Abstract
As opposed to other invasive pathogens that reside into host cells in a parasitic mode, Shigella, the causative agent of bacillary dysentery, invades the colonic mucosa but does not penetrate further to survive into deeper tissues. Instead, Shigella invades, replicates, and disseminates within the colonic mucosa. Bacterial invasion and spreading in intestinal epithelium lead to the elicitation of inflammatory responses responsible for the tissue destruction and shedding in the environment for further infection of other hosts. In this article, we highlight specific features of the Shigella arsenal of virulence determinants injected by a type III secretion apparatus (T3SA) that point to the targeting of intestinal epithelial cells as a discrete route of invasion during the initial event of the infectious process.
Shigella causes ∼100 million cases of dysentery each year. Using a type III secretion apparatus, it injects effectors into colonic mucosal cells, leading to an intense inflammatory reaction responsible for tissue destruction.
Shigella spp. are Gram-negative enteroinvasive bacteria responsible for diarrheal diseases. Shigella dysenteria and Shigella flexneri are responsible for bacillary dysentery, associated with epidemics and the endemic form of the disease, respectively, mostly in developing countries in children under the age of 5 with an estimated 100 million cases yearly. There is no known reservoir of these enteropathogenic bacteria, and transmission has been mostly reported from human-to-human through feco–oral contamination. On ingestion, Shigella invades the colonic mucosa in which it elicits an intense inflammatory reaction responsible for tissue destruction. In its most severe forms, Shigella infections are associated with the dysenteric syndrome with the emission of bloody, mucopurulent stools, and eventually death. Other intestinal complications include renal failure associated with the hematouremic syndrome and bacterial expression of the Shiga toxin, as well as rheumatoid arthritis.
PART I. THE INFECTIOUS STRATEGY OF SHIGELLA
Shigella, a Pathogenic Escherichia coli Adapted to Intracellular Lifestyle
Although related to enteroinvasive E. coli responsible for similar symptoms, phylogenetic analysis indicates that Shigella has evolved from a commensal E. coli, through horizontal gene transfer and genomic mutations in a pathoadaptative process. Hence, as opposed to E. coli commensals, Shigella presents mutations in genes encoding enzymes, such as spermidine acetylase, lysine decarboxylase, and arginine acetyltransferase, which regulates the polyamine contents in bacteria shown detrimental to the infection process (Bliven and Maurelli 2012; Prosseda et al. 2012). Also, Shigella does not express any known adhesin or curlin, does not form biofilm, and is nonmotile because of the lack of flagellum expression. Instead, Shigella expresses IcsA, a surface protein allowing intracellular motility, required for bacterial spreading in intestinal epithelial cells (Bliven and Maurelli 2012). Importantly, Shigella carries a large virulence plasmid encoding the majority of the virulence determinants, including a T3SA allowing the injection of bacterial effectors into host cells to divert cellular processes.
Environmental Cues for the Regulation of Virulence Genes
How Shigella persists in the environment is not known. There are, however, environmental conditions that turn on virulence genes, allowing bacteria to invade and to replicate within the colonic mucosa. Under low temperature and low osmolarity conditions, corresponding to environmental conditions, the H-NS repressor inhibits the expression of the VirF master transcriptional regulator, and thereby the expression of virulence genes (Dorman et al. 2001). On ingestion by the host, the temperature shift leads to the activation of VirF, which induces the expression of another transcriptional regulator VirB (Dorman et al. 2001). VirB directly controls the synthesis of virulence genes, including that of the type III secretion system (T3SS). The acidic pH conditions encountered in the stomach activate the RpoS-dependent expression of periplasmic proteins allowing acid resistance, as well as the CpxA/CpxR two-component system, which, together with the temperature and osmolarity, control the expression of VirF/VirB (Marteyn et al. 2012). The conditions encountered in the colon trigger the expression of genes involved in anaerobic energy metabolism, modification of the LPS and outer membrane layers, resistance to oxidative stress, as well as that of various transcriptional regulators, including FNR. FNR was shown to regulate the synthesis of the T3SA genes, resulting in secretion apparatus with elongated needles, presumably primed but repressed for injection of effectors. At the close vicinity of colonic intestinal epithelial cells, the microaerophilic environment leads to FNR repression and activation of secretion to promote bacterial invasion (Marteyn et al. 2010). In the intracellular environment, carbon source and iron limitation may also regulate the expression of VirF/VirB-dependent genes.
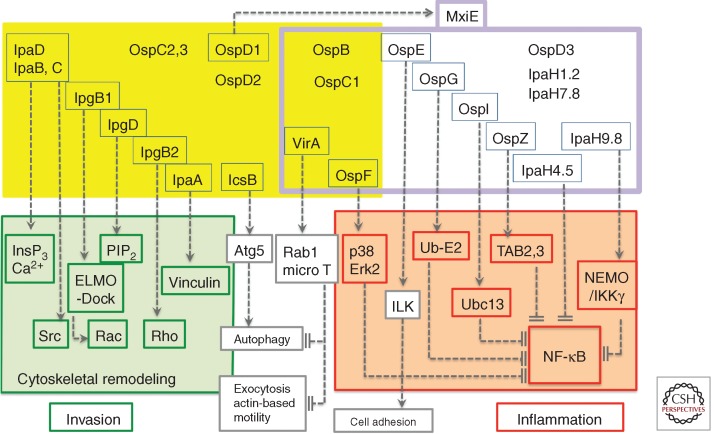
The expression of T3SA substrates is subjected to another level of regulation dependent on the secretion activity. Among the 25 identified T3S effectors, 16 are constitutively expressed at the basal state (Fig. 1) (Parsot 2009). These include the Ipa proteins implicated in invasion, which on cell contact, are injected to induce cytoskeletal reorganization. Thirteen effectors, however, are up-regulated following induction of secretion under intracellular growth conditions. Transcriptional control of these genes is performed by MxiE. MxiE senses the secretory state of the T3SA through its binding to IpgC, a chaperone of IpaB and IpaC, as well as to the T3SA substrate OspD1 (Parsot et al. 2005). On activation of the T3SA, secretion of IpaB and IpaC leads to the release of free IpgC in the bacterial cytoplasm (Mavris et al. 2002). Secretion of OspD1 that sequesters MxiE at basal state, leads to increased levels of free MxiE, which presumably in association with IpgC, up-regulate the transcription of a second wave of type III secretion (T3S) effectors. As will be developed further, the function of these second wave effectors for those characterized, is consistent with a strategy of a “discrete” intracellular replication, where the bacteria prevents inflammation and alert signals.
Figure 1.
