Background: Obesity-induced inflammation is characterized by macrophage migration and polarization; signaling regulation therein remains poorly understood.
Results: Lipid-induced fetuin-A from adipose tissue acts as chemoattractant for macrophage migration and also polarizes adipose tissue M2 macrophages to proinflammatory M1 subtype.
Conclusion: Adipocyte fetuin-A is a novel signaling molecule in lipid-induced tissue inflammation.
Significance: These findings have revealed an unseen area of inflammation.
Keywords: Adipose Tissue, Inflammation, Lipids, Macrophages, Obesity
Abstract
Macrophage infiltration into adipose tissue during obesity and their phenotypic conversion from anti-inflammatory M2 to proinflammatory M1 subtype significantly contributes to develop a link between inflammation and insulin resistance; signaling molecule(s) for these events, however, remains poorly understood. We demonstrate here that excess lipid in the adipose tissue environment may trigger one such signal. Adipose tissue from obese diabetic db/db mice, high fat diet-fed mice, and obese diabetic patients showed significantly elevated fetuin-A (FetA) levels in respect to their controls; partially hepatectomized high fat diet mice did not show noticeable alteration, indicating adipose tissue to be the source of this alteration. In adipocytes, fatty acid induces FetA gene and protein expressions, resulting in its copious release. We found that FetA could act as a chemoattractant for macrophages. To simulate lipid-induced inflammatory conditions when proinflammatory adipose tissue and macrophages create a niche of an altered microenvironment, we set up a transculture system of macrophages and adipocytes; the addition of fatty acid to adipocytes released FetA into the medium, which polarized M2 macrophages to M1. This was further confirmed by direct FetA addition to macrophages. Taken together, lipid-induced FetA from adipocytes is an efficient chemokine for macrophage migration and polarization. These findings open a new dimension for understanding obesity-induced inflammation.
Introduction
Evidences obtained in recent years indicate an association between obesity, insulin resistance, and immunity (1–3). Adipose tissue inflammation has been increasingly recognized as the primary cause of obesity-induced insulin resistance (2, 4, 5). In fact, accumulation of a considerable amount of macrophages in adipose tissue greatly contributes to obesity-induced inflammation (6, 7). Polarity of adipose tissue macrophages (ATM)3 is inclined toward proinflammatory or classically activated M1 phenotype from anti-inflammatory or alternatively activated M2 subtype (8–10). Proinflammatory cytokines from M1, in turn, adversely affect insulin activity in insulin target cells. This is an area where excess of lipid might create an environment for linking immunity with insulin resistance. Three issues are necessary in the dynamics of adipose tissue inflammation: (i) the first issue is the lipid-induced proinflammatory status of adipose tissue; (ii) this process attracts M2 macrophages to be recruited into adipose tissue; and (iii) after being recruited there, the M2 subpopulation eventually transforms to the M1 subtype. If we briefly scan the available literature on these three issues, we will find that there are many convincing studies on fatty acid (FA)-induced adipose tissue inflammation (3, 11–14). We have recently demonstrated that FA-induced adipocyte inflammation is not direct; it acts through fetuin-A (FetA), an endogenous ligand for TLR4. FetA binds to FA and presents it to TLR4, which triggers elevated release of proinflammatory cytokines through TLR4-NF-κB pathway, causing insulin resistance (15). Many investigators have reported that FA-induced inflammation of adipose tissue caused macrophage infiltration (8, 10). Monocyte chemoattractant protein-1 (MCP-1) has been suggested to be involved in this migration (16). However, a recent study shows that only about 40% macrophage infiltration occurs with MCP-1 (17). This information suggests the possible occurrence of other factor(s) for macrophage infiltration into adipose tissue. However, above this point, our understanding is indeed very poor. The most conspicuous gap that remains in our understanding is with macrophage polarization; we cannot yet identify the factor(s) that regulates ATM polarization from M2 to M1 subtype. However, Oh et al. (17) have suggested that the signaling molecule for ATM polarization most likely exists within adipose tissue itself; this appears to be a proposition closer to reality. Based on our observations on adipose tissue FetA, where FetA is abundantly available and consistently increased due to excess lipid intake, we thought that FetA could be another chemokine in addition to MCP-1. In this study, we show that FetA increases macrophage migration into adipose tissue. We also demonstrate that FA induces FetA gene and protein expressions in adipocytes followed by its excess release, which causes M2 to M1 polarization.
