Abstract
Defining mechanisms by which Plasmodium virulence is regulated is central to understanding the pathogenesis of human malaria. Serial blood passage of Plasmodium through rodents1-3, primates4 or humans5 increases parasite virulence, suggesting that vector transmission regulates Plasmodium virulence within the mammalian host. In agreement, disease severity can be modified by vector transmission6-8, which is assumed to ‘reset’ Plasmodium to its original character3. However, direct evidence that vector transmission regulates Plasmodium virulence is lacking. Here we utilise mosquito transmission of serially blood passaged (SBP) Plasmodium chabaudi chabaudi9 to interrogate regulation of parasite virulence. Analysis of SBP P.c. chabaudi before and after mosquito transmission demonstrates that vector transmission intrinsically modifies the asexual blood-stage parasite, which in turn, modifies the elicited mammalian immune response, which in turn, attenuates parasite growth and associated pathology. Attenuated parasite virulence associates with modified expression of the pir multi-gene family. Vector transmission of Plasmodium therefore regulates gene expression of probable variant antigens in the erythrocytic cycle, modifies the elicited mammalian immune response, and thus regulates parasite virulence. These results place the mosquito at the centre of our efforts to dissect mechanisms of protective immunity to malaria for the development of an effective vaccine.
The definitive host for mammalian Plasmodium is the Anopheline mosquito. Within this vector, a complex series of developmental events, including fertilisation and meiosis, culminates in invasion of the salivary glands by infective sporozoites, which are transmitted to the mammalian host via mosquito bite. Sporozoites deposited in the dermis migrate to the liver, invade hepatocytes and undergo further developmental processes prior to the release of merozoites that invade erythrocytes. The subsequent erythrocytic cycle is entirely responsible for the morbidity and mortality associated with malaria. The complexity of the Plasmodium life cycle has led to much of the basic biology of the blood-stage infection being studied in isolation, with in vivo experiments largely initiated via direct injection of infected erythrocytes. However, serial blood passage of Plasmodium increases parasite virulence1-5, suggesting that regulation of Plasmodium virulence is an inherent consequence of vector transmission3. This could result indirectly from vector control of inoculum size or the passage of large parasite populations through extreme bottlenecks, although these consequences of mosquito transmission are not thought to be major determinants of disease severity8,10. Alternatively, vector transmission may regulate Plasmodium virulence by intrinsically modifying the parasite and its interaction with the mammalian host. In this context, the immune response elicited by Plasmodium influences disease severity11, and can therefore dictate parasite virulence. The interrelationship between the vector, parasite and mammalian immune system could thus underpin the pathogenesis of malaria.
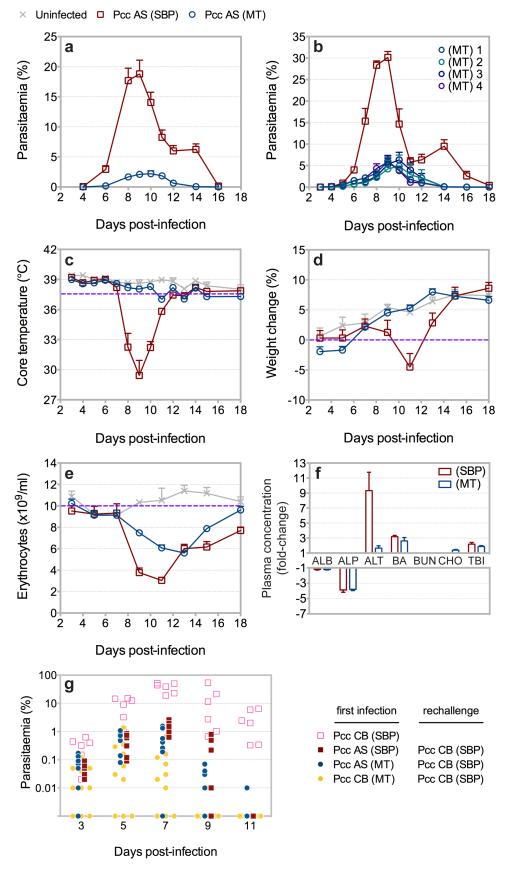
