Summary
Mechanical stimulation affects many biological aspects in living cells through mechanotransduction. In myogenic precursor cells (MPCs), mechanical stimulation activates p38 mitogen-activated protein kinase (MAPK), a key regulator of myogenesis, via activating TNFα-converting enzyme (TACE, also known as ADAM17), to release autocrine TNFα. However, the signaling mechanism of mechanical activation of TACE is unknown. Because TACE possesses the structural features of substrates of the non-receptor tyrosine kinase Src, we tested the hypothesis that Src mediates mechanical activation of TACE in MPCs. We observed that mechanical stretch of C2C12 or primary rat myoblasts rapidly activates Src, which in turn interacts and colocalizes with TACE, resulting in tyrosine phosphorylation and activation of TACE. Particularly, Src activates TACE via the phosphorylation of amino acid residue Tyr702 in the intracellular tail of TACE, resulting in increased TNFα release and p38 activation. Src inhibition or deficiency blocks stretch activation of the TACE–p38-MAPK signaling, resulting in impaired myogenic gene expression. In response to functional overloading, Src and TACE are activated in mouse soleus muscle. Further, overloading-induced myogenesis and regeneration are impaired in the soleus of Src+/− mice. Therefore, Src mediates mechano-activation of TACE and myogenesis.
Key words: TACE, ADAM17, Src, Mechanotransduction, p38 MAPK, Myogenic gene expression, Muscle regeneration
Introduction
Mechanical stimuli applied on living cells induce various cellular responses by triggering mechanotransduction. The major cellular components that mediate mechanotransduction include the integrins, cytoskeleton, G proteins, tyrosine kinases, mitogen-activated protein kinases (MAPKs) and stretch-activated ion channels, which form complex signaling networks (Wang and Thampatty, 2006). Notwithstanding, our understanding of the mechanotransduction that regulates various cellular functions remains very limited.
Mechanical stimulation induces myogenesis by provoking activation, proliferation and differentiation of muscle precursor cells (MPCs), known as satellite cells, to form new muscle fibers (Tidball, 2005; Wozniak et al., 2005). However, the signaling mechanisms through which satellite cells convert mechanical stimulation to biochemical signaling that mediates the myogenic responses are poorly understood. For example, studies in muscle have shown that mechanical stimulation activates p38 MAPK (Boppart et al., 2001; Martineau and Gardiner, 2001), a necessary and sufficient ‘switch’ that turns on myogenic gene expression as a key component of epigenetic regulation of myogenic loci (Guasconi and Puri, 2009; Lluís et al., 2006). Yet, the mechanism through which mechanical stimulation activates p38 MAPK in MPCs is not well understood. To gain insights into the related mechanism of mechanotransduction, we showed previously that, in response to mechanical stimulation, myoblasts release autocrine TNFα, which is crucial to myogenic activation of the MKK6 (also known as MAP2K6) to p38 MAPK pathway and ensuing myogenic differentiation (Zhan et al., 2007). Moreover, we found that TNFα-converting enzyme (TACE, also known as ADAM17), the disintegrin metalloproteinase (Black, 2002) that cleaves plasma membrane-anchored pro-TNFα (26 kDa) to release free TNFα (17 kDa), is rapidly activated by mechanical stimulation and is rate limiting for mechano-activation of p38 MAPK (Zhan et al., 2007). These findings revealed a new signaling paradigm through which myogenic cues are transduced to activate myogenic gene expression via the activation of TACE release of autocrine TNFα. However, the mechanism through which mechanical stimulation activates TACE is unknown.
TACE is constitutively expressed in most tissues including skeletal muscle (Black et al., 1997). The extracellular domains of TACE are involved in interaction with the integrins, substrate recognition and substrate cleavage. The cytoplasmic tail (amino acid residues 695–828) interacts with intracellular signaling molecules that regulate TACE activity. For example, Ser819 is a target of growth-factor-induced phosphorylation via the ERK1/2 MAPK, whereas Ser791 undergoes dephosphorylation in response to growth factor stimulation (Fan et al., 2003). Thr735 is also phosphorylated by ERK (Díaz-Rodríguez et al., 2002; Soond et al., 2005), which might mediate TACE protein trafficking (Soond et al., 2005).
Src, a non-receptor tyrosine kinase that is anchored to the inner face of plasma membrane (Thomas and Brugge, 1997), has been shown to play a role in the transduction of mechanical stimulation into biochemical signaling in a variety of non-skeletal muscle cells (Han et al., 2004; Sai et al., 1999; Wang et al., 2005). In cells that are mechanically stimulated, Src undergoes phosphorylation and dephosphorylation rapidly, which activates Src; activated Src in turn activates its downstream effectors through tyrosine phosphorylation (Sai et al., 1999). It is well established that mechanical activation of Src leads to the activation of kinases that are sensitive to mechanical stimulation, such as p38 MAPK (Aikawa et al., 2002), JNK MAPK (Nadruz et al., 2005), ERK1/2 MAPK (Plotkin et al., 2005) and AKT (Jin et al., 2005), in various types of non-skeletal muscle cells. However, the signaling mechanisms that mediate the activation of these serine/threonine kinases by the tyrosine kinase Src remain largely unknown. Src phosphorylation of its substrates requires an interaction between the Src homology 3 (SH3) domain of Src and the substrate (Alexandropoulos and Baltimore, 1996). Interestingly, TACE possesses the structural features of Src substrates – the intracellular tail of TACE contains a potential SH3-binding motif (P731APQTPGR738), which is adjacent to a putative tyrosine phosphorylation motif (K696KLDKQYESL705) (Moss et al., 1997). These structural features of TACE suggest that it might be a substrate of Src. Therefore, we hypothesized that, in MPCs, Src mediates mechanical activation of TACE through the phosphorylation of Tyr702 within the putative tyrosine phosphorylation motif, which in turn activates p38 MAPK and myogenesis through releasing autocrine TNFα. In the present study, we tested this hypothesis. We now present evidence that Src mediates mechanical activation of myogenesis by activating TACE.
