Background: How sperm sterol removal enables acrosome exocytosis (AE) is unclear; phospholipase B (PLB) is enriched in sperm membrane rafts.
Results: Sterol removal leads to proteolytic activation of PLB, stimulating the initiation of changes leading to AE.
Conclusion: PLB activation plays an important role in fertilization.
Significance: We identify a mechanism for how sterol removal enables AE, consistent with new, zona pellucida-independent models for membrane fusion.
Keywords: Fertilization, Membrane Lipids, Phospholipase B, Serine Protease, Sperm, Acrosome Exocytosis, Acrosome Reaction, Capacitation, Raft
Abstract
Despite a strict requirement for sterol removal for sperm to undergo acrosome exocytosis (AE), the mechanisms by which changes in membrane sterols are transduced into changes in sperm fertilization competence are poorly understood. We have previously shown in live murine sperm that the plasma membrane overlying the acrosome (APM) contains several types of microdomains known as membrane rafts. When characterizing the membrane raft-associated proteomes, we identified phospholipase B (PLB), a calcium-independent enzyme exhibiting multiple activities. Here, we show that sperm surface PLB is activated in response to sterol removal. Both biochemical activity assays and immunoblots of subcellular fractions of sperm incubated with the sterol acceptor 2-hydroxypropyl-β-cyclodextrin (2-OHCD) confirmed the release of an active PLB fragment. Specific protease inhibitors prevented PLB activation, revealing a mechanistic requirement for proteolytic cleavage. Competitive inhibitors of PLB reduced the ability of sperm both to undergo AE and to fertilize oocytes in vitro, suggesting an important role in fertilization. This was reinforced by our finding that incubation either with protein concentrate released from 2-OHCD-treated sperm or with recombinant PLB peptide corresponding to the catalytic domain was able to induce AE in the absence of other stimuli. Together, these results lead us to propose a novel mechanism by which sterol removal promotes membrane fusogenicity and AE, helping confer fertilization competence. Importantly, this mechanism provides a basis for the newly emerging model of AE in which membrane fusions occur during capacitation/transit through the cumulus, prior to any physical contact between the sperm and the oocyte's zona pellucida.
Introduction
Mammalian sperm undergo functional maturation in the female reproductive tract. This process, known as “capacitation” (1, 2), is a prerequisite for sperm to undergo acrosome exocytosis (AE).2 Although there are some differences among species, several stimuli for capacitation are highly conserved, including the influx of bicarbonate and calcium and the removal of sterols, sometimes referred to as sperm sterol efflux (3). Multiple downstream events in capacitation have been described (4). However, the mechanisms by which stimuli, such as sterol removal, are transduced into changes in fertilizing ability have remained unclear.
This question is of particular importance because of the recent emergence of a new model for AE. Previously, it was thought that the physical interaction of a capacitated sperm with the egg's zona pellucida (ZP) triggered fusion of the plasma membrane overlying the acrosome (APM) with the outer acrosomal membrane. This change from an “acrosome-intact” to an “acrosome-reacted” state resulted in release of the contents of the acrosome, facilitating sperm penetration of the ZP. This binary model has been challenged by data generated from experiments on sperm expressing green fluorescent protein in the acrosome. Using this tool, it was first found that contents of the acrosomal matrix were exposed and released in a series of regulated steps suggesting multiple fusion events (5). More recently, another group has followed the fluorescence status of the fertilizing sperm during in vitro fertilization. Jin et al. (6) found that the fertilizing sperm lost fluorescence in the acrosome (i.e. they had “acrosome-reacted” in the terminology of the old model) before they encountered the ZP. These findings have led to a new model for AE that raises substantive new questions regarding the molecular nature of the trigger for these membrane fusion events.
AE can be triggered by plasma membrane accumulation of lysophosphatidylcholine that is generated by phospholipase A2 (PLA2) activity in the presence of calcium (7, 8). A recent study demonstrated that group X calcium-dependent PLA2 is present in the acrosome, is secreted, and is involved in spontaneous AE in damaged sperm (9). However, the nature of the phospholipase(s) involved in physiologically stimulated AE has remained uncertain.
Because of the importance of lipids in regulating sperm function, we and others have focused much attention on their organization in the sperm plasma membrane. Briefly, it is known that the APM is highly enriched in sterols and the ganglioside GM1 (10, 11), yet this region is not homogeneous; rather, it is composed of multiple, dynamic microdomains (12), including membrane rafts, that are highly enriched in sterols, gangliosides, and functional proteins (13). Membrane rafts play important roles in the regulation of diverse cellular processes (14, 15). We have shown not only that murine sperm possess membrane rafts (16) but also that there are at least three raft subtypes differing reproducibly in their lipid and protein compositions (17).
In our proteomic characterization of these raft subtypes, we identified PLB on the basis of multiple specific peptide sequences (18). PLB is an integral membrane enzyme that is able to hydrolyze both the sn-1 and sn-2 acyl ester bonds of glycerophospholipids, displaying calcium-independent PLA1, PLA2, and lysophospholipase activities (19). To date, PLB expression has been reported in several mammalian tissues, such as intestine (20), epididymis (21), epidermis (22), and testis (23). Although studies of pancreatic insufficiency in rats indirectly suggested participation of PLB in the digestion of dietary lipids (24), physiological functions of mammalian PLB have remained understudied. In contrast, it has been shown in a pathogenic fungus that PLB is concentrated in membrane rafts, that its activity is increased markedly upon release from those rafts, and that this activity could play an important role in adhesion and invasion of the pathogens to host cells (25). This constellation of factors prompted us to investigate the functional roles of PLB in murine sperm.
In this study, we demonstrate that PLB is present in sperm membranes and that its active catalytic domain is proteolytically released into the extracellular fluid in response to sterol removal. Using a competitive PLB inhibitor, we show that PLB activation plays an important role in murine AE and fertilization. We confirmed these findings by showing that recombinant active PLB stimulated AE in capacitated sperm. Together, our results provide new insight into the molecular mechanism through which sterol removal enables AE and promotes fertilizing ability in murine sperm. (Hereafter, PLA1 and PLA2 designate calcium-independent phospholipase A1 and A2 unless otherwise noted.)
EXPERIMENTAL PROCEDURES
Reagents and Animals
All reagents were purchased from Sigma, unless otherwise noted.
Selective substrates for PLA1 and PLA2 were purchased from Invitrogen (PED-A1 and PED6). Polyclonal antibody to the rat N-terminal region of PLB was purchased from Santa Cruz Biotechnology, Inc., and polyclonal antibody to the human internal region of recombinant PLB was from Abnova (Taipei City, Taiwan). Polyclonal antibody to testisin was purchased from Santa Cruz Biotechnology. “Complete” protease inhibitor mixture was purchased from Roche Applied Science. Furin-specific inhibitors, Dec-RVKR-CMK and hexa-d-arginine, were purchased from EMD Chemicals (Gibbstown, NJ). All animal work was performed with the approval of Cornell University's Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Testicular RNA Analysis
Mouse testes were collected from CD-1 neonates at postnatal days 4–29 and stored in liquid nitrogen. Total RNA was isolated using TRI reagent and was treated with DNase I to deplete genomic DNA. For RT-PCR, 0.3 μg of testis RNA was utilized for first strand synthesis using Superscript III (Invitrogen). For subsequent PCR, 60 ng of cDNA was used with Go Taq Green Master Mix (Promega). The PCR products were separated using a 1% agarose gel containing ethidium bromide and visualized by UV illuminator.
Sperm Collection
Murine sperm were collected from the cauda epididymides of retired breeder CD-1 mice by the swim-out procedure in a modified Whitten's medium (22 mm HEPES, 1.2 mm MgCl2, 100 mm NaCl, 4.7 mm KCl, 1 mm pyruvic acid, 4.8 mm lactic acid hemicalcium salt, pH 7.3) as described previously (26). All procedures of the collection and washing were performed at 37 °C using large orifice pipette tips to minimize damage to the sperm membranes.
Biochemical Characterization
Membrane linkage of PLB was examined through use of phosphatidylinositol-specific phospholipase C (PI-PLC), which cleaves glycosylphosphatidylinositol (GPI)-anchored proteins and a Triton X-114 phase separation assay (27). To obtain soluble fractions containing proteins previously anchored by GPI linkages, sperm (30 million) were incubated with 5 IU/ml PI-PLC for 30 min at 37 °C and ultracentrifuged at 100,000 × g for 1 h. The soluble fractions were boiled in Laemmli sample buffer (28) prior to SDS-PAGE and immunoblotted with antibodies against PLB (Santa Cruz Biotechnology) and testisin. For Triton X-114 phase separation, sperm (30 million) were sonicated at output 2 with 10 short bursts in PBS containing protease inhibitor mixture, using a Branson Sonifier 450 (Branson Ultrasonics Corp., Danbury, CT). The samples were centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatant was mixed with 10% (v/v) Triton X-114 to give 2% (v/v) final. The samples were incubated at 4 °C for 30 min and at 37 °C for 3 min sequentially. After partitioning of vesicles with associated lipid-interacting or transmembrane proteins from soluble/peripheral membrane proteins by centrifugation at 1,000 × g for 10 min, the proteins in the resultant aqueous and detergent-enriched fractions were extracted by protein precipitation with 10% (v/v) TCA and subjected to immunoblotting as described above. To confirm the presence of full-length PLB in sperm, whole sperm (10 million) were processed for SDS-PAGE as described above and immunoblotted with PLB antibody (Abnova).