To study regulation of Plasmodium virulence we developed routine mosquito transmission of SBP P.c. chabaudi9, a rodent malaria parasite that exhibits many characteristics associated with the pathogenesis of human infection12. This allowed us to directly compare SBP parasites before and after vector transmission. Accordingly, mice were infected with SBP P.c. chabaudi AS either by injection of parasitised erythrocytes (pE) or mosquito bite (see Methods Summary). Following mosquito transmission, asexual blood-stage parasite growth was attenuated (Fig. 1a), and a low-grade, recrudescing infection with extended chronicity was established (Supplementary Fig. 1). Attenuated parasite growth in the erythrocytic cycle was not influenced by dose (ref.9 and Supplementary Fig. 2) or, importantly, by the pre-erythrocytic-stages of infection, as attenuated parasite growth was similarly observed when mice were injected with pE derived from recently mosquito transmitted (MT) parasite lines (Fig. 1b). Similar results were observed with cloned parasites derived from SBP P.c. chabaudi AS (Supplementary Fig. 3), and with the hypervirulent P.c. chabaudi CB (Supplementary Fig. 4). Mosquito transmission therefore attenuated the asexual blood-stage parasite. As expected, serial blood passage of MT P.c. chabaudi AS rapidly increased parasite growth (Supplementary Fig. 5). Mice infected with P.c. chabaudi AS via mosquito bite did not exhibit the severe hypothermia, cachexia or hepatic cellular damage that was observed during the acute phase of infection in mice injected with SBP parasites, although they still exhibited severe anaemia despite attenuated parasite growth (Fig. 1c-f). Mosquito transmission therefore reduced disease severity in the mammalian host. Despite attenuated parasite growth and reduced pathogenicity, MT P.c. chabaudi AS elicited robust, long-term protection to reinfection with homologous or heterologous blood-stage parasites (Fig. 1g and Supplementary Fig. 6). Thus, vector transmission regulates the virulence of Plasmodium by intrinsically modifying the asexual blood-stage parasite, without influencing the capacity of the mammalian host to acquire robust immunity to reinfection.
Figure 1.
Mosquito transmission of P.c. chabaudi AS attenuates virulence. a, Parasitaemia of C57BL/6 mice injected with 105 SBP P.c. chabaudi AS (Pcc AS) or infected with Pcc AS via mosquito bite. b, Parasitaemia of C57BL/6 mice injected with 105 SBP Pcc AS or injected with 104 or 105 pE derived from one of four recently MT lines of Pcc AS. c-e, Temperature (c), weight (d) and erythrocyte count (e) of C57BL/6 mice injected with 105 SBP Pcc AS or infected with Pcc AS via mosquito bite. f, Liver enzyme concentration on day 10 post-infection in plasma of C57BL/6 mice injected with 105 SBP Pcc AS or infected with Pcc AS via mosquito bite. Data presented as fold-change relative to uninfected control mice. (ALBumin; ALkaline Phosphatase; ALanine amino Transferase; Bile Acids, Blood Urea Nitrogen; CHOlesterol; Total BIlirubin). g, Parasitaemia of C57BL/6 mice injected with 106 SBP P.c. chabaudi CB (Pcc CB) as a first infection (open symbols), or as a rechallenge (closed symbols) 90 days after injection with 105 SBP Pcc AS or infection with Pcc AS or CB via mosquito bite. (n = 3-20 mice per group; data presented as mean with SEM).
