Abstract
Possible etiologies of infection in the solid organ recipient are diverse, ranging from common bacterial and viral pathogens to opportunistic pathogens that cause invasive disease only in immunocompromised hosts. The recognition of infectious syndromes in this population is limited by alterations in the clinical manifestations by immunosuppression. The risk of serious infections in the organ transplant patient is determined by the interaction between the patients’ recent and distant epidemiological exposures and all factors that contribute to the patient’s net state of immune suppression. This risk is altered by antimicrobial prophylaxis and changes in immunosuppressive therapies. In addition to the direct effects of infection, opportunistic infections, and the microbiome may adversely shape the host immune responses with diminished graft and patient survivals. Antimicrobial therapies are more complex than in the normal host with a significant incidence of drug toxicity and a propensity for drug interactions with the immunosuppressive agents used to maintain graft function. Rapid and specific microbiologic diagnosis is essential. Newer microbiologic assays have improved the diagnosis and management of opportunistic infections. These tools coupled with assays that assess immune responses to infection and to graft antigens may allow optimization of management for graft recipients in the future.
In organ transplant recipients, the risk of infection depends on the infectious exposures of the recipient and donor, and the nature and intensity of immunosuppression. Sensitive molecular diagnostic tests are essential.
If one were to design an experiment to study the effects of infection following organ transplantation, it is unlikely that one would use as subjects an outbred population with multiple, severe comorbidities, uncontrolled infectious exposures, and a wide variety of regimens for immunosuppression undergoing major surgery with implantation of organs from an equally diverse donor pool. Nonetheless, as regimens for immunosuppression have “stabilized,” some general observations can be made based on this ongoing “experiment.” Important clinical observations regarding infection in transplantation include:
-
1.
The risk for infection is related to the nature and intensity of immunosuppression and the infectious exposures of the organ recipient and donor (Fishman and Rubin 1998; Fishman et al. 2007). An understanding of the relationship between the intensity of immunosuppression and infectious risk allows the development of effective prophylactic strategies.
-
2.
Recognition of infection is more difficult in transplant recipients than in individuals with normal immune function given diminished signs and symptoms of infection and the array of noninfectious etiologies of fever (e.g., graft rejection, drug toxicity) (Fishman and Rubin 1998; Fishman 2007). Nonspecific signs and symptoms must be appreciated.
-
3.
Infection progresses rapidly in the immunocompromised host. Thus, the prevention of infection is essential. Confronted with infection, early and effective therapy is required.
-
4.
The spectrum of potential pathogens is broad; multiple pathogens may be present at the same time (e.g., virus and molds). Rapid and specific diagnoses are needed to guide antimicrobial therapies and to limit avoidable drug interactions and toxicities. Rapid and improved microbiologic assays have greatly improved care. Surgical debridement and invasive diagnostic procedures are often required.
-
5.
There are no currently available assays that can determine an optimal immunosuppressive regimen while avoiding infection.
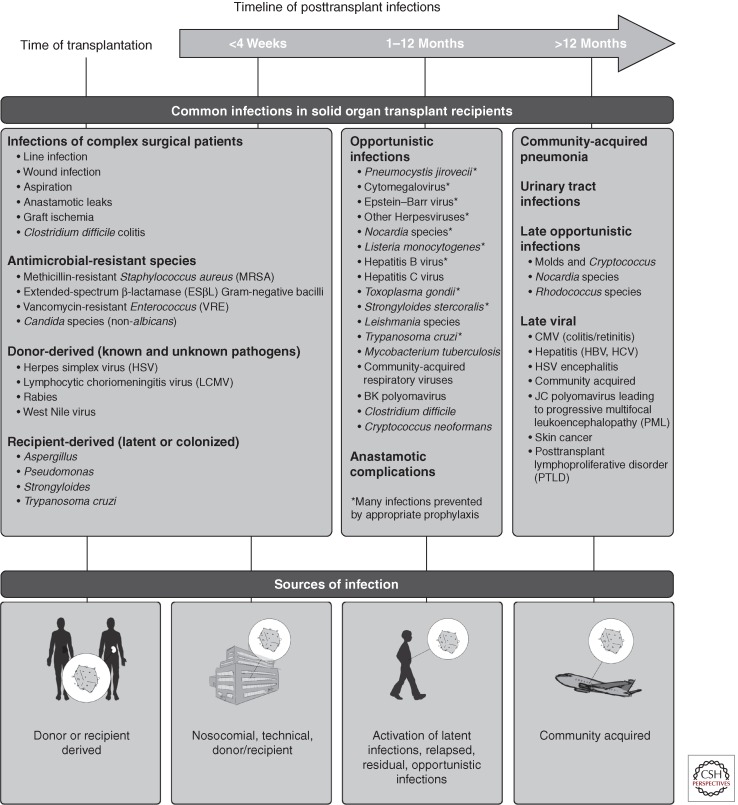
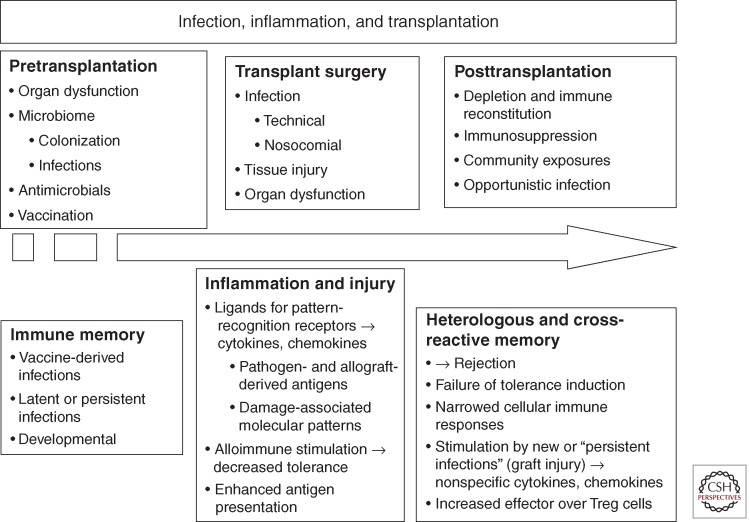
The nature of the clinical transplantation experiment has tended to obscure the impact of diverse infections on immune functions including tolerance induction and graft rejection. Recent studies in animal models have emphasized the importance of the microbiome, infectious exposures, and the innate immune system in determining aspects of immune function including the development of T-cell specificity and function, the risk for subsequent infections, and, to a degree, graft and patient survival (Fig. 1) (Selin et al. 1994; Yewdell and Bennink 1999; Adams et al. 2003a; Chong and Alegre 2012). This review focuses on more recent concepts in the impact of infection in solid organ transplantation.