The control of cell responses by Shigella T3S effectors. Shigella T3S effectors are shown in yellow and purple boxes. The T3S effectors boxed in blue represent those for which a function has been assigned. T3S effectors that are constitutively expressed (yellow box) or up-regulated following cell-contact induction of secretion (boxed in purple) and activation by MxiE. On cell contact, IpaB and IpaC insert into the host-cell membrane to form the “translocon,” required for the induction of InsP3-dependent Ca2+ responses. The targets of T3S effectors involved in Shigella invasion are boxed in green. IpaC, through its carboxy-terminal domain induces the recruitment and activation of the Src kinase. IpgB1 and IpgD, together with IpaC, participate in actin polymerization and membrane ruffle formation by targeting ELMO/Dock and hydrolyzing PIP2, respectively. IpgB2 that activates Rho, and IpaA that binds to vinculin, further reorganize the actin cytoskeleton to promote invasion. IcsB prevents autophagic responses by inhibiting binding of Atg5 to IcsA. The targets of T3S effectors that down-regulate inflammation are boxed in red. VirA prevents autophagy and IL-8 secretion through its GAP activity toward Rab1. VirA also inhibits microtubule polymerization to favor actin-based motility. Among the T3S effectors up-regulated by MxiE, OspF prevents the activation of the p38 and Erk2 MAP kinases via its phospho-threonine lyase activity. OspE reinforces cell adhesion by targeting ILK. The targets of OspG, OspI, OspZ, IpaH4.5, and IpaH9.8 that down-regulate NF-κB activation are indicated.
Shigella Invasion of the Colonic Epithelium via Multiple Routes
Shigella invasion of the colonic mucosa is associated with an intense inflammatory reaction, and experiments performed in rabbit ileal loops indicate that the inhibition of inflammation through the perfusion of IL-1 Ra, an antagonist of IL-1 receptor-mediated signaling, prevents not only tissular destruction but also bacterial dissemination in the intestinal mucosa (Phalipon and Sansonetti 2007). This has led to speculation that inflammation is triggered by the bacteria for efficient tissue colonization. Consistently, on ingestion by macrophage, Shigella was shown to induce cell death in a so-called “pyroptotic” process, involving the cleavage of caspase-1 and the release of proinflammatory IL-1β and IL-18 (Zychlinsky et al. 1992; Sansonetti et al. 2000). Using an icsA mutant deficient for cell-to-cell spreading, it was observed that Shigella invades at the levels of Peyer’s patches, through M-cells of the follicle-associated epithelium (Sansonetti and Phalipon 1999). Via this route of entry, Shigella-induced pyroptotic death of macrophage leads to the recruitment of polymorphonuclear cells and contributes to the destabilization of the epithelial layer, thus permitting de novo invasion of intestinal epithelial cells through a basolateral route. The rabbit ileal loop infection model, however, does not allow the study of primary events during Shigella infection. Although relatively inefficient in vitro, Shigella invasion can occur at the apical surface of polarized intestinal epithelial cells (Fig. 2). This infection route, which only involves discrete sites of the intestinal epithelial layer, may have been overlooked in studies using an icsA mutant deficient in cell-to-cell spreading. In recent years, the functional characterization of various T3S second wave effectors suggests that discrete invasion of intestinal epithelial cells by Shigella represents a route, in which the down-regulation of inflammation is required for bacterial replication and dissemination.
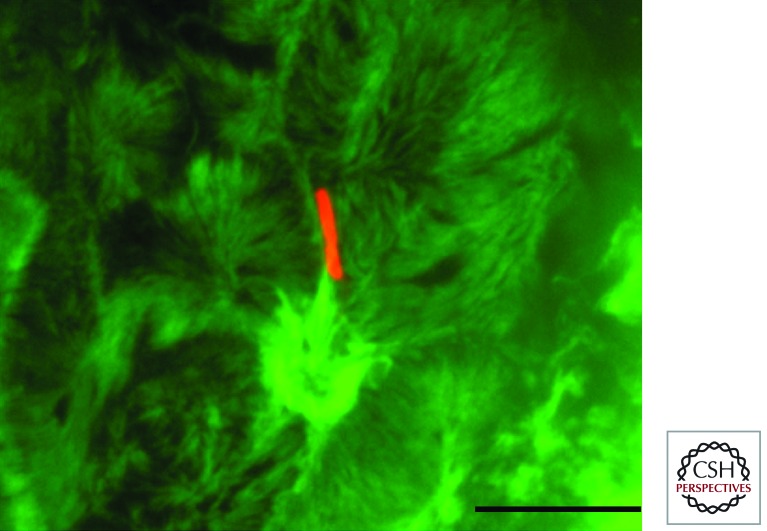
Figure 2.
Filopodial capture and Shigella invasion at the apical side of polarized Caco-2 cells. Shown is a Z projection of confocal fluorescence images of Shigella-induced actin foci at the apical side of polarized Caco-2 cells. Red, anti-LPS immunostaining; green, Alexa488-phalloidin staining. Z sections were acquired via spinning-disk confocal microscopy. Scale bar, 5 mm.
Following cell invasion, Shigella lyses the phagocytic vacuole to replicate intracellularly (Fig. 3). During intracellular replication, Shigella moves by polymerizing actin at one bacterial pole, forming actin comet tails, allowing the formation of bacteria-containing protrusions at the cell plasma membrane that invade adjacent cells. Following lysis of the donor and recipient cell membranes, bacteria reinitiate intracellular replication to disseminate into the epithelium. Bacterial intracellular replication occurs at a doubling time estimated at 10–15 min, emphasizing the fitness of the bacterium to the intracellular environment. Infected cells loaded with bacteria die a few hours following infection. The precise mechanism implicated in Shigella-induced epithelial cell death has not been solved but appears to correspond to a proinflammatory necrotic death associated with mitochondrial membrane damages (Carneiro et al. 2009).
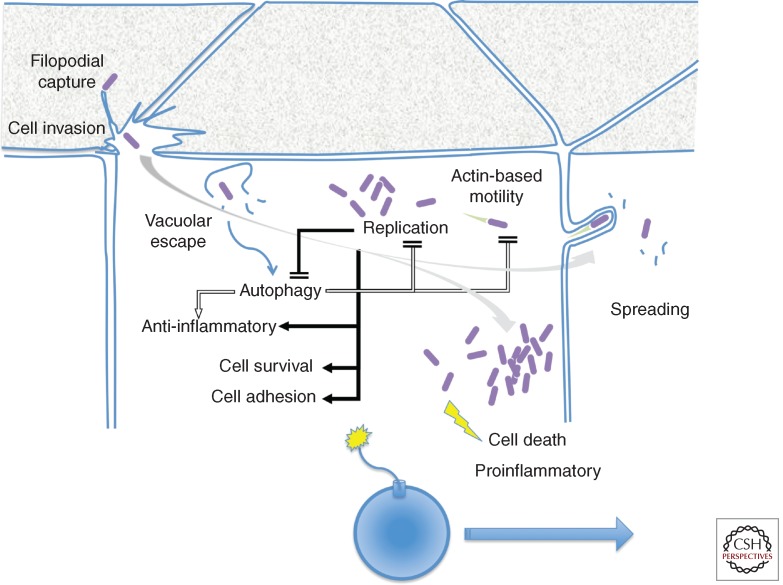
Figure 3.
