there is evidence that lipid rafts play a role in clathrin-dependent uptake mechanisms (15). Additionally, clathrin facilitates the entry of a variety of zippering bacteria, such as Listeria and Chlamydia trachomatis, into non-phagocytic cells (16, 17).
Dynamin, a member of the large (100-kDa) GTPase family, participates in several endocytic processes. This protein is required for endocytic events during membrane fusion and selectively regulates the assembly of endocytic vacuoles (18, 19). In particular, dynamin plays an important role in both clathrin-dependent endocytosis (20, 21) and caveolae-dependent uptake and endocytosis (22). In addition, dynamin is not only involved in the formation of endosomes at the plasma membrane but is also required for the vesiculation of endosome tubules (23).
Despite extensive study, the roles of clathrin and dynamin in B. abortus infection remain unclear. Although B. abortus interacts with host cells, such as macrophages and epithelial cells, through lipid rafts (1, 4, 12–14), little is known about the precise mechanisms by which this pathogen exploits the host entry and intracellular trafficking apparatus, including the mediators that enable this interaction, for its intracellular survival.
Here, we elucidated the mechanism underlying the clathrin-dependent entry and early intracellular trafficking of B. abortus following association with lipid rafts. Clathrin critically influenced the intracellular trafficking of B. abortus, which was associated with Rab5.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection Experiments
HeLa cells (ATCC 229) were grown at 37 °C in a 5% CO2 atmosphere in DMEM containing 10% heat-inactivated FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen). For all assays, cells were seeded (2 × 104 cells/well) in cell culture plates and incubated for 24 h before infection.
For transfection, siRNA targeting the clathrin heavy chain (HC oligo I) (AAC CUG CGG UCU GGA GUC AAC) and the non-targeting firefly (Photinus pyralis) luciferase siRNA (AAC GTT ACC GCG GAA TAC TTC GA) were obtained from Dharmacon. RNA targeting and formation of an siRNA duplex were performed as described previously (24). HeLa cells were transfected with clathrin heavy chain siRNA (60 pmol of RNA duplex) using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer. As a control, firefly luciferase siRNA was transfected into cells in parallel. The knockdown efficiency was determined by comparing protein levels in cells transfected with clathrin siRNA and control siRNA, as indicated by Western blot analysis using antibodies against the clathrin heavy chain (BD Transduction Laboratories) and β-actin (Cell Signaling Technology). Western blot signals were quantified using the National Institutes of Health ImageJ software program.
Bacterial Strains and Culture Conditions
B. abortus strains were derived from 544 (ATCC 23448), a smooth, virulent B. abortus biovar 1 strain. B. abortus organisms were stored as frozen aliquots in 80% (v/v) glycerol at −70 °C. Bacteria were grown in Brucella broth (Difco, BD Biosciences) at 37 °C with shaking incubation until they reached the stationary phase, and then the viable counting of bacteria was assessed by plating serial dilutions on Brucella agar.
Bacterial Infection
To analyze bacterial internalization efficiency, cultured cells were treated with various inhibitors or transfected with target siRNA prior to infection. Following treatment, bacteria were deposited onto cells at a multiplicity of infection (MOI) of 10, centrifuged at 150 × g for 10 min, and incubated at 37 °C in 5% CO2 for 0, 15, and 30 min. Cells were incubated in DMEM containing 10% FBS and gentamicin (30 μg/ml, Sigma-Aldrich) for 30 min to kill any remaining extracellular bacteria. To determine the intracellular replication efficacy, infected cells were incubated at 37 °C for 1 h, cultured in DMEM containing 10% FBS and gentamicin, and incubated with various inhibitors. After 2, 24, and 48 h, infected cells were lysed and spread on Brucella agar plates in triplicate, and the number of viable bacteria was determined by counting colony-forming units (CFUs).
Inhibitor Studies
Inhibitors targeting different lipid raft-associated molecules were used selectively. The viability of drug-treated cells was evaluated by staining single-cell preparations with trypan blue. Prior to infection, cultured cells were incubated at 37 °C with the following inhibitors for the indicated times: 12.5 μM chloropromazine (CPZ), a clathrin inhibitor, for 45 min; 80 μM dynasore, a dynamin inhibitor, for 30 min; and 5 mM methyl-β-cyclodextrin (MβCD), a lipid raft-associated cholesterol-depleting drug, for 30 min (all from Sigma-Aldrich). The viability values of cells treated with the above indicated concentrations of all drugs showed more than 98% compared with the untreated cells (100%).
Isolation of Lipid Rafts
Lipid rafts were isolated on ice as described previously (25), with some modifications. Briefly, the cells were scraped and lysed in base buffer and centrifuged at 1000 × g for 10 min. The postnuclear supernatant (0.84 ml) was adjusted to 35% OptiPrep (Sigma-Aldrich) by adding 60% OptiPrep (1.16 ml). Subsequently, 2 ml each of 30, 25, and 20% OptiPrep and 1 ml of base buffer (0%) were overlaid on top of the lysate (35% OptiPrep). The gradients were centrifuged at 52,000 × g for 3 h using an SW-41 rotor in a Beckman ultracentrifuge (Beckman Coulter) and fractionated into nine fractions (1 ml/fraction). Fractionated proteins were analyzed by Western blotting.