Figure 1.
The timeline of infections following organ transplantation. The risk for infection following organ transplantation follows a standard pattern with routine immunosuppression and infectious exposures. The potential pathogens for which the risk is modified by prophylaxis, including vaccinations and antimicrobial agents, are indicated (*). Individual risk is modified by events such as surgery, treatment of graft rejection, or malignancy. Note that graft rejection and drug reactions may be among noninfectious causes of fever in transplant recipients. (From Fishman 2007; modified, with permission, from the author.)
RISK FOR INFECTION AND THE TIMELINE OF INFECTION
The risk for infection in an individual transplant recipient is determined by two factors:
-
1.
The epidemiologic exposures of both the patient and the organ donor including those unrecognized by the patient or distant in time (Rubin et al. 1981; Fishman and Rubin 1998).
-
2.
The patient’s “net state of immunosuppression” including all factors contributing to the risk for infection (Table 1) (Fishman and Rubin 1998).
Table 1.
Factors contributing to the “net state of immunosuppression”
| Immunosuppressive therapy: Type, temporal sequence, intensity Prior therapies (chemotherapy or antimicrobials) Mucocutaneous barrier integrity (intravenous access and urinary catheters, surgical drains) Neutropenia, lymphopenia (often drug-induced) Underlying immune deficiency (e.g., adrenal insufficiency, systemic lupus, complement deficiencies) Metabolic conditions: Uremia, malnutrition, diabetes, alcoholism/cirrhosis Viral infection (CMV, hepatitis B and C, RSV) |
These factors are conceptual assessments similar to measuring the area under the curve of a formula relating epidemiology and immune function. At higher levels of immunosuppression, infection occurs at lower levels of infectious “exposure” or with organisms of lower levels of native virulence. With lower levels of immunosuppression, infection is less common, drug toxicities are less frequent, but graft rejection more prevalent. Specific immune deficits (genetic or acquired) and specific immunosuppressive regimens predispose to infection with specific classes of organisms (e.g., T-lymphocyte depletion activates latent cytomegalovirus [CMV] and allows viral replication to occur while corticosteroids predispose to Pneumocystis and other fungal infections) (Table 2) (Issa and Fishman 2009). Successful prophylaxis reduces the burden of organisms or protects against invasive infection (e.g., bacteremia) and allows more intensive immunosuppression. As a result, preventative strategies must be adapted to immunosuppression and to the individual’s risk factors for infection.
Table 2.
Immunosuppression and infection: Common associations
| Antilymphocyte globulins (lytic depletion) and alloimmune response |
| T lymphocytes: Activation of latent (herpes)viruses, fever, cytokines |
| B lymphocytes: Encapsulated bacteria |
| Plasmapheresis: Encapsulated bacteria |
| Costimulatory blockade: Unknown so far; possible increased risk for EBV/PTLD |
| Corticosteroids: Bacteria, PCP, hepatitis B, possibly hepatitis C |
| Azathioprine: Neutropenia, possibly papillomavirus |
| Mycophenolate mofetil (MMF): Early bacterial infection, B cells, late CMV |
| Calcineurin inhibitors: Enhanced viral replication (absence of immunity), gingival infection, intracellular pathogens |
| mTOR inhibitors: |
| Poor wound healing |
| Excess infections in combination with other agents |
| Idiosyncratic interstitial pneumonitis |
EPIDEMIOLOGY
Epidemiologic exposures can be divided into four categories: donor-derived (often unknown exposures), recipient-derived infections and colonization, endemic community-derived infections, and healthcare institution-derived pathogens. The importance of an otherwise unimportant organism on the skin of a transplant recipient is magnified in the face of surgery, devitalized tissues, hematoma or fluid collections, and immunosuppression.
Donor-Derived Infections
Infection is transmitted efficiently with the transplantation of viable tissues; transmission is likely enhanced by immunosuppression (Fishman 2007). Data on allograft-associated disease transmissions are limited by difficulties in distinguishing allograft-derived infections from recipient-derived infections (e.g., because of latent infections or nosocomial infections) and by the failure to recognize or to report these events (Fishman 2007, 2008; Fishman et al. 2009; Grossi and Strong 2009; Grossi et al. 2009). In immunosuppressed hosts, the incidence of donor-derived infection may be underestimated owing to the absence of leukocytosis, pain (in a denervated graft), or erythema.
Some infections generally preclude organ donation (e.g., uncontrolled sepsis, HIV or HTLV infection, West Nile virus, Rabies virus, LCMV). Guidelines precluding the use of organs from HIV or HTLV-infected individuals are being reconsidered in some countries (Huang and Fishman 2011). The potential donor with microbiologically undiagnosed and untreated infection (e.g., sepsis, meningitis, or encephalitis) and/or for whom resolution has not been documented may represent a significant risk to the recipient (Satoi et al. 2001). Some common pathogens may limit the use of donor organs (to infected or immune recipients) and are included in donor (and recipient) screening panels (Table 3). These include serologic and other assays for hepatitis B and C viruses (HBV, HCV). The interpretation of serologic tests from donors must include consideration of the potential for false-positive assays after blood transfusion or false-negative assays after hemodilution of blood samples following infusion of colloids and crystalloids (Eastlund 2000). Similarly, testing for viral antibodies from newborns less than 1 mo of age is unreliable given exposure to maternal antibodies and the inconsistent antibody responses of the immature immune system.
Table 3.
