Abstract
The sensitive and broadly reactive character of the innate immune system makes it liable to activation by stress factors other than infection. Thermal and metabolic stresses experienced during the transplantation procedure are sufficient to trigger the innate immune response and also augment adaptive immunity in the presence of foreign antigen on the donor organ. The resulting inflammatory and immune reactions combine to form a potent effector response that can lead to graft rejection. Here we examine the evidence that the complement and toll-like receptor systems are central to these pathways of injury and present a formidable barrier to transplantation. We review extensive information about the effector mechanisms that are mediated by these pathways, and bring together what is known about the damage-associated molecular patterns that initiate this sequence of events. Finally, we refer to two ongoing therapeutic trials that are evaluating the validity of these concepts in man.
Thermal and metabolic stresses during organ transplantation can trigger the innate immune response and augment adaptive immunity, leading to graft rejection. The complement and toll-like receptor systems are central to this process.
The innate arm of the immune system is geared to rapidly react with broad groups of invasive pathogens but is also triggered by a variety of physical and metabolic insults. The response consists of soluble and cellular mediators of inflammation, which resolve once the threat has been eliminated. However, failure of this response to resolve can result in chronic inflammation, loss of tissue parenchyma and development of tissue fibrosis. The presence of foreign antigen stimulates a more specific pattern of clonal expansion and affinity maturation by cells of the adaptive immune system, the recruitment of which is enhanced by the innate response. Thus the ability to acquire long-lasting immunity mediated by high-affinity T- and B-cell receptors with exquisite sensitivity for antigenic peptides is an integrated response between innate and adaptive arms of host defense.
Whereas the adaptive system ever changes with lifetime experience of infection, the innate response is fixed by evolution. The innate system is directed against shared pathogenic sequences that often include carbohydrate and lipid moieties. A set of pattern-recognition receptors that engage with these ligands is found on tissue-resident and migratory cells. They are also present on antigen-presenting cells, T cells, and B cells. These innate receptors can detect pathogen- or damage-associated molecular patterns. Other innate receptors may detect second signals amplified by the complement and coagulation cascades. In effect, tissue cells and infiltrating leukocytes are “hard-wired” to sense potentially dangerous signals in the local environment and accordingly can initiate intracellular signaling pathways that mobilize the inflammatory and immune responses.
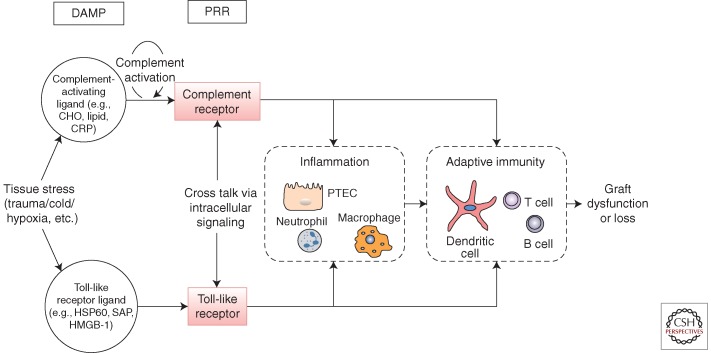
The complement and toll-like receptor (TLR) systems are well-characterized components of innate immunity. Their involvement in transplantation offers insight into the general principles outlined above. In addition, they illustrate how the innate immune response is layered into soluble and cellular components that integrate to provide immune surveillance at critical tissue sites. The purpose of this article is to understand how and to what extent these innate pathways can influence graft dysfunction and rejection. We shall also address whether manipulation of specific components at defined cellular locations can steer the response towards graft acceptance. A current perception of how these two pathways could interact is shown in Figure 1.
Figure 1.
Pathway of injury mediated by innate immune receptors. Toll-like receptors (TLR) are pattern-recognition receptors (PRR) that sense damage-associated molecular patterns (DAMPS). Complement receptors (CR) are PRR that sense complement effector molecules (e.g., C3a, C5a, C3b, iC3b, C3d) generated by DAMP-mediated activation of complement. Stress-induced signaling through PRRs on resident tissue cells and infiltrating leukocytes mediate tissue injury, and on antigen-presenting cells and T cells promote the donor-specific immune response. Effector responses against donor antigen are also PRR-signal dependent. Cross talk between CR and TLR may alter the cellular response in a complex biological system.
THE COMPLEMENT SYSTEM AND ITS ROLE IN ORGAN AND CELL TRANSPLANTATION
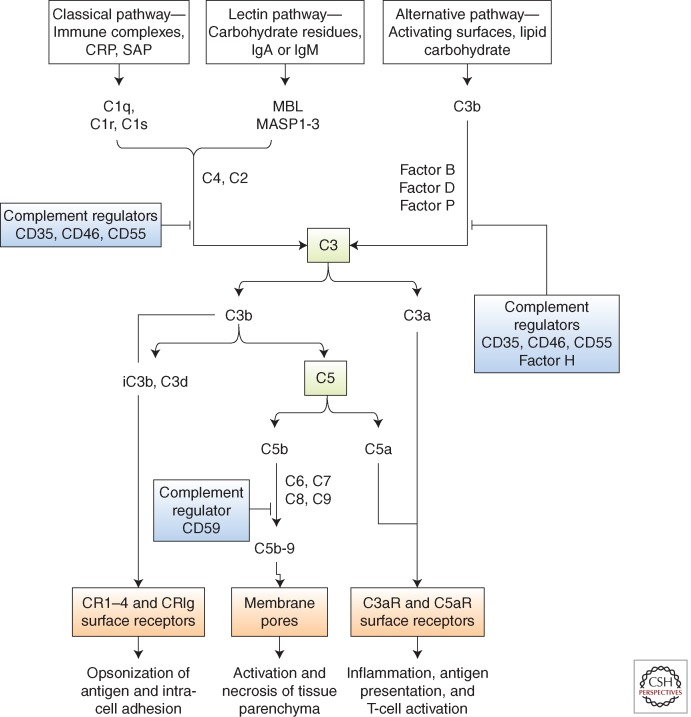
Complement proteins are expressed throughout the animal kingdom and function in antimicrobial defense. The pivotal protein C3 has a highly conserved thioester group, which enables covalent binding of the activated molecule on pathogen surfaces and elimination by phagocytic cells with receptors for activated C3. Covalently attached C3 also acts as a focal point for the conversion of C5 into an active form leading to the assembly of membrane attack complex, C5b-9 as lethal pores in the pathogen surface. These three basic mechanisms, namely the marking of cells for disposal, pore formation liberation of the proinflammatory fragments C3a and C5a, account for much of the injury to host tissue when the complement control mechanisms are overwhelmed or are defective. Injury of mammalian cells can therefore be related to direct membrane injury caused by C5b-9, or to cell activation mediated by specific receptors for C3a and C5a (C3aR and C5aR), or to interaction with leukocytes that have receptors (CR1-4) for the fragment C3b and its inactive metabolites iC3b and C3d attached to the target cell surface (Fig. 2).
Figure 2.