EXPERIMENTAL PROCEDURES
Animals and Treatments
Control (C57BLKS/6J) and db/db (BKS.Cgm+/+Lepr(db)/J, stock number 000642) male mice aged 12–18 weeks obtained from The Jackson Laboratory were conditioned with 12-h light/12-h dark cycle at 23 ± 2 °C and relative humidity 55 ± 5% along with access to standard diet (SD) ad libitum. A subset of control BALB/c mice was reared on high fat diet (HFD) (15). All animal experiments were performed with the approval of the Animal Ethics Committees of Visva-Bharati University and National Institute of Immunology following their guidelines. Partial hepatectomy of HFD mice was performed according to our earlier procedure (15).
Human Subjects
Visceral adipose tissue was obtained from nine non-obese, non-diabetic individuals (seven males and two females) and five obese, diabetic individuals (four males and one female), 53–68 years old, who were admitted to IPGMER-SSKM Hospital and underwent abdominal surgery. Following due clearance from the ethical committee of the IPGMER-SSKM Hospital, Kolkata, India, and approval of the Institutional Ethics Committee (IEC), the study was conducted after obtaining informed consent from all participants. Adipocytes were isolated from the collected adipose tissue (18).
Reagents and Antibodies
All tissue culture materials were obtained from Life Technologies, and [3H]leucine (specific activity 1000 Ci/mmol) was from GE Healthcare. We purchased phospho-NFκB p65 (pNFκB Ser-536), IL-6, TNFα, MCP-1, arginase-1, IL-10, PPARγ, and FetA antibodies from Santa Cruz Biotechnology. Alkaline phosphatase-conjugated secondary antibodies were purchased from Sigma, recombinant human FetA (RD 172037100) was from BioVendor R&D, mouse FetA (1563-PI-050) was from R&D Systems, human MCP-1 (14-8398-80) was from E-Biosciences, and CLI-095 was from InvivoGen.
Cell Culture and Treatments
Mouse 3T3-L1 preadipocytes, macrophage cell line RAW264.7, and human adipocytes were cultured as described previously (15). Human monocyte cell line THP1 was cultured and differentiated following earlier protocol (19). Culture of adipocytes and macrophages was performed, and treatment with FA and FetA including use of chemicals and siRNA transfections was carried out following our previously described procedure (15); deviations are described in legend to Fig. 3. At the end of incubation, medium or cell lysate was subjected to further analysis.
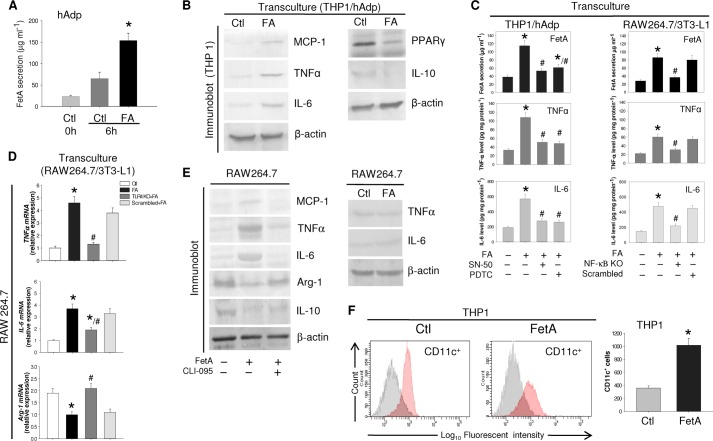
FIGURE 3.