Common screening tests for organ donorsa
| Human immunodeficiency virus antibody Hepatitis B (HBV) serologies including HBV surface antigen, core antibody, surface antibody, and hepatitis δ antigen and/or antibody in HBsAg-positive donors Hepatitis C antibody Nontreponemal and treponemal testing (rapid plasma reagin [RPR] + TPHA or TPPA or FTA-Abs) Human T-cell lymphotrophic virus (HTLV-I/II) antibody (less common currently given assay performance) Toxoplasma antibody (notably in cardiac donors) Cytomegalovirus antibody Epstein–Barr virus (EBV) antibody panel (EBV viral capsid antigen, ± early antigen, and nuclear antigen antibody levels) Herpes simplex virus antibody Varicella-zoster virus antibody Blood and urine cultures |
aMany procurement organizations supplement these tests with additional assays based on local epidemiology and/or use nucleic acid-based assays (NAT) (see also Grossi et al. 2009).
Known, asymptomatic infections of donors are commonly transmitted including cytomegalovirus (CMV), Epstein–Barr virus (EBV), herpes simplex virus (HSV), and varicella zoster virus (VZV). Donor screening is then useful to develop posttransplant preventative or monitoring strategies for recipients. These same pathogens may cause significant disease if infection is active (i.e., viremia) at the time of procurement. Some donor-derived pathogens may require posttransplant therapy: Treponema pallidum, M. tuberculosis, nontuberculous mycobacteria, Histoplasma capsulatum, Coccidioides immitis, Paracoccidioides spp., Blastomyces. Donors from endemic regions merit screening for parasites (Trypanosoma cruzi, Plasmodium spp., Strongyloides stercoralis, Schistosoma spp., Leishmania spp.) as well as epidemic or endemic viruses (Chikungunya virus, West Nile virus, and human T-cell lymphotropic virus, HTLV-I/II). Donors also may be screened for infectious agents of importance in the transplantation of specific organs (e.g., Toxoplasma gondii in cardiac recipients).
Unintentional transmission of infection occurs in at least 1%–2% of recipients (Len et al. 2008; Ison et al. 2011). Clusters of infection in recipients of allografts from a single donor have included Mycobacterium tuberculosis, Candida, and Aspergillus (and other fungal) species, herpes simplex virus (HSV) and human herpes virus 8, lymphocytic choriomeningitis virus (LCMV), rabies virus, Trypanosoma cruzi, HIV, and hepatitis C virus. Detection of transmission requires clinical suspicion, access to advanced microbiologic testing including nucleic acid amplification technologies (NAT), recognition of epidemiologic exposures, and, where appropriate, assistance by public health authorities.
Significant controversy exists over the optimal screening panel and specific tests (e.g., nucleic acid tests or NAT) for donors (Table 3). This is a reflection of at least three areas of uncertainty. First, the degree to which it is possible to reduce infection. Second, the advantages and disadvantages of NAT assays compared with serologic (antibody-based) tests. Third, the actual risk of transmission posed by individuals with possibly increased risk of disease transmission, notably for HCV, HBV, or HIV, is unclear. HIV infectious risk is inferred from the donor’s medical and social history, in addition to data from screening assays. The U.S. Public Health Service (PHS) published guidelines in 1994 describing the epidemiological risk factors identified for HIV transmission; those guidelines have also been applied widely for HCV and HBV prevention (USPH Service 1994). Given the low incidence of reported viral transmission associated with organ transplantation, these guidelines appear to have been effective, notably for HCV. Updated draft guidelines reflect the availability of, and controversy over, highly sensitive NAT and protein-based assays for HIV, HCV, and HBV (USPH Service 2011).
A limitation to serologic assays is the time required to develop positive assays compared with nucleic acid or protein detection assays. Transmission of infection may occur in this “window period” between donor infection and seroconversion (Schreiber et al. 1996; Pillonel et al. 1998; Kolk et al. 2002; Biswas et al. 2003; Fiebig et al. 2003; Kleinman et al. 2003; Busch et al. 2005; Kleinman and Busch 2006; Yao et al. 2007, 2008). HIV antibody assays can show seroconversion within approximately 22 d of infection (but can be as long as 6 mo), with NAT detection reducing the window period for detection from 5.6 to 10.2 d (i.e., 4–15 d in which infection is detected by NAT but not ELISA). HBV surface antigen (HBsAg) ELISA assays have a window period of 38.3 to 49.7 d, with NAT in the range of 20.4 to 25.7 d (Busch et al. 1995, 2005; Schreiber et al. 1996; Pillonel et al. 1998; Kolk et al. 2002; Biswas et al. 2003; Fiebig et al. 2003; Kleinman et al. 2003; Kleinman and Busch 2006; Yao et al. 2007, 2008). The use of HBV NAT testing may detect HBV infection in donors who are HBSAg negative. The HCV ELISA assays have window periods of 38 to 94 d, which is reduced to 6.1 to 8.7 d using NAT assays.
Decisions regarding the use of organs from donors with active or suspected infection take into account the urgency of transplantation for the recipient, microbiological data and treatment options for the donor or recipient, and the availability of alternatives. The key concept underlying donor screening is to provide data for clinicians and potential recipients that allow informed decisions balancing the risk of infection against the benefits of organ replacement.
Pretransplant Infections in Organ Recipients
Before donation, potential recipients (or donors) may become colonized by nosocomially acquired organisms carrying resistance to multiple antimicrobial agents (e.g., vancomycin-resistant Enterococcus [VRE], multidrug-resistant Klebsiella pneumoniae, azole-resistant Candida species). As a result, routine surgical prophylaxis may be inadequate to prevent transmission. The presence of, and microbiological susceptibility patterns for, such colonizing organisms should be discerned before transplantation when possible (notably in lung transplant candidates).
Any active infections in the recipient should be treated and, ideally, resolved before procurement or transplantation (Delmonico and Snydman 1998; Freeman et al. 1999; Lumbreras et al. 2001; Satoi et al. 2001; Singh 2002; D’Albuquerque et al. 2007; Fishman 2007; Nanni Costa et al. 2008). There are no data on which to base a recommendation for the optimal duration of therapy or the interval between resolution of infection and procurement. Clearance or control of infection should be documented and consideration given to surgical or posttransplant prophylaxis. The inflammation associated with bacterial peritonitis is a common source of diffuse intraoperative bleeding or of subsequent fibrosis in liver transplantation—increasing the surgical technical difficulty and the risk for infection. Spillage from infected renal or hepatic cysts may also contaminate a surgical field.