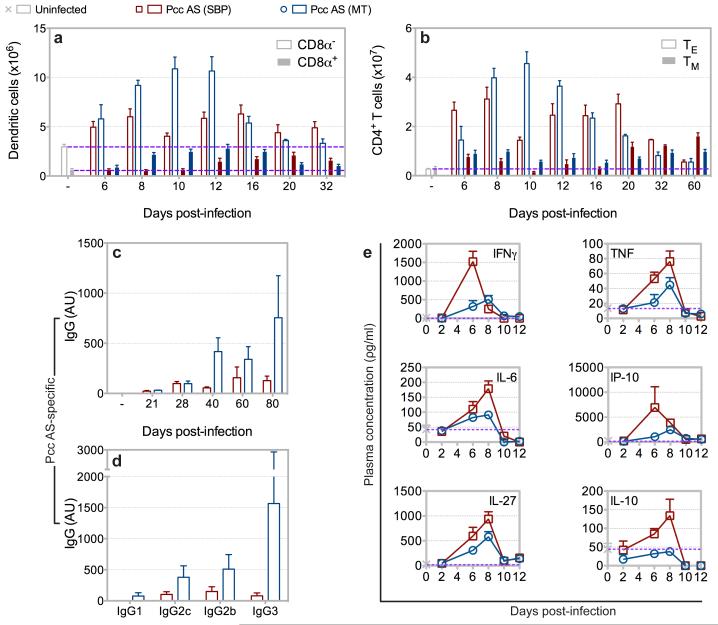
The pathogenesis of malaria is complex and influenced by the mammalian immune system; dysregulated immune reactions can directly promote severe disease11, whereas an apposite response can enhance parasite clearance without promoting pathology13. The immune response induced by Plasmodium can therefore define its virulence. Throughout the erythrocytic cycle the spleen is the major anatomical site associated with the developing immune response14, and mice infected with P.c. chabaudi AS via mosquito bite developed marked splenomegaly with rapid recruitment of inflammatory monocytes (Supplementary Fig. 7-8). Importantly, following mosquito transmission there was enhanced expansion of activated CD8α+ and CD8α− dendritic cells, which present malaria-specific antigens and stimulate CD4+ T cell proliferation15, in the acute phase of infection (Fig. 2a and Supplementary Fig. 9). Correspondingly, the magnitude of the effector CD4+ T cell response, which orchestrates innate and adaptive immune control of blood-stage parasite growth13, was also enhanced following mosquito transmission, and the memory CD4+ T cell population exhibited a predominantly effector memory phenotype (Fig. 2b and Supplementary Fig. 10). Infection with MT P.c. chabaudi AS also increased the magnitude of the class-switched malaria-specific antibody response, a central component of erythrocytic immunity11 (Fig. 2c-d). Mosquito transmission therefore enhanced antibody production in the chronic phase of infection, subsequent to enhanced innate and adaptive cellular responses early in infection. Conversely, mosquito transmission attenuated systemic inflammation during the acute phase response, with decreased circulating levels of pro-inflammatory cytokines and chemokines associated with severe disease11,16 (Fig. 2e). Thus, vector transmission intrinsically modifies the asexual blood-stage parasite and transforms the mammalian immune response elicited during the erythrocytic cycle.
Figure 2.
Mosquito transmission of P.c. chabaudi AS transforms the elicited mammalian immune response. a-b, Number of CD8α− (open bars) and CD8α+ (closed bars) dendritic cells (a), and effector (TE) (open bars) and memory (TM) (closed bars) CD4+ T cells (b) in spleens of C57BL/6 mice injected with 105 SBP Pcc AS or infected with Pcc AS via mosquito bite. c-d, Plasma concentration of total parasite-specific IgG throughout infection (c) and parasite-specific IgG subclasses on day 80 post-infection (d) in C57BL/6 mice injected with 105 SBP Pcc AS or infected with Pcc AS via mosquito bite. Data presented as arbitrary units (AU) relative to hyper-immune plasma. e, Plasma cytokine concentration in C57BL/6 mice injected with 105 SBP Pcc AS or infected with Pcc AS via mosquito bite. (n = 3-5 mice per group per time-point; data presented as mean with SEM).