Shigella invasion, intracellular replication, and spreading in epithelial cells. Filopodial capture initiates the invasion process, accompanied by localized membrane ruffles at the epithelial cell apical surface. Following invasion, bacterial escape from the vacuole triggers autophagic responses that dampen inflammatory signals and limit bacterial replication and actin-based motility. During the early stages of bacterial intracellular replication, T3S effectors counter autophagy, down-regulate inflammatory responses, promote cell survival, and reinforce cell adhesion (solid arrows) to preserve the epithelial cell integrity (grey arrow), to allow bacterial spreading. At late stages of intracellular replication, the increase in cytosolic Ca2+ concentration and mitochondrial damage lead to necrotic cell death and the emission of proinflammatory signals (yellow). Shigella invasion of epithelial cells can be seen as the ignition of a time bomb, in which bacterial spreading must occur before necrotic death of the primary infected cell.
Filopodial Capture Sets the Stage for Bacterial Invasion
Shigella is a highly efficient invasive pathogen, because 100–1000 ingested bacteria were estimated sufficient to cause the disease. The high in vivo invasion efficiency is in contrast to the relatively poor efficiency of epithelial cell invasion modeled in in vitro cultured polarized intestinal cells. One factor limiting bacterial invasion is the absence of constitutive cell-binding activity. This lack of cell-binding activity probably stresses the bacterial fitness to the intracellular lifestyle, and perhaps underlines a strategy of invasion associated with a lack of exposure at the cell surfaces during the initial stages of the infectious process.
Although Shigella does not express adhesin that would allow constitutive cell-binding activity, during the initial steps of cell infection, only a minute fraction of bacteria interacts with epithelial cells to trigger their internalization. This peculiar characteristic is linked to a sampling of invasive bacteria by finger-like cell extensions protruding from the cell surface termed filopodia, corresponding to sensory organelles probing the cell environment to establish adhesion structures (Fig. 2) (Romero et al. 2011). The interaction between one bacterial pole and the filopodial tip triggers the retraction of bacteria-bound extensions toward the cell body in which invasion occurs. Bacterial capture by filopodia is dependent on the T3SA and appears to result from direct binding of the T3SA tip complex to unidentified receptors at the filopodial tip. Measurements based on optical tweezers indicate that a discrete numbers of T3SA are sufficient to promote filopodial retraction, with a strength that is comparable to that induced by several thousands of integrin ligands (Romero et al. 2012). There is no evidence for active type III secretion during these initial events to promote retraction. The discrete number of T3SA sufficient to promote retraction probably reflects elementary events at the filopodial tip regulating the dynamics of actin assembly on the few tens of filaments in the filopodial shaft. Bacterial-induced filopodial retraction results from inhibition of actin polymerization at the filopodial tip, and from the action of the treadmilling of actin filaments in the cell cortex that pulls the embedded filopodial actin shaft inward. Retraction is controlled by the MAP kinase Erk that also controls the dynamics of bacterial capture by filopodia. Filopodial capture is increased during Shigella invasion, leading to the internalization of multiple bacteria at a single invasion site (Romero et al. 2011).
Vacuolar Lysis
Following internalization, Shigella rapidly lyses the phagocytic vacuole. Time-lapse fluorescence microscopy using markers of the vacuolar membrane or using a FRET sensor to detect bacterial access to the cytosol, indicate that vacuolar lysis occurs in the continuation of the invasion process, within minutes following bacterial-cell contact (Ehsani et al. 2012). Studies involving the complementation of mutants with orthologs from Salmonella typhimurium, an enteroinvasive bacterium that does not lyse, but replicates within an intracellular vacuole, point to a role for translocon components in vacuolar rupture (Hermant et al. 1995), but the precise mechanism remains undefined. As for invasion, efficient vacuolar rupture probably results from the concerted action of T3S effectors.
Autophagy and Inflammation
On vacuolar rupture, membrane remnants act as danger signals that are controlled by the autophagic responses. Proteins contained in membrane remnants are polyubiquinated and recruit autophagic markers such as LC3, as well as the Nalp3, Asc, and Ipaf inflammasome markers (Dupont et al. 2009). By targeting the inflammasome markers to degradation, autophagy acts as a rheostat controlling the levels of danger signals emitted by bacteria invading the cell cytosol (Fig. 3). Thus, during early phases of invasion, discrete events of vacuolar rupture controlled by the autophagic pathway may have a limited impact on inflammatory signals, in particular because T3S effectors also negatively regulate these signals (Ashida et al. 2011).
At later stages, this balance may switch to a proinflammatory context, when the accumulation of invading bacteria and vacuolar membrane remnants overwhelm the autophagy machinery. Bacteria intracellular replication leads to cytosolic accumulation of PAMPs (pathogen associated molecular patterns), such as muramyl dipeptide, which activates the NLR inflammasome. Increase in cytosolic calcium concentration leads to the activation of protease, such as calpain, and mitochondrial damage leads to the death of infected cells via a necrotic pathway (Fig. 3) (Carneiro et al. 2009; Bergounioux et al. 2012).
As for many pathogens that use actin to move intracellularly, such as Listeria or Shigella, activation of autophagy may control intracellular replication. For Shigella, autophagy is associated with rings of septins, described as molecular scaffolds implicated in cell division (Mostowy et al. 2010). Septins form “cages” entrapping Shigella, preventing intracellular motility and targeting bacteria to autophagy. To counter autophagy, Shigella secretes the IcsB T3S effector that prevents Atg5 recognition of IcsA and bacterial degradation (Figs. 1 and 3) (Ogawa et al. 2005).
Cell-to-Cell Spreading
In vitro experiments, using reconstituted motility assays with purified proteins, show that IcsA is necessary and sufficient for Shigella motility. IcsA binds to N-WASP (neuronal Wiskott–Aldrich syndrome protein), which in turn, activates the Arp2/3 actin nucleator complex (Egile et al. 1999). In cells, IcsA-mediated bacterial motility is also controlled by tyrosine kinases, such as Btk or Abl/Arg that phosphorylate N-WASP and allow its recruitment at the bacterial surface (Burton et al. 2005; Dragoi et al. 2012). Intracellular Shigella also recruits the TOCA-1 protein, necessary to relieve the autoinhibition of N-WASP (Leung et al. 2008). Actin-based motility is favored by the action of the T3S effector VirA that prevents the assembly of microtubules representing potential physical obstacles (Yoshida et al. 2006).