Community Exposures
A careful epidemiologic history may provide microbiologic clues to possible infectious presentations. Outbreaks of viral respiratory illness (e.g., influenza, respiratory syncytial virus) occur earlier in the season in immunocompromised individuals than in normal hosts. Travel, hobbies (gardening, hiking), or work (teachers) exposures may suggest exposures to contaminated food or water (Listeria, Cryptospoduals ridium), soil (Aspergillus or Nocardia), birds (Cryptococcus), or geographically restricted mycoses (Blastomyces dermatitidis, Coccidioides immitis, Paracoccidioides sp., and Histoplasma capsulatum).
Nosocomial Exposures
Nosocomial infections are of increasing importance in transplant donors and recipients. As patients wait for organs and may have undergone multiple procedures and antimicrobial exposures, colonization may occur with hospital-acquired antimicrobial-resistant pathogens. Among the common pathogens are vancomycin-resistant enterococci (VRE), methicillinresistant staphylococci (MRSA), fluconazole-resistant Candida species, Aspergillus species, and highly resistant Gram-negative bacteria (van Delden et al. 2009). Respiratory viral infections may be acquired from visitors and hospital staff.
THE NET STATE OF IMMUNOSUPPRESSION
The net state of immunosuppression is a conceptual measure of factors contributing to a patient’s risk for infection (Table 1) (Fishman 2007). Among these are:
-
1.
The specific immunosuppressive therapy, including dose, duration, and sequence of agents.
-
2.
Surgical issues resulting from the transplant procedure, resulting in leaks (blood, lymph, urine) and fluid collections, devitalized tissue, poor wound healing, and surgical drainage catheters.
-
3.
Prolonged ventilatory support.
-
4.
Broad-spectrum antibiotics.
-
5.
Posttransplant renal, hepatic, pulmonary or cardiac dysfunction, or diabetes.
-
6.
Prolonged use of urinary, vascular access, or dialysis catheters, surgical drains, or other breaks in skin or mucosal defenses.
-
7.
Viral infection with resulting local or systemic immunosuppression and increased risk for superinfection. Of special importance in transplantation are the herpesviruses (CMV, EBV, HSV, VZV), HBV or HCV, or HIV.
-
8.
Malnutrition.
-
9.
Neutropenia, which may be related to medications such as mycophenolate mofetil, azathioprine, ganciclovir, valganciclovir (rarely to trimethoprim-sulfamethoxazole). Overestimates of renal function based on serum creatinine levels are often associated with drug toxicity.
Individual drugs are associated with increased risk for certain infections (Table 2), although the degree of immunosuppression associated with a specific regimen varies between individuals relative to the risks of both graft rejection and for infection. Assays that measure the level of “immunity” against specific pathogens (e.g., cellular immune function or antibody levels against specific organisms) are not yet predictive of infectious risk. Serologic assays determine past exposures and latent infections (herpesviruses) but are poorly predictive of the efficacy of the immune response to specific pathogens in immunosuppressed hosts. Measures of “global” immune function remain relatively crude (Husain et al. 2009). Few data exist on the integrity of immune reconstitution against infection after T- or B-lymphocyte depletion or treatment with other antibody preparations (e.g., antibodies to tumor necrosis factor, costimulatory blockade). Immune deficits associated with biologic agents are generally more severe and persist longer (months to years) than is generally appreciated. Similarly, the effects of organ dysfunction (e.g., cirrhosis, renal dysfunction) on systemic immune function resist precise quantification.
Among underappreciated “immune defects” are breaches in cutaneous barriers. As was noted, the combination of “sticky” organisms such as VRE, Candida spp., or Staphylococcus aureus from colonized skin often associated with biofilms on in-dwelling catheters or surgical drains leads to increased risk for bacteremia. These effects are amplified by neutropenia caused by drug effects (e.g., ganciclovir, mycophenolate mofetil, allopurinol, trimethoprim-sulfamethoxazole) and nutritional deficits. Surgical, invasive radiologic, or gastrointestinal procedures used to repair technical complications such as bile leaks or biliary or ureteric strictures, risk dissemination of organisms from those sites with distant seeding and abscess formation in joints or ischemic regions of grafts.
TIMELINE OF INFECTION
With more standardized immunosuppressive regimens, common infections are observed in a relatively consistent pattern based on the time elapsed since transplantation (Fig. 2). This is a reflection of changing risk factors over time: surgery/hospitalization, immune suppression, acute and chronic rejection, emergence of latent infections, and exposures in the community (Fishman 2007). A key concept is that predictable changes in the risk for infection occur with unique epidemiologic exposures (e.g., returning to work) and with alterations in the drug regimen (interactions, treatment of graft rejection, neutropenia). Infections appear later than usual when delayed by effective prophylaxis and as allograft function improves.
Figure 2.
The impact of infectious exposures, inflammation, the microbiome, heterologous immunity, and innate immune stimulation on transplantation. The microbiome is the sum of colonizing organisms, acute and chronic infections, and reflects alterations in this flora by the immune system, vaccination, or antimicrobial agents. Antigens and activating molecular patterns from microbes or damaged tissues are released during all phases of transplantation and are present during graft rejection, immunosuppression, and/or immune reconstitution following lymphocyte depletion. These antigenic exposures and inflammatory mediators shape immune function via both the innate and adaptive immune systems and impact the outcome of transplantation (see also Chong and Alegre 2012).
The timeline reflects three overlapping periods of risk for infection: (1) the perioperative period to approximately 30 d after transplantation, (2) the period 1–6 mo after transplantation (depending on the use of antilymphocyte “induction” therapy and the level of immunosuppression), and (3) the period beyond the 6–12 mo after transplantation. The timeline may be used in a variety of ways: (1) to establish a differential diagnosis for the transplant patient suspected of having an infection, (2) as a clue to the presence of an excessive environmental hazard for the individual, either within the hospital or in the community, and (3) as a guide to the design of prophylactic antimicrobial strategies. Infections occurring outside the usual period or with unusual severity suggest the presence of an excessive epidemiologic hazard or of excessive immunosuppression (Fishman 2007). Routine preventative strategies from the Massachusetts General Hospital are outlined in Tables 4 and 5. It should be noted that such strategies serve only to delay the onset of infection in the face of epidemiologic pressure. The use of antibiotic prophylaxis, vaccines, and behavioral modifications (e.g., routine hand washing or advice against buying new kittens) may only result in a “shift to the right” of the timing of infection unless the intensity of immune suppression is reduced or immunity develops.