The complement cascade. Complement is activated by three major pathways: classical, lectin, and alternative. The classical pathway is triggered by the binding of C1 to immune surveillance molecules that are attached to the target sequence (e.g., immunoglobulin), C-reactive protein (CRP), and serum amyloid protein (SAP). The lectin pathway is initiated by mannose binding protein (MBL), which binds to carbohydrate residues on the pathogenic surface or IgA and IgM molecules. The alternative pathway is triggered by direct binding of C3b to activating surface. All three pathways progress to form enzyme complexes (classical or alternative pathway) that convert C3 and then C5 into active forms. This generates three groups of complement effectors. C3b is highly reactive and attaches to the activating surface. C3b and metabolites iC3b and C3d are ligands for receptors that are found on leukocytes and mediate inflammation, antigen uptake, and B-cell stimulation. C5b triggers the formation of C5b-9, a multimeric complex that creates a pore in the target cell membrane and induces cell activation and cell death. The small peptide fragments C3a and C5a interact with receptors on leukocytes and parenchymal cells to promote inflammation and, in the presence of foreign antigen, enhance T-cell stimulation. Regulators of complement activation are soluble (e.g., factor H) or membrane-associated, for example, CD35 (complement receptor 1, CR1), CD46 (membrane cofactor protein, MCP), and CD55 (decay-accelerating factor, DAF). The regulators bind C3b (and C4b) and increase its decay or proteolysis from the C3 and C5 convertases of the classical and alternative pathways. Factor H is unique to the alternative pathway. Other regulators inhibit the formation of C5b-9 (e.g., through binding of C3 by CD59).
The conversion of C3 into an active form is achieved by enzyme complexes that are assembled by the classical (antibody-mediated), alternative (hydrolytically mediated), and lectin (carbohydrate-mediated) pathways. There is much overlap in the molecular signatures that initiate these pathways. For example, the classical pathway may be triggered by nonimmunoglobulin immune surveillance molecules such as C-reactive protein (Kaplan and Volanakis 1974) and serum amyloid P (Ying et al. 1993). Carbohydrate residues on IgA and IgM molecules may trigger the lectin pathway (Zhang et al. 2006; Shi et al. 2009). The alternative pathway is constantly “ticking over” and may serve to amplify the amount of C3 that is deposited after classical or lectin pathway activation. Precise identification of the relevant pathways may therefore be problematic and, in any case, it may be suboptimal to inhibit just one of these pathways for therapeutic purposes.
Regulators (e.g., CD35, CD46, and CD55) that disrupt the stability of the converting enzyme complexes and consequently limit the cleavage of C3 on cell surfaces are cell protective. Genetic or acquired defects in the function of these regulators can lead to inappropriate activation of complement. Such defects underpin common disorders in which inflammation and immunity play a role (Walport 2001a,b). Complement-mediated injury can therefore result from undue stimulation of the pathways that activate C3 or defective regulation of C3 cleavage, or both.
Local production of complement proteins by many types of tissue-resident cells and infiltrating leukocytes can enhance the functions of innate immunity in health and disease. As a measure of this local capacity, intrarenal synthesis of C3 largely by proximal tubular epithelial cells (PTECs) contributes up to 15% of the circulating pool, the remainder of which is mainly generated by the liver (Tang et al. 1999). Damage or pathogen-related stress is a potent stimulus to this tissue production, increasing the number of copies of local product by several hundredfold (Springall et al. 2001; Farrar et al. 2006). Other essential components (e.g., lectin pathway proteins), are exclusively manufactured in the liver or by infiltrating macrophages.
Complement-Mediated Inflammatory Injury in the Early Course of Transplantation
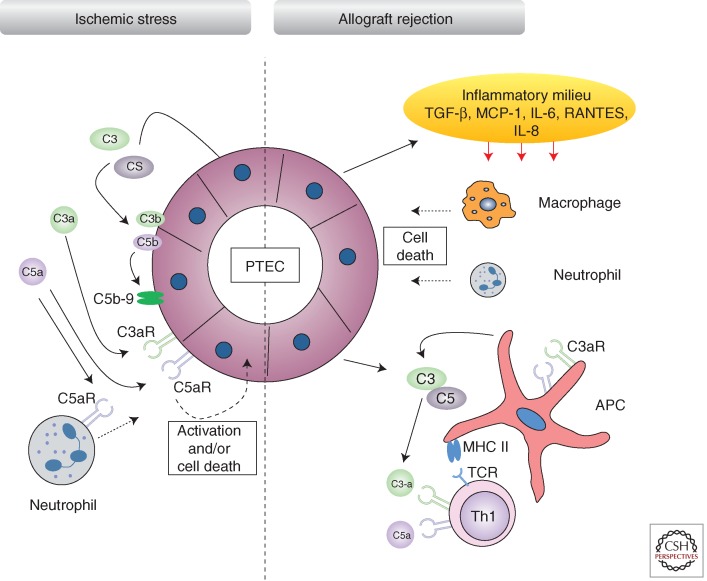
Complement activation within the donor organ can occur at any stage of the transplant procedure (e.g., activation as a result of severe brain injury in the donor). However, two peaks of activity are recognized, the first coinciding with ischemia-reperfusion (I/R) injury (Farrar et al. 2006) and the second during acute rejection (Pratt et al. 2000), as outlined in Figure 3. Complement activation in the pretransplant kidney biopsy is associated with poor midterm function of the transplanted organ, suggesting a causal relationship with innate immune expression.
Figure 3.
Kidney immune activation against ischemia-reperfusion and transplant injury. Ischemic stress to the kidney induces the local synthesis and secretion of complement proteins directly by proximal tubule epithelial cells. Subsequent activation of the complement cascade leads to C3b and C5b deposition and engagement of receptors by terminal pathway effector molecules such as C5a, which signals via C5aR. Deposition of membranes attack complex (C5b-9) and engagement of C5aR by C5a can lead to PTEC activation and/or cell death. During allograft rejection, chemokines secreted by perturbed PTECs, such as TGF-β, result in an inflammatory phenotype within the interstitium, where lymphocytes, neutrophils, macrophages, and antigen-presenting cells (APCs) enter. APCs express complement components and receptors following complement activation during the inflammatory response and can enhance DC-mediated T-cell priming and the generation of T helper 1 (Th1) cells. C3a and C5a recognition by naïve Th cells can also promote their differentiation to a Th1 phenotype. TCR, T-cell receptor; MHC II, major histocompatibility complex class II.
Animal experiments have shown a clear role for complement in organ reperfusion injury. Complement-deficient or depleted mice or rats are protected from I/R injury of native or transplanted organs, including heart (Jordan et al. 2001), lung (Eppinger et al. 1997), liver (Strey et al. 2003; Fondevila et al. 2008), intestine (Hart et al. 2005), pancreas (Tjernberg et al. 2008), and kidney (Zhou et al. 2000; Farrar et al. 2006). Loss of renal function is reduced by up to 50% in protected mice (Zhou et al. 2000). Cardiac infarct size is reduced by almost one-half (Schwaeble et al. 2011). Regarding mechanism, C5b-9 is known to mediate parenchymal injury within the kidney, heart, and gut, increasing the cellular release of proinflammatory and fibrotic factors and contributing to tissue infarction. A second mechanism of toxicity involves a direct parenchymal action of C5a and an indirect effect mediated by leukocytes (Peng et al. 2012). In contrast, C3a appears to have no major impact, either in cardiac (Busche and Stahl 2010) or renal reperfusion damage (E. Asgari, pers. comm.).