Purification of Bacteria-containing Phagosomes
Bacteria-containing phagosomes were purified as described previously described, with slight modifications (26). Infected cells (1 × 108 cells) were washed by centrifugation at 300 × g for 5 min at 4 °C and suspended and lysed in 1 ml of ice-cold homogenization buffer. Nuclei and intact cells were removed from the homogenate by centrifugation at 800 × g for 5 min at 4 °C. The supernatant was laid on top of a discontinuous sucrose gradient consisting of 2 ml 50% sucrose, 4 ml 37% sucrose, and 4 ml 25% sucrose and centrifuged at 100,000 × g for 1 h at 4 °C using an SW-41 rotor in a Beckman ultracentrifuge. The band between the 50 and 37% sucrose layers, which contained the bacteria-containing phagosomes, was collected and resuspended in 12 ml of cold PBS. The bacteria-containing phagosomes were pelleted by centrifugation at 40,000 × g for 15 min at 4 °C, and the resulting pellets were resuspended in 0.1 ml of homogenization buffer and subjected to Western blotting.
Western Blotting
Protein concentrations were determined using the Bradford protein assay (Richmond, CA), and proteins were loaded and separated by SDS-PAGE before transfer to PVDF membranes (Millipore). Blots were blocked for 1 h with 5% BSA in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and washed three times with TBS-T for 20 min. Blots were incubated with the appropriate primary antibodies overnight at 4 °C with gentle shaking and washed as described above. Binding of primary antibodies was visualized using HRP-conjugated secondary antibodies, and immunolabeling was detected using ECL (SurModics) according to the instructions of the manufacturer and exposure to x-ray film (Fujifilm).
Immunofluorescence Staining and Microscopy
HeLa cells were treated with inhibitors before infection as described above. After 10, 15, 30, and 60 min of infection with Alexa Fluor 405-conjugated B. abortus, the cells were fixed in 4% paraformaldehyde in PBS for 30 min at 37 °C, washed three times with PBS, and permeabilized with 0.1% Triton X-100 for 10 min at 22 °C. After 30 min of incubation with a blocking buffer (2% goat serum in PBS), the preparations were stained with different antibodies in blocking buffer. To stain F-actin, the cells were incubated with 0.5 μm phalloidin-TRITC for 30 min at 22 °C. To detect intracellular protein localization, the cells were incubated with primary antibodies against clathrin, dynamin (BD Transduction Laboratories), and Rab5 (Cell Signaling Technology) and the corresponding fluorescence-conjugated secondary antibodies. For LAMP-1 staining, after 2, 4, 8, 24, and 48 h of infection with unconjugated B. abortus, fixed cells were stained with an anti-B. abortus polyclonal antibody produced in rabbits immunized with B. abortus (12) and an anti-LAMP-1 antibody (Invitrogen). Finally, the preparations were washed and mounted with fluorescent mounting medium (DakoCytomation). Fluorescent images were collected using an Olympus FV1000 laser-scanning confocal microscope. Images were processed using Adobe Photoshop and National Institutes of Health ImageJ software. For LAMP-1 staining, 100 bacteria within macrophages were selected randomly, and the extent of bacterial LAMP-1 acquisition was determined.
Statistical Analysis
Data are expressed as mean ± S.D. for replicate experiments. Statistical analysis was performed using GraphPad Prism software, version 4.00 (GraphPad Software). Student's t test or a one-way analysis of variance followed by the Newman-Keuls test was used for statistical comparisons between groups. p < 0.05 was considered statistically significant.
RESULTS
Clathrin Affects the Entry and Intracellular Survival of B. abortus
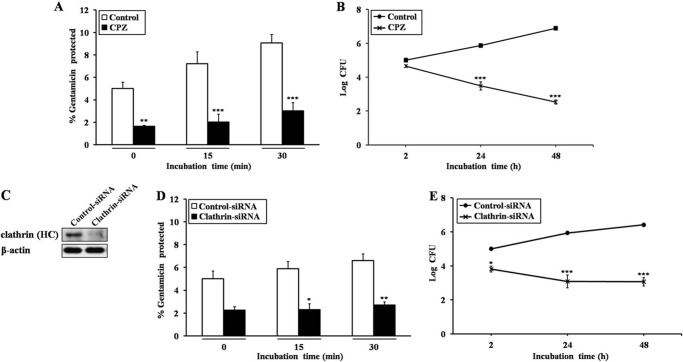
Several infection processes are dependent on functional clathrin (16, 17, 27), but the interaction between B. abortus and clathrin has yet to be elucidated. To investigate the role of clathrin in the entry and intracellular survival of B. abortus, HeLa cells were treated with 12.5 μM CPZ, a clathrin inhibitor. The entry and intracellular replication of B. abortus in CPZ-treated cells were significantly diminished in comparison with untreated cells (p < 0.001, Fig. 1, A and B). We used 60 pmol of clathrin-specific siRNA to silence clathrin expression, and the transfection efficiency in HeLa cells was adequate (98.32 ± 3.74%) (Fig. 1C). The entry and intracellular replication of B. abortus in clathrin knockdown cells were also significantly decreased compared with control cells (p < 0.01, Fig. 1, D and E). These results suggested that clathrin was essential for the entry and intracellular survival of B. abortus in epithelial cells.
FIGURE 1.