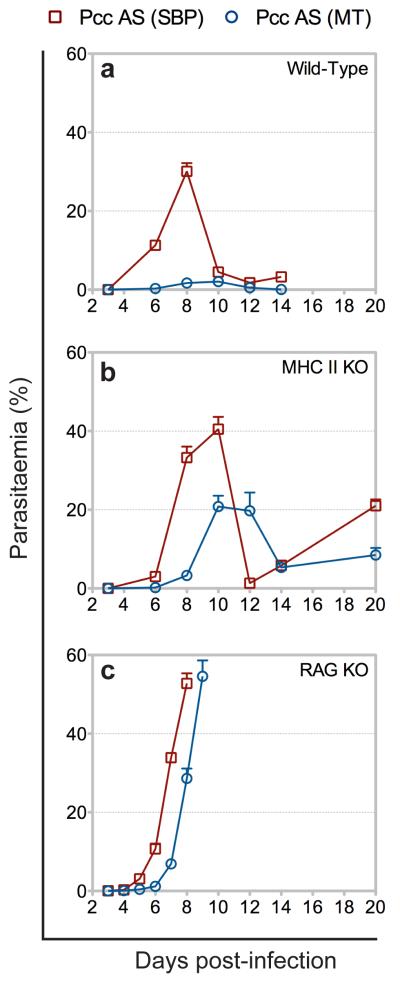
Parasite growth and pathogenicity are, in part, determined by host susceptibility. Infection of susceptible mouse strains with P.c. chabaudi AS via mosquito bite causes severe disease and death9, demonstrating that vector transmission does not limit the potential virulence of the asexual blood-stage parasite. We therefore addressed whether attenuated parasite virulence in C57BL/6 mice infected with MT P.c. chabaudi AS was a consequence of the transformed host immune response. Immunodeficient mice were injected with SBP P.c. chabaudi AS, or with an equivalent number of pE derived from a recently MT line. Disruption of the innate and adaptive immune responses, through depletion of CD4+ T cells, or depletion of the entire adaptive arm of the immune system, led to a virulent and fatal acute phase infection (Fig. 3). Attenuation of parasite virulence by mosquito transmission was therefore dependent upon an intact mammalian immune response and, furthermore, independent of parasite growth rate (Supplementary Fig. 11). Immune control of parasite virulence therefore resulted directly and exclusively from modified innate and adaptive immune responses elicited by, and directed against, the blood-stage parasite. Vector transmission of Plasmodium thus intrinsically modifies the asexual blood-stage parasite, which in turn, modifies the elicited mammalian immune response, which in turn, regulates parasite virulence.
Figure 3.
Transformed innate and adaptive immune responses attenuate P.c. chabaudi AS virulence. a-c, Parasitaemia of wild-type (a), CD4+ T cell deficient (MHC II KO) (b), and B and T cell deficient (RAG KO) (c) C57BL/6 mice injected with 105 SBP Pcc AS or injected with 105 pE derived from a recently MT line of Pcc AS. (n = 4-6 mice per group; data presented as mean with SEM).
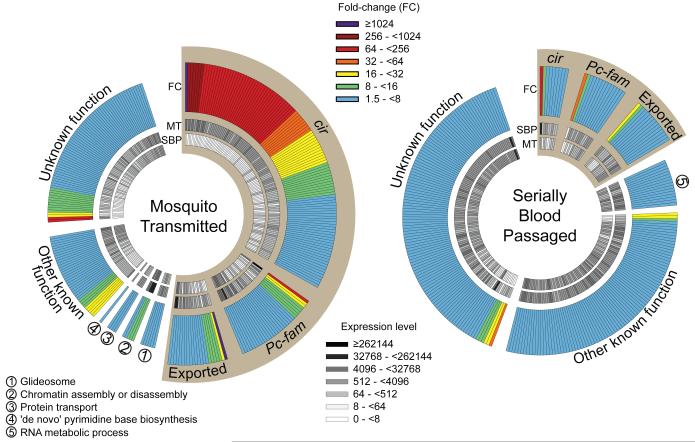
Defining parasite gene expression in the erythrocytic cycle after vector transmission is thus central to understanding the pathogenesis of malaria. We therefore performed genome-wide RNA sequencing on P.c. chabaudi AS, directly comparing blood-stage parasites before and after mosquito transmission. This allowed us to identify a set of Plasmodium virulence genes that direct the elicited mammalian immune response (Fig. 4). Vector transmission modified expression of approximately 10% of the entire genome in the late trophozoite stage parasite (Supplementary Tables). The majority of genes upregulated following mosquito transmission encoded exported proteins, with the potential to access and modulate the mammalian immune system. Importantly, parasite gene expression was most intensely regulated within the sub-telomeric large multi-gene families, with preferential regulation of the pir multi-gene family (termed cir in P.c. chabaudi) (Supplementary Fig. 12). 123 of 200 cir genes (61.5%) were differentially expressed following mosquito transmission, with 114 cir genes (57%) upregulated. Furthermore, the most upregulated gene following serial blood passage was identified as the most highly expressed cir gene (PCHAS_110030) in mice infected with SBP P.c. chabaudi AS (ref.17, Fig. 4 and Supplementary Fig. 12). Serial blood passage therefore selected for dominant cir gene expression, whilst mosquito transmission revoked the selected expression hierarchy and promoted a generalised increase in cir expression across the parasite population. We therefore uncover a direct association between pir gene expression and Plasmodium virulence, and demonstrate that vector transmission regulates expression of probable antigenic variants18, as previously hypothesised19,20. Vector transmission of Plasmodium thus regulates parasite gene expression in the erythrocytic cycle and, consequently, regulates immune control of Plasmodium virulence.