Table 4.
Prophylaxis for Pneumocystis jiroveci pneumonia (PCP)
| Background: Low dose trimethoprim-sulfamethoxazole prophylaxis (in adults: 1 single strength per day orally) is well tolerated and essentially eradicates Pneumocystis infection from this patient population. Lower doses (3 days per week) prevent PCP but may not prevent other infections such as urinary tract infection, including those attributable to susceptible Nocardia and Listeria, toxoplasmosis, and a variety of gastrointestinal and pulmonary infections. |
| Regimen: One single strength trimethoprim-sulfamethoxazole tablet (containing 80 mg trimethoprim, 400 mg sulfamethoxazole) po qhs for a minimum of 4–6 mo posttransplant. Patients infected with CMV, with chronic rejection, recurrent infections, and most lung, liver, and heart recipients may benefit from lifelong prophylaxis. |
| Alternative regimen: For patients proven not to tolerate trimethoprim-sulfamethoxazole, alternative regimens include: (1) a combination of atovaquone 1500 mg po with meals once daily plus levofloxacin (or equivalent fluoroquinolone without antianaerobic spectrum) 250 mg once daily, (2) pentamidine (300 mg iv or inhaled q 3–4 weeks), and (3) dapsone (100 mg po qd to biw) ± pyrimethamine. Each of these agents has toxicities that must be considered including hemolysis in G6PD-deficient hosts with dapsone. None of these alternative programs offer the same broad protection of TMP-SMX. |
Table 5.
Prophylaxis for herpes group virusesa
| CMV universal antiviral prophylaxisa | ||
|---|---|---|
| CMV serologic status ± antilymphocyte-globulin-induction therapy (ALG) | Prophylaxis | Monitoring (antigenemia) |
| D+/R− no ALG | Intravenous ganciclovir 5 mg/kg iv (loading dose) then po valganciclovir (900 mg/d corrected for renal function) or po ganciclovir (3 gm/d)c × 3 mo | Monthly for 6 mo after discontinuation of therapyb |
| D+ or R+ with ALG | Intravenous ganciclovir 5 mg/kg iv for first dose then either per renal function to discharge or switch to po valganciclovir (900 mg/d corrected for renal function) or po ganciclovir (3 gm/d)c × 6 mo | Monthly for 6 mo after discontinuation of therapyb |
| D−/R+ no ALG | Oral valganciclovir (900 mg/d corrected for renal function) × 3 mo | Symptoms only |
| D−/R− | Oral famciclovir 500 mg po qd × 3–4 mo (or valacyclovir 500 bid or acyclovir 400 tid). Use of CMV-negative or leukocyte-filtered blood | Symptoms, fever/neutropenia |
| Status unknown with ALG | Intravenous ganciclovir 5 mg/kg iv for first dose and QD (corrected for renal function) until sero-status determined. | As above |
The human herpes viruses are among the most important causes of infectious disease morbidity and mortality in the transplant recipient. Preventative regimens are determined by the clinical risk, the major determinants of which are the past experience of donor and recipient with the virus (as defined by the presence or absence of circulating antibody before transplant) and the nature of the immunosuppressive therapy. The dose of antiviral therapies are not, in general, reduced for neutropenia.
aPreemptive therapy: Preemptive therapy requires a carefully organized monitoring program and patient compliance. Either a molecular CMV viral load test or a pp65 antigenemia assay may be used for monitoring. Monitoring should be performed once weekly after transplantation for 12–24 wk. Infections indicated by positive assays are treated with either oral valganciclovir (900 mg 2 times a day) or intravenous ganciclovir (5 mg/kg 2 times a day). Full doses are used for loading after which dosing is corrected for renal function. Therapy is continued until viremia is undetectable. Mixed prophylaxis: Many centers prefer universal prophylaxis for highest risk recipients (D+/R− or R+ with lymphocyte depletion) and preemptive therapy for other groups.
bALG: Antilymphocyte antibodies include any of the lytic, lymphocyte-depleting antibody preparations.
cValacyclovir (8 gm/d) has been used as an alternative agent in renal transplant recipients.
Phase 1: 1 Month Posttransplantation
During the first month after transplantation, the types of infection are generally related to complications of the transplant surgery (fluid collections, sepsis), infections present in the donor or recipient before transplantation, or nosocomial infections. Graft rejection may occur but is uncommon. Early removal of lines and drains, appropriate prophylaxis for surgery based on knowledge of the patient’s colonization status, discontinuation of unneeded medications including antimicrobial agents, drainage of hematomas and other collections, and meticulous wound care are essential. Opportunistic infections are uncommon in this period despite intensive immunosuppression; sustained administration of immunosuppressive agents is generally required to allow organisms of low native virulence to establish invasive disease. When these occur, they reflect prior immune defects (pretransplant therapies) or environmental exposures.
In this period, the stage is also set for the emergence of a subgroup of patients—the “chronic ne’er do well”—individuals with early infectious complications and/or graft dysfunction and who may require sustained higher levels of immunosuppression. Such individuals show lifelong susceptibility to infection and merit prolonged (lifelong) prophylaxis (Tables 4 and 5).
Phase 2: 1 to 6 Months Posttransplant
The opportunistic infections emerge in the first 6 mo posttransplant coupled with technical issues remaining from the transplant admission. Use of anti-CMV therapy with broad antiherpes efficacy and trimethoprim–sulfamethoxazole (TMP-SMX) prophylaxis has altered the pattern of posttransplant infections. TMP-SMX eliminates P. jiroveci pneumonia (PCP) and, given daily, reduces the incidence of urinary tract infection and urosepsis, some respiratory and GI infections, L. monocytogenes meningitis, many Nocardia species infections, and Toxoplasma gondii. TMP-SMX also have activity against many strains of community-acquired MRSA. Other agents substituted for TMP-SMX in the sulfa-allergic or neutropenic patient do not provide the same spectrum of benefits. In practice, most “allergies” to TMP-SMX merit reconsideration unless well documented. Effective anti-CMV prophylaxis should prevent most CMV infections (and those caused by most herpesviruses) for the duration of therapy. Thus, the differential diagnosis of infectious syndromes in this period includes:
Graft rejection.