The role of clathrin in the entry and intracellular survival of B. abortus in non-professional phagocytes. A and B, HeLa cells were pretreated with 12.5 μM CPZ, a clathrin inhibitor, for 45 min prior to infection with B. abortus at an MOI of 10 for the indicated times. C–E, HeLa cells were transiently transfected with control or clathrin siRNA, whose optimal conditions were evaluated by Western blotting (C), and subsequently infected according to the procedure described above (D and E). Bacterial internalization and intracellular survival efficiency were determined by evaluating the protection of internalized bacteria from gentamicin killing and calculating the log10 CFU, respectively. The data represent the mean ± S.D. of triplicate trials from three independent experiments. Differences that were statistically significant compared with untreated samples are indicated. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The Lipid Raft-associated Molecules Dynamin and Cholesterol Play a Prominent Role in the Entry and Intracellular Survival of B. abortus
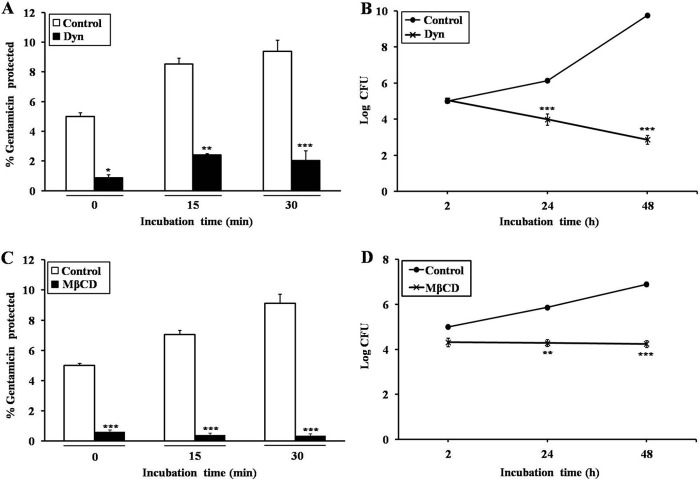
To confirm the role of lipid rafts in B. abortus infection, HeLa cells were pretreated with the dynamin inhibitor dynasore (80 μM) or the lipid raft-associated cholesterol-depleting drug MβCD (5 mM) and infected with B. abortus. The results indicated that the inhibition of dynamin (p < 0.001, Fig. 2, A and B) and cholesterol (p < 0.001, C and D) reduced bacterial entry and intracellular replication compared with control cells.
FIGURE 2.
The role of lipid raft-associated dynamin and cholesterol in the entry and intracellular survival of B. abortus in non-professional phagocytes. A–D, HeLa cells were pretreated with 80 μM dynasore (Dyn), a dynamin inhibitor (A and B), or 5 mM MβCD, a lipid raft-associated cholesterol-depleting drug (C and D), for 30 min prior to infection with B. abortus at an MOI of 10 for the indicated times. Bacterial internalization and intracellular survival efficiency were determined by evaluating the protection of internalized bacteria from gentamicin killing and calculating the log10 CFU, respectively. Data represent the mean ± S.D. of triplicate trials from three independent experiments. Differences that were statistically significant compared with untreated samples are indicated. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Clathrin Cooperates with Dynamin to Facilitate B. abortus Entry into Phagocytes
Because B. abortus internalization has been associated with the membrane sorting of lipid rafts within host cells (12, 13), we assessed whether clathrin and dynamin cooperated in the entry of B. abortus via lipid rafts. The results indicated that both clathrin and dynamin were recruited and concentrated into lipid rafts at 10 min post-infection. Additionally, the accumulation of clathrin in lipid raft fractions in both clathrin- and cholesterol-inhibited cells was reduced compared with untreated cells (Fig. 3A). The distribution of dynamin was similar in clathrin-, dynamin-, and cholesterol-inhibited cells (Fig. 3B). Consistent with evidence indicating that the overall recruitment of clathrin and dynamin to the plasma membrane is increased by infection with pathogens (17, 18, 35, 36), we observed that in infected cells, although clathrin and dynamin were mainly redistributed to lipid rafts (fraction 2–3) compared with uninfected controls, these molecules were also partially distributed to non-lipid rafts (fractions 7–9) (Fig. 3, A and B). Taken together, these findings clearly demonstrated that in B. abortus-infected phagocytes, clathrin and dynamin were associated with lipid rafts via membrane sorting and modification.
FIGURE 3.
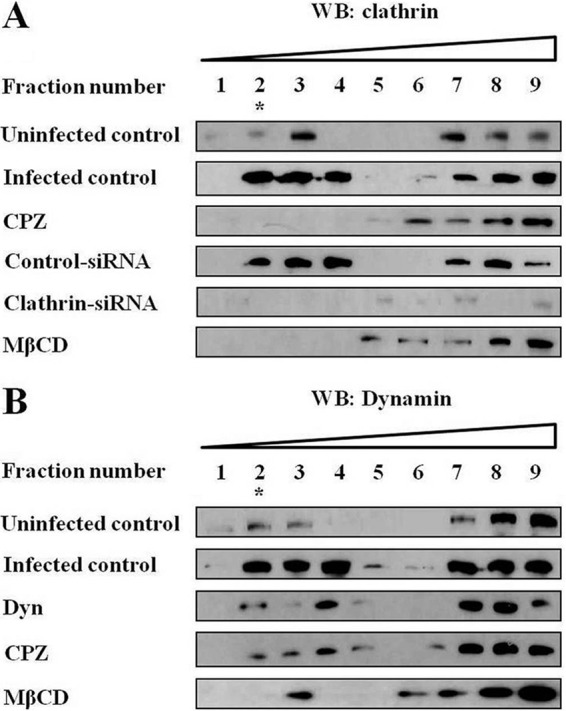
Cooperation of clathrin with dynamin during membrane sorting and the modification of lipid rafts in B. abortus-infected phagocytes. HeLa cells were pretreated with 12.5 μM CPZ, 5 mM MβCD, or 80 μM dynasore (Dyn) or transiently transfected with control or clathrin siRNA prior to infection with B. abortus at an MOI of 10 for 10 min. Cell membranes were fractionated using sucrose density gradient ultracentrifugation. A and B, individual fractions were analyzed for clathrin (A) and dynamin (B) by immunoblotting. The lipid raft fractions are indicated by an asterisk in fraction 2. WB, Western blot.