Preparation of Subcellular Fractions
Sperm (2 × 106 cells/300 μl) were incubated under non-capacitating conditions (modified Whitten's medium supplemented with 5.5 mm glucose) for 1 h. Sterol removal was induced by the addition of 3 mm 2-OHCD to the incubating condition. Separation of subcellular fractions was performed as described previously (29) with some modifications. After the incubation with 2-OHCD, sperm were ultracentrifuged at 100,000 × g for 1 h to separate a fraction consisting of released proteins (released). The remaining pellets of sperm and any membrane vesicles were resuspended and lysed via sonication in modified Whitten's medium plus protease inhibitor mixture. The cell lysates were centrifuged at 10,000 × g to separate a fraction representing cytoskeletal elements, organelles, and any remaining whole cells or cell fragments (pellet). The supernatants were ultracentrifuged at 100,000 × g for 1 h, yielding cytosolic and membrane fractions. Protein concentrations in the fractions were measured by the Bradford assay (Thermo Fisher Scientific) and normalized between replications if necessary.
To concentrate protein in the released fraction, 6 ml of this fraction were centrifuged using Amicon Ultra-4 centrifugal filter units (10,000 molecular weight cut-off; Millipore (Billerica, MA)) at 3,700 × g for 20 min. The protein concentrates were washed by resuspension with 4 ml of PBS and centrifugation. Protein amounts in the different fractions were quantified and utilized for immunoblots with PLB antibody (Abnova), gel-based enzyme extraction under non-reducing conditions, or AE assays, following normalization of protein amounts and concentration by dilution in PBS.
Fluorometric Assay of PLA1 and PLA2 Activities
Spectrofluorometry was utilized to assess PLA1 and PLA2 activities. PED-A1 and PED6 are conjugated with BODIPY at the sn-1 and sn-2 positions, respectively, and in both reagents, the fluorescence is inhibited by an adjacent quencher molecule. These reagents are designed to release a fluorescent metabolite only in response to specific hydrolysis by either PLA1 (30) or PLA2 (31) activity. The substrate stock solutions were prepared following the manufacturer's instructions and solubilized in a reaction buffer (50 mm Tris-HCl, 100 mm NaCl, and 1 mm EGTA, pH 8.0) at 5 μm final concentration for every use. Equal volumes (20 μl) of released (15 μg of protein), pellet (3 μg of protein), cytoplasm (2 μg of protein), or membrane (2 μg of protein) fractions were mixed with 30 μl of reaction buffer in a 96-well microplate. For 15 min following the addition of 50 μl of either substrate, we monitored fluorescence at 37 °C with scanning at every 2 min with a Safire microplate reader (Tecan, Mannedorf, Switzerland) with an excitation wavelength at 488 nm and an emission wavelength at 530 nm. All raw data from kinetic measurements were normalized by dividing them by the signal intensity obtained at the first reading point (1 min).
Separation and Extraction of Enzyme Activity with Polyacrylamide Gel Electrophoresis
PLB enzyme activity from the released fractions was extracted as described previously (32), with the exception that a native gel was used. Protein concentrates from the released fractions of sperm incubated under non-capacitating conditions with or without 2-OHCD were incubated under non-reducing conditions at 37 °C for 30 min and electrophoretically separated using a 7.5% polyacrylamide gel. When the dye front for each sample came to the bottom of the gel, the gel was taken out of the apparatus and cut into 17 pieces (6.4 mm each), which were individually minced and immersed in 200 μl of extraction buffer (0.25 m Tris-HCl, 0.25 m NaCl, pH 8.5). The samples were extracted overnight at 4 °C and then utilized for activity assays as described above, except that the hydrolytic reaction was performed using 50 μl of extract and monitored for 1 h.
Immunostaining
For immunohistochemistry, murine testicular sections were prepared as described previously (17). The samples were blocked with 10% normal goat serum for 1 h. The sections were then incubated with PLB antibody (Santa Cruz Biotechnology) in PBS (1:100) overnight at 4 °C, washed, and then incubated with Alexa Fluor-594 IgG for 1 h at room temperature. Nuclear staining was performed with SYTOX Green (Invitrogen). Coverslips were mounted using ProLong Gold Antifade (Invitrogen).
Subcellular localization of PLB was examined in sperm fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in PBS containing 5 mm calcium chloride. We have shown that this fixative preserves the integrity of sperm plasma membranes and the localization of lipid components of membrane domains (11). Because a previous study (23), as well as our present results, suggested that PLB is enriched in the acrosomal membranes, which are in close apposition with the APM, this fixative offers us the ability to distinguish localization to the plasma membrane versus intracellular membranes by contrasting fluorescence between intact and permeabilized cells. After washing or incubation for 1 h under the desired conditions, sperm were fixed for 30 min at room temperature and then blocked and labeled with PLB antibody (Santa Cruz Biotechnology) as described above. The samples were incubated with Alexa Fluor-594 IgG and Alexa Fluor-488 peanut agglutinin (PNA) for 1 h at room temperature. To permeabilize the cell membranes, fixed sperm were sonicated for three short bursts with a Branson Sonifier 450 prior to immunostaining. Cells were then examined using fluorescence microscopy (Axio Observer, Carl Zeiss, Gottingen, Germany).
To examine potential changes in PLB localization between non-capacitated sperm and sperm after sterol removal, at least 200 sperm per trial that were PNA-negative (consistent with intact APM domains) were utilized for quantification of PLB localization. Sperm were categorized as having one of the following four observed patterns: no signal, signal overlying the apical acrosomal region, signal in the APM, and signal that was diffuse over the sperm head. Results were converted into percentages for comparison among experiments and between groups and for plotting.
Preparation of Recombinant PLB Peptide
cDNA encoding the consensus catalytic domain of PLB (amino acids Met366–Ser711) was amplified using PCR and cloned in pcDNA 3.1/V5-His TOPO vector (Invitrogen) designed to insert a C-terminal V5 and polyhistidine tag. The cloned PLB fragment was also inserted into pcDNA 4/HisMax TOPO vector (Invitrogen) to add an N-terminal polyhistidine tag, with a stop codon introduced just after Ser711. Both constructs were validated by restriction enzyme digestion and sequencing and transfected into HEK293 cells (FreeStyle 293-F cells, Invitrogen). Transfection was also performed without the vectors for preparation of control protein. The cells were lysed in xTractor buffer (Clontech) at 72 h post-transfection, and the His-tagged proteins were purified using a nickel-nitrilotriacetic acid purification system (Invitrogen). Resultant fractions (cell lysate, flow-through, elution, and elution of control protein) were used for immunoblots to confirm the identity of the recombinant peptides. The purified peptides were concentrated using Amicon Ultra 0.5-ml centrifugal filter units (10,000 molecular weight cut-off; Millipore), and imidazole buffer was replaced with PBS.
AE Assay
The effects of inhibition of PLB in murine sperm were examined using palmitoyl dl-carnitine (dl-carnitine) and palmitoyl l-carnitine (l-carnitine). Note that although no truly specific inhibitors are yet available for studies of PLB, dl- and l-carnitine are preferred because they inhibit both the PLA1 and PLA2 activities of fungal PLB (33, 34). Sperm were incubated with 0, 0.5, 5, 50, or 100 μm dl-carnitine under non-capacitating or capacitating conditions for 50 min at 37 °C. The samples were treated with 20 μm progesterone (P4) for 20 min to induce AE and then fixed with 4% paraformaldehyde. The samples were washed with 100 mm ammonium acetate (pH 9.0), and air-dried on slides. The samples were stained with Coomassie Brilliant Blue G-250 and observed under a bright field microscope (×400). A total of at least 200 sperm for each sample were assessed for status of the acrosome as described (35). The results were converted into percentages of sperm that underwent AE to facilitate comparisons between groups and conditions.
To examine the effect of PLB on initiation of AE, sperm were incubated for 40 min under non-capacitating or capacitating conditions at 37 °C. The sperm suspensions were then incubated for an additional 20 min in the presence of protein concentrates (2.5 μg) from the released fraction of other sperm previously incubated under non-capacitating or capacitating conditions. After this incubation, the sperm were either fixed immediately or incubated further with 20 μm P4 for 20 min to induce AE as a positive control. In a similar set of experiments, sperm were capacitated for 40 min and then incubated with 5 μg of recombinant PLB catalytic domain tagged with N-terminal polyhistidine (N-PLB) or P4 for 20 min, followed by fixation. Coomassie Blue staining and assessment of acrosomal status were performed as described above.
In Vitro Fertilization (IVF)
Superovulation and oocyte collection were performed as described previously (36). Oocyte-cumulus complexes (15–25 oocytes) were placed in 200 μl of TYH medium (37) covered with mineral oil. Cauda epididymal sperm were collected from CD-1 mice and preincubated in TYH medium supplemented with or without 50 μm dl-carnitine for 1.5 h for capacitation at 37 °C, 5% CO2. Oocyte-cumulus complexes were inseminated with the capacitated sperm at 1 million sperm/ml final concentration in TYH medium supplemented with or without 50 μm dl-carnitine. After 6 h of co-incubation, eggs were rinsed briefly and further incubated in KSOM medium (38) for 16 h. Embryo development to a 2-cell stage was considered as successful fertilization. IVF and additional culture for early development were performed in a chamber with an atmosphere of 5% O2, 5% CO2, and 90% N2 at 37 °C.