Although actin-based motility allows Shigella to probe its intracellular environment, it is not sufficient for bacterial spreading. Cadherin-based junctions are required for Shigella cell-to-cell spread, presumably by providing the machinery necessary for protrusion formation by the donor cell, and its endocytosis by the recipient cell in a process requiring the activity of the T3SA and myosin II (Sansonetti et al. 1994; Schuch et al. 1999; Rathman et al. 2000). Recently, Shigella was observed to disseminate preferentially at multijunctions, corresponding to sites within the epithelium in which several cells intersect (Fukumatsu et al. 2012). Tricellulin, a marker of these multijunctions, has been involved in Shigella spreading, by participating in a noncanonical clathrin-dependent endocytic pathway (Fukumatsu et al. 2012).
Preserving the Epithelium Integrity to Allow Dissemination
As mentioned earlier, increased loads of bacteria linked to intracellular replication and multiple invasion events will lead infected epithelial cells to undergo necrotic death. The death of infected cells, however, may be counterproductive for the infectious process if occurring too early. Through the action of injected T3S effectors, Shigella preserves epithelial cells’ integrity by delaying cell detachment, thus increasing possibility to disseminate.
IpgD is a T3S effector that hydrolyzes phosphatidylinositol (PI) (4, 5) biphosphate (PIP2) to generate PI5P. PI5P activates a cell-survival pathway dependent on the PI3 kinase and the Akt kinase (Pendaries et al. 2006). Another T3S effector, OspE, binds to ILK (integrin-like kinase) to stabilize integrin-dependent attachment of the cell to the basal matrix (Fig. 1) (Kim et al. 2009). Reinforcement of cell adhesion to the matrix may represent an important factor during infection, by preventing their clearing in the intestinal lumen (Kim et al. 2009).
Shigella also induces calcium signals during the early phase of epithelial cell invasion, leading to the opening of connexin hemichannels at the plasma membrane (Tran Van Nhieu et al. 2003). ATP, released in the extracellular medium through the opening of hemichannels, signals in a paracrine manner to neighboring cells to enhance bacterial spreading, as well as invasion by stimulating Erk-dependent filopodial capture (Clair et al. 2008; Romero et al. 2011).
Silencing Inflammation and Danger Signals
Some constitutively expressed T3S effectors are up-regulated following activation of the T3SA and Shigella invasion (Parsot 2009). For these, it is possible that their activity is required early following the invasion process, as well as at later stages, during intracellular replication and spreading. Modulation of T3S effectors activity can occur consequently to endoproteolytic cleavage by Caspase 3 (Srikanth et al. 2010). T3S effectors and translocon components can have multiple activities. For example, in addition to its role in translocation, IpaB participates in the pyroptotic death observed in macrophages, and dissolution of the Golgi observed during epithelial cell invasion (Fig. 1) (Mounier et al. 2012). The disruption of the Golgi apparatus may impair processes such as exocytosis, involved in membrane receptor signaling, and the mounting of inflammatory responses. Along these lines, VirA shows GAP activity toward Rab1 and prevents the targeting of bacteria to autophagy, as well as the secretion of the proinflammatory cytokine IL-8 (Fig. 1) (Dong et al. 2012). Six T3S effectors have been reported to directly or indirectly inhibit the activation of the pro-inflammatory transcription factor NF-κ. OspF removes phosphothreonine residues on the p38 and Erk2 MAP kinases, preventing their activation (Arbibe et al. 2007; Li et al. 2007); OspG prevents the ubiquitinylation of IκBα and therefore its degradation, by targeting ubiquitinylated ubiquitin-conjugating enzymes (Kim et al. 2005); OspI deamidates Ubc13 to prevent its ubiquitin ligase activity and TRAF-6 signaling (Sanada et al. 2012); OspZ is related to the EPEC NleE T3S effector, that prevents NF-κ translocation through the cystein methylation of ubiquitin chain-sensing protein (Newton 2010; Zhang et al. 2011); the IpaH proteins are E3 ubiquitin ligases. IpaH4.5 ubiquitinates the p65 subunit of NF-κB (Wang et al. 2013); IpaH9.8 promotes the polyubiquitination of NEMO/IκB kinase and its degradation (Fig. 1) (Ashida et al. 2010).
PART II. MOLECULAR INSIGHTS INTO SHIGELLA INVASION OF EPITHELIAL CELLS
The Shigella Mxi-Spa T3SA
T3SAs are expressed by many of Gram-negative bacterial pathogens to mammals, fish, and plants (Mueller et al. 2008). These secretion systems are repressed under basal state conditions, and are activated on cell contact to allow the delivery of effectors, directly from the bacterial cytosol to the cell cytoplasm (Cossart and Sansonetti 2004; Münter et al. 2006; Matteï et al. 2011). This inducible feature of the secretory activity may be an important reason for the wide representation of T3SAs in bacterial pathogens, because it allows the masking of virulence determinants to host defense systems and a cis-acting activity of injected effectors. T3SAs present high levels of synteny, conserved structure, and function. These apparatus are related to flagellar systems and many of the general T3SS features have been inferred from studies on the Shigella T3SS.
The Shigella Mxi-Spa T3SA is composed of 21 different proteins forming a basal body embedded in the bacterial inner and outer membranes prolonged by a needle capped by a so-called “tip complex” (Blocker et al. 2008; Mueller et al. 2008). As for flagella, the basal body consists of a series of rings formed by the oligomerization of different T3SA components. Structural evidence indicates that at basal state, the tip complex corresponds to a homopentamer of IpaD (Epler et al. 2012). On binding to deoxycholate, which may correspond to the bile salts enriched environment encountered by Shigella during transit in the intestinal lumen, IpaD switches conformation to allow the tip presentation of IpaB (Dickenson et al. 2011). On contact with host cells, IpaC is secreted and together with IpaB insert into the host-cell plasma membrane to form the “translocon,” endowed with pore-forming activity and required for the translocation of injected effectors into the host cell cytosol.
How the tip complex senses host-cell membrane to trigger secretion remains an open question. Host-cell sensing has been proposed to involve interaction between IpaB and cell-surface receptors, such as hyaluronic acid receptor CD44. Interestingly, IpaD thought to connect IpaB and the MxiH-needle, also appears to control the various stages of secretion between early and late effectors on cell contact (Schiavolin et al. 2013). The precise mechanism controlling the switch from nonactive to active secretion following tip contact is not known. The change of configuration of the tip complex proteins associated with interaction with a cell-surface receptor may lead to the emission of signals, involving MxiC and perhaps a tip-to-base transduction of signals through change of configuration of MxiH (Blocker et al. 2008; Botteaux et al. 2009; Fujii et al. 2012).
The IpaB and IpaC Translocon Components and Tyrosine Kinase Signaling
Shigella invasion of epithelial cells is associated with the formation of actin-rich membrane ruffles at the site of bacterial contact that engulf the bacterium in a macropinocytic process. This invasion process is triggered by the cell-contact-dependent injection of type-III effectors acting in concert to reorganize the actin cytoskeleton (Figs. 1 and 2).