These pathological functions of complement in I/R injury are highly dependent on local production of C3. Mice that lack the ability to produce C3 within the donor organ were resistant to renal reperfusion damage, despite abundant C3 being produced by the recipient. Graft-derived complement is thus a potential target in strategies to limit the impact on reperfusion damage. More selective blockade may be possible by targeting the relevant activation pathway, but it is often uncertain which pathway is critical. Evidence suggests the lectin pathway plays a role in cardiac and renal models of postischemic injury, possibly triggered by carbohydrate motifs or binding of natural IgM to targets exposed as a result of tissue stress. This will need further resolution before committing to highly selective strategies for complement blockade.
Islet transplantation provides another example of the challenge posed by complement. Donor islets infused into the portal vein trigger the complement (and coagulation) systems (Ricordi and Strom 2004) and this is associated with marked reduction in cell mass. The protease inhibitor α1-antitrypsin (AAT), which includes activity against the complement and coagulation systems, had a protective effect, lowering the threshold number of islets required for successful engraftment (Koulmanda et al. 2012). At present, islets from more than one pancreas are often required for successful engraftment. Experiments are in progress to determine if islet sparing can be achieved by using more specific interventions directed at the complement and coagulation cascades.
So, complement provides a common target for modifying the biological responses of donor organs and cells used in transplantation on exposure to physical and metabolic stress. One therapeutic strategy has used C3 mRNA inhibition to limit production within the donor organ. Another strategy uses a therapeutic construct that is planted in the graft to inhibit the cleavage of C3 and prevent the generation of complement effectors. Having successfully completed preclinical and phase I studies, the construct is being evaluated in a phase II efficacy trial (Smith 2002). Our interest in complement has led to the design of a new imaging ligand based on the receptor CR2 (Badar et al. 2011); this detects organ-bound C3b and provides a means to quantify and monitor the effect of therapy on this specific pathway of injury.
Impact of Complement on Cell-Mediated Graft Rejection
Complement activation is a well-known feature of cell-mediated rejection, as detected by the measurement of activation products in the peripheral blood, urine, and the graft. Large increases in complement gene expression occur within the graft and correspond to cellular infiltration (Pratt et al. 2000, 2002) and release of T-cell cytokines that are known to regulate complement transcription (Gerritsma et al. 1996).
More than 10 years ago, mouse kidney transplant experiments confirmed that local production of complement was essential for allograft rejection. Most animals transplanted with donor kidney from C3-deficient mice were found to accept the grafts for > 100 days (Pratt et al. 2002). In contrast, recipients of wild-type organs rejected their kidney transplants rapidly, even when the recipient was complement-deficient. Heart transplant studies illustrated a similar principle using donor mice that were deficient in the complement regulator CD55. Here, uncontrolled activation of the complement cascade led to enhanced T-cell reactivity and promoted allograft rejection (Pavlov et al. 2008). These experiments highlight the requirement for complement to generate potent T-cell responses against alloantigen.
The mechanisms by which complement mediates T-cell alloreactivity have been investigated. To a large extent, C3a and C5a explain the effects of complement on the T-cell response. These small peptide fragments are usually associated with the inflammatory reaction in severe allergy or sepsis. However, C3a and C5a have also been shown to enhance the function of antigen-presenting cells (APC) (Peng et al. 2008, 2009) and T cells, providing essential costimulatory signals for donor-specific immune recognition (Lalli et al. 2008; Strainic et al. 2008). Receptor signaling induced by C3a and C5a increased the capacity of donor APC to generate Th1 response to alloantigen (Peng et al. 2008, 2009). In addition, C3a and C5a act directly on T cells increasing the differentiation of naïve T helper (Th0) cells to Th1, which mediate graft rejection. C3aR and C5aR signaling were also found to inhibit the development of T-regulatory cells, further directing the immune response down a pathway of rejection (Peng et al. 2006; Strainic et al. 2012; Kwan et al. 2013). Finally, donor parenchymal cells that are coated with complement were more strongly interactive than nonopsonized cells with donor-specific T cells, suggesting that intercell adhesion mediated by complement was an important factor for graft destruction by antigen-experienced T cells (Li et al. 2004). In principle, complement has multiple sites of interaction with the afferent and efferent limbs of the immune response that participate in graft rejection.
Further work has illuminated our understanding of which intracellular signaling pathways mediate the effect of C3a and C5a on antigen presentation. These not only include the expected effects on PI3-kinase and NF-κB signaling, which regulate MHC and costimulatory molecule expression on APC, but also negative regulation of cAMP, an intracellular second messenger with immunosuppressive actions (Li et al. 2008). Thus, both positive and negative signals within APC could transmit the effects of complement-induced signaling on the alloimmune response. In addition, it is possible that signaling via the mammalian target of rapamycin (mTOR) is induced by C5a, because the immunosuppressive effects of C5aR blockade were profoundly enhanced by rapamycin (Strainic et al. 2012). If verified, C5a blockade could provide a possible means to increase the immunosuppressive action of rapamycin.
Cell-autonomous production enhances these immunoregulatory functions of complement. For instance, APC derived from a variety of sources and species express complement components that lead to the generation of C3a and C5a (Li et al. 2011, 2012; Peng et al. 2006, 2009; Zhou et al. 2006). APC with specific complement defects were found to have low levels of MHC and costimulatory molecules on the cell surface and showed poor capacity for T-cell priming; instead these deficient APC promoted the development of Foxp3+ T regulatory function (Peng et al. 2006). And as already noted, donor kidney epithelial cells that express complement components normally are much better targets for donor-specific T cells. None of this is surprising given the impact of donor-derived complement on allograft rejection, at least in mice. It is as if cellular emission of complement marks out perturbed cells for the attention of the immune system.
Complement as a Marker and Activator of Humoral Rejection
The complement system was so named because it complements the ability of antibody and macrophages to clear pathogens from the blood. The effector role of complement in acute antibody-mediated rejection (AMR) is relatively well understood (Colvin and Smith 2005). Formed by classical pathway activation at the site of endothelium-bound anti-donor antibody, the terminal components C5a and C5b-9 mediate the acute inflammatory response. Inflammation of the vessel wall together with the pro-thrombotic effects of complement stimulates the coagulation cascade, and this result in vessel occlusion and distal tissue infarction.
Capillary wall C4d is a relatively stable marker of classical pathway activity. The detection of C4d is an integral part of the Banff classification of AMR and serves as a diagnostic and prognostic aid (Solez et al. 2008). The development of a C4d-based assay offers a further step toward improved recognition in serum of pathogenic antibodies against HLA antigens. Given the mounting significance of complement-fixing antibodies in allograft rejection, accurate markers and diagnostic tests with established specificity and sensitivity are likely to be a help in future trials of complement inhibitors. One caveat however is in the context of C5 blockade, which has already shown success in the prevention or reversal of acute AMR (Stegall et al. 2011). C4d will not be directly affected by anti-C5 treatment, because it is formed upstream of C5 in the complement cascade. Therefore C4d may not be a reliable marker in every setting.