Persistent infection from the perisurgical period including relapsed C. difficile colitis, inadequately treated pneumonia, or infection related to graft-specific technical problems (e.g., pleural effusion, urine leak, cholangitis, lymphocele, infected hematoma, airway necrosis).
Viral infections including CMV, herpes simplex virus (HSV), shingles (localized or disseminated zoster attributable to varicella zoster virus, VZV), human herpesvirus 6 or 7, BK polyomavirus, EBV, relapsed hepatitis (HBV, HCV), and the community-acquired respiratory viruses (adenovirus, influenza, parainfluenza, respiratory syncytial virus, metapneumovirus). Secondary postviral infections (bacterial or fungal) are common.
Opportunistic infection as a result of Pneumocystis jiroveci, Listeria monocytogenes, Toxoplasma gondii, Nocardia species (in the absence of TMP-SMX), Aspergillus species, and other agents. The specific opportunistic infections that occur reflect the specific immunosuppressive regimen used and the presence or absence of immunomodulating viral infection.
Phase 3: More than 6 to 12 Months Posttransplant
After the first 6–12 mo, transplant recipients with good graft function and reduced maintenance immunosuppression remain at some risk for reactivation of latent infections (e.g., VZV) or for primary CMV infection (socially acquired) but have community-acquired exposures as their main risk, which may predispose to more serious opportunistic superinfections. Respiratory viruses or infections related to underlying diseases (e.g., skin infections in diabetes) are common. The major challenges include EBV and posttransplant lymphoproliferative disorders, persistent BK polyomavirus infection, hepatitis C virus in liver recipients, and papillomavirus (warts, anogenital, and skin cancers). Advanced therapies for HIV and HCV have dramatically altered the impact of these infections. Viral infections are often the major factor in risk for infections or graft rejection.
The patients with less satisfactory allograft function or with renal dysfunction or diabetes as a result of calcineurin inhibitor toxicity tend to be relatively over-immunosuppressed. This group includes those identified early in the posttransplant course caused by delayed or poor initial graft function or complications and is at persistent risk for opportunistic infections such as Pneumocystis, Zygomycetes, or Cryptococcus (Fishman 2007). Lesions identified in this group such as of skin or lungs should be biopsied to exclude fungal infection or malignancy.
“INDIRECT EFFECTS,” THE MICROBIOME, AND IMMUNE FUNCTION
Latent or acute infections are associated with adverse outcomes in the transplant recipient including an increased risk for opportunistic infection and graft rejection. The existence of these “indirect effects” has been implied based on clinical observations and the effects of effective prophylaxis. For CMV, in addition to an increased rate of opportunistic infections (fungal including aspergillosis, accelerated HCV infection), effects include coronary vaculopathy in heart recipients, bronchiolitis obliterans syndrome in lung recipients, and an increased rate of EBV-associated posttransplant lymphoproliferative disorders (PTLD) and increased mortality in the absence of prophylaxis. In liver transplant recipients with recurrent HCV hepatitis, there is an increased rate of infections, posttransplant diabetes, and mortality (Hodson et al. 2005; Kalil et al. 2005; Bloom and Lake 2006; Small et al. 2006). HCV infection may also enrich the hepatic regulatory T cell population, which may persist after liver transplantation (Spangenberg et al. 2005; Nellore and Fishman 2011).
These effects may be mediated by innate or adaptive immune mechanisms and should not be confused with “chronic allograft rejection” but are rather viral effects on cellular proliferation, functions of immune cells and inflammatory networks, and modulation of cellular gene activities in both the graft and host. These remain an important area for investigation.
Infection and the microbiome control many aspects of alloimmunity in transplantation (Chong and Alegre 2012). Thus, consistent with experience, “dirty” transplantation (e.g., lung or intestine) would be expected to have lower graft survival than cleaner (e.g., kidney, heart) procedures without exposure to organisms and the environment. This may also be a reflection of technical difficulty and differing immunologic challenges.
Heterologous immunity is the concept used to describe immune memory responses to previously encountered pathogens that alter subsequent immune responses to unrelated pathogens or to grafts. Some of these effects are attributed to the generation of immunological cross reactivity between viral epitopes and graft and other antigens. Virally induced alloreactive memory may create a barrier to transplantation tolerance or induce graft rejection or autoimmunity (Braciale et al. 1981; Adams et al. 2003a,b). The observed effects may be mediated via memory CD8+ cells following viral infection or more broadly by inflammatory mediators such as TNF-α and IFN-γ. Such virally induced alloreactive memory may create a barrier to transplantation tolerance or induce graft rejection or autoimmunity. Such effects have been shown for both CMV and EBV. Viral reactivation may also compress the T-cell repertoire and diminish responses to new antigens with an increased risk for opportunistic infections (Yewdell and Bennink 1999; Brehm et al. 2002). All limbs of the host immune response may be altered by infection, including adaptive as well as NK cell, neutrophil, and macrophage responses, and may contribute to the risk for graft injury and opportunistic infections.
GENERAL APPROACHES TO INFECTION IN TRANSPLANT RECIPIENTS
The spectrum of infection in the immunocompromised host is broad. Given the potential toxicity of antimicrobial agents and the need for rapid interruption of infection, early and specific diagnosis is essential in this population. Advances in diagnostic modalities, including advanced imaging and molecular microbiologic techniques, greatly assist in this process. However, the need for invasive diagnostic approaches cannot be overemphasized. Whereas these approaches carry some risk, the failure to achieve a specific diagnosis often necessitates broad, empiric therapy without clear endpoints for therapy. Initial, often empiric, therapy will target a broad range of potential pathogens with rapid narrowing of the antimicrobial spectrum as data become available.
A central consideration for the patient with an “infectious syndrome” is whether to reduce the intensity of immunosuppression as a part of therapy, risking graft rejection or an immune reconstitution syndrome (IRIS). Given that infection and rejection are often linked, clear distinctions between these processes may be difficult. For example, recurrent HCV infection may increase the risk for liver graft rejection. Pneumonia, including aspiration, community-acquired respiratory viruses, CMV, and bacterial infections, will increase the rate of obliterative bronchiolitis in lung recipients (Bando et al. 1995; Kroshus et al. 1997; Avery 2006). CMV also contributes to cardiac and renal allograft rejection (Lowance et al. 1999; Potena and Valantine 2007). In practice, it is often possible to decrease the intensity of immune suppression in the face of significant infection; however, as the patient improves, rejection may occur. The selection of the specific agents to be reduced should depend on the organisms isolated—and often is not the calcineurin inhibitor. No prospective studies of the efficacy of reduced immunosuppression have been performed. Reversal of immune deficits (neutropenia, hypogammaglobulinemia) may be possible with adjunctive therapies (colony stimulating factors or antibody). Coinfection with virus (CMV) is a universal concern and merits therapy.