Clathrin Associated with Lipid Rafts Contributes to Actin-dependent B. abortus Entry
Actin filaments interact with membrane-associated coat, adaptor, and accessory proteins to assist carrier biogenesis in endocytic pathways (28). The collaboration of clathrin with the actin cytoskeleton is a critical event for bacterial entry into non-professional phagocytes (17, 28, 29). Thus, we investigated whether the actin rearrangements needed for B. abortus entry mediated the action of lipid raft-associated clathrin. To test this premise, the recruitment of clathrin and F-actin during the entry of B. abortus into clathrin-depleted cells was assessed using confocal microscopy. The results indicated that F-actin polymerization in clathrin- and cholesterol-inhibited cells was attenuated compared with untreated cells and that clathrin recruitment at sites where B. abortus was bound was reduced in clathrin-inhibited cells (Fig. 4). These observations suggested that the entry of B. abortus through a lipid raft- and clathrin-dependent pathway might be accompanied by actin polymerization.
FIGURE 4.
Actin polymerization and clathrin rearrangement associated with lipid rafts during the entry of B. abortus into non-professional phagocytes. A–C, F-actin polymerization, bacterial colocalization, and clathrin rearrangement were observed in HeLa cells pretreated with medium (Control) (A), 12.5 μM CPZ (B), or 5 mM MβCD (C) at 10 min p.i. The cells were fixed and stained with rhodamine-conjugated phalloidin to visualize F-actin (red), Alexa Fluor 405-labeled B. abortus (blue), and a FITC-labeled clathrin antibody (green). All images shown are representative of three separate experiments. Scale bars = 5 μm as indicated.
The Interaction of Rab5 with BCVs Requires the Clathrin-dependent Pathway
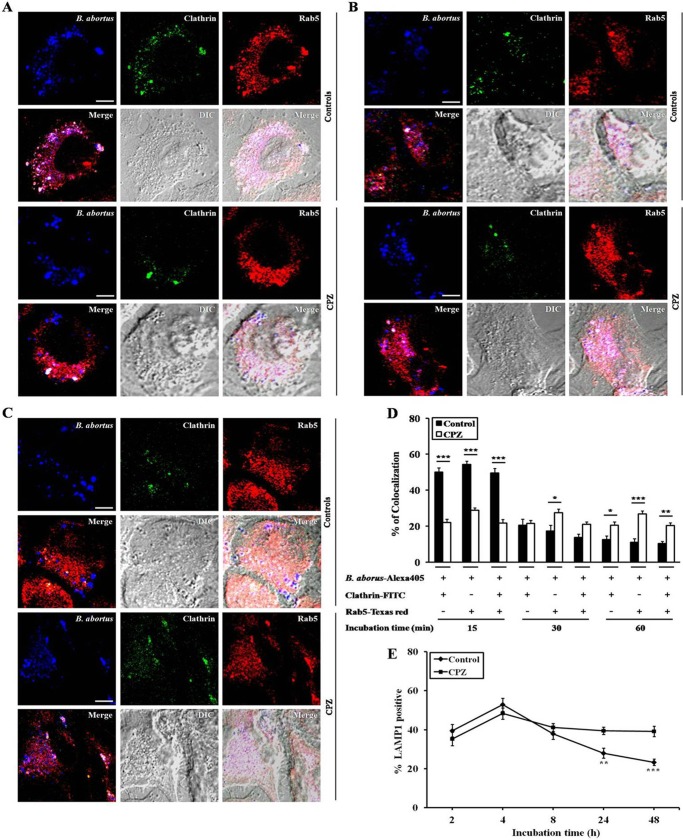
The small GTPase Rab5 is commonly linked with early endosomes and plays a fundamental role in the intracellular trafficking of several pathogens, including Brucella (30, 31). In the endocytic pathway, Rab5 plays a key role in the transport of vesicles to their acceptor compartments (32). We determined whether clathrin was recruited to Rab5-associated BCVs and affected the interaction of Rab5 with BCVs. High levels of clathrin and Rab5 were colocalized in BCVs 15 min post infection (p.i.) in control cells (Fig. 5A), but clathrin recruitment to Rab5-associated BCVs was dramatically reduced at later time points (B and C) (49.5 ± 2.59%, 13.75 ± 1.75%, and 10.25 ± 1.25% colocalization at 15, 30, and 60 min p.i., respectively) (D). In contrast, clathrin-inhibited cells had markedly lower levels of colocalization than control cells at 15 min p.i. (Fig. 5A), but BCVs continued to fuse with early endosomes, and clathrin colocalized with Rab5-associated BCVs at later time points (B and C) (21.75 ± 1.93%, 21.00 ± 1.29%, and 20.25 ± 1.65% colocalization at 15, 30, and 60 min p.i., respectively) (D). These findings suggest that B. abortus was contained within clathrin-coated vesicles in association with Rab5. However, 30 min after infection, there was a considerable reduction of clathrin and Rab5 association with the Brucella-containing vesicles.