Another important observation concerns the mechanism of endothelial resistance to complement attack. “Accommodation” refers to the acquired resistance of graft endothelial cells to injury, despite the presence of pathogenic antibody and complement, as first described in ABO-incompatible transplantation (Park et al. 2003). Although the molecular basis for this resistance remains uncertain, several observers have noted that expression of CD46, CD55, and CD59 is increased in “accommodated” cardiac and renal allografts, suggesting that endothelial stability is maintained through resistance to complement and inducible cell death (Gonzalez-Stawinski et al. 2008; Griesemer et al. 2009; Tan et al. 2009). If confirmed, this would support current attempts to induce longer periods of graft acceptance by increasing the number of regulatory molecules on the endothelial surface. Targeted delivery of therapeutic complement and coagulation regulators to the vessel wall provides a feasible means to achieve this (Smith et al. 2007).
It is also conceivable that prophylactic treatment to reduce complement activation on the vessel wall may help to reduce the sensitization of the recipient against donor HLA antigen. The initiation of IgG production against donor major histocompatibility complex antigen was found to be complement dependent, in common with other IgG responses to different antigens (Marsh et al. 2001). Opsonization with C3 is known to improve antigen retention in lymphoid tissue and increases the sensitivity of B cells to antigenic stimulation (Dempsey et al. 1996), giving a plausible explanation for the effect of complement on alloantibody production. Blockade of this afferent limb of the antibody response may have value in preventing sensitization against a range of alloantigens.
THE TOLL-LIKE RECEPTOR SYSTEM AND ITS ROLE IN ORGAN INJURY AND TRANSPLANTATION
Toll-like receptors were first discovered in 1998, in mice displaying endotoxin resistance but a high susceptibility to gram-negative bacterial infection (Poltorak et al. 1998). TLRs are an evolutionarily conserved group of trans-membrane proteins of which to date, 11 have been identified in humans and 13 in mice (Table 1). These innate receptors have a central role in immunity against invading pathogens by virtue of their ability to transduce signals in response to ligation of distinctive molecular motifs termed pathogen-associated molecular patterns (PAMPs). They are a major group of pattern- recognition receptors and are ubiquitous, being expressed on a host of both immune and nonimmune cells (Arumugam et al. 2009). TLR-PAMP interactions lead to downstream effects such as cytokine and chemokine release and augmentation of costimulatory molecule expression (Sobek et al. 2004). All TLRs mediate signal transduction via the adapter molecule myeloid differentiation factor 88 (MyD88), apart from TLR3, which is dependent on the adapter molecule Toll/IL-1R domain-containing adapter-inducing IFN-β (Trif) and TLR4 through which signaling is dependent on both Trif and MyD88 (Robson 2009). The observation that TLRs are expressed on parenchymal cells suggests that they may have functions unrelated to immune-mediated destruction of pathogens. Indeed, it is now apparent that endogenous, cell-derived ligands (DAMPs) derived from both intracellular and extracellular sources during inflammation and tissue damage are capable of binding TLRs (Yu et al. 2010). DAMPs are released from cells and displayed on the cell surface following cellular injury such as hypoxia; therefore, under normal conditions, these molecules are not expressed and invisible to the immune system. A variety of endogenous DAMPs have been described that can engage TLRs, such as heat shock protein (Dempsey et al. 1996) (HSP60) (Ohashi et al. 2000), purines, heparan sulphate and degradation products of fibronectin, the EDA domain (Okamura et al. 2001). More recently, immune response to allografts has displayed an association of TLRs with heat shock proteins and high mobility group box 1 (HMGB1) (Kruger et al. 2010).
Table 1.
TLRs—their microbial, endogenous ligands and cellular distribution
| Receptor | Microbial ligand(s) | Endogenous ligands | Cellular expression |
|---|---|---|---|
| TLR1 | Triacyl lipopeptides | B cells, monocytes, macrophages, and certain dendritic cells | |
| TLR2 | Peptodoglycan, zymosan, lipoteichoic acid, and glycolipids | HSP60, HSP70, hyaluronan, HMGB1 | Monocytes and macrophages, mast cells and myeloid dendritic cells |
| TLR3 | Double-stranded RNA, poly I:C | Messenger RNA (mRNA) | B cells, dendritic cells, and fibroblasts |
| TLR4 | LPS | Fibrinogen, HSPs, surfactant protein A, b-defensin 2, hyaluronan, fibronectin extra domain A, heparin sulfate, HMGB-1 | Monocytes and macrophages, mast cells, certain dendritic cells, B cells; intestinal epithelium and hepatocytes (low) |
| TLR5 | Flagellin | Monocytes and macrophages, subset of dendritic cells; intestine | |
| TLR6 | Multiple diacyl lipopeptides on mycoplasma | B cells, mast cells, and macrophages | |
| TLR7 | Single-stranded RNA imidazoquinolines | RNA and protein complexes | Plasmacytoid dendritic cells, monocytes, and macrophages; B cells |
| TLR8 | Single-stranded RNA imidazoquinolines and small synthetic compounds | Monocytes and macrophages; subset of dendritic cells; mast cells | |
| TLR9 | CpG oligodeoxynucleotide DNA | Monocytes, macrophages, and plasmacytoid dendritic cells | |
| TLR10 | Undefined | B cells, monocytes, and regulatory T cells | |
| TLR11 | Profilin | Kidney and urinary bladder epithelium | |
| TLR12 | Profilin | Macrophages, neurons, and dendritic cells | |
| TLR13 | Conserved bacterial 23S ribosomal RNA (rRNA) sequence | Monocytes, macrophages, and dendritic cells |
Data based on Robson (2009).
TLR-Mediated I/R Injury in Solid Organs
Organ procurement is often associated with a significant period of cold ischemia, which has a well-documented deleterious impact on graft survival. The period of ischemia and subsequent reperfusion leads to rapid complement activation as discussed earlier. Likewise, TLRs are rapidly upregulated during I/R injury. In animal models of renal I/R injury, in the absence of any alloimmune events, expression of both TLR2 (Shigeoka et al. 2007) and TLR4 has been characterized, with demonstrable upregulation of TLR4 in the outer medulla of ischemic kidney just 4 h postischemia, with extensive expression on proximal tubular epithelial cells (PTECs) 24 h after injury (Chen et al. 2011). MyD88-dependent signaling via TLR4 is required for full development of I/R injury as both TLR4 and MyD88 knockout mice are resistant to renal I/R injury (Wu et al. 2007). Conversely, TLR4 mediates hepatic I/R injury in an MyD88-independent fashion, showing the diversity and complexity of the signaling mechanisms in different organ models (Zhai et al. 2004). Indeed, the absence of TLR4 signaling in the donor organ is required to reduce I/R injury in a mouse liver transplantation model (Shen et al. 2007).
Mice that are either deficient for TLR2 or receive antisense TLR2 oligonucleotide therapy display significant protection from renal I/R injury (Leemans et al. 2005). This protective effect of therapeutic blockade of TLR2 is also observed after cardiac ischemia (Arslan et al. 2010). In the context of solid organ transplantation, both donor and recipient cells have the capacity to express TLR2. Notably, ablation of the recipient pool of TLR2 alone with a therapeutic agent directed at murine TLR2 conferred protection from transplantation-associated ischemic injury in an isograft model (Farrar et al. 2012), suggesting that recipient leukocyte expression of TLR2 is an important mediator of the observed injury and constitutes a viable therapeutic target. The acute kidney injury was reduced by up to 60% in terms of renal function. The mechanism of inflammatory damage resulting from TLR-ligand engagement is complex and not completely understood (Leventhal and Schroppel 2012). Evidence to date suggests a complex interaction between the induction of proinflammatory cytokines, complement activation, and TLR signaling. This potential cross talk between complement and TLR systems will be discussed in more detail later in this chapter.