Figure 4.
Mosquito transmission of P.c. chabaudi AS modifies parasite gene expression in the erythrocytic cycle. C57BL/6 mice were injected with 105 SBP Pcc AS or infected with Pcc AS via mosquito bite. Parasites were isolated after 6 cycles of the blood-stage infection, and at the late trophozoite stage of development (98.3% (0.76%) and 97.0% (1.41%) trophozoites for SBP and MT samples, respectively (mean with SD)). Total parasite RNA was extracted and sequenced. Those genes differentially expressed between SBP and MT Pcc AS were determined; genes identified as significantly upregulated in blood-stage parasites following mosquito transmission (left) versus serial blood passage (right) are shown. Each segment represents one gene, and genes are categorised according to the function of their product and ranked based on fold-change (outer circle). The DESeq-normalised expression levels for each gene are also shown (inner circles). Sepia wedges highlight genes whose products are predicted to be exported, or otherwise accessible to the mammalian immune system.
Vector transmission will inherently regulate Plasmodium virulence within the mammalian host. Recombination of distinct parasite genotypes within the mosquito is likely to be fundamental for the evolution of virulence21. The results of this study reveal that vector transmission also regulates Plasmodium virulence by modifying parasite gene expression, and therefore the mammalian immune response, in the erythrocytic cycle. This is likely the outcome of a combination of distinct regulatory processes acting at multiple stages of the parasite life cycle, in both the mosquito vector and mammalian host. It is therefore important to delineate the timing and mechanism(s) of regulation of parasite gene expression, in the context of the complete Plasmodium life cycle, to understand the molecular regulation of parasite virulence. Attenuation of parasite virulence following mosquito transmission associates with modified expression of the pir multi-gene family, which is conserved from rodent to human Plasmodium17. Importantly, vector transmission of cultured P. falciparum similarly modifies the composition and frequency of var gene expression22. Regulation of antigenic variants by vector transmission is therefore universal19,20,22, and vector transmission will therefore universally regulate immune control of Plasmodium virulence. The interrelationship between the vector, parasite and mammalian immune system thus underpins the pathogenesis of malaria.
Methods
Mice
Inbred wild-type, major histocompatibility complex class II knock-out (MHC II KO)23 and recombination activation gene-1 knock-out (RAG KO)24 C57BL/6 mice were bred under specific pathogen-free conditions at NIMR. All experiments were performed in accordance with UK Home Office regulations (PPL 80/2358) and approved by the ethical review panel at NIMR. Experimental mice were age- and sex-matched, housed under reverse light conditions (light 19.00-07.00: dark 07.00-19.00) at 20-22°C, and had continuous access to mouse breeder diet and water. Measurements of clinical pathology were taken at 16.00 hours: core body temperature was measured with a rectal thermometer; body weight was calculated relative to a baseline measurement taken on day −2; and erythrocyte density was determined on a VetScan HMII haematology system (Abaxis). To measure liver enzymes, plasma was analysed on a VetScan Chemistry Analyzer, using a Mammalian Liver Profile reagent rotor (Abaxis).
Enumeration of blood-stage parasites by Real-Time PCR
Whole blood was isolated 20 hours after liver merozoite egress, when parasites were at the late trophozoite stage of development and within the first cycle of schizogony. Total RNA was extracted by acid guanidinium thiocyanate-phenol-chloroform extraction25, and reverse transcribed by PCR at 42°C using 75U MuLV Reverse Transcriptase and 2.5μM Random Hexamer primers (both Applied Biosystems) per sample. Parasites were quantified by Real-Time PCR, comparing P.c. chabaudi AS 18S rRNA copy number between samples and a standard curve of pE prepared at the late trophozoite stage of development. The reaction mix contained TaqMan Universal PCR Master Mix (Applied Biosystems), 300ηM forward primer (5′-AAGCATTAAATAAAGCGAATACATCCTTAT-3′), 300ηM reverse primer (5′-GGGAGTTTGGTTTTGACGTTTATGCG-3′) and 50ηM probe ([6FAM]CAATTGGTTTACCTTTTGCTCTTT[TAM]). Real-Time PCR amplification was performed on an ABI Prism 7000 Sequence Detection System (Applied Biosystems), with a temperature profile as follows: 50°C for 2 minutes, followed by 95°C for 10 minutes, and then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Parasite numbers were determined per 100μl whole blood; total circulating parasites were then calculated for each mouse based on their weight and, therefore, their estimated circulating blood volume.