FIGURE 5.
Clathrin-dependent interaction of Rab5 with BCVs during the early stage of infection. HeLa cells were pretreated with 12.5 μM CPZ and infected with B. abortus at an MOI of 10 for the indicated times. A–C, at 15 (A), 30 (B), and 60 (C) min p.i., the cells were fixed and stained with Texas red-conjugated Rab5 (red), Alexa Fluor 405-labeled B. abortus (blue), and FITC-labeled clathrin (green) antibodies. All images shown are representative of three separate experiments. Scale bars = 5 μm as indicated. D and E, the number of BCVs that colocalized with clathrin and/or Rab5 (D) or LAMP-1 (E) was determined by analyzing 100 BCVs (combined from at least five random fields) from three separate monolayers for each time point. Data represent the mean ± S.D. of triplicate trials from three independent experiments. Differences that were statistically significant compared with untreated samples are indicated. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
In addition, we determined whether clathrin was involved in the interaction of BCVs with late endosomal/lysosomal glycoproteins. In control cells, LAMP-1-positive BCVs increased until 4 h p.i. and gradually declined thereafter. In contrast, the BCVs of clathrin-inhibited cells displayed higher levels of LAMP-1 than control cells after 8 h p.i. Indeed, they were continuously retained within late endosomes, showing significant increases (1.41-fold and 1.67-fold) at 24 and 48 h p.i., respectively (p < 0.001, Fig. 5E).
Clathrin Affects the Recruitment of Functional Proteins Associated with the Endocytic Pathway of BCVs
We investigated whether clathrin-associated dynamin, lipid rafts, and actin cytoskeletal proteins collaborated in the establishment of BCVs by investigating the association of these molecules with Rab5 during the early stages of the endocytic entry of B. abortus. We isolated BCVs after infection, and the presence of target proteins in BCVs was evaluated by immunoblotting (Fig. 6A). High levels of dynamin, caveolin (22), and clathrin were detected in BCVs at 15 min p.i. (2.11-fold, 5.76-fold, and 2.88-fold higher than uninfected cells, respectively (Fig. 6B)). In addition, BCVs from infected cells also contained 2.43-fold more Rab5 than those of uninfected cells at 15 min p.i. (Fig. 6B).
FIGURE 6.
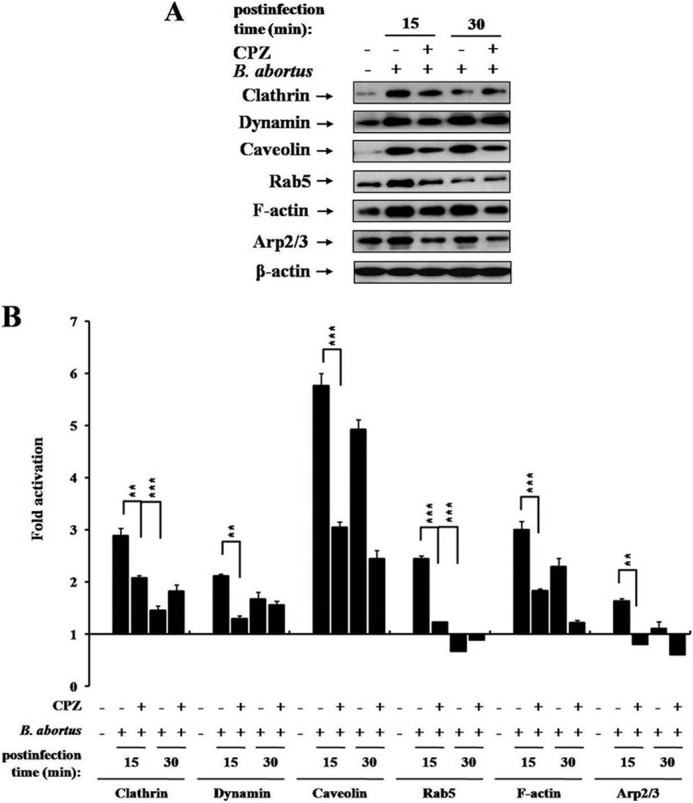
The role of clathrin in the recruitment of functional proteins associated with the endocytic pathway to BCVs. A, immunoblot analysis of clathrin, dynamin, caveolin, Rab5, F-actin, Arp2/3, and β-actin in BCVs isolated from HeLa cells pretreated with or without 12.5 μM CPZ at the indicated times p.i. The images shown are representative of three independent experiments. B, immunoblot ECL signals were quantified using National Institutes of Health ImageJ software, and the densitometry ratios of the β-actin signal to individual target protein signals are shown. Data represent the mean ± S.D. of triplicate trials from three independent experiments. Differences that were statistically significant compared with untreated samples are indicated. **, p < 0.01; ***, p < 0.001.