FetA-induced polarization of macrophage from M2 to M1 phenotype. A, hAdp were incubated without or with FA, and FetA released into the medium was estimated by ELISA. B, THP-1 and hAdp were transcultured with or without FA, and THP1 cell lysate was immunoblotted with anti-MCP-1, TNFα, IL-6, PPARγ, and IL-10 antibodies. β-Actin was used as a loading control. C, THP1 and SN-50 or pyrrolidine dithiocarbamate (PDTC)-treated hAdp or RAW264.7 and NF-κB KO 3T3-L1 adipocytes were transcultured with or without FA. FetA secretion was estimated by ELISA, whereas TNFα and IL-6 were determined in macrophage cell lysates by ELISA. D, TLR4 KO RAW264.7 and 3T3-L1 adipocytes were transcultured with or without FA, and relative mRNA expression levels of TNFα, IL-6, and Arg-1 were determined by qPCR. Results of C and D are expressed as means ± S.E. (n = 3). *, p < 0.001(versus Ctl); #, p < 0.01(versus FA). E, RAW264.7 cells were treated with or without FetA or CLI-095+FetA. Media and cell lysates were immunoblotted with anti-MCP-1, TNFα, IL-6, Arg-1, and IL-10 antibodies. RAW264.7 cells were incubated with or without FA, and media were immunoblotted against anti-TNFα and IL-6 antibodies. F, THP1 cells were incubated with or without FetA, and conversion to CD11c+ was determined by FACS analysis. Results are expressed as means ±S.E. (n = 3). *, p < 0.001 (versus Ctl).
Removal of Endotoxin Contamination
We removed any endotoxin contamination from FA as mentioned earlier (15).
[3H]Leucine Incorporation Study
This was performed with 3T3-L1 adipocytes or SD mice adipocytes by following our earlier procedure (18).
Transwell System of Co-culture
Indirect co-culture was performed by incubating human adipocytes (1 × 106 cells) or differentiated 3T3-L1 adipocytes (1 × 106 cells) in 0.4-μm pore size cell culture inserts (Thermo Nunc) and placing them in 6-well plates containing THP1 macrophages (1 × 106 cells) or RAW264.7 macrophages (1 × 106 cells), respectively. Co-cultures were incubated for 6 h with or without FA (0.75 mm).
Cell Migration Assay
The THP1/RAW264.7 cell migration assay was performed in a Boyden chamber system (Millipore QCM 24-well colorimetric cell migration assay kit) by the addition of FetA (100 μg ml−1) or MCP-1 (100 ng ml−1) or cytokines or antibodies in the lower chamber. The procedure is also provided in the legend to Fig. 2 and under “Results.”
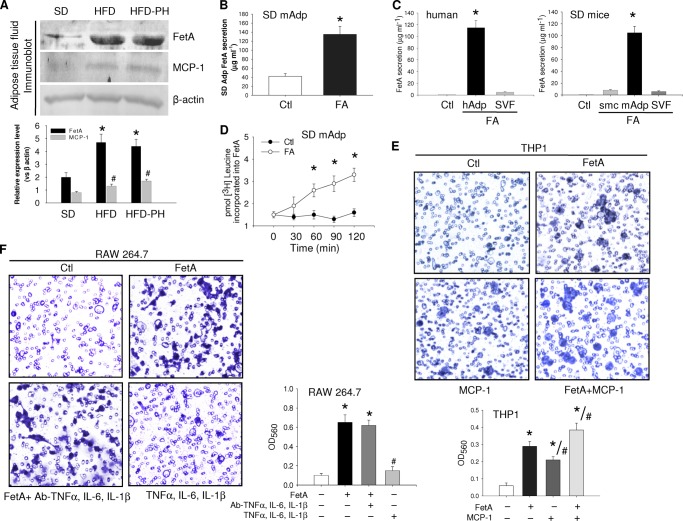
FIGURE 2.