Flow cytometry
Single cell suspensions of splenocytes were prepared, erythrocytes lysed, and cells enumerated on a haemocytometer. Cells were stained with monoclonal antibodies (CD3ε Biotin or PerCP-Cy5.5 (145-2C11); CD4 Pacific Blue (RM4-5); CD8α Pacific Blue (53-6.7); CD11b Pacific Blue (M1/70); CD11c APC (N418); CD44 FITC (IM7); I-Ab FITC (AF6-120.1); Ly-6G PE (1A8); NK-1.1 Biotin (PK136); TER-119 Biotin (all from BioLegend)) (CD19 Biotin (1D3); CD62L APC (MEL-14); CD127 PE (A7R34) (all from eBioscience)) (Ly-6C ALEXA FLUOR 647 (ER-MP20) (from AbD Serotec)) or irrelevant isotype-matched monoclonal antibodies as negative controls. PerCP/Cy5.5-conjugated Streptavidin (BD Biosciences) was used secondary to biotinylated antibodies. For phenotypic analysis, samples were acquired on a CyAn (Beckman Coulter), and data were analysed with FlowJo software (TreeStar).
Antibodies and cytokines
Malaria-specific antibodies were measured in plasma by ELISA: 96 well PolySorp plates (Nunc) were coated with 50μg/ml parasite lysate prepared from pE isolated from C57BL/6 mice infected with SBP P.c. chabaudi AS; 2-fold serial dilutions of plasma from uninfected and hyper-immune mice were used as negative and positive controls, respectively, for experimental samples; alkaline phosphatase-conjugated goat anti-mouse IgG, IgG1, IgG2c, IgG2b and IgG3 (all from SouthernBiotech) were used for detection. Samples were developed with 1mg/ml 4-Nitrophenyl phosphate disodium salt hexahydrate (Sigma) and OD was measured at 405ηm. Antibody concentrations are presented as arbitrary units (AU) relative to hyper-immune plasma. Cytokines were measured in plasma by LEGENDplex Luminex custom assay (BioLegend).
Plasmodium RNA preparation
C57BL/6 mice were infected with SBP P.c. chabaudi AS via injection of infected erythrocytes, or via mosquito bite. Parasites were isolated at exactly 20 hours into the seventh cycle of the blood-stage infection, at the late trophozoite stage of development, as follows: whole blood was depleted of leukocytes by Plasmodipur filtration (EuroProxima); erythrocytes were centrifuged at 400xg for 10 minutes and lysed with 0.15% (w/v) saponin (Sigma); samples were centrifuged at 1000×g for 5 minutes and washed with PBS; parasites were resuspended in TRIzol (Life Technologies) and snap-frozen on dry ice. We prepared 3 biological replicates of SBP P.c. chabaudi AS from 8 mice each, and 2 biological replicates of MT P.c. chabaudi AS from 30 mice each. RNA was extracted as described26, resuspended in water and DNA removed with a TURBO DNA-free Kit (Applied Biosystems), according to the manufacturer’s instructions. RNA quantity/quality was determined on an Agilent 2100 Bioanalyzer RNA 6000 Nano chip.