T Cells Facilitate TLR-Mediated I/R Organ Damage
Although innate immune-driven I/R injury may develop in syngeneic grafts, ex vivo, or under sterile conditions, T cells, particularly of CD4 phenotype, are indispensable for the activation of local TLR-mediated proinflammatory immune sequel. The observation that systemic immunosuppression (CsA, FK506) attenuated peritransplant hepatocellular damage provided initial indirect evidence for T-cell involvement in the pathophysiology of organ I/R injury (Suzuki et al. 1993). Studies in T-cell-deficient (nude) and CD4-deficient mouse systems have proven the pivotal function of CD4 T cell in the process (Zwacka et al. 1997; Rabb et al. 2000; Burne et al. 2001; Shen et al. 2002). The question arises as to how T cells may function in the predominantly I/R-triggered innate response and in the absence of exogenous antigen stimulation?
The pathogenic role of T-cell costimulation was initially shown in a study in which CD28 blockade with CTLA-4Ig protected rat kidneys from local I/R damage (Takada et al. 1997). Consistent with the essential function of both CD28 and CD154 molecules to activate TLR-mediated inflammation cascade, livers in CD154 KO or CD28 KO mice and in WT mice treated with anti-CD154 or CTLA-4Ig are all protected from I/R damage (Shen et al. 2002). Indeed, Th1-type cells are critical in the process, as Stat4 KO (deficient in Th1 development) but not Stat6 KO, mice are I/R-injury resistant, whereas reconstitution of nude mice with T cells from Stat6KO, but not Stat4KO, mice restores cardinal features of IR damage (Shen et al. 2003).
Although the role of CD154 has been attributed to its costimulatory T-cell function, CD40 ligation on DC or macrophages by T-cell-derived CD154 represents, first of all, the critical activating signal to innate immune cells. It is plausible that endogenous ligands that trigger I/R injury may be insufficient to fully activate and sustain proinflammatory phenotype in I/R-stressed organ. For instance, Kupffer cells (KC) in the liver allograft may be less sensitive to TLR4 stimulation than peripheral macrophages because of the exposure to portal-drained and gut-derived endotoxin. Liver DC have also lower TLR4 expression levels and are less susceptible to LPS stimulation as compared with their spleen counterparts (De et al. 2005). In fact, conventional DC may exert immune-regulatory functions during I/R by producing IL-10 via a TLR9-mediated mechanism (Bamboat et al. 2010).
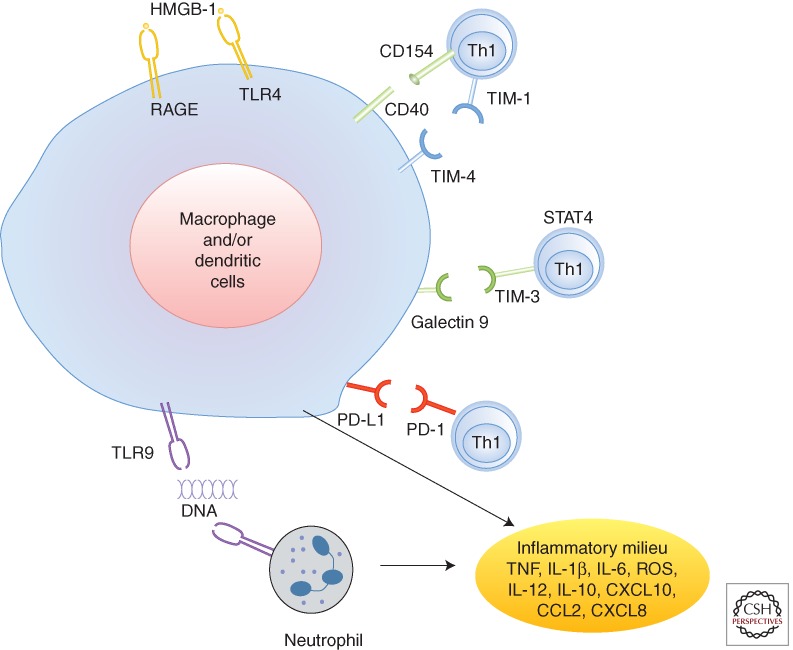
Interactions between the T-cell immunoglobulin mucin (TIM) family of costimulatory proteins constitute a novel molecular signaling pathway of T-cell—macrophage regulation at the innate—adaptive interface. Treatment of mice with anti-TIM-1 mAb ameliorates the hepatocellular damage, accompanied by decreased local neutrophil infiltration/activation, inhibition of T lymphocyte/macrophage sequestration and diminished homing of TIM-1 ligand-expressing TIM-4 cells in the ischemic liver (Uchida et al. 2010a). The induction of proinflammatory cytokine and chemokine programs was also blunted, data supported by findings from a renal I/R injury mouse model (Rong et al. 2011). The TIM-3–Gal-9, on the other hand, constitutes a “negative” T-cell costimulation signal, as TIM-3 blockade worsens tissue damage, along with increased IFN-γ and reciprocally depressed IL-10 expression in I/R-stressed organs (Uchida et al. 2010b). The PD-1 (B7)-PD-L1 (H1) “negative” T-cell pathway has been also shown to promote I/R cytoprotection (Ji et al. 2010; Ueki et al. 2011). Thus, multiple T-cell costimulatory pathways, both positive and negative, may function in a two-way traffic fashion to promote vs. inhibit TLR-dependent innate immune responses against I/R-insult (Fig. 4).
Figure 4.
Liver immune activation against ischemia-reperfusion injury. The ischemia insult induces initial cell death, which results in diverse “danger” molecules, such as HMGB1, DNA fragments, and histones activating TLR4, RAGE, and TLR9 signaling on macrophages and/or dendritic cells and neutrophils. CD4+ Th1 effectors might also facilitate and regulate local innate immune activation via CD154–CD40, TIM-1–TIM3, TIM-4–galectin 9, and PD-L1 pathways. The proinflammatory milieu, composed of TNF, IL-1β, IL-5, IL-12, CXCL10, CCL2, CXCL8, and ROS, further activates local immune cells and recruits circulating immune cells, culminating in inflammatory reperfusion injury. HMGB1, high-mobility group protein B1; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; RAGE, receptor for advanced glycation end products; STAT4, signal of transducer and activator of transcription 4; Th1, T-helper type 1 cell; TIM, T cell, immunoglobulin, mucin-containing molecules; TLR, Toll-like receptor. (Image adapted from data from a review by Zhai et al. 2013.)