Because F-actin polymerization is associated with clathrin-dependent endocytosis (28, 33), we evaluated the levels of F-actin and Arp2/3, a protein necessary for the actin filament network (34). Levels of F-actin (3.00-fold) and Arp2/3 (1.63-fold) were also increased in BCVs at 15 min p.i., which is consistent with microscopic observations and results from previous studies (33, 35). However, the observed levels of clathrin and Rab5, but not dynamin, caveolin, F-actin, or Arp2/3, were reduced in BCVs at 30 min p.i. compared with BCVs at 15 min p.i. In contrast, the levels of all tested proteins in the BCVs of clathrin-inhibited cells at 15 min p.i. were lower than those of control cells (p < 0.001, Fig. 6B). Collectively, these findings indicated that clathrin cooperates with functional proteins, which are necessary for the formation of lipid rafts and the function of the actin cytoskeleton, in the early endocytic events that lead to the establishment of BCVs.
DISCUSSION
Clathrin-mediated endocytosis is a primary endocytic pathway that is mediated through clathrin-coated pits that are assembled from cytosolic coat proteins that subsequently invaginate and pinch off of the membrane to form a clathrin-coated vesicle (36). During Clathrin-mediated endocytosis, dynamin is highly associated with clathrin, and both protein-dependent internalization processes are essential for the entry of large particles, including pathogenic bacteria, fungi, and large viruses (16, 17, 27, 37). However, few studies have addressed the interaction of B. abortus with clathrin, and the roles of clathrin coats and clathrin-associated functional proteins in the intracellular trafficking of B. abortus have remained elusive. The interaction of bacteria with host cells is on the basis of the recruitment of multiple signaling molecules to lipid rafts (38). The exploitation of lipid rafts is a common mechanism for immune subversion by pathogens, suggesting that the lipid raft-mediated pathway may allow for escape from the endocytic pathway and subsequent lysosomal degradation (39, 40). Although several studies have described lipid raft-dependent B. abortus pathogenesis (12, 13), the interaction of clathrin with B. abortus remains unclear. Consistent with a previous report (41), we demonstrated that clathrin was concentrated in lipid rafts during B. abortus entry. In addition, clathrin inhibition caused a shift of clathrin and dynamin out of raft fractions. These findings suggested that B. abortus internalization via clathrin- and dynamin-dependent pathways proceeded via lipid rafts. Thus, we propose that the primary pathway that mediates phagocytosis of B. abortus is lipid raft-mediated entry via clathrin-coated pits.
B. abortus invades a variety of cell types, including macrophages (3, 5), epitheloid HeLa cells, NIH3T3 fibroblasts, Vero cells, and Madin-Darby bovine kidney (MDBK) cells (4, 6). The phagocytic strategies used by this bacterium have been elucidated by analyzing internalization and BCV trafficking in macrophage and epithelial cell models (1–7). Previous studies with B. abortus have illuminated the diverse processes involved in the invasion of non-professional phagocytic cells, such as HeLa cells (1, 6, 7). Several studies investigated the role of actin in clathrin-mediated endocytosis, primarily in eukaryotic cells (35), and demonstrated that the actin cytoskeleton participates directly in membrane dynamics during clathrin-mediated endocytosis. In this study, we found that clathrin inhibition disrupted the recruitment of actin and clathrin to sites of bacteria-host cell interaction. Our results are consistent with previous evidence indicating that clathrin recruitment is necessary for actin polymerization during bacterial entry (16, 17) and suggest that clathrin plays an important role in actin polymerization-dependent phagocytosis during B. abortus entry into phagocytes.
Intracellular bacterial pathogens interact with Rab GTPases on the membranes of the host cell vacuoles that they occupy and manipulate Rab functions to exploit host cell trafficking pathways and establish their replicative niches (42). Studies exploring the intracellular trafficking mechanisms of these bacteria reported that Rab5 modulates fusion events between bacteria-containing vacuoles and early endosomes (43, 44). Although the association of B. abortus with early endosomes is an essential prerequisite for escaping phagosome-lysosome fusion (1, 5), no evidence is currently available concerning the involvement of clathrin in the interaction of Rab5 with B. abortus in non-professional phagocytes. In B. abortus-infected cells, the recruitment of both Rab5 and clathrin was observed as early as 15 min p.i., with a significant difference compared with clathrin-inhibited cells, but the majority of BCVs lacked both proteins by 30 min. These observations indicated that BCVs were concentrated in clathrin-coated vesicles associated with early endosomes, but this association was transient, and BCVs rapidly segregated from the early endocytic pathway. We propose that clathrin, together with Rab5, ensured proper endocytic sorting to promote BCV escape from fusion with lysosomes. Our findings are consistent with reported temporary interactions between various intracellular bacteria (45, 46) and Rab5 in early endosomes that facilitate pathogen survival within phagocytes.
The early stage of BCV formation following B. abortus entry is a critical event that determines the progression to late replicative BCVs (13, 31). In addition, it has been suggested that the bilayered clathrin coats on early endosomes are involved in protein sorting to lysosomes (47). Consistent with these data, we observed the presence of differing levels of clathrin in the endosomal vacuoles of normal and B. abortus-infected cells. In B. abortus-infected cells, there was a transient accumulation of a high level of clathrin at 15 min p.i., and clathrin levels were subsequently reduced in BCVs. In chlorpromazine-treated cells, however, the level of clathrin in BCVs was decreased at 15 min p.i., and only minor differences were observed at later time points. This finding is consistent with a previous study demonstrating that chlorpromazine prevented the uncoating of the clathrin coat after vesicle formation (48).