Amplification-free RNA-seq libraries
PolyA+ transcripts were selected from 10μg total RNA using Sera-Mag Oligo(dT)-coated Magnetic Particles (Thermo Scientific). RNA was diluted with water to a volume of 130μl and fragmented to approximately 200 nucleotides using Covaris Adaptive Focused Acoustics technology (settings: 5% Duty Cycle; Intensity 5; 200 Cycles per burst for 60 seconds). The RNA was ethanol precipitated and resuspended in 10μl water. First strand cDNA was synthesised with Random Hexamer primers and SuperScript II Reverse Transcriptase (Life Technologies), following the manufacturer’s instructions. Second strand cDNA synthesis, end repair and dA-tailing were performed using the NEBNext mRNA library kit for Illumina (New England Biolabs), eluting in a final volume of 15μl. Sequencing templates were prepared by mixing 15μl cDNA, 5μl 33μM adaptors (based on the published adaptor27 with the addition of barcode sequences; oligos supplied by Integrated DNA Technologies), 25μl Quick Ligation buffer and 5μl Quick DNA ligase (both from New England Biolabs) and incubating for 15 minutes at 25°C. Excess adaptors were removed with 2 rounds of clean up with 50μl of Agencourt AMPure XP Beads (Beckman Coulter). Final libraries were eluted in 30μl water, visualised on an Agilent Bioanalyzer 2100 High Sensitivity DNA chip and quantified by qPCR. A pool of the 5 indexed libraries was sequenced on an Illumina HiSeq2000, with 100bp paired-end reads.
Analysis of RNA expression
Paired-end RNA sequencing reads were mapped to the P.c. chabaudi AS reference genome (September 2012 release: ftp://ftp.sanger.ac.uk/pub/pathogens/P_chabaudi/September_2012/) using Tophat28 v1.4.1, with appropriate fragment size parameters and maximum intron size 10000. Read counts per gene were calculated using in-house Perl scripts and non-uniquely mapping reads were excluded. Six genes with less than 20% unique coding sequence (kmer = 100) were excluded from the analysis (PCHAS_073210; PCHAS_083750; PCHAS_100020; PCHAS_113280; PCHAS_113290; PCHAS_130130). Differential gene expression between SBP and MT P.c. chabaudi AS was determined using DESeq29; the three SBP P.c. chabaudi AS replicates were compared against the two MT P.c. chabaudi AS replicates to determine genes upregulated in blood-stage parasites following mosquito transmission, and vice versa to determine genes upregulated following serial blood passage. In both cases a corrected p-value cutoff of 0.01 was applied. The resulting gene lists were categorised into ‘cir’ (based on published annotation17), ‘Pc-fam’ (based on GeneDb annotation by Ulrike Boehme at the Wellcome Trust Sanger Institute, with minor reannotation), ‘Exported’ (based on known biology or ExportPred30 prediction), ‘Other known function’ and ‘Unknown function’. For those genes within the category ‘Other known function’, we sub-categorised genes based on enriched biological process GO terms using TopGO31; a p-value cutoff of 0.01 was applied. We independently added ‘glideosome’ as a sub-category.
Supplementary Material
Acknowledgements
This work was supported by the Medical Research Council (U117584248) and the Wellcome Trust (089553 and 098051). P.J.S. is the recipient of a Leverhulme Trust Early Career Fellowship. The authors thank R. Sinden, K. Baker and M. Tunnicliff for provision of Anopheles stephensi. M. Blackman, G. Kassiotis and G. Stockinger are thanked for critical reading of the manuscript.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions | P.J.S., W.J. and J.L. designed the study. P.J.S., W.J., P.L., L.C. and T.B. performed the experiments. P.J.S. and A.J.R. analysed the data. M.S. and M.B. provided project management. P.J.S. wrote the manuscript.
RNA-seq datasets have been deposited in ArrayExpress with accession number E-ERAD-95.
The authors declare no competing financial interests.