TLR-Mediated Allograft Rejection
TLR-mediated I/R injury is likely to increase the immunogenicity of a solid organ graft by augmentation of the inflammatory response of innate myeloid cells and lymphocytes that mediate adaptive immune responses (Fig. 1). To this end, it could be argued that TLRs have no direct effect on antigen-specific responses to solid organ transplants but merely exacerbate rejection by increasing the damage associated purely with IR events, for example, through production and interaction of TLR4 and HGMB1 (Kruger et al. 2009). However, there is growing evidence suggesting TLR can directly affect the antigen-specific immune response. TLRs have high expression on DC and ligation of DC-expressed-TLR leads to increased expression of costimulatory molecules (Medzhitov 2001). Mice deficient for the TLR signal adapter molecule MyD88 have impaired CD4+ T-cell function characterized by a skewing towards a Th2 phenotype following immunization with bacterial extracts (Jankovic et al. 2002), suggesting MyD88 is crucial for appropriate development of Th1 or Th2 T cells. Organ transplantation in the presence or absence of TLR and/or specific adapter molecules required for full signaling has yielded mixed results. The absence of MyD88 prolongs skin graft survival in the minor-histocompatibility (HY) model (Goldstein et al. 2003). However, in more robust models, fully MHC-mismatched skin and cardiac transplants show impaired Th1 responses with concomitant reduction in DCs in the absence of MyD88, but with no prolongation of graft survival (Tesar et al. 2004).
Islet transplantation has provided insight as to the role of TLR4 in the rejection process. TLR4 is upregulated after islet transplantation and recipient TLR4 deficiency prolonged allograft survival (Zhang et al. 2010). The islet isolation procedure leads to increased TLR4 expression, a process that can be attenuated by prior exposure of donor islets to carbon monoxide, resulting in prolonged islet allograft survival (Goldberg et al. 2007). In attempts to elucidate how TLR-induced production of endogenous DAMPs mediates allograft rejection, breaking tolerance induced by costimulatory blockade may be just one mechanism. Tolerance to skin grafts induced by donor-specific transfusion and anti-CD154 immunotherapy can be overcome by activation of TLR using TLR agonists, thereby preventing apoptotic clearance of CD8+ effector T cells, which then reject the graft rapidly (Thornley et al. 2006). There is also a contributory T-regulatory element, highlighted by the observation that DAMP production during the alloimmune and concurrent TLR2 and TLR4 activation, leads to downregulation of Foxp3+ regulatory T-cell production (Lal et al. 2011).
CROSS TALK BETWEEN COMPLEMENT AND TLR PATHWAYS
Acute renal failure (ARF) in mice can be circumvented by blocking TLR2 (Farrar et al. 2012). The study tentatively links TLR2-mediated renal injury with complement activation as decreased deposition of activated complement component C3d was observed in the protected kidneys. The lectin complement pathway can be activated during renal I/R injury (Farrar et al. 2009), mediated through an interaction of MBL-MASP-2 complexes bound to DAMPs that are expressed following transplantation. As the TLR system can be activated by engagement of DAMPs, one may speculate there will be a degree of cross talk between the two sets of activation pathways. Indeed, it has been suggested that the two systems may be capable of synergy (Damman et al. 2011a). MBL has been proposed as a TLR4 ligand, again indicative of close interaction between the two systems (Wang et al. 2011) and furthermore, MBL deficiency confers protection from renal I/R injury, an effect associated with lower renal expression of C3 (Moller-Kristensen et al. 2005). Mice that are deficient for both factor B and TLR2 develop severe acute renal failure even though knockout mice for the individual genes display curtailed injury (Amura et al. 2012), suggesting a protective mechanism may be mediated through either TLR2 or complement, or that there may be a regulatory link between the two pathways. Mice deficient in the membrane-bound complement regulator decay-accelerating factor (DAF, CD55) produce large amounts of proinflammatory cytokines in response to TLR4 agonist. In this model, regulation of TLR pathway activity by complement was completely abrogated in double-deficient mice (DAF and C3), an effect indicating strong dependence on complement activation (Zhang et al. 2007). Complement may regulate TLR4-mediated injury in the intestine where I/R injury-induced deposition of activated complement product occurs in a TLR4-dependent fashion (Pope et al. 2010).
Points at which there is cross talk between the two systems could prove attractive for designing targeted therapeutics. DAMPs, such as HGMB1 (Leventhal and Schroppel 2012) and MAP kinase (Zhang et al. 2007), may be key bridging points between the two systems. At present, no such studies in humans have analyzed the extent of cooperation between the two activation pathways (Damman et al. 2011a). However, in rodent models, targeting of DAMPs in islet allografts using a specific anti-HGMB1 or TLR has shown improved graft function and survival (Matsuoka et al. 2010).
CLINICAL RELEVANCE OF COMPLEMENT AND TLR MECHANISMS OF I/R INJURY
Hyperexpression of the molecular components of the complement and TLR pathways in human transplantation (Tang et al. 1999; Kruger et al. 2009; Naesens et al. 2009; Damman et al. 2011b) provides circumstantial evidence of their pathological relevance and also support the experimental data derived in rodent studies. However, more significant proof of their clinical importance will require specific intervention by therapeutic trial. Reassuringly, at least two such trials are in progress. One is addressing the role of therapeutic complement regulator (mirococept) in the prevention of delayed graft function (DGF) in renal transplantation—an important manifestation of ischemia reperfusion injury with implication for acute rejection rate and long-term graft survival. Another study is assessing the benefit of TLR2 blockade with monoclonal antibody (OPN-305), also in the context of DGF. These two different studies, one aimed to protect the tubular epithelium against complement and the other targeting the function of migratory leukocytes in the evolution of postischemic damage, are primarily designed to assess patient benefit through control of innate immunity. But they are also much needed to establish the validity of two target pathways that are thought to be important in human disease.