The pattern of clathrin association with BCVs appears to be similar to that of Rab5 during the early stages of infection. Clathrin coats associated with early endosomes appear to contribute to BCV formation and trafficking. In addition, dynamin, actin, and the Arp2/3 complex have all been shown to be transiently associated with clathrin-coated pits in mammalian cells (33, 35). We also found concomitant relative increases in clathrin-associated dynamin and lipid rafts as well as actin cytoskeletal proteins in BCVs at 15 min p.i. Unlike the transient interaction of clathrin and Rab5 with BCVs, increased levels of dynamin, caveolin, F-actin, and Arp2/3 were observed in BCVs for an extended period of time. These results may indicate that these components have a variety of functions related to the transport and sorting of endocytic vesicles in addition to their roles in the formation of clathrin-coated vesicles (49, 50). The association of BCVs with early endosomes is dependent on Rab5 and clathrin and accompanied by interactions with lipid rafts, dynamin, and cytoskeletal components.
We conclude that clathrin is a fundamental molecule that facilitates the interaction of BCVs with Rab5, thereby regulating fusion between BCVs and intracellular compartments and allowing bacteria to reside within a safe replicative subcellular location.
This work was supported by Basic Science Research Program Grants 2012-1032 and 2010-0009080 through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology and by National Veterinary Research and Quarantine Service Grant 0468-2010002.
- BCV
- B. abortus-containing vacuoles
- MOI
- multiplicity of infection
- CPZ
- chloropromazine
- TRITC
- tetramethylrhodamine isothiocyanate
- MβCD
- methyl-β-cyclodextrin
- p.i.
- post-infection.
REFERENCES
- 1. Gorvel J. P., Moreno E. (2002) Brucella intracellular life. From invasion to intracellular replication. Vet. Microbiol. 90, 281–297 [DOI] [PubMed] [Google Scholar]
- 2. Garnham P. C. (1958) Zoonoses or infections common to man and animals. J. Trop. Med. Hyg. 61, 92–94 [PubMed] [Google Scholar]
- 3. Baldwin C. L., Winter A. J. (1994) Macrophages and Brucella. Immunol. Ser. 60, 363–380 [PubMed] [Google Scholar]
- 4. Detilleux P. G., Deyoe B. L., Cheville N. F. (1990) Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58, 2320–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celli J., de Chastellier C., Franchini D. M., Pizarro-Cerda J., Moreno E., Gorvel J. P. (2003) Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198, 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Comerci D. J., Martínez-Lorenzo M. J., Sieira R., Gorvel J. P., Ugalde R. A. (2001) Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol. 3, 159–168 [DOI] [PubMed] [Google Scholar]
- 7. Starr T., Ng T. W., Wehrly T. D., Knodler L. A., Celli J. (2008) Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9, 678–694 [DOI] [PubMed] [Google Scholar]
- 8. Brown D. A., London E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14, 111–136 [DOI] [PubMed] [Google Scholar]
- 9. Gatfield J., Pieters J. (2000) Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288, 1647–1650 [DOI] [PubMed] [Google Scholar]
- 10. Wang M., Hajishengallis G. (2008) Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell Microbiol. 10, 2029–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naroeni A., Porte F. (2002) Role of cholesterol and the ganglioside GM(1) in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 70, 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watarai M., Makino S., Fujii Y., Okamoto K., Shirahata T. (2002) Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol. 4, 341–355 [DOI] [PubMed] [Google Scholar]
- 13. Kim S., Watarai M., Makino S., Shirahata T. (2002) Membrane sorting during swimming internalization of Brucella is required for phagosome trafficking decisions. Microb. Pathog. 33, 225–237 [DOI] [PubMed] [Google Scholar]
- 14. Kim S., Watarai M., Suzuki H., Makino S., Kodama T., Shirahata T. (2004) Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb. Pathog. 37, 11–19 [DOI] [PubMed] [Google Scholar]
- 15. Abrami L., Liu S., Cosson P., Leppla S. H., van der Goot F. G. (2003) Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160, 321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veiga E., Guttman J. A., Bonazzi M., Boucrot E., Toledo-Arana A., Lin A. E., Enninga J., Pizarro-Cerdá J., Finlay B. B., Kirchhausen T., Cossart P. (2007) Invasive and adherent bacterial pathogens co-opt host clathrin for infection. Cell Host Microbe 2, 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veiga E., Cossart P. (2006) The role of clathrin-dependent endocytosis in bacterial internalization. Trends Cell Biol. 16, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Praefcke G. J., McMahon H. T. (2004) The dynamin superfamily. Universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 5, 133–147 [DOI] [PubMed] [Google Scholar]
- 19. Sever S., Damke H., Schmid S. L. (2000) Garrotes, springs, ratchets, and whips. Putting dynamin models to the test. Traffic 1, 385–392 [DOI] [PubMed] [Google Scholar]