Invasion of epithelial cells was shown to depend on cholesterol-rich microdomains, presumably because they represent signaling platforms readily mobilized that are proficient for bacterial-induced cytoskeletal reorganization. Targeting of microdomains may occur through direct preferential binding of the IpaB translocon components to cholesterol, or through IpaB interaction with cell-surface receptors such as CD44, that triggers its mobilization to microdomains (Lafont et al. 2002). On cell contact, IpaC is secreted and together with IpaB form the translocon within the host-cell plasma membrane, required for the injection of T3S effectors. In addition to its role in translocation, IpaC participates in the early stages of the invasion process by inducing actin polymerization. IpaC-mediated actin polymerization involves the recruitment and activation of the Src tyrosine kinase, which allows further amplification of actin polymerization and ruffle formation (Fig. 1) (Mounier et al. 2009). During Shigella invasion, Src mediates the phosphorylation of cortactin, an F-actin binding protein that can promote Arp2/3 complex-dependent actin polymerization. Tyrosyl-phosphorylated cortactin is then recruited at the plasma membrane, via interaction with the Crk adaptor to amplify actin polymerization and membrane ruffle formation (Bougnères et al. 2004). Tyrosine kinase signaling may also further allow the amplification of actin polymerization at invasion sites by acting upstream of the Rac GTPase. In addition to its role in the recruitment of cortactin, Crk was shown to be a target for Abl/Arg tyrosine kinases that are activated and required during Shigella invasion (Burton et al. 2003). Because in other systems Crk has been implicated in the recruitment and activation of Rac, the Abl/Arg kinases may maintain and amplify Rac activation at entry sites (Vuori and Ruoslahti 1995). Interestingly, Abl/Arg kinases are also activated downstream from Src kinases (Plattner et al. 1999). Thus, tyrosine kinases via Crk may feed in a loop that amplifies signals and actin polymerization leading to ruffle formation at Shigella invasion sites.
The Role of IpgB1 and IpgD in Shigella-Induced Actin Polymerization
Among the T3S injected effectors, IpgB1 has also been implicated in Shigella invasion of epithelial cells. IpgB1 belongs to the so-called “WXXXE” type-III effector family, formerly proposed to act as mimics of RhoGTPases, but for which there are structural and enzymatic activity evidence that this family rather corresponds to GEFs (guanosine exchange factor) for small GTPases (Fig. 1) (Alto et al. 2006; Bulgin et al. 2010; Orchard and Alto 2012). The picture is blurred by evidence that the same WXXXE effectors recruit GEFs for the GTPase. For example, IpgB1 first described as a mimic for Rac, may also recruit the ELMO-Dock180 complex with GEF activity toward Rac (Handa et al. 2007). Rac activation leads to actin polymerization mediated by the activation of the Arp2/3 nucleator complex. Although it is clear that Rac-dependent actin polymerization occurs in the absence of IpgB1, IpgB1-mediated activation increases ruffle formation at entry sites (Mounier et al. 2009).
IpgD also participates in the induction of membrane ruffling at Shigella invasion sites, although its precise mode of action is unclear. PIP2-binding proteins are involved in the anchoring of cortical actin to the plasma membrane. Through PIP2 hydrolysis, IpgD may help free cortical actin to favor de novo polymerization at invasion sites (Fig. 1) (Niebuhr et al. 2000). It is possible, however, that IpgD participates through different means in Shigella invasion. The Salmonella ortholog SopB/ SigD, also hydrolyzes PIP2. SopB, however, was also shown to act as an inositolpolyphosphate (InsP) phosphatase leading to the production of InsP4, shown to stimulate Rac-dependent membrane ruffling (Zhou et al. 2001). More recently, SopB has been involved in the activation of the Abi/Wave complex through the stimulation of PIP3 production and the activation of the ARNO GEF. SopB activity may synergize with that of the SopE GEF for Rac to produce actin polymerization, or alternatively act in a SopE-independent pathway to induce bacterial invasion in a myosin-II-dependent process (Hänisch et al. 2011; Humphreys et al. 2012).
The Role of IpgB2 and IpaA in Shigella-Induced Cytoskeletal Reorganization
Although Rac and actin polymerization are required for ruffle formation and the initiation of Shigella invasion, Rho activation appears to be required for the recruitment of components such as ezrin, involved in later stages of the invasion process (Duménil et al. 2000). Other lines of evidence implicate Rho at later stages of Shigella invasion through the action of two injected T3S effectors, IpaA and IpgB2 (Fig. 1) (Alto et al. 2006; Demali et al. 2006; Klink et al. 2010). IpaA binds to the focal adhesion protein vinculin via its carboxy-terminal domain that mimics talin (Izard et al. 2006). Talin is also a component of adhesion structures that binds to the cytosolic domain of the integrin β1 subunit and activates vinculin. Following activation, vinculin unfolds and its carboxy-terminal domain associates with actin filaments. The talin–vinculin interaction is used by cells to reinforce cytoskeletal anchorage to integrin receptors (del Rio et al. 2009; Parsons et al. 2010). In the absence of classical receptor–ligand interaction explaining how Shigella recruits focal adhesion proteins, it is possible that signaling occurs through injected IpaA. Consistent with its vinculin-binding activity, IpaA allows the anchorage of bacteria within membrane ruffle (Tran Van Nhieu et al. 1997). Surprisingly, however, not only does IpaA promote this bacterial anchorage activity, but IpaA also permits the depolymerization of actin filaments during the completion of the invasion process (Bourdet-Sicard et al. 1999). The mechanism underlying IpaA-mediated actin depolymerization is still unclear. The amino-terminal part of IpaA has been proposed to induce the activation of Rho by an undefined mechanism which, together with IpaA-mediated inhibition of talin association with β1 integrin, leads to the disappearance of actin stress fibers (Demali et al. 2006). IpaA in association with vinculin was also shown to regulate the dynamics of actin polymerization/depolymerization by partially capping the barbed end of actin filaments (Ramarao et al. 2007).
IpgB2, first described as a Rho mimic, may also activate Rho through its GEF activity, or through the activation of GEF-H1 (Alto et al. 2006; Fukazawa et al. 2008). A role for IpgB2 in Shigella invasion is observed in polarized but not in nonpolarized cells and only in combination with IpgB1 (Hachani et al. 2008). Because polarized cells differ from nonpolarized cells by the establishment of cell–cell junctions, it is tempting to speculate that the combined action of IpgB1 and IpgB2 serves to regulate Rac- and Rho-dependent junctional structures such as cadherin-based junctions (Harris and Tepass 2012).