ACKNOWLEDGMENTS
S.H.S. and C.A.F. are supported by the Medical Research Council (U.K.) Centre for Transplantation. Their research is also supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the investigators and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Editors: Laurence A. Turka and Kathryn J. Wood
Additional Perspectives on Transplantation available at www.perspectivesinmedicine.org
REFERENCES
- Amura CR, Renner B, Lyubchenko T, Faubel S, Simonian PL, Thurman JM 2012. Complement activation and toll-like receptor-2 signaling contribute to cytokine production after renal ischemia/reperfusion. Mol Immunol 52: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F, Smeets MB, O’Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, et al. 2010. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 121: 80–90 [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM 2009. Toll-like receptors in ischemia-reperfusion injury. Shock 32: 4–16 [DOI] [PubMed] [Google Scholar]
- Badar A, DeFreitas S, McDonnell JM, Yahya N, Thakor D, Razavi R, Smith R, Sacks S, Mullen GE 2011. Recombinant complement receptor 2 radiolabeled with [99mTc(CO)3]+: A potential new radiopharmaceutical for imaging activated complement. PLoS ONE 6: e18275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP 2010. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest 120: 559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne MJ, Daniels F, El GA, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H 2001. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MN, Stahl GL 2010. Role of the complement components C5 and C3a in a mouse model of myocardial ischemia and reperfusion injury. Ger Med Sci 8: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, John R, Richardson JA, Shelton JM, Zhou XJ, Wang Y, Wu QQ, Hartono JR, Winterberg PD, Lu CY 2011. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int 79: 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RB, Smith RN 2005. Antibody-mediated organ-allograft rejection. Nat Rev Immunol 5: 807–817 [DOI] [PubMed] [Google Scholar]
- Damman J, Daha MR, van Son WJ, Leuvenink HG, Ploeg RJ, Seelen MA 2011a. Cross talk between complement and Toll-like receptor activation in relation to donor brain death and renal ischemia-reperfusion injury. Am J Transplant 11: 660–669 [DOI] [PubMed] [Google Scholar]
- Damman J, Seelen MA, Moers C, Daha MR, Rahmel A, Leuvenink HG, Paul A, Pirenne J, Ploeg RJ 2011b. Systemic complement activation in deceased donors is associated with acute rejection after renal transplantation in the recipient. Transplantation 92: 163–169 [DOI] [PubMed] [Google Scholar]
- De CA, Abe M, Lau H, Hackstein H, Raimondi G, Thomson AW 2005. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol 174: 2037–2045 [DOI] [PubMed] [Google Scholar]
- Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT 1996. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science 271: 348–350 [DOI] [PubMed] [Google Scholar]
- Eppinger MJ, Deeb GM, Bolling SF, Ward PA 1997. Mediators of ischemia-reperfusion injury of rat lung. Am J Pathol 150: 1773–1784 [PMC free article] [PubMed] [Google Scholar]
- Farrar CA, Zhou W, Lin T, Sacks SH 2006. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J 20: 217–226 [DOI] [PubMed] [Google Scholar]
- Farrar CA, Asgari E, Lynch N, Roscher S, Stover CM, Schwaeble WJ, Sacks SH 2009. Mannan binding lectin associated serine protease-2 (MASP-2) is a critical player in the pathophysiology of renal ischaemia reperfusion (I/R) injury and mediates tissue injury in absence of complement C4. Mol Immunol 46: 2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar CA, Keogh B, McCormack W, O’Shaughnessy A, Parker A, Reilly M, Sacks SH 2012. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB J 26: 799–807 [DOI] [PubMed] [Google Scholar]
- Fondevila C, Shen XD, Tsuchihashi S, Uchida Y, Freitas MC, Ke B, Busuttil RW, Kupiec-Weglinski JW 2008. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl 14: 1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsma JS, Gerritsen AF, van KC, van Es LA, Daha MR 1996. Interleukin-1α enhances the biosynthesis of complement C3 and factor B by human kidney proximal tubular epithelial cells in vitro. Mol Immunol 33: 847–854 [DOI] [PubMed] [Google Scholar]
- Goldberg A, Parolini M, Chin BY, Czismadia E, Otterbein LE, Bach FH, Wang H 2007. Toll-like receptor 4 suppression leads to islet allograft survival. FASEB J 21: 2840–2848 [DOI] [PubMed] [Google Scholar]
- Goldstein DR, Tesar BM, Akira S, Lakkis FG 2003. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 111: 1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Stawinski GV, Tan CD, Smedira NG, Starling RC, Rodriguez ER 2008. Decay-accelerating factor expression may provide immunoprotection against antibody-mediated cardiac allograft rejection. J Heart Lung Transplant 27: 357–361 [DOI] [PubMed] [Google Scholar]
- Griesemer AD, Okumi M, Shimizu A, Moran S, Ishikawa Y, Iorio J, Arn JS, Yamada K 2009. Upregulation of CD59: Potential mechanism of accommodation in a large animal model. Transplantation 87: 1308–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL 2005. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol 174: 6373–6380 [DOI] [PubMed] [Google Scholar]
- Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A 2002. In the absence of IL-12, CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10−/− setting. Immunity 16: 429–439 [DOI] [PubMed] [Google Scholar]
- Ji H, Shen X, Gao F, Ke B, Freitas MC, Uchida Y, Busuttil RW, Zhai Y, Kupiec-Weglinski JW 2010. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology 52: 1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan JE, Montalto MC, Stahl GL 2001. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation 104: 1413–1418 [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Volanakis JE 1974. Interaction of C-reactive protein complexes with the complement system. I: Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol 112: 2135–2147 [PubMed] [Google Scholar]
- Koulmanda M, Bhasin M, Fan Z, Hanidziar D, Goel N, Putheti P, Movahedi B, Libermann TA, Strom TB 2012. α1-Antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci 109: 15443–15448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, et al. 2009. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci 106: 3390–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger B, Yin N, Zhang N, Yadav A, Coward W, Lal G, Zang W, Heeger S, Bromberg JS, Murphy B, et al. 2010. Islet-expressed TLR2 and TLR4 sense injury and mediate early graft failure after transplantation. Eur J Immunol 40: 2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan WH, van der TW, Paz-Artal E, Li MO, Heeger PS 2013. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med 210: 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal G, Yin N, Xu J, Lin M, Schroppel S, Ding Y, Marie I, Levy DE, Bromberg JS 2011. Distinct inflammatory signals have physiologically divergent effects on epigenetic regulation of Foxp3 expression and Treg function. Am J Transplant 11: 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS 2008. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 112: 1759–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der PF, Weening JJ, Florquin S 2005. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal JS, Schroppel B 2012. Toll-like receptors in transplantation: Sensing and reacting to injury. Kidney Int 81: 826–832 [DOI] [PubMed] [Google Scholar]
- Li K, Patel H, Farrar CA, Hargreaves RE, Sacks SH, Zhou W 2004. Complement activation regulates the capacity of proximal tubular epithelial cell to stimulate alloreactive T cell response. J Am Soc Nephrol 15: 2414–2422 [DOI] [PubMed] [Google Scholar]
- Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, Wang N, Sacks SH, Zhou W 2008. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood 112: 5084–5094 [DOI] [PubMed] [Google Scholar]
- Li K, Fazekasova H, Wang N, Sagoo P, Peng Q, Khamri W, Gomes C, Sacks SH, Lombardi G, Zhou W 2011. Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol 48: 1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Fazekasova H, Wang N, Peng Q, Sacks SH, Lombardi G, Zhou W 2012. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology 217: 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH 2001. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation 72: 1310–1318 [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, et al. 2010. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest 120: 735–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R 2001. Toll-like receptors and innate immunity. Nat Rev Immunol 1: 135–145 [DOI] [PubMed] [Google Scholar]
- Moller-Kristensen M, Wang W, Ruseva M, Thiel S, Nielsen S, Takahashi K, Shi L, Ezekowitz A, Jensenius JC, Gadjeva M 2005. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol 61: 426–434 [DOI] [PubMed] [Google Scholar]
- Naesens M, Li L, Ying L, Sansanwal P, Sigdel TK, Hsieh SC, Kambham N, Lerut E, Salvatierra O, Butte AJ, et al. 2009. Expression of complement components differs between kidney allografts from living and deceased donors. J Am Soc Nephrol 20: 1839–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H 2000. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164: 558–561 [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF III 2001. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233 [DOI] [PubMed] [Google Scholar]