Macrophage migration due to FetA. A, adipose tissue fluid collected from SD or HFD or HFD-PH (PH = partially hepatectomized) mice was immunoblotted for FetA and MCP-1 by using the respective antibodies. B, adipocytes from SD mice (mAdp) were incubated without or with FA, and FetA release was measured by ELISA. C, FetA secretion in Ctl (media without cells) and hAdp or stromal vascular fraction (SVF) or mouse skeletal muscle cells (smc) or mouse Adp incubated with FA was measured by ELISA. D, SD mice adipocytes were incubated with FA in the presence of [3H]leucine for different time periods. On termination, medium was immunoprecipitated with anti-FetA antibody and subjected to radioactive counting. E, THP1 macrophages were added to the upper chamber of the Boyden chamber and allowed to migrate through the porous membrane into the lower chamber containing medium alone or medium with FetA (100 μg ml−1) or MCP-1(100 ng ml−1) or both. F, similar migration of RAW264.7 cells was observed in the Boyden chamber containing medium with FetA (100 μg ml−1) or FetA plus anti-TNFα, IL-6, and IL-1β antibodies (2 μg ml−1) and TNFα, IL-6, and IL-1β (2 μg ml−1). Migrated cells on the lower side of the membrane were stained and observed under a microscope. Dye was further extracted following the manufacturer's protocol, and optical density (OD) was measured at 560 nm. Data are expressed as means ± S.E. (n = 3). *, p < 0.001 (versus Ctl); #, p < 0.01 (versus FetA).
Immunoblotting
Immunoblotting experiments were carried out as described earlier (15).
RT-PCR and Quantitative PCR
Total RNA was extracted following a previous protocol (18). Primers used for qPCR are: FetA (PPM32919B, 170 bp), Il-6 (PPM03015A, 178 bp), Tnfα (PPM03113F, 93 bp), Arg-1(PPM 31770B (139 bp), and Gapdh (PPM02946E (140 bp). Primers used for RT-PCR are: FetA (forward) 5′-CACCGAACTTACCACGACCT-3′; (reverse) 3′ATGTCCTGTCTGCCAAAACC-5′; Gapdh (forward) 5′-CCACCCATGGCAAATTCCATGGCA-3′; (reverse) 5′-TCTAGACGGCAGGTCAGGTCCACC-3′.
ChIP Assay
ChIP was performed by using a ChIP assay kit (Upstate Biotech Millipore) in human adipocytes (hAdp) by using anti-NF-κBp65 and primers for human fetuin-A promoter by following our previous procedure (18).
ELISA
Cytokine levels were measured in cell culture medium/lysates using human/mouse TNFα and human/mouse IL-6 ELISA kits (RayBiotech Inc.) and a human/mouse FetA ELISA kit (R&D Systems) following the manufacturers' protocols.
FACS
THP1 cells treated with FetA (100 μg ml−1) were washed and resuspended in 50 μl of PBS containing 0.5% BSA and 2 mmol l−1 EDTA and incubated with phycoerythrin-conjugated CD11c antibody (BD Pharmingen) or with their isotype control (Santa Cruz Biotechnology) for 30 min at 4 °C. Labeled cells were washed with PBS and analyzed by flow cytometry using a BD FACSAriaTM II and the FACSDiva Software.
Statistical Analyses
Data were analyzed by using one-way analysis of variance where the F value indicated significance, and means were compared by a post hoc multiple range test. All values were means ± S.E.
RESULTS
Lipid-induced FetA Synthesis and Secretion from Adipocytes
Based on the assumption made in previous studies that chemotactic signals affecting influx of monocytes to adipose tissue could be from tissue origin (17), we hypothesized that FetA might be one of such molecules. This possibility was implied by our observations where adipose tissue of obese diabetic mice (db/db) showed more than 3-fold higher FetA level in comparison with their lean non-diabetic control (Fig. 1A). A significant increase of FetA occurred in adipose tissue of HFD-fed mice and obese diabetic subjects (Fig. 1A). These results suggest that excess lipid in adipose tissue probably influences FetA level as FA is known to induce FetA expression (18). To examine this, we incubated adipocytes from non-obese SD-fed mice with FA, i.e. palmitate, and found a dose-dependent increase of FetA release into the medium (Fig. 1B). Because liver is known to be the only organ for FetA gene expression and protein secretion, we made an in-depth study of this unusual observation.