Results
Src mediates mechanical activation of TACE in myoblasts
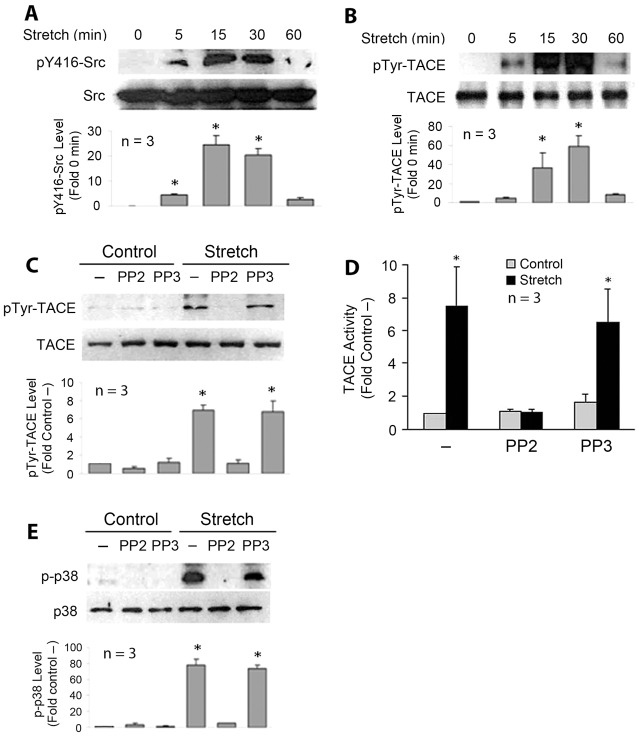
We previously showed that mechanical stretch of myoblasts rapidly activates TACE (Zhan et al., 2007). To assess whether Src mediates TACE activation by mechanical stretch, we first investigated whether there is a causal relationship between Src activity and TACE phosphorylation and activation in mechanically stretched myoblasts. C2C12 myoblasts grown on silicon membrane in serum-rich growth medium were subjected to 10% single-strain global stretch for various time periods. Because Src activation involves phosphorylation at its Tyr416 residue (Thomas and Brugge, 1997), western blot analysis using an antibody specific for Src with phosphorylated Tyr416 was performed to monitor Src activity. A rapid augmentation of activated Src was seen in cell lysates prepared from C2C12 myoblasts that had been stretched for 5 minutes, and the Src activation peaked at around 30 minutes of stretch before rapidly subsiding at 60 minutes (Fig. 1A). To determine the tyrosine phosphorylation state of TACE, TACE was immunoprecipitated from the lysate of stretched C2C12 myoblasts and analyzed by western blotting using an antibody against phosphorylated tyrosine. We observed an increase in tyrosine phosphorylation of TACE at 5 minutes of stretch, and the phosphorylation peaked at around 30 minutes, followed by rapidly subsiding at 60 minutes (Fig. 1B), which highly resembles the time course of the activation of Src. To assess whether stretch-induced TACE phosphorylation and activation are dependent on the activity of Src, myoblasts were pre-treated with the pharmacological inhibitor of Src family kinases (SFK), PP2 (Hanke et al., 1996) or its inactive analog PP3. PP2, but not PP3, blocked stretch-induced tyrosine-phosphorylation of TACE (Fig. 1C). Utilizing an enzymatic assay to monitor the cleavage of a peptide containing the TACE-specific cleavage site in pro-TNFα we observed an increase of 7.5-fold in TACE activity at 30 minutes of stretch, which was blocked by PP2 (Fig. 1D). Consequently, stretch activation of p38 MAPK, which was previously shown dependent on the activation of TACE-mediated TNFα release (Zhan et al., 2007), was abolished by PP2 (Fig. 1E). These results suggest that Src mediates mechanical activation of TACE and its down stream signaling events via tyrosine phosphorylation in myoblasts.
Fig. 1.