References
- 1.Dearsly AL, Sinden RE, Self IA. Sexual development in malarial parasites: gametocyte production, fertility and infectivity to the mosquito vector. Parasitology. 1990;100(Pt 3):359–368. doi: 10.1017/s0031182000078628. [DOI] [PubMed] [Google Scholar]
- 2.Mackinnon MJ, Read AF. Selection for high and low virulence in the malaria parasite Plasmodium chabaudi. Proc Biol Sci. 1999;266:741–748. doi: 10.1098/rspb.1999.0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoeli M, Hargreaves B, Carter R, Walliker D. Sudden increase in virulence in a strain of Plasmodium berghei yoelii. Ann Trop Med Parasitol. 1975;69:173–178. doi: 10.1080/00034983.1975.11686998. [DOI] [PubMed] [Google Scholar]
- 4.Hartley EG. Increased virulence of Plasmodium cynomolgi bastianellii in the rhesus monkey. Trans R Soc Trop Med Hyg. 1969;63:411–412. doi: 10.1016/0035-9203(69)90023-6. [DOI] [PubMed] [Google Scholar]
- 5.Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am J Trop Med Hyg. 1968;17:355–358. doi: 10.4269/ajtmh.1968.17.355. [DOI] [PubMed] [Google Scholar]
- 6.Alger NE, Branton M, Harant J, Silverman PH. Plasmodium berghei NK65 in the inbred A-J mouse: variations in virulence of P. berghei demes. J Protozool. 18:598–601. doi: 10.1111/j.1550-7408.1971.tb03382.x. [DOI] [PubMed] [Google Scholar]
- 7.Knowles G, Walliker D. Variable expression of virulence in the rodent malaria parasite Plasmodium yoelii yoelii. Parasitology. 1980;81:211–219. doi: 10.1017/s0031182000055165. [DOI] [PubMed] [Google Scholar]
- 8.Mackinnon MJ, Bell A, Read AF. The effects of mosquito transmission and population bottlenecking on virulence, multiplication rate and rosetting in rodent malaria. Int J Parasitol. 2005;35:145–153. doi: 10.1016/j.ijpara.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Spence PJ, Jarra W, Lévy P, Nahrendorf W, Langhorne J. Mosquito transmission of the rodent malaria parasite Plasmodium chabaudi. Malar J. 2012;11:407. doi: 10.1186/1475-2875-11-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynn JR, Collins WE, Jeffery GM, Bradley DJ. Infecting dose and severity of falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:281–283. doi: 10.1016/0035-9203(95)90540-5. [DOI] [PubMed] [Google Scholar]
- 11.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 12.Stephens R, Culleton RL, Lamb TJ. The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol. 2012;28:73–82. doi: 10.1016/j.pt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spence PJ, Langhorne J. T cell control of malaria pathogenesis. Curr Opin Immunol. 2012;24:444–448. doi: 10.1016/j.coi.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Del Portillo HA, et al. The role of the spleen in malaria. Cell Microbiol. 2012;14:343–355. doi: 10.1111/j.1462-5822.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 15.Sponaas AM, et al. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J Exp Med. 2006;203:1427–1433. doi: 10.1084/jem.20052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain V, et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar J. 2008;7:83. doi: 10.1186/1475-2875-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawton J, et al. Characterization and gene expression analysis of the cir multi-gene family of Plasmodium chabaudi chabaudi (AS) BMC Genomics. 2012;13:125. doi: 10.1186/1471-2164-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham D, Lawton J, Jarra W, Preiser P, Langhorne J. The pir multigene family of Plasmodium: antigenic variation and beyond. Mol Biochem Parasitol. 2010;170:65–73. doi: 10.1016/j.molbiopara.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Brannan LR, McLean SA, Phillips RS. Antigenic variants of Plasmodium chabaudi chabaudi AS and the effects of mosquito transmission. Parasite Immunol. 1993;15:135–141. doi: 10.1111/j.1365-3024.1993.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 20.McLean SA, Phillips RS, Pearson CD, Walliker D. The effect of mosquito transmission of antigenic variants of Plasmodium chabaudi. Parasitology. 1987;94(Pt 3):443–449. doi: 10.1017/s0031182000055797. [DOI] [PubMed] [Google Scholar]
- 21.Manske M, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters J, et al. High diversity and rapid changeover of expressed var genes during the acute phase of Plasmodium falciparum infections in human volunteers. Proc Natl Acad Sci U S A. 2002;99:10689–10694. doi: 10.1073/pnas.162349899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madsen L, et al. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 26.Kyes S, Pinches R, Newbold C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol Biochem Parasitol. 2000;105:311–315. doi: 10.1016/s0166-6851(99)00193-0. [DOI] [PubMed] [Google Scholar]
- 27.Kozarewa I, et al. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nat Methods. 2009;6:291–295. doi: 10.1038/nmeth.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sargeant TJ, et al. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 2006;7:R12. doi: 10.1186/gb-2006-7-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexa A, Rahnenfuhrer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22:1600–1607. doi: 10.1093/bioinformatics/btl140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.