CONCLUSION
Transplant infectious disease has increasingly become a field characterized by preventative approaches and early therapies based on sensitive molecular diagnostic tests. The prevention of CMV, other herpesviruses, and Pneumocystis have been important advances in transplantation. Infection is often preventable with correction of technical problems (e.g., drainage of fluid collections before infection). Patients with viremia are over immunosuppressed relative to their immune systems. In the absence of assays useful for the individualization of global immunosuppression, sensitive molecular tests, and assays for pathogen-specific immune function may guide the modulation of the intensity of the individual’s regimen. In the future, host risk factors will be amenable to investigation including genetic polymorphisms (e.g., toll-like receptors) and immunoregulatory elements to allow the individualization of transplant immunosuppression.
Footnotes
Editors: Laurence A. Turka and Kathryn J. Wood
Additional Perspectives on Transplantation available at www.perspectivesinmedicine.org
REFERENCES
- Adams AB, Pearson TC, Larsen CP 2003a. Heterologous immunity: An overlooked barrier to tolerance. Immunol Rev 196: 147–160 [DOI] [PubMed] [Google Scholar]
- Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, et al. 2003b. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 111: 1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RK 2006. Infections after lung transplantation. Semin Respir Crit Care Med 27: 544–551 [DOI] [PubMed] [Google Scholar]
- Bando K, Paradis IL, Komatsu K, Konishi H, Matsushima M, Keena RJ, Hardesty RL, Armitage JM, Griffith BP 1995. Analysis of time-dependent risks for infection, rejection, and death after pulmonary transplantation. J Thorac Cardiovasc Surg 109: 49–57; discussion 57-49 [DOI] [PubMed] [Google Scholar]
- Biswas R, Tabor E, Hsia CC, Wright DJ, Laycock ME, Fiebig EW, Peddada L, Smith R, Schreiber GB, Epstein JS, et al. 2003. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion 43: 788–798 [DOI] [PubMed] [Google Scholar]
- Bloom RD, Lake JR 2006. Emerging issues in hepatitis C virus-positive liver and kidney transplant recipients. Am J Transplant 6: 2232–2237 [DOI] [PubMed] [Google Scholar]
- Braciale TJ, Andrew ME, Braciale VL 1981. Simultaneous expression of H-2-restricted and alloreactive recognition by a cloned line of influenza virus-specific cytotoxic T lymphocytes. J Exp Med 153: 1371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol 3: 627–634 [DOI] [PubMed] [Google Scholar]
- Busch MP, Lee LL, Satten GA, Henrard DR, Farzadegan H, Nelson KE, Read S, Dodd RY, Petersen LR 1995. Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: Implications for screening of blood and tissue donors. Transfusion 35: 91–97 [DOI] [PubMed] [Google Scholar]
- Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, Pappalardo B, Kleinman SH 2005. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion 45: 254–264 [DOI] [PubMed] [Google Scholar]
- Chong AS, Alegre ML 2012. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol 12: 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Albuquerque LA, Gonzalez AM, Filho HL, Copstein JL, Larrea FI, Mansero JM, Peron G Jr, Ribeiro MA Jr, Oliveira e Silva A 2007. Liver transplantation from deceased donors serologically positive for Chagas disease. Am J Transplant 7: 680–684 [DOI] [PubMed] [Google Scholar]
- Delmonico FL, Snydman DR 1998. Organ donor screening for infectious diseases: Review of practice and implications for transplantation. Transplantation 65: 603–610 [DOI] [PubMed] [Google Scholar]
- Eastlund T 2000. Hemodilution due to blood loss and transfusion and reliability of cadaver tissue donor infectious disease testing. Cell Tissue Bank 1: 121–127 [DOI] [PubMed] [Google Scholar]
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, et al. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS 17: 1871–1879 [DOI] [PubMed] [Google Scholar]
- Fishman JA 2007. Infection in solid-organ transplant recipients. N Engl J Med 357: 2601–2614 [DOI] [PubMed] [Google Scholar]
- Fishman JA 2008. Rapporto dell’OMS sulla valutazione del programma nazionale trapianti in Italia. Trapianti 12: 37–53 [Google Scholar]
- Fishman JA, Rubin RH 1998. Infection in organ-transplant recipients. N Engl J Med 338: 1741–1751 [DOI] [PubMed] [Google Scholar]
- Fishman JA, Greenwald MA, Kuehnert MJ 2007. Enhancing transplant safety: A new era in the microbiologic evaluation of organ donors? Am J Transplant 7: 2652–2654 [DOI] [PubMed] [Google Scholar]
- Fishman JA, Strong DM, Kuehnert MJ 2009. Organ and tissue safety workshop 2007: Advances and challenges. Cell Tissue Bank 10: 271–280 [DOI] [PubMed] [Google Scholar]
- Freeman RB, Giatras I, Falagas ME, Supran S, O’Connor K, Bradley J, Snydman DR, Delmonico FL 1999. Outcome of transplantation of organs procured from bacteremic donors. Transplantation 68: 1107–1111 [DOI] [PubMed] [Google Scholar]
- Grossi P, Strong DM 2009. The role of testing in determining suitability of donors and tissues. In Tissue and cell donation: An essential guide (ed. Warwick DFRM, Brubaker SA, Eastlund T). Blackwell, New York [Google Scholar]
- Grossi PA, Fishman JA, AST Infectious Disease Community of Practice 2009. Donor-derived infections in solid organ transplant recipients. Am J Transplant 9: S19–S26 [DOI] [PubMed] [Google Scholar]
- Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K, Vimalachandra D, Craig JC 2005. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: A systematic review of randomised controlled trials. Lancet 365: 2105–2115 [DOI] [PubMed] [Google Scholar]
- Huang RC, Fishman JA 2011. Screening of deceased organ donors: No easy answers. Transplantation 91: 146–149 [DOI] [PubMed] [Google Scholar]