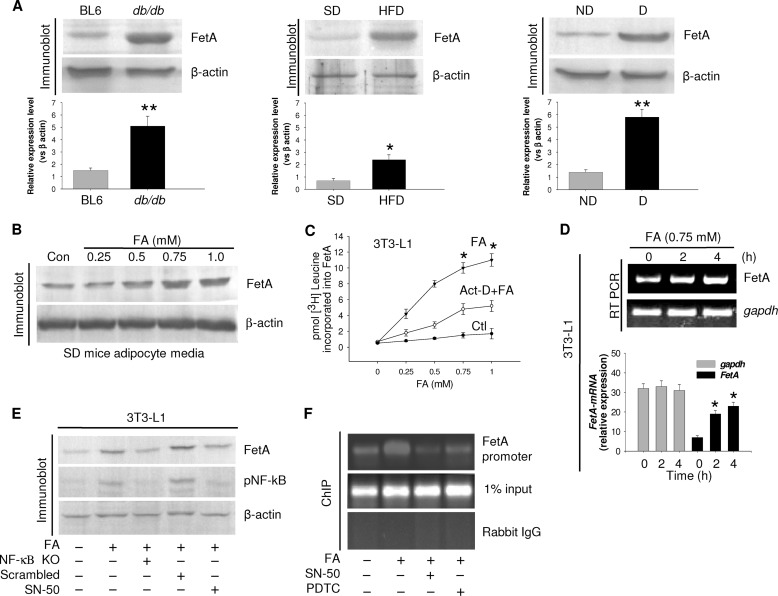
FIGURE 1.
FA-induced FetA secretion from adipocytes. A, adipose tissue collected from control (Ctl) or db/db mice, SD, or HFD mice and obese diabetic (D) or non-diabetic (ND) human subjects was immunoblotted for FetA. Results are expressed as means ± S.E. (n = 5). *, p < 0.01, **, p < 0.001 (versus BL6 or SD or non-diabetic). B, SD mice adipocytes with varied concentrations of palmitate (FA) were incubated for 4 h, and medium was immunoblotted for FetA. Con, control. C, 3T3-L1 adipocytes were incubated with increasing concentrations of FA in the presence of [3H]leucine without or with actinomycin D. Media were immunoprecipitated by anti-FetA antibody and subjected for radioactive counting. *, p < 0.001 (versus Ctl). D, RNA extracted from 3T3-L1 adipocytes was incubated with FA for different periods and subjected to RT-PCR and qPCR. Data are expressed as means ± S.E. (n = 3). *, p < 0.001 (versus Ctl). E, Ctl or NF-κB siRNA (NF-κB KO) transfected or SN-50-treated 3T3-L1 cells were incubated with FA and immunoblotted with indicated antibodies. pNF-kB, phospho-NF-κB. F, human adipocytes were incubated with FA or FA+SN-50 or pyrrolidine dithiocarbamate (PDTC). NF-κB binding to the FetA promoter was determined by ChIP assay.
We therefore examined whether FA can induce FetA synthesis as was observed with liver (18). Incubation of 3T3-L1 adipocytes with FA in the presence of [3H]leucine followed by radiolabeled FetA immunoprecipitation showed dose-dependent increase in FetA synthesis in response to FA (Fig. 1C), coinciding with elevated FetA mRNA expression (Fig. 1D). As in liver, FA-induced adipocyte FetA expression was also found to be NF-κB-dependent; FA failed to express FetA in NF-κB KO cells or in the presence of NF-κB translocation inhibitor, SN-50 (Fig. 1E). This was further evident from the ChIP assay where FA increased NF-κB binding to adipocyte FetA promoter, effecting its activation; this was inhibited by NF-κB inhibitors (Fig. 1F). These results indicate that FetA expression in adipocytes is similarly regulated as observed with liver cells (18).