Statistical Analysis
Statistical analysis for multiple comparisons was performed with the Tukey-Kramer method when Bartlett's test and the Kolmogorov-Smirnov/Lilliefor test confirmed both the equal variance and normality assumptions. Pairwise comparisons were performed with the Kolmogorov-Smirnov test.
RESULTS
Localization and Characterization of PLB in Murine Sperm
Although PLB was previously localized to the developing acrosome in rat round spermatids (23), to date there have been no biochemical or functional characterizations of PLB during spermatogenesis or in mature sperm. Previously, our proteomic study found that PLB localized in sperm membrane raft subtypes isolated without the use of detergent (18), consistent with a role for PLB in sperm membrane function. Our immunohistochemistry with murine testis confirmed that PLB localized to the developing acrosome (Fig. 1A, n = 3 trials), consistent with the rat. PLB also localized to residual cytoplasmic droplets observed in the lumens of seminiferous tubules (Fig. 1A). The presence of PLB in sperm was confirmed by immunoblots showing a 165 kDa immunoreactive band (Fig. 1B, n = 4 trials), consistent with the size of the full-length protein described in a previous study (23). In morphologically mature sperm, indirect immunofluorescence revealed that PLB also resided in the APM (Fig. 1C, n = 3 trials). Plasma membrane integrity in those sperm was confirmed by use of fluorescent PNA (39). As expected from the localization during germ cell development, PLB was also detected in the acrosome when the plasma membrane was disrupted by sonication (Fig. 1C).
FIGURE 1.
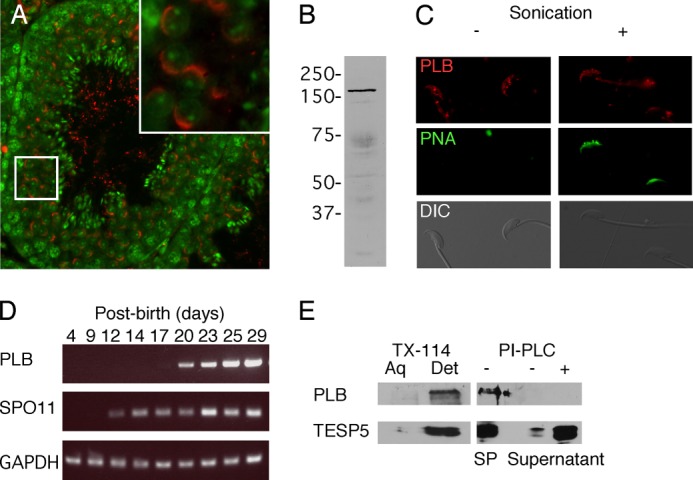
Characterization of PLB in murine germ cells. PLB localized to the acrosome in round spermatids (A). The presence of PLB in a cell lysate from whole murine sperm was verified by immunoblot, showing a specific protein band around 165 kDa (B). Localization of PLB in the APM macrodomain of the plasma membrane was determined in sperm fixed with a protocol shown to leave the plasma membrane intact (11). In this experiment, sperm with intact membranes were negative for PNA staining but showed signal for PLB in the APM and, to varying degrees, in the midpiece. Fixed sperm were briefly sonicated to permeabilize the membranes, revealing PNA fluorescence (C). RT-PCR of RNA collected from mouse testes at various days of postnatal development revealed that expression of PLB mRNA was first faintly detectable on day 17 (D), temporally consistent with the appearance of round spermatids. The nature of PLB linkage to membranes was evaluated by Triton X-114 (TX-114) phase separation and PI-PLC cleavage assay (E). PLB and testisin (TESP5) were tightly associated with membranes, appearing in the detergent (Det) fraction of a Triton X-114 phase separation assay. However, partitioning of PLB to the sperm pellet (SP) in a PI-PLC cleavage assay showed that PLB behaved as a transmembrane protein, compared with the GPI-linked TESP5 (42). DIC, differential interference contrast.
The timing of PLB mRNA expression was determined by RT-PCR using RNA extracted from neonatal mice at different days post-parturition (n = 3 trials). Our results (Fig. 1D) showed that mRNA expression of PLB started at postnatal day 17, in accordance with the appearance of the developing acrosome (40). As a control, SPO11 was expressed at postnatal day 12, consistent with a previous report (41).
The nature of PLB membrane linkage was examined by comparing the results of Triton X-114 phase separation and PI-PLC cleavage assays. Testisin, a GPI-anchored protein abundant in sperm (42), was used as a control (n = 3 trials). When sperm membranes and soluble proteins were treated with Triton X-114, almost all of both PLB and testisin partitioned into the detergent-enriched fraction containing proteins that are either integral to the membranes or tightly associated with them (Fig. 1E). However, unlike testisin, the PI-PLC treatment did not release PLB into the supernatant (Fig. 1E). Because PLB is also highly expressed in the epididymis (21), where sperm acquire several peripheral membrane proteins, we also performed protein extraction with high salt conditions (1 m NaCl or 1 m KCl) to remove most peripheral membrane proteins. In these experiments, PLB immunoreactivity was again not released into the supernatant (data not shown). These results suggest that PLB is a transmembrane protein, in agreement with a previous study of mammalian PLB (32) and in contrast to the GPI-linked fungal forms (43).
Detection of PLA1 and PLA2 Activities in Subcellular Fractions
Capacitation is a prerequisite for sperm to acquire the ability to undergo AE. To investigate whether PLB might play a role in this process, we prepared subcellular fractions after incubating sperm under either non-capacitating or capacitating conditions. We then performed kinetic measurements of PLA1 and PLA2 activities using selective fluorescent substrates (n = 4 trials) (19, 44, 45). There were slight but statistically significant differences in the pellet, with capacitated sperm showing higher activity versus sperm incubated under non-capacitating conditions or sperm that were not incubated (Fig. 2) (p < 0.05, later than 7 min). Similarly, the membrane fraction of sperm incubated under non-capacitating conditions showed higher PLA2 activity than the membranes of capacitated sperm (p < 0.05, later than 9 min), although in this case, the difference was not significant when compared with sperm without incubation (Fig. 2). However, there was a significant increase in PLA2 activity that was released from capacitated sperm in comparison with non-capacitated sperm or sperm assayed without incubation (p < 0.05, between 3 and 7 min; p < 0.005, later than 9 min). Similar results were obtained for all fractions when PLA1 activity was measured (data not shown). These results suggested that most of the active PLB was released into the extracellular fluid during capacitation, although a small subset of active PLB was present in the pellet and membrane fractions.
FIGURE 2.
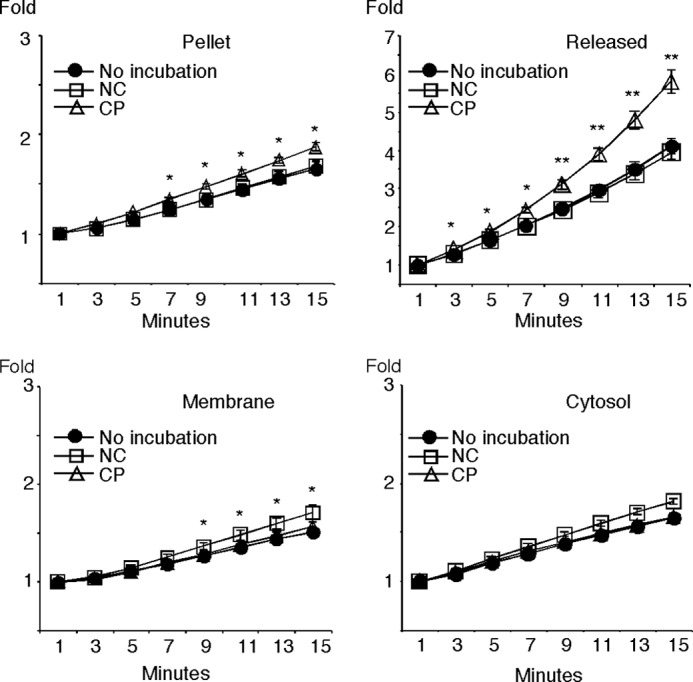
PLA2 activity in subcellular fractions. Murine sperm were incubated for 1 h under non-capacitating conditions (NC) or capacitating conditions (CP, which included bicarbonate and 2-OHCD) and then fractionated into pellet, released, membrane, and cytosol fractions, as indicated. Sperm taken at the start of the experiment that were not incubated under any condition were processed as a control (No incubation). The assay was performed with a fluorescent PLA2 substrate in the presence of EGTA, a Ca2+ chelator, to confirm the Ca2+-independent nature of the enzyme activity. *, p < 0.05; **, p < 0.005 in the comparison between non-capacitating and capacitating conditions.