InsP3-Dependent Local Calcium Signals during Shigella Invasion
How precisely initial events leading to bacterial-induced actin polymerization during Shigella invasion are triggered is not known. There is evidence indicating that this early signaling is directly linked to insertion of the translocon in the plasma membrane. First, although inactivation of the above-mentioned injected T3S effectors only lead to a partial decrease of bacterial invasion, mutations in the translocon component IpaC that does not prevent effector translocation totally abolishing bacterial-induced actin polymerization and Shigella uptake (Mounier et al. 2009). Interestingly, Shigella induces calcium responses localized at the site of bacterial invasion that, unlike bacterial-induced global calcium responses, are required for actin polymerization at entry sites (Tran Van Nhieu et al. 2013). These calcium responses can occur in the absence of extracellular calcium and are elicited by InsP3-mediated release of intracellular pools, in a process implicating PLC-β1 and PLC-δ1. As for actin polymerization, Shigella-induced InsP3-mediated signaling does not appear to depend on injected T3S effectors, and is totally abolished in mutants for the translocon component IpaC (Tran Van Nhieu et al. 2013). As described for pore-forming toxins, insertion of the Shigella translocon in the plasma membrane may lead to the destabilization of lipid bilayers leading to the activation of PLCs, that hydrolyze PIP2 to induce InsP3-mediated signaling (García-Sáez et al. 2011). Future works will be required to investigate the relationship between this Insp3-mediated signaling, tyrosine kinase activation, and stimulation of Rac-dependent actin polymerization during Shigella invasion.
CONCLUDING REMARKS
Shigella can be considered as a primitive, poorly evolved pathogen because it invades the colonic mucosa, without persisting in deeper tissues, and elicits an intense inflammation and tissue destruction that may kill its host. We propose a scheme where Shigella invasion occurs at discrete sites of the intestinal epithelium, timing its intracellular replication to ensure spreading before the death of the primary infected cell. This discrete route of invasion plants the seeds leading to the massive inflammation and tissue destruction observed at the late stages of infection.
In this article, we have focused on intestinal epithelial cells as the spreading ground for this pathogen. Clearly, this is a reductionist view, as the role of other players such as macrophages and M cells, Paneth cells secreting defensins, or the enteric nervous system, shown to be important in the control of Shigella infection has not been developed. While compared with other pathogens, the invasion and destruction of the colonic mucosa could appear as a reflection of a primitive adaptation to its host, the extent of sophistication developed by Shigella through the action of multiple effectors dedicated to the timely subversion of host-cell processes keeps revealing facets of the complexity of the infectious process.
ACKNOWLEDGMENTS
The authors thank the Collège de France, the Institut National de la Santé et de la Recherche Médical, the Centre National de la Recherche Scientifique, and l’Agence Nationale de la Recherche for funding. N.C. is a Marie Curie IRG grant recipient.
Footnotes
Editors: Pascale Cossart and Stanley Maloy
Additional Perspectives on Bacterial Pathogenesis available at www.perspectivesinmedicine.org
REFERENCES
- Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, McMahon SA, Ghosh P, Hughes TR, Boone C, et al. 2006. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124: 133–145 [DOI] [PubMed] [Google Scholar]
- Arbibe L, Kim DW, Batsche E, Pedron T, Mateescu B, Muchardt C, Parsot C, Sansonetti PJ 2007. An injected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses. Nat Immunol 8: 47–56 [DOI] [PubMed] [Google Scholar]
- Ashida H, Kim M, Schmidt-Supprian M, Ma A, Ogawa M, Sasakawa C 2010. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKγ to dampen the host NF-κB-mediated inflammatory response. Nat Cell Biol 12: 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida H, Ogawa M, Kim M, Suzuki S, Sanada T, Punginelli C, Mimuro H, Sasakawa C 2011. Shigella deploys multiple countermeasures against host innate immune responses. Curr Opin Microbiol 14: 16–23 [DOI] [PubMed] [Google Scholar]
- Bergounioux J, Elisee R, Prunier AL, Donnadieu F, Sperandio B, Sansonetti P, Arbibe L 2012. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium’s epithelial niche. Cell Host Microbe 11: 240–252 [DOI] [PubMed] [Google Scholar]
- Bliven KA, Maurelli AT 2012. Antivirulence genes: Insights into pathogen evolution through gene loss. Infect Immun 80: 4061–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker AJ, Deane JE, Veenendaal AK, Roversi P, Hodgkinson JL, Johnson S, Lea SM 2008. What’s the point of the type III secretion system needle? Proc Natl Acad Sci 105: 6507–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteaux A, Sory MP, Biskri L, Parsot C, Allaoui A 2009. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol Microbiol 71: 449–460 [DOI] [PubMed] [Google Scholar]
- Bougnères L, Girardin SE, Weed SA, Karginov AV, Olivo-Marin JC, Parsons JT, Sansonetti PJ, Tran Van Nhieu G 2004. Cortactin and Crk cooperate to trigger actin polymerization during Shigella invasion of epithelial cells. J Cell Biol 166: 225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdet-Sicard R, Rüdiger M, Jockusch BM, Gounon P, Sansonetti PJ, Tran Van Nhieu G 1999. Binding of the Shigella protein IpaA to vinculin induces F-actin depolymerization. EMBO J 18: 5853–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgin R, Raymond B, Garnett JA, Frankel G, Crepin VF, Berger CN, Arbeloa A 2010. Bacterial guanine nucleotide exchange factors SopE-like and WxxxE effectors. Infect Immun 78: 1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EA, Plattner R, Pendergast AM 2003. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J 22: 5471–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EA, Oliver TN, Pendergast AM 2005. Abl kinases regulate actin comet tail elongation via an N-WASP-dependent pathway. Mol Cell Biol 25: 8834–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro LA, Travassos LH, Soares F, Tattoli I, Magalhaes JG, Bozza MT, Plotkowski MC, Sansonetti PJ, Molkentin JD, Philpott DJ, et al. 2009. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe 5: 123–136 [DOI] [PubMed] [Google Scholar]
- Clair C, Combettes L, Pierre F, Sansonetti P, Tran Van Nhieu G 2008. Extracellular-loop peptide antibodies reveal a predominant hemichannel organization of connexins in polarized intestinal cells. Exp Cell Res 314: 1250–1265 [DOI] [PubMed] [Google Scholar]
- Cossart P, Sansonetti PJ 2004. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science 304: 242–248 [DOI] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP 2009. Stretching single talin rod molecules activates vinculin binding. Science 323: 638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demali KA, Jue AL, Burridge K 2006. IpaA targets β1 integrins and ρ to promote actin cytoskeleton rearrangements necessary for Shigella entry. J Biol Chem 281: 39534–39541 [DOI] [PubMed] [Google Scholar]