- Husain S, Raza K, Pilewski JM, Zaldonis D, Crespo M, Toyoda Y, Shutt K, Spichty K, Bentlejewski C, Pakstis DL, et al. 2009. Experience with immune monitoring in lung transplant recipients: Correlation of low immune function with infection. Transplantation 87: 1852–1857 [DOI] [PubMed] [Google Scholar]
- Ison MG, Llata E, Conover CS, Friedewald JJ, Gerber SI, Grigoryan A, Heneine W, Millis JM, Simon DM, Teo CG, et al. 2011. Transmission of human immunodeficiency virus and hepatitis C virus from an organ donor to four transplant recipients. Am J Transplant 11: 1218–1225 [DOI] [PubMed] [Google Scholar]
- Issa NC, Fishman JA 2009. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis 48: 772–786 [DOI] [PubMed] [Google Scholar]
- Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG 2005. Meta-analysis: The efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 143: 870–880 [DOI] [PubMed] [Google Scholar]
- Kleinman SH, Busch MP 2006. Assessing the impact of HBV NAT on window period reduction and residual risk. J Clin Virol 36: S23–S29 [DOI] [PubMed] [Google Scholar]
- Kleinman SH, Kuhns MC, Todd DS, Glynn SA, McNamara A, DiMarco A, Busch MP 2003. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: Implications for transfusion transmission and donor screening. Transfusion 43: 696–704 [DOI] [PubMed] [Google Scholar]
- Kolk DP, Dockter J, Linnen J, Ho-Sing-Loy M, Gillotte-Taylor K, McDonough SH, Mimms L, Giachetti C 2002. Significant closure of the human immunodeficiency virus type 1 and hepatitis C virus preseroconversion detection windows with a transcription-mediated-amplification-driven assay. J Clin Microbiol 40: 1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM 3rd 1997. Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg 114: 195–202 [DOI] [PubMed] [Google Scholar]
- Len O, Gavalda J, Blanes M, Montejo M, San Juan R, Moreno A, Carratala J, de la Torre-Cisneros J, Bou G, Cordero E, et al. 2008. Donor infection and transmission to the recipient of a solid allograft. Am J Transplant 8: 2420–2425 [DOI] [PubMed] [Google Scholar]
- Lowance D, Neumayer HH, Legendre CM, Squifflet JP, Kovarik J, Brennan PJ, Norman D, Mendez R, Keating MR, Coggon GL, et al. 1999. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. N Engl J Med 340: 1462–1470 [DOI] [PubMed] [Google Scholar]
- Lumbreras C, Sanz F, Gonzalez A, Perez G, Ramos MJ, Aguado JM, Lizasoain M, Andres A, Moreno E, Gomez MA, et al. 2001. Clinical significance of donor-unrecognized bacteremia in the outcome of solid-organ transplant recipients. Clin Infect Dis 33: 722–726 [DOI] [PubMed] [Google Scholar]
- Nanni Costa A, Grossi P, Gianelli Castiglione A, Grigioni WF 2008. Quality and safety in the Italian donor evaluation process. Transplantation 85: S52–S56 [DOI] [PubMed] [Google Scholar]
- Nellore A, Fishman JA 2011. NK cells, innate immunity and hepatitis C infection after liver transplantation. Clin Infect Dis 52: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillonel J, Saura C, Courouce AM 1998. Prevalence of HIV, HTLV, and hepatitis B and C viruses in blood donors in France, 1992–1996 (in French). Transfus Clin Biol 5: 305–312 [DOI] [PubMed] [Google Scholar]
- Potena L, Valantine HA 2007. Cytomegalovirus-associated allograft rejection in heart transplant patients. Curr Opin Infects Dis 20: 425–431 [DOI] [PubMed] [Google Scholar]
- Rubin RH, Wolfson JS, Cosimi AB, Tolkoff-Rubin NE 1981. Infection in the renal transplant recipient. Am J Med 70: 405–411 [DOI] [PubMed] [Google Scholar]
- Satoi S, Bramhall SR, Solomon M, Hastings M, Mayer AD, de Goyet JV, Buckels JA, McMaster P, Mirza DF 2001. The use of liver grafts from donors with bacterial meningitis. Transplantation 72: 1108–1113 [DOI] [PubMed] [Google Scholar]
- Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ 1996. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med 334: 1685–1690 [DOI] [PubMed] [Google Scholar]
- Selin LK, Nahill SR, Welsh RM 1994. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med 179: 1933–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N 2002. Impact of donor bacteremia on outcome in organ transplant recipients. Liver Transpl 8: 975–976 [DOI] [PubMed] [Google Scholar]
- Small LN, Lau J, Snydman DR 2006. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: Meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis 43: 869–880 [DOI] [PubMed] [Google Scholar]
- Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsacker F, Blum HE, Thimme R 2005. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology 42: 828–837 [DOI] [PubMed] [Google Scholar]
- USPH Service 1994. PHS guideline for preventing transmission of HIV through transplantation of human tissue and organs. In Morbidity and mortality weekly report (MMWR), pp. 1–17 [PubMed]
- USPH Service 2011. (Draft) Public Health Service guideline for reducing transmission of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) through solid organ transplantation (ed. DoHaH Services), Docket No. CDC–2011–0011. CDC Federal Register
- van Delden C, Blumberg EA, AST Infectious Diseases Community of Practice 2009. Multidrug resistant Gram-negative bacteria in solid organ transplant recipients. Am J Transplant 9: S27–S34 [DOI] [PubMed] [Google Scholar]
- Yao F, Seed C, Farrugia A, Morgan D, Cordner S, Wood D, Zheng MH 2007. The risk of HIV, HBV, HCV and HTLV infection among musculoskeletal tissue donors in Australia. Am J Transplant 7: 2723–2726 [DOI] [PubMed] [Google Scholar]
- Yao F, Seed C, Farrugia A, Morgan D, Wood D, Zheng MH 2008. Comparison of the risk of viral infection between the living and nonliving musculoskeletal tissue donors in Australia. Transpl Int 21: 936–941 [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol 17: 51–88 [DOI] [PubMed] [Google Scholar]