Regulatory Mechanism of PLB Activation
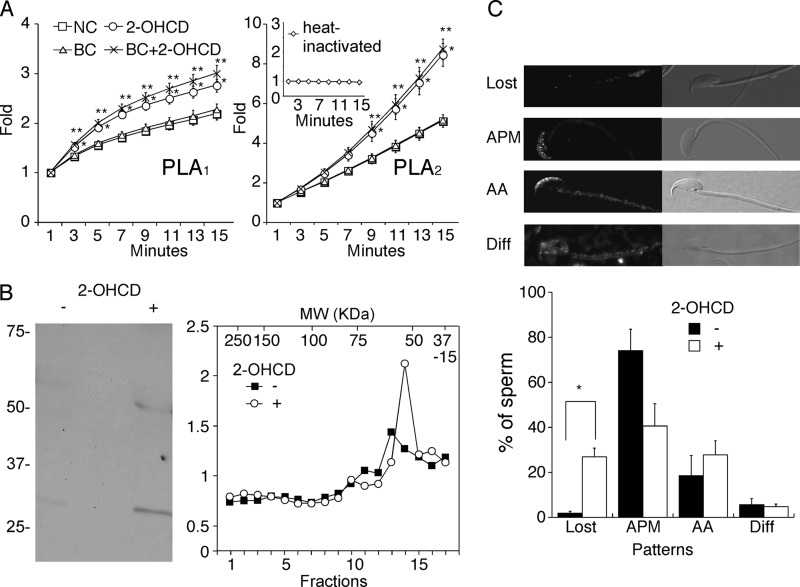
The release of both PLA1 and PLA2 activities suggested that PLB was the origin of these activities. We therefore sought to understand the mechanism of release and the identity of the enzyme(s) responsible in more detail. Because bicarbonate and 2-OHCD can act on different pathway components to stimulate capacitation, we examined whether these stimuli could individually activate PLB by measuring PLA1 and PLA2 activities in the released fractions, using selective substrates (n = 7 trials). We found that both PLA1 and PLA2 activities were higher in fractions from sperm incubated with 2-OHCD when compared with released fractions from sperm incubated in its absence (Fig. 3A, p < 0.05). When the fraction of sperm incubated with 2-OHCD alone was heat-inactivated (90 °C for 30 min), PLA2 activity was completely abolished, confirming that the activity originated from a protein (Fig. 3A; PLA1 activity was also abolished; data not shown). Of interest, the kinetics of the PLA1 and PLA2 activities were different, and this point is discussed below. These data demonstrated that sterol removal was a common trigger for release of both PLA1 and PLA2 activities.
FIGURE 3.
Regulation of PLB activation by sterol removal. The released fractions from sperm incubated under different conditions (non-capacitating (NC), with bicarbonate (BC), with 2-OHCD, or with bicarbonate + 2-OHCD) were utilized for fluorescent Ca2+-independent PLA1 and PLA2 assays (A, *, p < 0.05 between non-capacitating and 2-OHCD; **, p < 0.05 between bicarbonate and bicarbonate + 2-OHCD). Immunoblot and gel-based extraction for the presence of PLB were performed on the fractions released from sperm with or without 2-OHCD treatment (B). We then sectioned the gel into 17 pieces corresponding with different molecular weight ranges and assayed for PLA2 enzyme activity in the different sections. The results showed Ca2+-independent PLA2 enzyme activity in the section containing the 50 kDa immunoreactive band. Immunostaining for PLB was performed on sperm treated with or without 2-OHCD (C). Sperm were fixed using methods to preserve plasma membrane integrity. The patterns of PLB localization were categorized according to the following criteria: no signal (Lost), plasma membrane overlying the acrosome (APM), apical acrosome (AA), and diffuse (Diff). *, p < 0.05. Error bars, S.E.
To investigate further both the nature of the enzyme and mechanism of release/activation, we performed gel-based size exclusion separation on the fraction released in the presence or absence of 2-OHCD. We examined different molecular weight ranges, looking for both enzyme activity and immunoreactivity (n = 3 trials). Interestingly, PLB immunoreactivity was found at ∼27 and 50 kDa in the fraction from sperm incubated with 2-OHCD alone, whereas both of those bands were absent in the released fraction from non-capacitated sperm (Fig. 3B). Furthermore, when we investigated the different molecular weight ranges for PLA2 activity (n = 3 trials), we found prominent activity in the 50 kDa fraction derived from sperm incubated with 2-OHCD (Fig. 3B). In contrast to this, there was no major increase in the enzyme activity corresponding to the 27-kDa fragment (Fig. 3B), suggesting that the 50-kDa PLB fragment contained a catalytic site but the 27-kDa fragment did not. The molecular sizes of both of these products were much smaller than the full-length PLB found in whole, uncapacitated sperm (Fig. 1B). We saw no evidence of the full-length protein being released or representing activity. Together, these data suggest that sterol removal proteolytically released an activated 50-kDa fragment of PLB.
If active PLB were being released from the APM, we hypothesized that this loss might be detectable using indirect immunofluorescence localization. We therefore examined sperm fixed and labeled with PLB antibody (Santa Cruz Biotechnology) and fluorescent PNA as described above (n = 5 trials). To avoid potential complications caused by PLB within the acrosome, at least 200 sperm that had no PNA signal (signifying an intact plasma membrane) were counted to categorize them into four distinct patterns for PLB, as shown in Fig. 3C. When sperm were incubated with 2-OHCD, PLB signal was lost in more sperm (26.9 ± 4.0%) than in those incubated in its absence (1.8 ± 0.8%, p < 0.05). Although there was some change in APM and apical acrosomal patterns between the treatments, these differences were not statistically significant.
Our results suggesting proteolytic cleavage resulting in release of active PLB fragments are consistent with data from the epididymis (21) and from the intestine (32). However, the endogenous proteases involved have yet to be identified in any tissues. To examine whether the activation of sperm PLB required proteolysis, we incubated sperm in the presence of various protease inhibitors during sterol removal and analyzed PLA1 and PLA2 activities in the released fractions (n = 6 trials). As seen above, both PLA1 and PLA2 activities were significantly increased in fractions released from sperm capacitated in the absence of inhibitors (Fig. 4, p < 0.05). The addition of benzamidine, trypsin inhibitor, and leupeptin did not inhibit the increase of either activity. However, when PMSF or Dec-RVKR-CMK, a furin-specific inhibitor, was present during capacitation, no increase in either activity was found (p < 0.05). Hexa-d-arginine, a furin-specific inhibitor, had this same effect on PLA1 and PLA2 activities (data not shown). Solvent controls showed no effects on activities at the concentrations used (ultrapure, 18-megaohm water for benzamidine, trypsin inhibitor, and leupeptin; isopropyl alcohol for PMSF; DMSO for Dec-RVKR-CMK).
FIGURE 4.
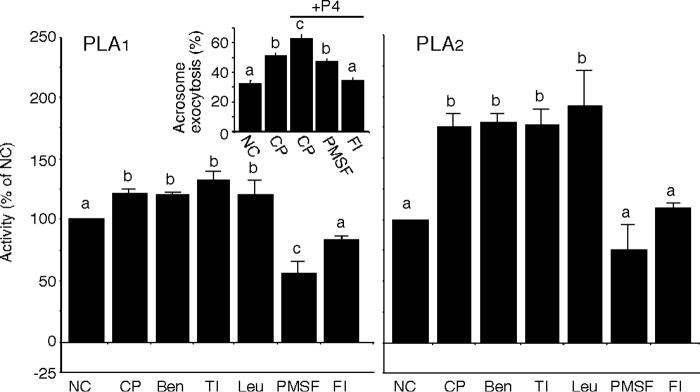
Selective inhibition of PLB activation. Capacitation was initiated in the presence of several protease inhibitors (benzamidine (Ben), trypsin inhibitor (TI), leupeptin (Leu), PMSF, and Dec-RVKR-CMK (FI; a furin-specific inhibitor)). The released fractions were collected and utilized for Ca2+-independent PLA1 and PLA2 activity assays. The addition of PMSF or Dec-RVKR-CMK inhibited the increase of both activities, whereas benzamidine, trypsin inhibitor, and leupeptin did not. Induction of AE in sperm capacitated in the presence of PMSF or Dec-RVKR-CMK and stimulated with P4 was evaluated (inset). Columns with different letters are significantly different (p < 0.05). Error bars, S.E.
To examine the effect of the inhibition of PLA1 and PLA2 activities on the ability of sperm to undergo AE, we evaluated the acrosome status in at least 200 sperm that were capacitated and stimulated with P4, with or without PMSF and Dec-RVKR-CMK (n = 7 trials). The results showed that capacitated sperm underwent a greater percentage of AE (48.7 ± 1.6%) as compared with non-capacitated sperm (32.4 ± 1.8%). When capacitated sperm were stimulated with P4, 62% of sperm underwent AE. However, when sperm were capacitated in the presence of PMSF or Dec-RVKR-CMK, AE was decreased in response to P4 stimulation (Fig. 4, inset, p < 0.05). These results suggested that proteolysis was required for the activation of PLB as well as AE induced by P4 stimulation.
Functional Role of PLB in Murine Fertilization
To examine a potential functional role for PLB during fertilization more directly, we investigated the ability of sperm to undergo AE or to fertilize an egg in the presence of the PLB inhibitor dl-carnitine or l-carnitine (n = 6 trials, respectively). These reagents have been shown to inhibit PLB activities in pathogenic fungi (33, 34). We found in sperm that these inhibitors did not affect sperm motility or tyrosine phosphorylation at concentrations of 100 μm or less (data not shown). When sperm were capacitated and stimulated with P4 in the absence of dl-carnitine, the percentage of sperm that underwent AE (52.8 ± 4.4%) was significantly higher than sperm incubated under non-capacitating or capacitating conditions alone (29.9 ± 2.4 or 40.9 ± 4.4%, respectively; Fig. 5A). Stimulation of AE was inhibited in a dose-dependent fashion by the presence of dl-carnitine. Maximal inhibition in terms of effect on AE was reached at concentrations of 50 μm or higher. Importantly, the percentages of AE seen in the presence of 50 and 100 μm concentrations of the inhibitors were not statistically different from those of sperm incubated under non-capacitating or capacitating conditions without P4 stimulation, suggesting that PLB activity is important in P4-stimulated AE. Similarly, supplementation of l-carnitine also inhibited AE to levels consistent with those when dl-carnitine was added (data not shown). These results suggest a role for PLB activity in induction of AE in response to this physiological stimulation.