Mechanical stretch activates TACE through Src-mediated phosphorylation in C2C12 myoblasts. C2C12 myoblasts were statically stretched for the indicated time period using the Flexcell® FX-5000™ Tension System. Cell lysate was prepared and subjected to western blotting or immunoprecipitation analysis. (A) Mechanical stretch activates Src. Antibodies specific for Src or Src that is phosphorylated at the Tyr416 residue (pY416) were used in western blot analysis to assess Src activity. (B) Mechanical stretch activates tyrosine phosphorylation of TACE. TACE was immunoprecipitated from cell lysate and analyzed for tyrosine phosphorylation by western blot analysis using antibodies against phosphorylated tyrosine (pTyr) or TACE. (C) The SFK inhibitor PP2 blocks mechanical activation of TACE phosphorylation. C2C12 myoblasts were stretched for 30 minutes with or without pretreatment with 10 µM PP2 or PP3. TACE was immunoprecipitated from cell lysate and analyzed by western blotting with antibodies against phosphorylated tyrosine or TACE. (D) PP2 blocks mechanical activation of TACE. C2C12 myoblasts were stretched for 30 minutes with or without pretreatment with 10 µM PP2 or PP3. TACE activity in cell lysates was analyzed by measuring the rate of cleavage of the peptide containing the TACE-specific cleavage site in pro-TNFα. (E) PP2 blocks mechanical activation of myogenic marker p38 MAPK. On the basis of the previously determined time course of stretch-activation of p38 (Zhan et al., 2007), C2C12 myoblasts were stretched for 120 minutes with or without 10 µM PP2 or PP3. Cell lysate was analyzed for p38 activation using western blotting. Western blots were quantified by densitometry. Data (means±s.e.) were analyzed by ANOVA (A,B,C and E) or Student's t-test (D); *P<0.05 compared with the non-stretched control.
Src mediates mechanical activation of TACE by phosphorylation of the Tyr702 residue
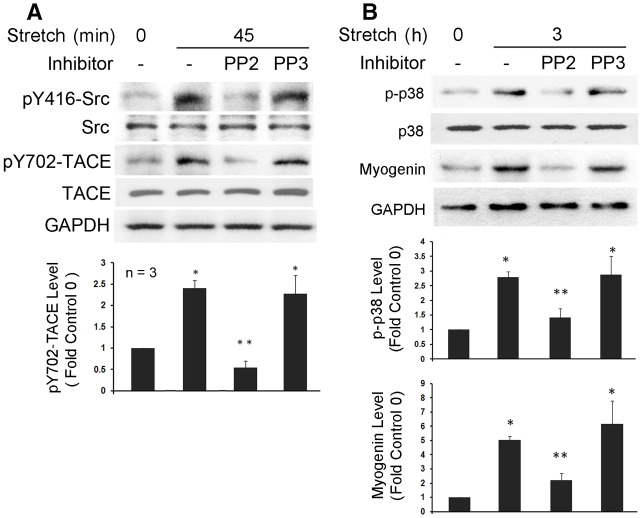
On the basis of the above data, we postulated that Src activates TACE by phosphorylating the potential tyrosine phosphorylation motif (K696KLDKQYESL705) with the tyrosine residue (Tyr702) in its intracellular tail. To assess whether Tyr702 is a functional phosphorylation site targeted by Src, a polyclonal antibody against this motif with phosphorylated Tyr702 was raised. Utilizing this antibody, we attempted to verify whether mechanically activated Src mediates TACE phosphorylation at Tyr702 in primary rat myoblasts. In stretched primary rat myoblasts, we observed rapid activation of Src (Fig. 2A) and TACE phosphorylation at Tyr702 (Fig. 2B), which peaked around 30 minutes, similar to the time course of Src activation and TACE phosphorylation observed above in stretched C2C12 myoblasts. Activation of TACE, as judged by measuring TNFα release into culture medium in an enzyme-linked immunosorbent assay (ELISA) (Fig. 2C), and activation of p38 MAPK, a TNFα-dependent event (Fig. 2D), ensued. By contrast, in the presence of PP2, stretch activation of phosphorylation of TACE on Tyr702 and p38 MAPK were blocked (Fig. 3A), resulting in impaired expression of the myogenic transcription factor myogenin (Fig. 3B). Therefore, Src activation appears crucial to mechanical activation of TACE phosphorylation on Tyr702 and myogenesis.
Fig. 2.