FetA Affects Macrophage Migration
Increased resident macrophage is a characteristic feature of adipose tissue in obese human and mice (6, 7). MCP-1 is known to be the chemokine that drives macrophage into adipose tissue (16). Recent studies show that the effect of MCP-1 is insufficient, suggesting the involvement of other factor(s) (17, 20). When we observed that FetA content in HFD mice was significantly elevated, whereas it was only marginal with MCP-1 and partial hepatectomy did not alter FetA level (Fig. 2A), we thought that this excess FetA due to FA could originate from adipose tissue itself. To examine this further, we isolated adipocytes from SD mice by following the method described previously (15, 18). Incubating them with FA produced 3-fold increase in FetA release (Fig. 2B). Fig. 2C shows that in adipose tissue, except in adipocytes, the stromal vascular fraction could not produce FetA due to FA. The addition of [3H]leucine in adipocyte incubation showed a time-dependent increase of radiolabeled FetA release (Fig. 2D). These observations suggest that the hyperlipidemic microenvironment of adipose tissue is favorable for FetA availability in excess. We then performed experiments to examine whether FetA could act as a chemoattractant for macrophage infiltration to adipose tissue by using a Boyden chamber system. Both FetA and MCP-1 affected THP1 migration through 5-μm pores of Boyden chambers. Most likely, in inflammatory adipose tissue where ATM is a co-inhabitant, both FetA and MCP-1 may be released concomitantly and produce an additional effect. Such a possibility is reflected from the results obtained with FetA and MCP-1 combination (Fig. 2E).We validated these results by observing whether the chemoattractive effect of proinflammatory cytokines, if any, share results produced by FetA. Immunodepletion of TNFα, IL-6, or IL-1β or direct addition of these did not alter FetA-stimulated macrophage migration. These findings show that the effect of FetA on macrophage movement is not influenced by other related factors.
FetA Induces Macrophage Polarization
Considering the situation that prevails in obese and hyperlipidemic conditions when adipocytes and macrophages co-exist in white adipose tissue, we hypothesized that FA-induced FetA release from adipocytes might have a role in macrophage polarity. Because our idea was to simulate the microenvironment that exists during inflammatory condition, co-culture of adipocytes and macrophages in the Transwell system appeared logical where cell-to-cell communication through signaling molecules could be mediated via culture media. THP1 macrophages were seeded on the bottom, whereas hAdp were cultured onto the membrane of Transwell cell culture inserts, and FA was added to these cells. Fig. 3A demonstrates that FA addition to hAdp greatly enhanced FetA release into the medium. In the transculture system, excess release of FetA from hAdp in response to FA is expected, but what is interesting here is overexpression of M1 markers in THP1 macrophage, such as TNFα, IL-6, and MCP-1, and significant decline of M2 markers, i.e. PPARγ and IL-10 (Fig. 3B). Elevated FetA release due to FA also enhanced TNFα and IL-6 expression in THP1 and RAW macrophages; suppression of NF-κB reduced the effect of FetA on macrophage polarization because this also subdued FetA secretion (Fig. 3C). Because FetA is a ligand for TLR4, we examined whether the effect of FetA is mediated through TLR4. Fig. 3D exhibits that the effect of FetA on macrophage polarization required TLR4 because FA-induced FetA augmented TNFα and IL-6 but decreased Arg-1 mRNA expression, whereas in TLR4 KO cells, the effect of FA was not observed, suggesting the occurrence of FA-FetA-TLR4 pathway in this process.
Taking cues from the results of THP1/hAdp or RAW264.7/3T3-L1 co-culture experiments, which indicated FetA involvement in M1 polarization, we directly incubated RAW264.7 cells with FetA. FetA triggered proinflammatory cytokine expressions with corresponding decrease in anti-inflammatory cytokines, whereas FA direct addition had no effect. FetA failed to elicit such responses in the presence of CLI-095, an inhibitor of TLR4 pathway (Fig. 3E), implicating the involvement of FetA-TLR4 signaling for M1 polarization. However, this requires further investigation to clarify the underlying mechanism. FetA influence on macrophage polarization toward M1 was further studied by using another important marker, i.e. CD11c. FACS analysis demonstrated that FetA could significantly alter macrophage polarity as the majority of macrophages were converted to CD11c+ cells, indicating M1 phenotype attainment (Fig. 3F).