FIGURE 5.
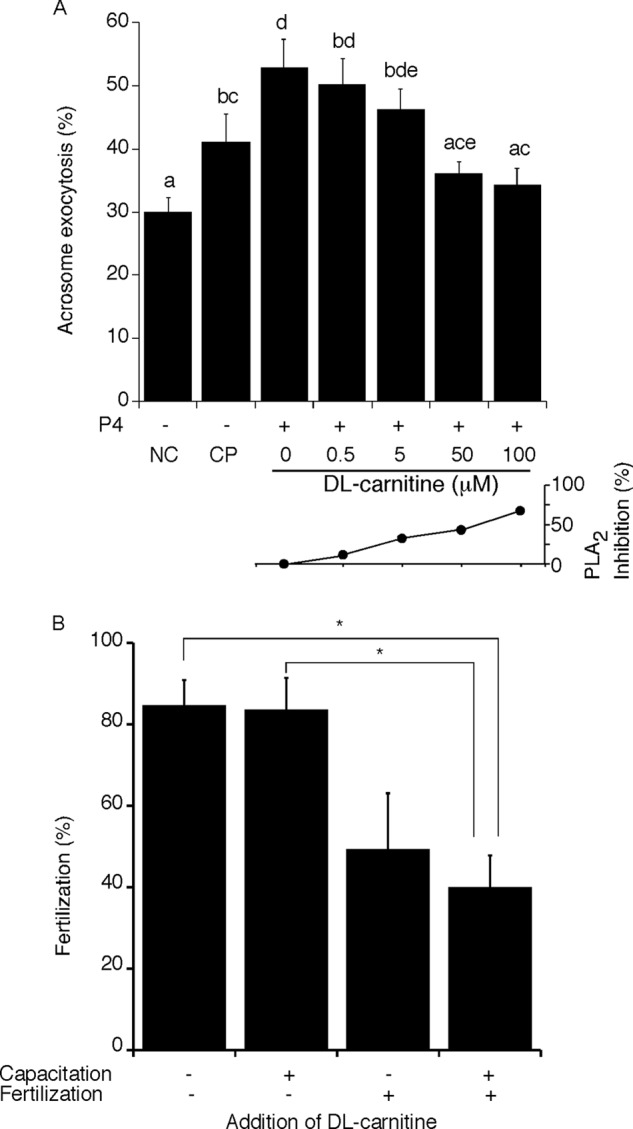
Roles of PLB in AE and fertilization. Sperm were incubated under non-capacitating (NC) or capacitating (CP) conditions, in the presence of 0–100 μm dl-carnitine, a competitive PLB inhibitor, and then stimulated with P4. The status of the acrosome was examined using Coomassie Blue staining. We found that P4-induced AE was inhibited at concentrations of 50–100 μm (A, top). Columns with different letters are significantly different (p < 0.05), whereas columns with the same letter are not significantly different. Multiple letters above a column reflect the comparative statistical relationships between that column and all other columns. Dose-dependent inhibition of Ca2+-independent PLA2 activity was observed in the presence of dl-carnitine (A, bottom). The impact of PLB inhibition on fertilization competence was examined by IVF. Sperm were preincubated for capacitation and then utilized for insemination in the presence (+) or absence (−) of 50 μm dl-carnitine. Fertilization success was halved when the inhibitor was present during both capacitation and fertilization (B). *, p < 0.05. Error bars, S.E.
Based on these results, we performed IVF in the presence or absence of 50 μm dl-carnitine. We exposed the sperm to this inhibitor either during a capacitating preincubation alone, during the fertilization time period alone, or during both the capacitating incubation and the time allowed for fertilization. When dl-carnitine was added during the preincubation period alone, there was no difference in the fertilization rate (83.3 ± 8.0%), compared with a control (88.4 ± 4.1%; Fig. 5, bottom). However, fertilization was inhibited when the inhibitor was present during sperm-egg co-incubation (56.9 ± 12.2%) and was inhibited at a statistically significant level when present both during preincubation and insemination (42.8 ± 8.0%, p < 0.05). To rule out the possibility that the oocyte or events of egg activation were affected, we also examined the developmental competence of those embryos that were produced in the presence of dl-carnitine. We found that more than 90% of 2-cell embryos developed to the blastocyst stage (data not shown). These data suggest that PLB exerted an important role in fertilization by facilitating AE.
Underlying Mechanism for the Involvement of PLB Activity in Initiation of AE
The decrease in P4-stimulated AE by PLB inhibitors suggested that PLB activation is involved in induction of AE by this physiological agonist. Recent studies investigating membrane changes during capacitation showed that membrane fusion events occur late during capacitation and that AE involves multiple steps, culminating in the differential release of acrosomal contents (5, 46). To understand better the mechanisms underlying capacitation-associated changes in membrane fusibility, we examined roles for PLB activity in the initial process of AE. To do this, we evaluated the acrosome status in at least 200 sperm that were capacitated in the presence of proteins released from other sperm that were themselves incubated under non-capacitating or capacitating conditions (n = 11 trials). AE in capacitated sperm without any supplementation was significantly increased (49.4 ± 2.4%), compared with that of sperm incubated under non-capacitating conditions (35.0 ± 3.0%; Fig. 6). When sperm were capacitated in the presence of protein concentrate released from non-capacitated sperm, there was no significant difference in AE (56.0 ± 2.3%) from that of capacitated sperm without supplementation (49.4 ± 2.4%). In contrast, a significant increase was observed when sperm were capacitated in the presence of proteins released from 2-OHCD-treated sperm (64.8 ± 2.2%). When sperm that were capacitated without any released fractions were stimulated with P4, AE was also increased (59.9 ± 1.9%), compared with capacitated sperm of no supplementation (49.4 ± 2.4%). There was no significant increase when sperm were capacitated and stimulated with P4 in the presence of the proteins released from non-capacitated sperm (62.4 ± 2.0%). Sperm that were capacitated in the presence of proteins released from 2-OHCD-treated sperm and stimulated with P4 had very similar rates of AE (70.7 ± 2.1%) when compared with sperm similarly treated except lacking the P4 (64.8 ± 2.2%). Results from this experiment alone suggest that one or more factors released from sperm plays an important role in initiation of AE. In conjunction with our previous results (above), these data are consistent with released PLB activity being an important component in stimulation of AE.
FIGURE 6.
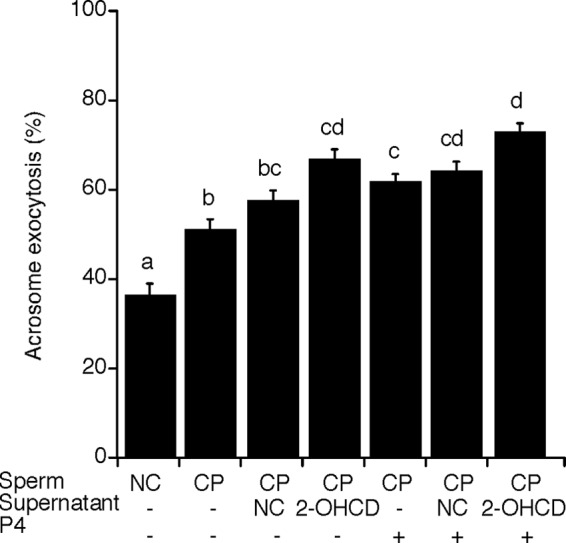
Supernatants from sperm that underwent sterol removal increased AE in other sperm. Sperm were initially incubated under non-capacitating (NC) or capacitating (CP) conditions for 40 min and then incubated for 20 min in the presence of proteins released from sperm that were themselves incubated under non-capacitating conditions or in the presence of 2-OHCD. The status of the acrosome was examined before or after P4 stimulation for 20 min. For non-capacitating sperm, the incubation was continued for 80 min total. Columns with different letters are significantly different (p < 0.05), whereas columns with the same letter are not significantly different. Multiple letters above a column reflect the comparative statistical relationships between that column and all other columns. Error bars, S.E.
However, from those data alone, it cannot be ruled out that released proteins other than PLB might contribute to the initiation of AE in conjunction with or separate from the PLB activity. To test whether PLB activity would be sufficient on its own to enable or induce AE, we generated N-PLB. We also made a recombinant PLB with a C-terminal tag, but this had lower enzymatic activity, so it was not used for further experimentation (data not shown). Immunoblots confirmed the expression, correct size, and identity of the N-PLB peptide (Fig. 7A). We tested whether this peptide, which corresponds approximately to the predicted proteolytic, catalytically active fragment (32), could stimulate the initiation of AE by evaluating the acrosome status of at least 200 sperm (n = 7 trials). When sperm that were capacitated for 40 min were treated with P4 or N-PLB for 20 min, the percentages of sperm undergoing AE were significantly increased (56.3 ± 2.3 and 58.9 ± 2.0%, respectively; Fig. 7B), compared with sperm capacitated for 60 min (47.8 ± 1.7%). No increase was observed when capacitated sperm were treated with control proteins prepared from transfected cells with no vector (45.5 ± 1.6%). These results corroborated our finding that activation of PLB is required for initiation of AE during capacitation.