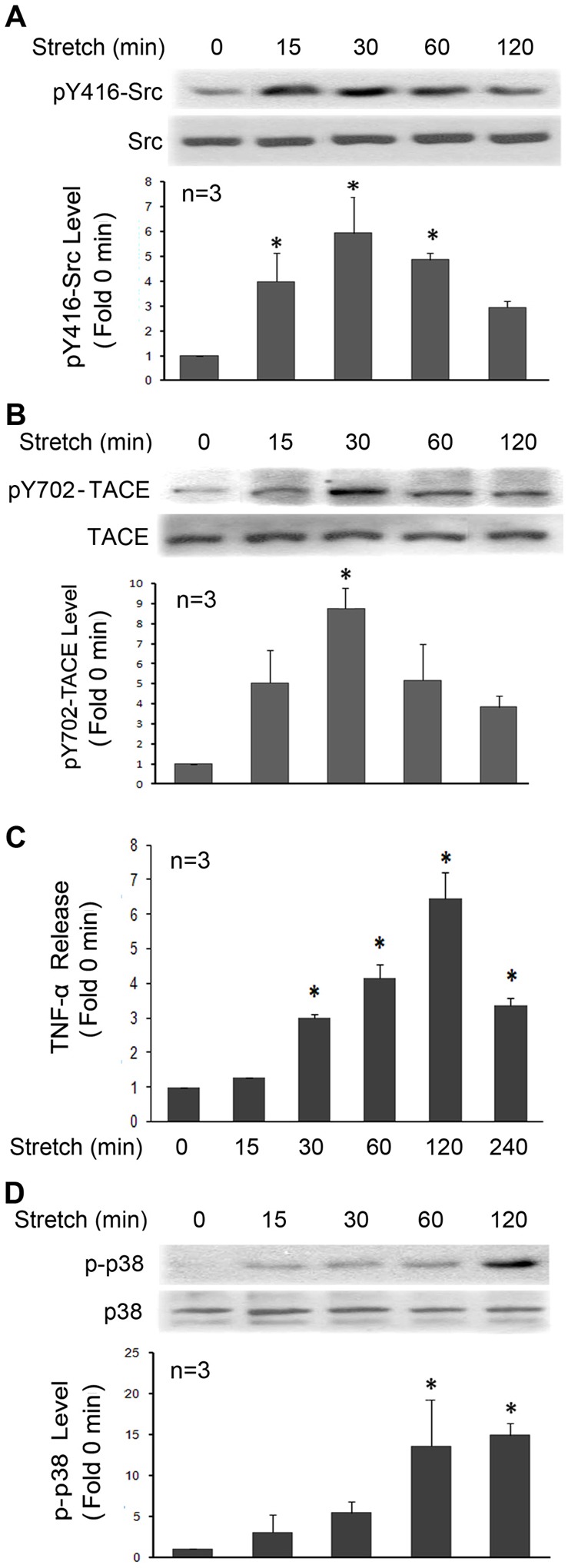
Mechanical stretch activates Src, TACE phosphorylation at Tyr702, TNFα release and p38 MAPK activity in primary rat myoblasts. Primary myoblasts prepared from newborn rats were subjected to static stretch for the indicated time periods. Activation of Src (A), TACE phosphorylation at Tyr702 (B) and p38 MAPK (D) in cell lysate was analyzed by western blotting. TACE activity was measured by determining the concentration of TNFα released into cell culture medium using ELISA (C). Data were analyzed by ANOVA; *P<0.05 compared with the non-stretched control.
Fig. 3.
Mechanical stretch-activated myogenesis in primary rat myoblasts is dependent on Src activation. Primary rat myoblasts were pre-incubated with PP2 or PP3 and stretched for the indicated time periods. Western blotting was performed to analyze Src activation and TACE phosphorylation on Tyr702 (A), or p38 MAPK activation and myogenin expression (B). Optical density data was analyzed by ANOVA. *P<0.05 compared with the non-stretched myoblasts (0 minute); **P<0.05 compared with the stretched myoblasts that were not treated with PP2 or PP3.
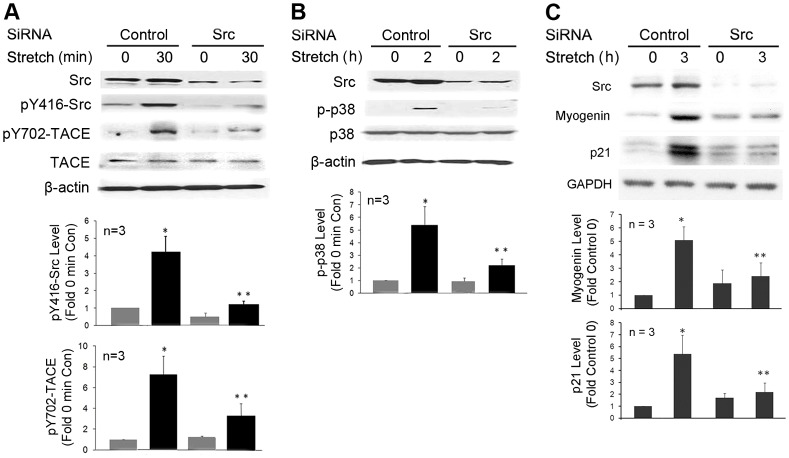
To determine whether phosphorylation of the Tyr702 residue in TACE is crucial for stretch activation of TACE, we transduced C2C12 myoblasts with a recombinant adenovirus encoding a TACE mutant in which Tyr702 had been replaced by alanine (TACE-Y702A). Overexpression of this TACE mutant blocked stretch activation of p38 MAPK (Fig. 4), a TACE-dependent event crucial to myogenesis (Zhan et al., 2007). To verify whether mechanical activation of TACE and its phosphorylation at Tyr702 indeed requires Src, Src expression was knocked down in C2C12 myoblasts by utilizing siRNA. Stretch activation of Src and TACE phosphorylation at Tyr702 were blocked in Src-deficient myoblasts (Fig. 5A). Consequently, stretch activation of p38 was blocked (Fig. 5B), which resulted in impairment of the expression of myogenic markers myogenin and p21 (Fig. 5C). These data suggest that mechanical stretch of MPCs activates TACE and myogenesis through Src-mediated phosphorylation of its Tyr702 residue.
Fig. 4.