- Park WD, Grande JP, Ninova D, Nath KA, Platt JL, Gloor JM, Stegall MD 2003. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant 3: 952–960 [DOI] [PubMed] [Google Scholar]
- Pavlov V, Raedler H, Yuan S, Leisman S, Kwan WH, Lalli PN, Medof ME, Heeger PS 2008. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol 181: 4580–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Li K, Patel H, Sacks SH, Zhou W 2006. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol 176: 3330–3341 [DOI] [PubMed] [Google Scholar]
- Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, Sacks SH, Zhou W 2008. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood 111: 2452–2461 [DOI] [PubMed] [Google Scholar]
- Peng Q, Li K, Wang N, Li Q, Asgari E, Lu B, Woodruff TM, Sacks SH, Zhou W 2009. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol 183: 6058–6068 [DOI] [PubMed] [Google Scholar]
- Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, Lu B, Sacks SH, Zhou W 2012. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 1474–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van HC, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282: 2085–2088 [DOI] [PubMed] [Google Scholar]
- Pope MR, Hoffman SM, Tomlinson S, Fleming SD 2010. Complement regulates TLR4-mediated inflammatory responses during intestinal ischemia reperfusion. Mol Immunol 48: 356–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JR, Abe K, Miyazaki M, Zhou W, Sacks SH 2000. In situ localization of C3 synthesis in experimental acute renal allograft rejection. Am J Pathol 157: 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JR, Basheer SA, Sacks SH 2002. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med 8: 582–587 [DOI] [PubMed] [Google Scholar]
- Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, Tang WW 2000. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 279: F525–F531 [DOI] [PubMed] [Google Scholar]
- Ricordi C, Strom TB 2004. Clinical islet transplantation: Advances and immunological challenges. Nat Rev Immunol 4: 259–268 [DOI] [PubMed] [Google Scholar]
- Robson MG 2009. Toll-like receptors and renal disease. Nephron Exp Nephrol 113: e1–e7 [DOI] [PubMed] [Google Scholar]
- Rong S, Park JK, Kirsch T, Yagita H, Akiba H, Boenisch O, Haller H, Najafian N, Habicht A 2011. The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol 22: 484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, Dudler T, Parent B, Lhotta K, Wallis R, et al. 2011. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci 108: 7523–7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, Kupiec-Weglinski JW 2002. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation 74: 315–319 [DOI] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, Busuttil RW, Kupiec-Weglinski JW 2003. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology 37: 296–303 [DOI] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, Busuttil RW, Kupiec-Weglinski JW 2007. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl 13: 1435–1443 [DOI] [PubMed] [Google Scholar]
- Shi T, Moulton VR, Lapchak PH, Deng GM, le Lucca JJ, Tsokos GC 2009. Ischemia-mediated aggregation of the actin cytoskeleton is one of the major initial events resulting in ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 296: G339–G347 [DOI] [PubMed] [Google Scholar]
- Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB 2007. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 178: 6252–6258 [DOI] [PubMed] [Google Scholar]
- Smith RA 2002. Targeting anticomplement agents. Biochem Soc Trans 30: 1037–1041 [DOI] [PubMed] [Google Scholar]
- Smith RAG, Koffman G, Chowdhury P, Smith KCG, Watson CJ, Nicholson ML, Zhou W, Sacks SH. 2007. Membrane-localising complement inhibitors—clinical progress. Mol Immunol 44: 3915 [Google Scholar]
- Sobek V, Birkner N, Falk I, Wurch A, Kirschning CJ, Wagner H, Wallich R, Lamers MC, Simon MM 2004. Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res Ther 6: R433–R446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, et al. 2008. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760 [DOI] [PubMed] [Google Scholar]
- Springall T, Sheerin NS, Abe K, Holers VM, Wan H, Sacks SH 2001. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat Med 7: 801–806 [DOI] [PubMed] [Google Scholar]
- Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FJ, Gandhi MJ, Kremers W, Gloor JM 2011. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11: 2405–2413 [DOI] [PubMed] [Google Scholar]
- Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME 2008. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28: 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strainic MG, Shevach EM, An F, Lin F, Medof ME 2012. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol 14: 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD 2003. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med 198: 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D 1993. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 55: 1265–1272 [DOI] [PubMed] [Google Scholar]
- Takada M, Chandraker A, Nadeau KC, Sayegh MH, Tilney NL 1997. The role of the B7 costimulatory pathway in experimental cold ischemia/reperfusion injury. J Clin Invest 100: 1199–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CD, Sokos GG, Pidwell DJ, Smedira NG, Gonzalez-Stawinski GV, Taylor DO, Starling RC, Rodriguez ER 2009. Correlation of donor-specific antibodies, complement and its regulators with graft dysfunction in cardiac antibody-mediated rejection. Am J Transplant 9: 2075–2084 [DOI] [PubMed] [Google Scholar]
- Tang S, Zhou W, Sheerin NS, Vaughan RW, Sacks SH 1999. Contribution of renal secreted complement C3 to the circulating pool in humans. J Immunol 162: 4336–4341 [PubMed] [Google Scholar]
- Tesar BM, Zhang J, Li Q, Goldstein DR 2004. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll-like receptor signal adaptor protein. Am J Transplant 4: 1429–1439 [DOI] [PubMed] [Google Scholar]
- Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini A, Greiner DL 2006. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J Immunol 176: 1561–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjernberg J, Ekdahl KN, Lambris JD, Korsgren O, Nilsson B 2008. Acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation 85: 1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ke B, Freitas MC, Ji H, Zhao D, Benjamin ER, Najafian N, Yagita H, Akiba H, Busuttil RW, et al. 2010a. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology 51: 1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, Najafian N, Kupiec-Weglinski JW 2010b. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology 139: 2195–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki S, Castellaneta A, Yoshida O, Ozaki K, Zhang M, Kimura S, Isse K, Ross M, Shao L, Stolz DB, et al. 2011. Hepatic B7 homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology 54: 216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walport MJ 2001a. Complement. First of two parts. N Engl J Med 344: 1058–1066 [DOI] [PubMed] [Google Scholar]
- Walport MJ 2001b. Complement. Second of two parts. N Engl J Med 344: 1140–1144 [DOI] [PubMed] [Google Scholar]
- Wang M, Chen Y, Zhang Y, Zhang L, Lu X, Chen Z 2011. Mannan-binding lectin directly interacts with Toll-like receptor 4 and suppresses lipopolysaccharide-induced inflammatory cytokine secretion from THP-1 cells. Cell Mol Immunol 8: 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ 2007. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SC, Gewurz AT, Jiang H, Gewurz H 1993. Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14–26 and 76–92 of the A chain collagen-like region of C1q. J Immunol 150: 169–176 [PubMed] [Google Scholar]
- Yu L, Wang L, Chen S 2010. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med 14: 2592–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW 2004. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol 173: 7115–7119 [DOI] [PubMed] [Google Scholar]
- Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW 2013. Ischaemia-reperfusion injury in liver transplantation–from bench to bedside. Nat Rev Gastroenterol Hepatol 10: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, et al. 2006. Identification of the target self-antigens in reperfusion injury. J Exp Med 203: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC 2007. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 110: 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Kruger B, Lal G, Luan Y, Yadav A, Zang W, Grimm M, Waaga-Gasser AM, Murphy B, Bromberg JS, et al. 2010. Inhibition of TLR4 signaling prolongs Treg-dependent murine islet allograft survival. Immunol Lett 127: 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH 2000. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 105: 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Patel H, Li K, Peng Q, Villiers MB, Sacks SH 2006. Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood 107: 2461–2469 [DOI] [PubMed] [Google Scholar]
- Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF 1997. CD4+ T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest 100: 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]