FIGURE 7.
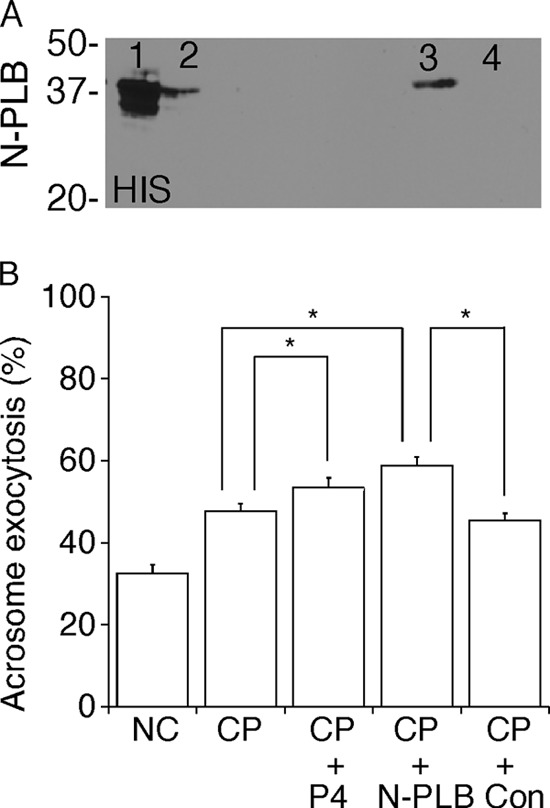
Stimulation of AE by recombinant PLB peptides. Constructs of the consensus catalytic domain of PLB (Met366–Ser711) were made with a N-PLB. Immunoblots were performed on the following fractions: cell lysates (lane 1), flow-through (lane 2), elution (lane 3), and elution from control cell lysates (lane 4). The N-PLB construct was verified using immunoblots to confirm immunoreactivity to anti-His antibody (A) and molecular size. Sperm were capacitated for 40 min and then incubated with P4, 5 μg of N-PLB, or 5 μg of protein from control cells for 20 min (B). A total of at least 200 sperm were assessed for evaluation of acrosome status for each tested condition, and a total of seven trials were performed. The results showed that N-PLB stimulated AE in capacitated sperm, whereas the control protein did not. *, p < 0.05.
DISCUSSION
Despite a strict requirement for sterol removal for sperm to undergo AE and fertilize an egg, how this change in membrane lipid composition changes sperm function has remained poorly understood. Results presented here on the activity of PLB in sperm not only address this longstanding question but also provide a mechanism for a controversial new model by which physiological AE is induced in the absence of physical contact with the ZP.
Our previous finding that PLB is present in sperm membrane rafts (18) suggested the possible involvement of this enzyme. Although functional roles for PLB remain unclear in any mammalian cell, PLB plays a central role in membrane fusion for several pathogenic bacteria and fungi (19, 47, 48). Of special relevance to our hypothesized role in sperm AE, a subtype of PLB, PLB1, localizes to membrane rafts and is activated by sterol removal with release into the extracellular space (25). Although there are both similarities and differences in the molecular characteristics of PLB among kingdoms and species, these data led us to investigate roles of PLB in sperm during fertilization.
Here, we demonstrated the presence of PLB in both the acrosome and plasma membrane overlying the acrosome, positioning it appropriately to take part in AE. We found that sterol removal resulted in the extracellular release of PLB fragments showing both calcium-independent PLA1 and PLA2 activities. As noted above, the kinetic curves for these activities were different; one possible explanation for this difference would be facilitation of the PLA2 activity by the PLA1 activity. Little is known about PLB, and future investigation of the regulation and nature of its activities would be of interest.
As a first test for functional role(s) of these released fragments, we assessed the ability of sperm to undergo AE and fertilize oocytes in the presence or absence of dl- or l-carnitine. These long-chain acyl carnitines have been widely used as potent competitive inhibitors for both PLA1 and PLA2 activities of fungal PLB (33, 34). These compounds have a high structural similarity to dipalmitoyl phosphocholine but cannot be hydrolyzed by phospholipases (33). Dipalmitoyl phosphocholine is highly abundant in lung tissue in mammals and is known as a one of the preferred lipid substrates for both fungal (49) and mammalian PLB (32).
The data from our studies with inhibitors and our finding that proteins released from sperm in response to sterol removal were able to induce AE in other sperm were both highly supportive of an important role for PLB. As we noted above, one might still argue that other factors were also released in response to sterol removal, and it was those unknown factors (also inhibited by the carnitines) that impacted sperm function. We therefore verified a specific functional ability for the PLB fragment through the use of a recombinant peptide.
Our findings combine to suggest strongly that PLB is important at transducing the stimulus of sterol removal into changes in the fusibility of sperm membranes, a critical late step in capacitation that promotes AE. Beyond that, these findings help address a controversy regarding the initiation, timing, and process of AE itself. Previously, binding to the ZP was thought to be requisite for induction of AE in sperm. However, a recent study monitoring sperm penetration during IVF demonstrated that AE began prior to contact with ZP, as sperm moved through the cumulus cells (6). That finding strengthened the developing model that AE is not a binary, “all or nothing” event but is rather a multistep process composed of intermediate stages of membrane fusion and then differential release of acrosomal contents (5, 50–52). To our knowledge, our finding that sterol removal stimulates the induction of AE via activation and release of PLB is the first report to provide a mechanism for this new model of AE induction.
Combining the results of our present study with the known characteristics of PLB and other results from the literature, we have synthesized a working model regarding how sterol removal might be transduced into initiation of AE in murine sperm. The schematic diagram depicts the APM and the outer acrosomal membrane in close apposition (Fig. 8). The APM contains numerous membrane rafts that are enriched in PLB. Sterol acceptors, such as HDLs or albumin, initiate reorganization and disruption of rafts (Fig. 8, 1), which allows PLB to approach proteases (2). PLB undergoes proteolytic cleavage and releases its catalytic domain (3). The active fragment degrades lipids of the outer bilayer (4). Resultant changes in membrane curvature and fluidity facilitate the formation of stalk structures at the APM (5) and fusion pores (6). These fusion events gradually progress during capacitation in vivo, leading toward full exocytosis. This model could serve as a starting point for future investigations of the induction of ZP-independent AE.
FIGURE 8.
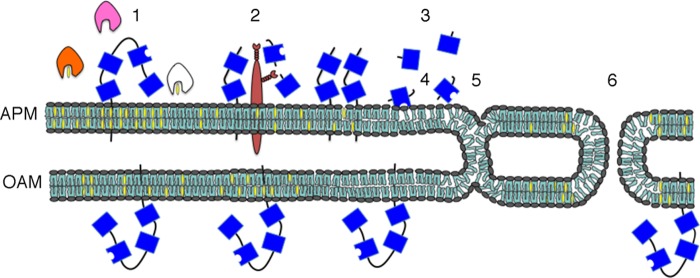
A working hypothesis for the regulation and the function of sperm PLB. Schematic diagram of the APM and the underlying outer acrosomal membrane (OAM). The APM possesses numerous membrane rafts enriched in PLB (sterols are shown in yellow). Sterol acceptors, such as HDLs or albumin (pink and orange and then white when having bound a sterol), initiate reorganization of rafts (1), which allows PLB (blue) to contact proteases (brown) (2). PLB undergoes proteolytic cleavage, releasing a catalytic, active fragment (free blue box with concave side) (3). The active fragment hydrolyzes phospholipids of the outer bilayer (4). The resultant changes in membrane curvature and fluidity facilitate the formation of stalk structures (5) and fusion pores (6). These point fusion events would occur during capacitation as a late event leading toward AE.
The importance of membrane instability for induction of AE has been shown by the report that group X calcium-dependent PLA2 can be released and stimulate AE in a paracrine fashion (9). The same authors carefully showed that treatment with this calcium-dependent PLA2 lowered progressive velocity in sperm with poor motility (53). In contrast to this, we did not find any changes in motility when incubating sperm with protein concentrates from different released fractions or with N-PLB (data not shown). These differences, as well as the fundamental difference of dependence upon calcium, suggest that group X PLA2 and PLB probably play different roles in sperm function.
Although several studies have shown proteolytic activation of PLB (21, 22, 32, 54), the endogenous protease(s) involved has yet to be identified. Our pharmacological experiments showed that PMSF and furin inhibitors (Dec-RVKR-CMK and hexa-d-arginine) inhibited the activation of PLB during sterol removal, whereas benzamidine, trypsin inhibitor, and leupeptin did not. Furin belongs to the proprotein convertase subtilisin/kexin type (PCSK) family consisting of nine serine endoproteases (55). Studies with transcriptome analysis and immunolocalization identified that PCSK3 (furin), PCSK4, and PCSK7 (56–58) are expressed in the testis. The furin inhibitors we used were able to inhibit multiple PCSK activities (59–62), so future studies will be needed to identify the specific protease(s) responsible for cleavage and activation of PLB. Recently, a study using mutant mice demonstrated that PCSK4 localizes to the sperm APM and proteolytically cleaves ADAM2 in response to sterol removal (63, 64), positioning it appropriately for this role.