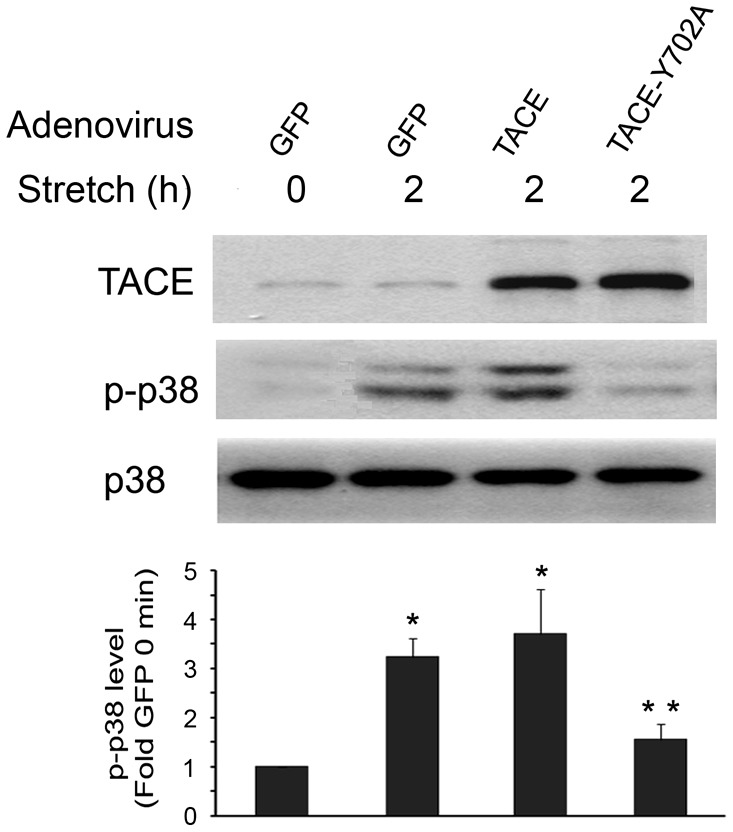
Tyr702 phosphorylation of TACE is crucial to its activation by mechanical stretch. C2C12 myoblasts were transduced with adenovirus encoding GFP, TACE or a TACE mutant in which Tyr702 was replaced with alanine (TACE-Y702A). Myoblasts were stretched for 2 hours before assessment of p38 activation. Optical density data was analyzed by ANOVA, *P<0.05 compared with the non-stretched myoblasts (0 minutes) that were transduced with GFP-encoding adenovirus; **P<0.05 compared with the stretched myoblasts that were transduced with GFP-encoding adenovirus.
Fig. 5.
Src is required for mechanical activation of TACE phosphorylation at Tyr702. C2C12 myoblasts were transfected with Src-specific siRNA or scrambled siRNA as control, and stretched for 30 minutes to assess Src activity and TACE phosphorylation at Tyr702 (A), 2 hours to assess p38 activation (B) or 3 hours to assess myogenin and p21 expression (C) using western blotting. Optical density data were analyzed by ANOVA. *P<0.05 compared with the non-stretched myoblasts (0 minute) that were transfected with control siRNA, **P<0.05 compared with the stretched myoblasts that were transfected with control siRNA.
Src interacts and clusters with TACE upon mechanical activation
TACE is a transmembrane protein and Src is anchored to the inner face of cellular membrane. For Src to phosphorylate TACE at Tyr702, it must interact with the intracellular tail of TACE. To investigate whether stretch activates Src interaction with TACE, TACE was immunoprecipitated from cell lysate of mechanically stretched C2C12 myoblasts, and co-precipitation of Src with TACE was examined by western blotting. As shown in Fig. 6A, co-precipitation of Src with TACE was induced by stretch with a time course similar to that of the stretch activation of Sac and TACE shown above. To verify whether stretch-activated Src colocalizes with stretch-activated TACE, C2C12 myoblasts were subjected to immunofluorescence staining with antibodies against activated Src (phosphorylated at Tyr416) and activated TACE (phosphorylated at Tyr702). We observed that a 30 minute stretch dramatically increased colocalization of activated Src and activated TACE in clusters, while the myoblasts became polarized (Fig. 6B). Therefore, mechanical stimulation of myoblasts induces Src interaction and clustering with TACE, which allows the activation of TACE by Src.
Fig. 6.