- Dickenson NE, Zhang L, Epler CR, Adam PR, Picking WL, Picking WD 2011. Conformational changes in IpaD from Shigella flexneri upon binding bile salts provide insight into the second step of type III secretion. Biochemistry 50: 172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Zhu Y, Lu Q, Hu L, Zheng Y, Shao F 2012. Structurally distinct bacterial TBC-like GAPs link Arf GTPase to Rab1 inactivation to counteract host defenses. Cell 150: 1029–1041 [DOI] [PubMed] [Google Scholar]
- Dorman CJ, McKenna S, Beloin C 2001. Regulation of virulence gene expression in Shigella flexneri, a facultative intracellular pathogen. Int J Med Microbiol 291: 89–96 [DOI] [PubMed] [Google Scholar]
- Dragoi AM, Talman AM, Agaisse H 2012. Bruton’s tyrosine kinase regulates Shigella flexneri dissemination in HT-29 intestinal cells. Infect Immun 81: 598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duménil G, Sansonetti P, Tran Van Nhieu G 2000. Src tyrosine kinase activity down-regulates ρ-dependent responses during Shigella entry into epithelial cells and stress fibre formation. J Cell Sci 113: 71–80 [DOI] [PubMed] [Google Scholar]
- Dupont N, Lacas-Gervais S, Bertout J, Paz I, Freche B, Tran Van Nhieu G, van der Goot FG, Sansonetti PJ, Lafont F 2009. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe 6: 137–149 [DOI] [PubMed] [Google Scholar]
- Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol 146: 1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsani S, Santos JC, Rodrigues CD, Henriques R, Audry L, Zimmer C, Sansonetti P, Tran Van Nhieu G, Enninga J 2012. Hierarchies of host factor dynamics at the entry site of Shigella flexneri during host cell invasion. Infect Immun 80: 2548–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epler CR, Dickenson NE, Bullitt E, Picking WL 2012. Ultrastructural analysis of IpaD at the tip of the nascent MxiH type III secretion apparatus of Shigella flexneri. J Mol Biol 420: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Cheung M, Blanco A, Kato T, Blocker AJ, Namba K 2012. Structure of a type III secretion needle at 7-A resolution provides insights into its assembly and signaling mechanisms. Proc Natl Acad Sci 109: 4461–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa A, Alonso C, Kurachi K, Gupta S, Lesser CF, McCormick BA, Reinecker HC 2008. GEF-H1 mediated control of NOD1 dependent NF-κB activation by Shigella effectors. PLoS Pathog 4: e1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumatsu M, Ogawa M, Arakawa S, Suzuki M, Nakayama K, Shimizu S, Kim M, Mimuro H, Sasakawa C 2012. Shigella targets epithelial tricellular junctions and uses a noncanonical clathrin-dependent endocytic pathway to spread between cells. Cell Host Microbe 11: 325–336 [DOI] [PubMed] [Google Scholar]
- García-Sáez AJ, Buschhorn SB, Keller H, Anderluh G, Simons K, Schwille P 2011. Oligomerization and pore formation by equinatoxin II inhibit endocytosis and lead to plasma membrane reorganization. J Biol Chem 286: 37768–37777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachani A, Biskri L, Rossi G, Marty A, Ménard R, Sansonetti P, Parsot C, Tran Van Nhieu G, Bernardini ML, Allaoui A 2008. IpgB1 and IpgB2, two homologous effectors secreted via the Mxi-Spa type III secretion apparatus, cooperate to mediate polarized cell invasion and inflammatory potential of Shigella flexenri. Microbes Infect 10: 260–268 [DOI] [PubMed] [Google Scholar]
- Handa Y, Suzuki M, Ohya K, Iwai H, Ishijima N, Koleske AJ, Fukui Y, Sasakawa C 2007. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat Cell Biol 9: 121–128 [DOI] [PubMed] [Google Scholar]
- Hänisch J, Kölm R, Wozniczka M, Bumann D, Rottner K, Stradal TE 2011. Activation of a RhoA/myosin II-dependent but Arp2/3 complex-independent pathway facilitates Salmonella invasion. Cell Host Microbe 9: 273–285 [DOI] [PubMed] [Google Scholar]
- Harris TJ, Tepass U 2012. Adherens junctions: From molecules to morphogenesis. Nat Rev Mol Cell Biol 11: 502–514 [DOI] [PubMed] [Google Scholar]
- Hermant D, Ménard R, Arricau N, Parsot C, Popoff MY 1995. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol Microbiol 17: 781–789 [DOI] [PubMed] [Google Scholar]
- Humphreys D, Davidson A, Hume PJ, Koronakis V 2012. Salmonella virulence effector SopE and Host GEF ARNO cooperate to recruit and activate WAVE to trigger bacterial invasion. Cell Host Microbe 11: 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard T, Tran Van Nhieu G, Bois PR 2006. Shigella applies molecular mimicry to subvert vinculin and invade host cells. J Cell Biol 175: 465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Lenzen G, Page AL, Legrain P, Sansonetti PJ, Parsot C 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc Natl Acad Sci 102: 14046–14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Ogawa M, Fujita Y, Yoshikawa Y, Nagai T, Koyama T, Nagai S, Lange A, Fässler R, Sasakawa C 2009. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature 459: 578–582 [DOI] [PubMed] [Google Scholar]
- Klink BU, Barden S, Heidler TV, Borchers C, Ladwein M, Stradal TE, Rottner K, Heinz DW 2010. Structure of Shigella IpgB2 in complex with human ρA: Implications for the mechanism of bacterial guanine nucleotide exchange factor mimicry. J Biol Chem 285: 17197–17208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F, Tran Van Nhieu G, Hanada K, Sansonetti P, van der Goot FG 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J 21: 4449–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung Y, Ally S, Goldberg MB 2008. Bacterial actin assembly requires toca-1 to relieve N-wasp autoinhibition. Cell Host Microbe 3: 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, Chen S, Zhou JM, Shao F 2007. The phosphothreonine lyase activity of a bacterial type III effector family. Science 315: 1000–1003 [DOI] [PubMed] [Google Scholar]
- Marteyn B, West NP, Browning DF, Cole JA, Shaw JG, Palm F, Mounier J, Prévost MC, Sansonetti P, Tang CM 2010. Modulation of Shigella virulence in response to available oxygen in vivo. Nature 465: 355–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteyn B, Gazi A, Sansonetti P 2012. Shigella: A model of virulence regulation in vivo. Gut Microbes 3: 104–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteï PJ, Faudry E, Job V, Izoré T, Attree I, Dessen A 2011. Membrane targeting and pore formation by the type III secretion system translocon. FEBS J 278: 414–426 [DOI] [PubMed] [Google Scholar]
- Mavris M, Sansonetti PJ, Parsot C 2002. Identification of the cis-acting site involved in activation of promoters regulated by activity of the type III secretion apparatus in Shigella flexneri. J Bacteriol 184: 6751–6759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Bonazzi M, Hamon MA, Tham TN, Mallet A, Lelek M, Gouin E, Demangel C, Brosch R, Zimmer C, et al. 2010. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 8: 433–444 [DOI] [PubMed] [Google Scholar]