Further work with the active fragment released is also warranted. Full-length PLB contains four tandem repeat domains (labeled I–IV starting from the N terminus). The high similarity observed in the structure and protein sequence between mammals is suggestive that its function is highly preserved among species. In our present study, we demonstrated, using immunoblot and gel-based separation, that a 50-kDa proteolytic product was enzymatically active, whereas a 27-kDa fragment showed no prominent activity. In studies of recombinant PLB from the rat and guinea pig, it was demonstrated that the N-terminal domain I is proteolytically cleaved as a pro-region that otherwise suppresses PLB activity (23, 54).
Although speculative, our results are consistent with the 27-kDa product corresponding to domain I. PLB is a nucleophilic lipase, in which all of its hydrolytic activities belong to a single catalytic triad in domain II, which has a molecular mass of ∼35 kDa (32, 65). Treatment of guinea pig PLB with trypsin resulted in a 97-kDa active product that mainly corresponded to domains II and III (54). Although there is a difference in molecular size between that and the present study, this probably reflects a difference between trypsin and the physiological protease.
In summary, we demonstrated in murine sperm that sterol removal resulted in release and activation of PLB by means of cleavage by a PCSK family protease. The active fragments produced play an important role in sperm function, particularly through promotion of AE, enabling fertilization. Our finding that a recombinant peptide containing the PLB catalytic domain initiated AE in the absence of ZP suggested that this enzyme might provide a mechanism for new models of AE induction.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-HD-045664 and DP-OD-006431 (to A. J. T.). This work was also supported by the Baker Institute for Animal Health.
- AE
- acrosome exocytosis
- PLB
- phospholipase B
- 2-OHCD
- 2-hydroxypropyl-β-cyclodextrin
- ZP
- zona pellucida
- APM
- plasma membrane overlying acrosome
- PLA1 and PLA2
- phospholipase A1 and A2
- GM1
- Galβ3GalNAcβ4(Neu5Acα3)Galα4GlcCer
- PI-PLC
- phosphatidylinositol-phospholipase C
- GPI
- glycosylphosphatidylinositol
- PNA
- peanut agglutinin
- dl-carnitine
- palmitoyl dl-carnitine
- l-carnitine
- palmitoyl l-carnitine
- P4
- progesterone
- N-PLB
- recombinant PLB catalytic domain tagged with N-terminal polyhistidine
- IVF
- in vitro fertilization
- PCSK
- proprotein convertase subtilisin/kexin type
- Dec
- decanoyl
- CMK
- chloromethylketone.
REFERENCES
- 1. Chang M. C. (1951) Fertilizing Capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697–698 [DOI] [PubMed] [Google Scholar]
- 2. Austin C. R. (1951) Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B 4, 581–596 [DOI] [PubMed] [Google Scholar]
- 3. Visconti P. E., Bailey J. L., Moore G. D., Pan D., Olds-Clarke P., Kopf G. S. (1995) Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121, 1129–1137 [DOI] [PubMed] [Google Scholar]
- 4. Travis A. J., Kopf G. S. (2002) The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Invest. 110, 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim K. S., Gerton G. L. (2003) Differential release of soluble and matrix components. Evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev. Biol. 264, 141–152 [DOI] [PubMed] [Google Scholar]
- 6. Jin M., Fujiwara E., Kakiuchi Y., Okabe M., Satouh Y., Baba S. A., Chiba K., Hirohashi N. (2011) Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. U.S.A. 108, 4892–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roldan E. R., Shi Q. X. (2007) Sperm phospholipases and acrosomal exocytosis. Front. Biosci. 12, 89–104 [DOI] [PubMed] [Google Scholar]
- 8. Roldan E. R. (1998) Role of phospholipases during sperm acrosomal exocytosis. Front. Biosci. 3, D1109–D1119 [DOI] [PubMed] [Google Scholar]
- 9. Escoffier J., Jemel I., Tanemoto A., Taketomi Y., Payre C., Coatrieux C., Sato H., Yamamoto K., Masuda S., Pernet-Gallay K., Pierre V., Hara S., Murakami M., De Waard M., Lambeau G., Arnoult C. (2010) Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Invest. 120, 1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visconti P. E., Ning X., Fornés M. W., Alvarez J. G., Stein P., Connors S. A., Kopf G. S. (1999) Cholesterol efflux-mediated signal transduction in mammalian sperm. Cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev. Biol. 214, 429–443 [DOI] [PubMed] [Google Scholar]
- 11. Selvaraj V., Asano A., Buttke D. E., McElwee J. L., Nelson J. L., Wolff C. A., Merdiushev T., Fornés M. W., Cohen A. W., Lisanti M. P., Rothblat G. H., Kopf G. S., Travis A. J. (2006) Segregation of micron-scale membrane sub-domains in live murine sperm. J. Cell. Physiol. 206, 636–646 [DOI] [PubMed] [Google Scholar]
- 12. Selvaraj V., Asano A., Buttke D. E., Sengupta P., Weiss R. S., Travis A. J. (2009) Mechanisms underlying the micron-scale segregation of sterols and GM1 in live mammalian sperm. J. Cell. Physiol. 218, 522–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simons K., Gerl M. J. (2010) Revitalizing membrane rafts. New tools and insights. Nat. Rev. Mol. Cell Biol. 11, 688–699 [DOI] [PubMed] [Google Scholar]
- 14. Simons K., Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 15. Pike L. J. (2006) Rafts defined. A report on the Keystone symposium on lipid rafts and cell function. J. Lipid Res. 47, 1597–1598 [DOI] [PubMed] [Google Scholar]
- 16. Travis A. J., Merdiushev T., Vargas L. A., Jones B. H., Purdon M. A., Nipper R. W., Galatioto J., Moss S. B., Hunnicutt G. R., Kopf G. S. (2001) Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and guinea pig spermatozoa. Dev. Biol. 240, 599–610 [DOI] [PubMed] [Google Scholar]
- 17. Asano A., Selvaraj V., Buttke D. E., Nelson J. L., Green K. M., Evans J. E., Travis A. J. (2009) Biochemical characterization of membrane fractions in murine sperm. Identification of three distinct sub-types of membrane rafts. J. Cell. Physiol. 218, 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asano A., Nelson J. L., Zhang S., Travis A. J. (2010) Characterization of the proteomes associating with three distinct membrane raft sub-types in murine sperm. Proteomics 10, 3494–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghannoum M. A. (2000) Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 13, 122–143, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gassama-Diagne A., Fauvel J., Chap H. (1989) Purification of a new, calcium-independent, high molecular weight phospholipase A2/lysophospholipase (phospholipase B) from guinea pig intestinal brush-border membrane. J. Biol. Chem. 264, 9470–9475 [PubMed] [Google Scholar]
- 21. Delagebeaudeuf C., Gassama-Diagne A., Nauze M., Ragab A., Li R. Y., Capdevielle J., Ferrara P., Fauvel J., Chap H. (1998) Ectopic epididymal expression of guinea pig intestinal phospholipase B. Possible role in sperm maturation and activation by limited proteolytic digestion. J. Biol. Chem. 273, 13407–13414 [DOI] [PubMed] [Google Scholar]
- 22. Maury E., Prévost M. C., Nauze M., Redoulès D., Tarroux R., Charvéron M., Salles J. P., Perret B., Chap H., Gassama-Diagne A. (2002) Human epidermis is a novel site of phospholipase B expression. Biochem. Biophys. Res. Commun. 295, 362–369 [DOI] [PubMed] [Google Scholar]
- 23. Takemori H., Zolotaryov F. N., Ting L., Urbain T., Komatsubara T., Hatano O., Okamoto M., Tojo H. (1998) Identification of functional domains of rat intestinal phospholipase B/lipase. Its cDNA cloning, expression, and tissue distribution. J. Biol. Chem. 273, 2222–2231 [DOI] [PubMed] [Google Scholar]
- 24. Tchoua U., Ito M., Okamoto M., Tojo H. (2000) Increased intestinal phospholipase A2 activity catalyzed by phospholipase B/lipase in WBN/Kob rats with pancreatic insufficiency. Biochim. Biophys. Acta 1487, 255–267 [DOI] [PubMed] [Google Scholar]
- 25. Siafakas A. R., Wright L. C., Sorrell T. C., Djordjevic J. T. (2006) Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot. Cell 5, 488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Travis A. J., Jorgez C. J., Merdiushev T., Jones B. H., Dess D. M., Diaz-Cueto L., Storey B. T., Kopf G. S., Moss S. B. (2001) Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J. Biol. Chem. 276, 7630–7636 [DOI] [PubMed] [Google Scholar]
- 27. Bordier C. (1981) Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256, 1604–1607 [PubMed] [Google Scholar]