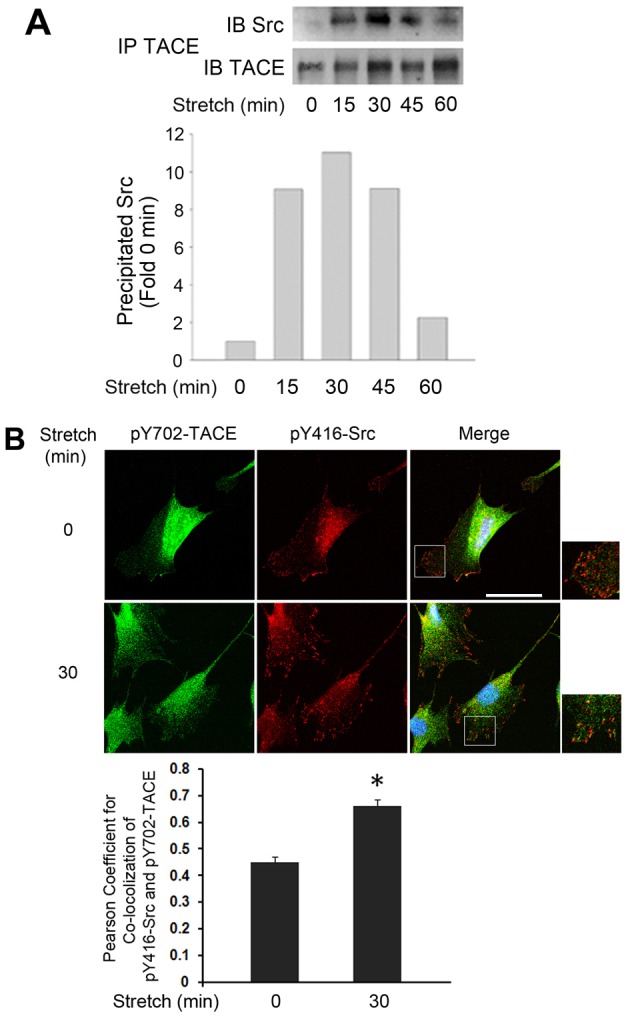
Mechanically activated Src and TACE interact and colocalize in clusters. (A) Mechanical stretch induces interaction between Src and TACE. C2C12 myoblasts were stretched for the indicated time period. Cell lysate was subjected to immunoprecipitation with an antibody against TACE and analyzed by western blotting for Src co-precipitation with TACE. (B) Mechanical stretch induces colocalization of Src and TACE into clusters. C2C12 myoblasts were stretched for 30 minutes and then subjected to immunofluorescence staining with antibodies against pY416-Src (red) and pY702-TACE (green). Scale bar: 20 µm. The Pearson coefficient for the colocalization in control and stretched cells was analyzed by Student's t-test, *P<0.01 compared with the non-stretched control.
Src is crucial to overloading-induced myogenesis and regeneration in mouse soleus
To evaluate the in vivo role of Src in mediating mechanical activation of myogenesis, we used a functional overloading model involving bilateral ablation of gastrocnemius to increase the loading on soleus and plantaris (Flück et al., 1999). This model has been shown previously to induce not only hypertrophy but also regeneration characterized by satellite cell activation (Phelan and Gonyea, 1997; Rosenblatt et al., 1994) and differentiation (Liu et al., 2010; Serrano et al., 2008). As shown in Fig. 7A, overloading increased the level of active Src dramatically, as well as total Src level to a lesser extent, which started from day 1 and peaked around day 5 with a 4-fold increase in the ratio of active Src over total Src. The level of active TACE increased similarly without an increase in total TACE level (Fig. 7B). To evaluate myogenic differentiation in the overloaded soleus, we observed activation of p38 MAPK from day 3 (Fig. 7C) and expression of myogenin from day 5 (Fig. 7D), indicating the activation of myogenesis. To investigate whether the Src activation takes place in satellite cells, cross sections of soleus were stained with immunohistofluorescence using antibodies against active Src (phosphorylated Tyr416) and markers of satellite cells (Pax7 or MyoD). As shown in Fig. 7E, active Src was not detected in quiescent satellite cells (Pax7-positive) from sham-operated mice. However, active Src increased dramatically in activated satellite cells (MyoD-positive) in mice overloaded for 5 days. These data reveal that, in skeletal muscle, mechanical stimulation activates Src in satellite cells, which in turn activates TACE. This signaling process precedes the activation of myogenic differentiation as indicated by myogenin protein expression, suggesting a physiological role of Src in myogenesis.
Fig. 7.
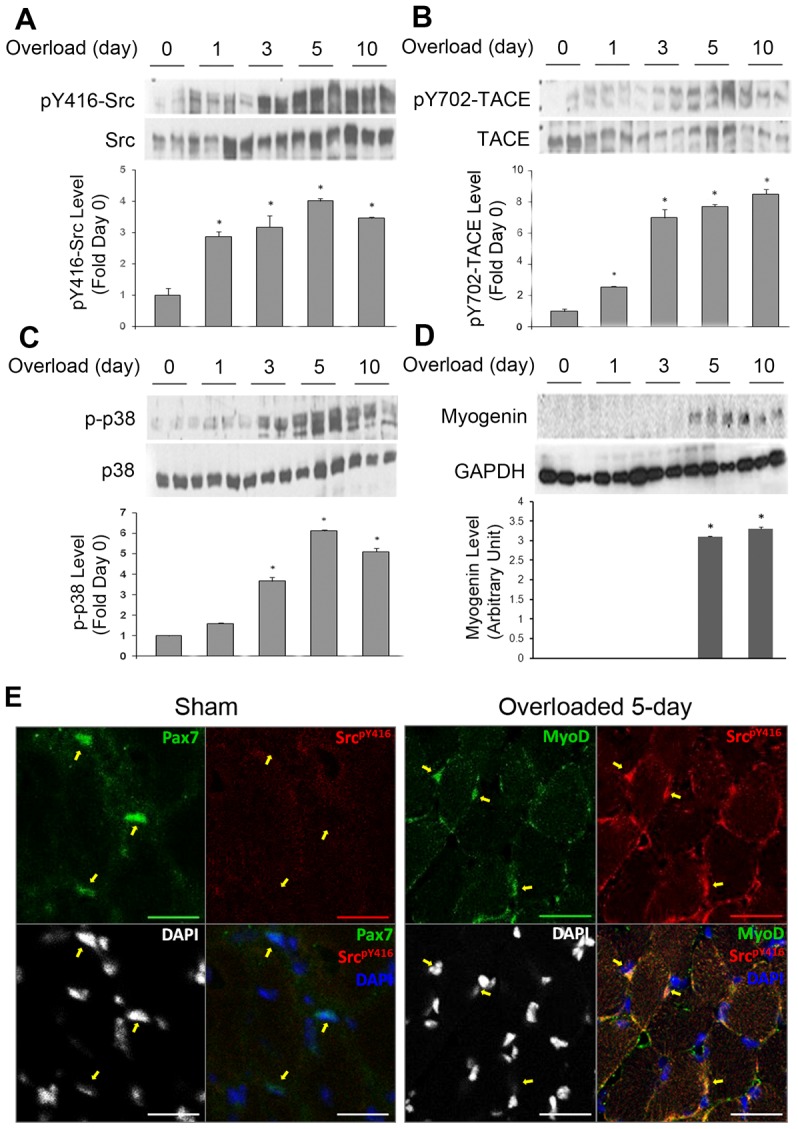