- 20. Loerke D., Mettlen M., Yarar D., Jaqaman K., Jaqaman H., Danuser G., Schmid S. L. (2009) Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 7, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mettlen M., Pucadyil T., Ramachandran R., Schmid S. L. (2009) Dissecting dynamin's role in clathrin-mediated endocytosis. Biochem. Soc. Trans. 37, 1022–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henley J. R., Krueger E. W., Oswald B. J., McNiven M. A. (1998) Dynamin-mediated internalization of caveolae. J. Cell Biol. 141, 85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicoziani P., Vilhardt F., Llorente A., Hilout L., Courtoy P. J., Sandvig K., van Deurs B. (2000) Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor. Mol. Biol. Cell 11, 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E. J. (2003) Effect of clathrin heavy chain- and α-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 278, 45160–45170 [DOI] [PubMed] [Google Scholar]
- 25. Macdonald J. L., Pike L. J. (2005) A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46, 1061–1067 [DOI] [PubMed] [Google Scholar]
- 26. Lührmann A., Haas A. (2000) A method to purify bacteria-containing phagosomes from infected macrophages. Methods Cell Sci. 22, 329–341 [DOI] [PubMed] [Google Scholar]
- 27. Veiga E., Cossart P. (2005) Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 7, 894–900 [DOI] [PubMed] [Google Scholar]
- 28. Anitei M., Hoflack B. (2012) Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat. Cell Biol. 14, 11–19 [DOI] [PubMed] [Google Scholar]
- 29. Clemente R., de la Torre J. C. (2009) Cell entry of Borna disease virus follows a clathrin-mediated endocytosis pathway that requires Rab5 and microtubules. J. Virol. 83, 10406–10416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alvarez-Dominguez C., Barbieri A. M., Berón W., Wandinger-Ness A., Stahl P. D. (1996) Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271, 13834–13843 [DOI] [PubMed] [Google Scholar]
- 31. Chaves-Olarte E., Guzmán-Verri C., Méresse S., Desjardins M., Pizarro-Cerdá J., Badilla J., Gorvel J. P., Moreno E. (2002) Activation of Rho and Rab GTPases dissociates Brucella abortus internalization from intracellular trafficking. Cell Microbiol. 4, 663–676 [DOI] [PubMed] [Google Scholar]
- 32. McLauchlan H., Newell J., Morrice N., Osborne A., West M., Smythe E. (1998) A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 8, 34–45 [DOI] [PubMed] [Google Scholar]
- 33. Yarar D., Waterman-Storer C. M., Schmid S. L. (2005) A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell 16, 964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weaver A. M., Young M. E., Lee W. L., Cooper J. A. (2003) Integration of signals to the Arp2/3 complex. Curr. Opin. Cell Biol. 15, 23–30 [DOI] [PubMed] [Google Scholar]
- 35. Merrifield C. J., Feldman M. E., Wan L., Almers W. (2002) Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4, 691–698 [DOI] [PubMed] [Google Scholar]
- 36. Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. (2004) Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 [DOI] [PubMed] [Google Scholar]
- 37. Hernaez B., Alonso C. (2010) Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry. J. Virol. 84, 2100–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Triantafilou M., Miyake K., Golenbock D. T., Triantafilou K. (2002) Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115, 2603–2611 [DOI] [PubMed] [Google Scholar]
- 39. Lafont F., Abrami L., van der Goot F. G. (2004) Bacterial subversion of lipid rafts. Curr. Opin. Microbiol. 7, 4–10 [DOI] [PubMed] [Google Scholar]
- 40. Mañes S., del Real G., Martínez A. C. (2003) Pathogens. Raft hijackers. Nat. Rev. Immunol. 3, 557–568 [DOI] [PubMed] [Google Scholar]
- 41. Shen-Tu G., Schauer D. B., Jones N. L., Sherman P. M. (2010) Detergent-resistant microdomains mediate activation of host cell signaling in response to attaching-effacing bacteria. Lab. Invest. 90, 266–281 [DOI] [PubMed] [Google Scholar]
- 42. Brumell J. H., Scidmore M. A. (2007) Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol. Mol. Biol. Rev. 71, 636–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sturgill-Koszycki S., Schaible U. E., Russell D. G. (1996) Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15, 6960–6968 [PMC free article] [PubMed] [Google Scholar]
- 44. Perskvist N., Roberg K., Kulyté A., Stendahl O. (2002) Rab5a GTPase regulates fusion between pathogen-containing phagosomes and cytoplasmic organelles in human neutrophils. J. Cell Sci. 115, 1321–1330 [DOI] [PubMed] [Google Scholar]
- 45. Baldeón M. E., Ceresa B. P., Casanova J. E. (2001) Expression of constitutively active Rab5 uncouples maturation of the Salmonella-containing vacuole from intracellular replication. Cell Microbiol. 3, 473–486 [DOI] [PubMed] [Google Scholar]
- 46. Rzomp K. A., Scholtes L. D., Briggs B. J., Whittaker G. R., Scidmore M. A. (2003) Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect. Immun. 71, 5855–5870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sachse M., Urbé S., Oorschot V., Strous G. J., Klumperman J. (2002) Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell 13, 1313–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang L. H., Rothberg K. G., Anderson R. G. (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hölttä-Vuori M., Vainio S., Kauppi M., Van Eck M., Jokitalo E., Ikonen E. (2012) Endosomal actin remodeling by coronin-1A controls lipoprotein uptake and degradation in macrophages. Circ. Res. 110, 450–455 [DOI] [PubMed] [Google Scholar]
- 50. Gopaldass N., Patel D., Kratzke R., Dieckmann R., Hausherr S., Hagedorn M., Monroy R., Krüger J., Neuhaus E. M., Hoffmann E., Hille K., Kuznetsov S. A., Soldati T. (2012) Dynamin A, Myosin IB and Abp1 couple phagosome maturation to F-actin binding. Traffic 13, 120–130 [DOI] [PubMed] [Google Scholar]