DISCUSSION
Obesity-induced inflammation of adipose tissue and accumulation of macrophage therein are characteristic features of chronic inflammation that causes insulin resistance and other metabolic disorders (3, 5–7, 22, 23). Three critical issues in this area are poorly understood. (i) Inflamed adipose tissue becomes a site of attraction for macrophages; accordingly, they migrate and infiltrate there. Besides MCP-1, we asked what other chemokine(s) could be as the effect of MCP-1 has recently been found to be insufficient (17, 20). (ii) Infiltrating M2 macrophages in adipose tissue are progressively transformed to proinflammatory M1 subtype (8–10, 17), which further worsens the inflammatory condition. It is still unclear which factor(s) or signal(s) are responsible for this polarization. (iii) Because this occurs within adipose tissue, it is speculated that this cue(s) may originate from the tissue (17). This issue will remain unresolved until we can detect such a factor in adipose tissue.
In this investigation, we have addressed these three important unresolved issues. When we observed that adipocytes of adipose tissue could express FetA gene and secrete FetA protein in response to lipid, we speculated that FetA may be one of such cues because FetA is a known biomarker of chronic inflammatory diseases (5, 15, 24, 25). Our hypothesis has been substantiated by the following findings: FetA contributes to macrophage migration and to their M2 to M1 polarization. By organizing transculture experiments, we attempted to simulate the situation prevailing in adipose tissue when adipocytes and macrophages exist in the same location. Results obtained from these experiments show that FA-induced FetA release from adipocytes influences M2 to M1 polarization.
Intriguingly, the influence of FetA on macrophage migration and polarization during adipose tissue inflammation appears to be independent of its source from liver. FA could efficiently stimulate FetA synthesis and secretion from adipocytes. We have checked other residual cells of adipose tissue after isolating adipocytes by incubating them with FA to see whether they could secrete FetA protein and found that its response is specific to adipocytes. FA-induced FetA synthesis in adipocytes is similar to hepatocytes. FA activates NF-κB, which binds to FetA promoter that in turn up-regulates FetA gene expression. Because inflamed adipose tissue harbors excess lipid, up-regulation of FetA expression may be constitutive, and that indicates the possibility of the sustained effect of FetA on ATM.
In conclusion, it may be stated that three new details have been obtained from this investigation: (i) FetA origin and secretion within adipose tissue microenvironment, (ii) contribution of FetA in macrophage infiltration, and (iii) contribution of FetA in macrophage polarization. The chemoattractant effect of FetA would be helpful to assess macrophage infiltration because the effect of MCP-1 has recently been found to be insufficient (17, 20). For M1 polarization in adipose tissue, there may be other factors apart from FetA, but in the absence of such information, detection of FetA as a potent effector for M2 to M1 polarization originating in the same niche would provide a better understanding of lipid-induced inflammation.
Acknowledgments
We thank Dr. Gobardhan Das, International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India for THP1 and Dr. Samit Chattopadhyay, National Centre For Cell Science (NCCS), Pune, India for RAW 264.7 cell lines. We acknowledge Dr. Santu Bandyopadhyay, Council of Scientific and Industrial Research-Indian Institute of Chemical Biology (CSIR-IICB), Kolkata, India for FACS analysis. We thank Hemanta Jadab for help in animal experiments. We duly acknowledge the Head of the Department of Zoology, Visva-Bharati University, Santiniketan, India for providing necessary facilities.
This work was supported by the Department of Science and Technology, Ministry of Science and Technology (Grant VI-D&P/413/2012-13/TDT) and Council of Scientific and Industrial Research (Grant CSC 207).