Src and TACE are activated in overloaded mouse soleus. The gastrocnemius of adult male mice (C57BL/6) was severed bilaterally to create functional overloading of soleus and plantaris. Sham-operated mice were used as control (day 0). At the indicated number of days after the surgery, the soleus was collected for western blot analysis of activation of Src (A), TACE (B) and p38 (C), as well as expression of myogenin (D). Each sample shown represents one mouse. Bar graphs summarize optical density data of the activated forms of the proteins normalized against the total protein or GAPDH. Data were analyzed by ANOVA. *P<0.05 compared with control (day 0). For D, the bar graph used arbitrary units owing to the zero expression of myogenin on day 0. (E) Cross sections of soleus prepared from mice that had been sham-operated or overloaded for 5 days were examined by immunohistofluorescence staining to visualize the localization of active Src (Red). Scale bars: 20 µm.
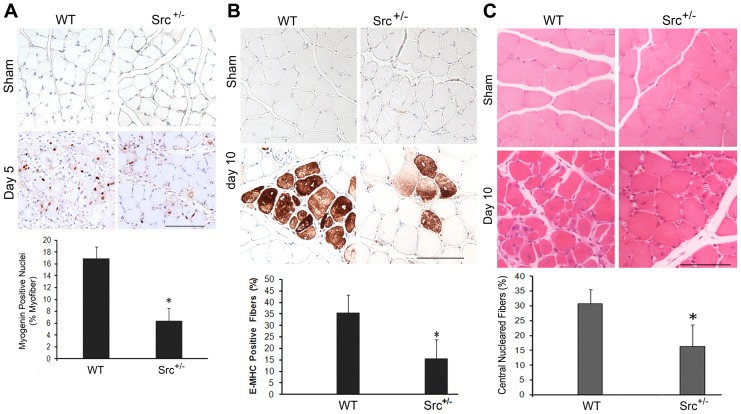
To determine whether Src activation is crucial for overloading-induced myogenesis, we utilized Src+/− mice in the overloading study (Src−/− mice die within a few weeks after birth). Utilizing immunohistochemistry, we observed fewer myogenin-positive nuclei on day 5 (Fig. 8A) and embryonic myosin heavy chain (MHC)-expressing myofibers on day 10 of overloading (Fig. 8B) in the soleus of Src+/− mice, indicating impaired myogenesis owing to Src-deficiency. Finally, H&E-stained soleus sections revealed that on day 10 of overloading, muscle regeneration, as determined by the presence of centralized nuclei, was significantly weaker in Src+/− soleus as compared with that of the wild-type counterpart (Fig. 8C). Therefore, Src is a key mediator of mechanical activation of myogenesis and regeneration in skeletal muscle.
Fig. 8.
Src is essential for overloading induced TACE activation, myogenic differentiation and regeneration in mouse soleus. Adult Src+/− mice and wild-type (WT) littermates were subjected to bilateral ablation of the gastrocnemius. Myogenin expression on day 5 (A) and embryonic MHC (eMHC) expression on day 10 (B) of overloading were compared by immunohistochemistry of cross sections of soleus. The percentage of myofibers that express myogenin or eMHC in overloaded soleus are quantified in the bar graph. Cross sections of soleus that had been overloaded for 10 days were stained by H&E (C). Centralized nuclei were counted as an indication of regeneration. Scale bars: 100 µm. Data were analyzed by Student's t-test, *P<0.05 compared with wild-type soleus.
Discussion
In the present study, we provide the first evidence that Src mediates mechanical activation of myogenesis in MPCs through the activation of TACE-mediated release of autocrine TNFα, which activates p38 MAPK. Even though TACE possesses the structural features of Src substrates, a physical and functional connection between these two regulatory proteins had not been previously examined in any cell type. We show that in mechanically stimulated MPCs, activated Src interacts with TACE and phosphorylates the Tyr702 residue within a typical tyrosine phosphorylation motif in the intracellular tail of TACE, which, in turn, activates TACE to release TNFα. Thereby, Src mediates the rapid activation of p38 MAPK by mechanical stimulation in MPCs. The significant increase in active Src, as well as total Src in overloaded soleus and the dependence of overloading-induced myogenesis and regeneration on Src, confirm that Src is an important signaling molecule in mechanotransduction in skeletal muscle.