- Mounier J, Popoff MR, Enninga J, Frame MC, Sansonetti PJ, Tran Van Nhieu G 2009. The IpaC carboxy terminal effector domain mediates Src-dependent actin polymerization during Shigella invasion of epithelial cells. PLoS Pathog 5: e1000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J, Boncompain G, Senerovic L, Lagache T, Chrétien F, Perez F, Kolbe M, Olivo-Marin JC, Sansonetti PJ, Sauvonnet N 2012. Shigella effector IpaB-induced cholesterol relocation disrupts the Golgi complex and recycling network to inhibit host cell secretion. Cell Host Microbe 12: 381–389 [DOI] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Cornelis GR 2008. The type III secretion system tip complex and translocon. Mol Microbiol 68: 1085–1095 [DOI] [PubMed] [Google Scholar]
- Münter S, Way M, Frischknecht F 2006. Signaling during pathogen infection. Sci STKE 2006: pre5. [DOI] [PubMed] [Google Scholar]
- Newton HJ, Pearson JS, Badea L, Kelly M, Lucas M, Holloway G, Wagstaff KM, Dunstone MA, Sloan J, Whisstock JC, et al. 2010. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-κB p65. PLoS Pathog 6: e1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr K, Jouihri N, Allaoui A, Gounon P, Sansonetti PJ, Parsot C 2000. IpgD, a protein secreted by the type III secretion machinery of Shigella flexneri, is chaperoned by IpgE and implicated in entry focus formation. Mol Microbiol 38: 8–19 [DOI] [PubMed] [Google Scholar]
- Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C 2005. Escape of intracellular Shigella from autophagy. Science 307: 727–731 [DOI] [PubMed] [Google Scholar]
- Orchard RC, Alto NM 2012. Mimicking GEFs: A common theme for bacterial pathogens. Cell Microbiol 14: 10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, Schwartz MA 2010. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11: 633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C 2009. Shigella type III secretion effectors: How, where, when, for what purposes? Curr Opin Microbiol 12: 110–116 [DOI] [PubMed] [Google Scholar]
- Parsot C, Ageron E, Penno C, Mavris M, Jamoussi K, d’Hauteville H, Sansonetti P, Demers B 2005. A secreted anti-activator, OspD1, and its chaperone, Spa15, are involved in the control of transcription by the type III secretion apparatus activity in Shigella flexneri. Mol Microbiol 56: 1627–1635 [DOI] [PubMed] [Google Scholar]
- Pendaries C, Tronchère H, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B 2006. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J 25: 1024–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalipon A, Sansonetti PJ 2007. Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: A tool box for survival? Immunol Cell Biol 85: 119–129 [DOI] [PubMed] [Google Scholar]
- Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM 1999. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev 13: 2400–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosseda G, Di Martino ML, Campilongo R, Fioravanti R, Micheli G, Casalino M, Colonna B 2012. Shedding of genes that interfere with the pathogenic lifestyle: The Shigella model. Res Microbiol 163: 399–406 [DOI] [PubMed] [Google Scholar]
- Ramarao N, Le Clainche C, Izard T, Bourdet-Sicard R, Ageron E, Sansonetti PJ, Carlier MF, Tran Van Nhieu G 2007. Capping of actin filaments by vinculin activated by the Shigella IpaA carboxyl-terminal domain. FEBS Lett 581: 853–857 [DOI] [PubMed] [Google Scholar]
- Rathman M, de Lanerolle P, Ohayon H, Gounon P, Sansonetti P 2000. Myosin light chain kinase plays an essential role in S. flexneri dissemination. J Cell Sci 113: 3375–3386 [DOI] [PubMed] [Google Scholar]
- Romero S, Grompone G, Carayol N, Mounier J, Guadagnini S, Prevost MC, Sansonetti PJ, Tran Van Nhieu G 2011. ATP-mediated Erk1/2 activation stimulates bacterial capture by filopodia, which precedes Shigella invasion of epithelial cells. Cell Host Microbe 9: 508–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S, Quatela A, Bornschlög T, Guadagnini S, Bassereau P, Tran Van Nhieu G 2012. Filopodium retraction is controlled by adhesion to its tip. J Cell Sci 125: 4999–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada T, Kim M, Mimuro H, Suzuki M, Ogawa M, Oyama A, Ashida H, Kobayashi T, Koyama T, Nagai S, et al. 2012. The Shigella flexneri effector OspI deamidates UBC13 to dampen the inflammatory response. Nature 483: 623–626 [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Phalipon A 1999. M cells as ports of entry for enteroinvasive pathogens: Mechanisms of interaction, consequences for the disease process. Semin Immunol 11: 193–203 [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Mounier J, Prévost MC, Mège RM 1994. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell 76: 829–839 [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A 2000. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12: 581–590 [DOI] [PubMed] [Google Scholar]
- Schiavolin L, Meghraoui A, Cherradi Y, Biskri L, Botteaux A, Allaoui A 2013. Functional insights into the Shigella type III needle tip IpaD in secretion control and cell contact. Mol Microbiol 88: 268–282 [DOI] [PubMed] [Google Scholar]
- Schuch R, Sandlin RC, Maurelli AT 1999. A system for identifying post-invasion functions of invasion genes: Requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol Microbiol 34: 675–689 [DOI] [PubMed] [Google Scholar]
- Srikanth CV, Wall DM, Maldonado-Contreras A, Shi HN, Zhou D, Demma Z, Mumy KL, McCormick BA 2010. Salmonella pathogenesis and processing of secreted effectors by caspase-3. Science 330: 390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G, Ben-Ze’ev A, Sansonetti PJ 1997. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J 16: 2717–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L 2003. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol 5: 720–726 [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G, Kai Liu B, Zhang J, Pierre F, Prigent S, Sansonetti P, Erneux C, Kuk Kim J, Suh PG, Dupont G, et al. 2013. Actin-based confinement of calcium responses during Shigella invasion. Nat Commun 4: 1567. [DOI] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E 1995. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem 270: 22259–22262 [DOI] [PubMed] [Google Scholar]
- Wang F, Jiang Z, Li Y, He X, Zhao J, Yang X, Zhu L, Yin Z, Li X, Wang X, et al. 2013. Shigella flexneri T3SS effector IpaH4.5 modulates the host inflammatory response via interaction with NF-κB p65 protein. Cell Microbiol 15: 474–485 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Handa Y, Suzuki T, Ogawa M, Suzuki M, Tamai A, Abe A, Katayama E, Sasakawa C 2006. Microtubule-severing activity of Shigella is pivotal for intercellular spreading. Science 314: 985–989 [DOI] [PubMed] [Google Scholar]
- Zhang L, Ding X, Cui J, Xu H, Chen J, Gong YN, Hu L, Zhou Y, Ge J, Lu Q, et al. 2011. Cysteine methylation disrupts ubiquitin-chain sensing in NF-κB activation. Nature 481: 204–208 [DOI] [PubMed] [Google Scholar]
- Zhou D, Chen LM, Hernandez L, Shears SB, Galán JE 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol 39: 248–259 [DOI] [PubMed] [Google Scholar]
- Zychlinsky A, Prevost MC, Sansonetti PJ 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358: 167–169 [DOI] [PubMed] [Google Scholar]