- 28. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 29. Travis A. J., Foster J. A., Rosenbaum N. A., Visconti P. E., Gerton G. L., Kopf G. S., Moss S. B. (1998) Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol. Biol. Cell 9, 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darrow A. L., Olson M. W., Xin H., Burke S. L., Smith C., Schalk-Hihi C., Williams R., Bayoumy S. S., Deckman I. C., Todd M. J., Damiano B. P., Connelly M. A. (2011) A novel fluorogenic substrate for the measurement of endothelial lipase activity. J. Lipid Res. 52, 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hendrickson H. S. (1999) Phospholipase A2 and phosphatidylinositol-specific phospholipase C assays by HPLC and TLC with fluorescent substrate. Methods Mol. Biol. 109, 1–6 [DOI] [PubMed] [Google Scholar]
- 32. Tojo H., Ichida T., Okamoto M. (1998) Purification and characterization of a catalytic domain of rat intestinal phospholipase B/lipase associated with brush border membranes. J. Biol. Chem. 273, 2214–2221 [DOI] [PubMed] [Google Scholar]
- 33. Chen S. C., Wright L. C., Golding J. C., Sorrell T. C. (2000) Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 347, 431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santangelo R. T., Nouri-Sorkhabi M. H., Sorrell T. C., Cagney M., Chen S. C., Kuchel P. W., Wright L. C. (1999) Biochemical and functional characterisation of secreted phospholipase activities from Cryptococcus neoformans in their naturally occurring state. J. Med. Microbiol. 48, 731–740 [DOI] [PubMed] [Google Scholar]
- 35. Larson J. L., Miller D. J. (1999) Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 52, 445–449 [DOI] [PubMed] [Google Scholar]
- 36. Selvaraj V., Asano A., Page J. L., Nelson J. L., Kothapalli K. S., Foster J. A., Brenna J. T., Weiss R. S., Travis A. J. (2010) Mice lacking FABP9/PERF15 develop sperm head abnormalities but are fertile. Dev. Biol. 348, 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Toyoda Y., Yokoyama M., Hoshi T. (1971) Studies on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperm. Jpn. J. Anim. Reprod. 16, 147–151 [Google Scholar]
- 38. Erbach G. T., Lawitts J. A., Papaioannou V. E., Biggers J. D. (1994) Differential growth of the mouse preimplantation embryo in chemically defined media. Biol. Reprod. 50, 1027–1033 [DOI] [PubMed] [Google Scholar]
- 39. Cross N. L., Lambert H., Samuels S. (1986) Sperm binding activity of the zona pellucida of immature mouse oocytes. Cell Biol. Int. Rep. 10, 545–554 [DOI] [PubMed] [Google Scholar]
- 40. Bellvé A. R., Cavicchia J. C., Millette C. F., O'Brien D. A., Bhatnagar Y. M., Dym M. (1977) Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 74, 68–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shannon M., Richardson L., Christian A., Handel M. A., Thelen M. P. (1999) Differential gene expression of mammalian SPO11/TOP6A homologs during meiosis. FEBS Lett. 462, 329–334 [DOI] [PubMed] [Google Scholar]
- 42. Honda A., Yamagata K., Sugiura S., Watanabe K., Baba T. (2002) A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 277, 16976–16984 [DOI] [PubMed] [Google Scholar]
- 43. Djordjevic J. T., Del Poeta M., Sorrell T. C., Turner K. M., Wright L. C. (2005) Secretion of cryptococcal phospholipase B1 (PLB1) is regulated by a glycosylphosphatidylinositol (GPI) anchor. Biochem. J. 389, 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugatani J., Okumura T., Saito K. (1980) Studies of a phospholipase B from Penicillium notatum. Substrate specificity and properties of active site. Biochim. Biophys. Acta 620, 372–386 [PubMed] [Google Scholar]
- 45. Saito K., Sugatani J., Okumura T. (1991) Phospholipase B from Penicillium notatum. Methods Enzymol. 197, 446–456 [DOI] [PubMed] [Google Scholar]
- 46. Buffone M. G., Kim K. S., Doak B. J., Rodriguez-Miranda E., Gerton G. L. (2009) Functional consequences of cleavage, dissociation and exocytotic release of ZP3R, a C4BP-related protein, from the mouse sperm acrosomal matrix. J. Cell Sci. 122, 3153–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hruskova-Heidingsfeldova O. (2008) Secreted proteins of Candida albicans. Front. Biosci. 13, 7227–7242 [DOI] [PubMed] [Google Scholar]
- 48. Köhler G. A., Brenot A., Haas-Stapleton E., Agabian N., Deva R., Nigam S. (2006) Phospholipase A2 and phospholipase B activities in fungi. Biochim. Biophys. Acta 1761, 1391–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wright L. C., Santangelo R. M., Ganendren R., Payne J., Djordjevic J. T., Sorrell T. C. (2007) Cryptococcal lipid metabolism. Phospholipase B1 is implicated in transcellular metabolism of macrophage-derived lipids. Eukaryot. Cell 6, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim K. S., Foster J. A., Gerton G. L. (2001) Differential release of guinea pig sperm acrosomal components during exocytosis. Biol. Reprod. 64, 148–156 [DOI] [PubMed] [Google Scholar]
- 51. Buffone M. G., Zhuang T., Ord T. S., Hui L., Moss S. B., Gerton G. L. (2008) Recombinant mouse sperm ZP3-binding protein (ZP3R/sp56) forms a high order oligomer that binds eggs and inhibits mouse fertilization in vitro. J. Biol. Chem. 283, 12438–12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harper C. V., Cummerson J. A., White M. R., Publicover S. J., Johnson P. M. (2008) Dynamic resolution of acrosomal exocytosis in human sperm. J. Cell Sci. 121, 2130–2135 [DOI] [PubMed] [Google Scholar]
- 53. Escoffier J., Pierre V. J., Jemel I., Munch L., Boudhraa Z., Ray P. F., De Waard M., Lambeau G., Arnoult C. (2011) Group X secreted phospholipase A2 specifically decreases sperm motility in mice. J. Cell. Physiol. 226, 2601–2609 [DOI] [PubMed] [Google Scholar]
- 54. Nauze M., Gonin L., Chaminade B., Perès C., Hullin-Matsuda F., Perret B., Chap H., Gassama-Diagne A. (2002) Guinea pig phospholipase B, identification of the catalytic serine and the proregion involved in its processing and enzymatic activity. J. Biol. Chem. 277, 44093–44099 [DOI] [PubMed] [Google Scholar]
- 55. Gyamera-Acheampong C., Mbikay M. (2009) Proprotein convertase subtilisin/kexin type 4 in mammalian fertility. A review. Hum. Reprod. Update 15, 237–247 [DOI] [PubMed] [Google Scholar]
- 56. Mbikay M., Tadros H., Ishida N., Lerner C. P., De Lamirande E., Chen A., El-Alfy M., Clermont Y., Seidah N. G., Chrétien M., Gagnon C., Simpson E. M. (1997) Impaired fertility in mice deficient for the testicular germ-cell protease PC4. Proc. Natl. Acad. Sci. U.S.A. 94, 6842–6846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakayama K., Kim W. S., Torii S., Hosaka M., Nakagawa T., Ikemizu J., Baba T., Murakami K. (1992) Identification of the fourth member of the mammalian endoprotease family homologous to the yeast Kex2 protease. Its testis-specific expression. J. Biol. Chem. 267, 5897–5900 [PubMed] [Google Scholar]
- 58. Bergeron F., Leduc R., Day R. (2000) Subtilase-like pro-protein convertases. From molecular specificity to therapeutic applications. J. Mol. Endocrinol. 24, 1–22 [DOI] [PubMed] [Google Scholar]
- 59. Wang X., Pei D. (2001) Shedding of membrane type matrix metalloproteinase 5 by a furin-type convertase. A potential mechanism for down-regulation. J. Biol. Chem. 276, 35953–35960 [DOI] [PubMed] [Google Scholar]
- 60. Basak A., Lotfipour F. (2005) Modulating furin activity with designed mini-PDX peptides. Synthesis and in vitro kinetic evaluation. FEBS Lett. 579, 4813–4821 [DOI] [PubMed] [Google Scholar]
- 61. Denault J. B., D'Orléans-Juste P., Masaki T., Leduc R. (1995) Inhibition of convertase-related processing of proendothelin-1. J. Cardiovasc. Pharmacol. 26, S47–S50 [PubMed] [Google Scholar]
- 62. Basak A., Shervani N. J., Mbikay M., Kolajova M. (2008) Recombinant proprotein convertase 4 (PC4) from Leishmania tarentolae expression system. Purification, biochemical study and inhibitor design. Protein Expr. Purif 60, 117–126 [DOI] [PubMed] [Google Scholar]
- 63. Gyamera-Acheampong C., Vasilescu J., Figeys D., Mbikay M. (2010) PCSK4-null sperm display enhanced protein tyrosine phosphorylation and ADAM2 proteolytic processing during in vitro capacitation. Fertil. Steril. 93, 1112–1123 [DOI] [PubMed] [Google Scholar]
- 64. Gyamera-Acheampong C., Tantibhedhyangkul J., Weerachatyanukul W., Tadros H., Xu H., van de Loo J. W., Pelletier R. M., Tanphaichitr N., Mbikay M. (2006) Sperm from mice genetically deficient for the PCSK4 proteinase exhibit accelerated capacitation, precocious acrosome reaction, reduced binding to egg zona pellucida, and impaired fertilizing ability. Biol. Reprod. 74, 666–673 [DOI] [PubMed] [Google Scholar]
- 65. Lu T., Ito M., Tchoua U., Takemori H., Okamoto M., Tojo H. (2001) Identification of essential residues for catalysis of rat intestinal phospholipase B/lipase. Biochemistry 40, 7133–7139 [DOI] [PubMed] [Google Scholar]