- ATM
- adipose tissue macrophages
- FetA
- fetuin-A
- MCP-1
- monocyte chemoattractant protein-1
- TLR4
- toll-like receptor 4
- hAdp
- human adipocytes
- FA
- fatty acid
- HFD
- high fat diet
- SD
- standard diet
- qPCR
- quantitative PCR
- PPARγ
- peroxisome proliferator-activated receptor γ
- Ctl
- control.
REFERENCES
- 1. Kahn S. E., Hull R. L., Utzschneider K. M. (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 [DOI] [PubMed] [Google Scholar]
- 2. Lumeng C. N., Saltiel A. R. (2011) Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glass C. K., Olefsky J. M. (2012) Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 15, 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregor M. F., Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 [DOI] [PubMed] [Google Scholar]
- 5. Johnson A. M. F., Olefsky J. M. (2013) The origins and drivers of insulin resistance. Cell 152, 673–684 [DOI] [PubMed] [Google Scholar]
- 6. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., Chen H. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007a) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lumeng C. N., Deyoung S. M., Bodzin J. L., Saltiel A. R. (2007b) Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 [DOI] [PubMed] [Google Scholar]
- 10. Nguyen M. T., Favelyukis S., Nguyen A. K., Reichart D., Scott P. A., Jenn A., Liu-Bryan R., Glass C. K., Neels J. G., Olefsky J. M. (2007) A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282, 35279–35292 [DOI] [PubMed] [Google Scholar]
- 11. Hotamisligil G. S. (1999) Mechanisms of TNF-α-induced insulin resistance. Exp. Clin. Endocrinol. Diabetes 107, 119–125 [DOI] [PubMed] [Google Scholar]
- 12. Dey D., Basu D., Roy S. S., Bandyopadhyay A., Bhattacharya S. (2006) Involvement of novel PKC isoforms in FFA induced defects in insulin signaling. Mol. Cell Endocrinol. 246, 60–64 [DOI] [PubMed] [Google Scholar]
- 13. Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fève B., Bastard J. P. (2009) The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 5, 305–311 [DOI] [PubMed] [Google Scholar]
- 15. Pal D., Dasgupta S., Kundu R., Maitra S., Das G., Mukhopadhyay S., Ray S., Majumdar S. S., Bhattacharya S. (2012) Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 [DOI] [PubMed] [Google Scholar]
- 16. Yu R., Kim C. S., Kwon B. S., Kawada T. (2006) Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity 14, 1353–1362 [DOI] [PubMed] [Google Scholar]
- 17. Oh D. Y., Morinaga H., Talukdar S., Bae E. J., Olefsky J. M. (2012) Increased macrophage migration into adipose tissue in obese mice. Diabetes 61, 346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dasgupta S., Bhattacharya S., Biswas A., Majumdar S. S., Mukhopadhyay S., Ray S., Bhattacharya S. (2010) NF-κB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem. J. 429, 451–462 [DOI] [PubMed] [Google Scholar]
- 19. Takashiba S., Van Dyke T. E., Amar S., Murayama Y., Soskolne A. W., Shapira L. (1999) Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor κB. Infect. Immun. 67, 5573–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inouye K. E., Shi H., Howard J. K., Daly C. H., Lord G. M., Rollins B. J., Flier J. S. (2007) Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56, 2242–2250 [DOI] [PubMed] [Google Scholar]
- 21.Deleted in proof [Google Scholar]
- 22. Després J. P., Lemieux I. (2006) Abdominal obesity and metabolic syndrome. Nature 444, 881–887 [DOI] [PubMed] [Google Scholar]
- 23. Schenk S., Saberi M., Olefsky J. M. (2008) Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Invest. 118, 2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mori K., Emoto M., Yokoyama H., Araki T., Teramura M., Koyama H., Shoji T., Inaba M., Nishizawa Y. (2006) Association of serum fetuin-A with insulin resistance in type 2 diabetic and non-diabetic subjects. Diabetes Care 29, 468. [DOI] [PubMed] [Google Scholar]
- 25. Ix J. H., Sharma K. (2010) Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J. Am. Soc. Nephrol. 21, 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]