The mechanosome concept, developed in studies of bone cells, indicates that Src is a key component of mechanosomes, which convert mechanical stress into biochemical signals (Bidwell and Pavalko, 2010; Rangaswami et al., 2010). Our observation that in mechanically stimulated MPCs, activated Src colocalizes with activated TACE in clusters suggests that TACE is a component of mechanosomes. Because TACE is constitutively expressed in most tissues (Black et al., 1997), Src might also mediate TACE activation in diverse types of load-sensitive cells. TNFα is a pleotropic cytokine involved in inflammation, cell death and cell remodeling in various load-sensitive organs, including bone (Blair et al., 2005), blood vessel (Ungvari et al., 2006) and heart (Mann, 2003). Therefore, the Src–TACE–TNFα–p38-MAPK mechanotransduction pathway shown in the present study might have broad physiological and/or pathological implications in various tissues.
Activation of p38 MAPK in MPCs is a key signal for the activation of myogenic gene expression (Guasconi and Puri, 2009; Lluís et al., 2006). Autocrine TNFα mediates p38 MAPK activation during MPC differentiation induced by diverse stimuli, including serum withdraw or injury (Chen et al., 2005; Chen et al., 2007; Palacios et al., 2010). We previously found that the increase in TACE activity during serum-withdraw-induced differentiation in myoblasts is mediated by a downregulation in the expression of the endogenous TACE inhibitor TIMP3 through an miR-206-mediated process (Liu et al., 2010). Serum-withdraw-induced downregulation of TIMP3 in MPCs takes at least 10 hours (Liu et al., 2010). In contrast, mechanical activation of TACE in MPCs only takes several minutes. Our data presented here indicate that the rapid activation of TACE by mechanical stimulation is mediated by a distinct mechanism involving Src.
Mechano-activation of p38 MAPK is uniquely dependent on Src-mediated TACE activation. Although Src also mediates mechanical activation of JNK MAPK (Nadruz et al., 2005), ERK1/2 MAPK (Plotkin et al., 2005) and AKT (Jin et al., 2005) in various types of load-sensitive cells, we do not expect TACE to be a key mediator of Src activation of these kinases, because we showed previously that unlike stretch activation of p38 MAPK, stretch activation of these kinases are not dependent on autocrine TNFα in MPCs (Zhan et al., 2007).
Overloading of soleus allowed us to examine the in vivo role of Src in myogenesis induced by mechanical stimulation owing to the fact that soleus has a relatively high density of satellite cells and therefore it is more responsive to load increases. We demonstrated the time course of Src and TACE activation, which are consistent with a role for these events in myogenesis. Because Src−/− mice are deficient in bone and tooth development, and die within a few weeks after birth, we used Src+/− mice to evaluate the role of Src in mechanical activation of myogenesis. The reduction of Src expression in Src+/− mice resulted in attenuated myogenesis and regeneration in overloaded Src+/− soleus, which is consistent with our in vitro data and supports a physiological role of Src in mediating mechanical activation of myogenesis.
There have been mixed reports on the role of Src in myogenesis. A physiological role of Src in myogenesis is suggested by a previous report showing that it is activated in differentiating L6 myoblasts, and that its inhibition prevents myogenin expression and myotube formation (Lu et al., 2002). By contrast, overexpression of v-Src, a constitutively active oncogenic mutant of Src, in myoblasts inhibits myoblast differentiation (Falcone et al., 1991; Falcone et al., 2003; Yoon and Boettiger, 1994). The ectopically overexpressed v-Src might not necessarily represent the physiological role of endogenous Src, owing to such factors as location, timing and level of expression. The RNAi and gene knockout technologies used here allowed us to reveal the physiological role of Src in myogenesis. Our in vitro and in vivo data consistently depict a key role of endogenous Src in mediating myogenesis activated by mechanical stimulation. Taken together, our data link Src to TACE in a mechanotransduction scheme that activates myogenesis in skeletal muscle.
Materials and Methods
Culture and stretch of MPCs
Murine C2C12 myoblasts [American Type Culture Collection (ATCC), Rockville, MD] were cultured as previously described (Zhan et al., 2007). Myoblasts were plated at ∼1×105 cells per well and allowed to proliferate to ∼85% confluence in six-well Bioflex® plates (Flexcell International, Hillsborough, NC) that were coated with collagen I. At this point, cells were placed in fresh growth medium for 2 hours before a constant 10% global stretch was initiated using the Flexcell® FX-5000™ Tension System (Flexcell International, Hillsborough, NC). Parallel sets of non-stretched myoblasts were used as controls. Stretch was maintained for a designated period of time under normal cell culture conditions (37°C with 5% CO2 in a humidified incubator). When indicated, the SFK inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-days]pyrimidine (PP2) or its negative control PP3 (Sigma-Aldrich), dissolved in DMSO, was added to the medium (final concentration 10 µM) 30 minutes prior to the stretch and were maintained during the stretch; DMSO (0.1% final concentration) was added to the medium for the control. After the completion of the stretch, myoblasts were collected by scraping into ice-cold PBS. Primary myoblasts were prepared from neonatal rats (2–4 days old) as previously described (Chen et al., 2007).
Animal use
Experimental protocols were approved in advance by the Institutional Animal Welfare Committee (AWC) at University of Texas Health Science Center at Houston. Male mice of C57BL/6 or Src+/− in the C57BL/6 background at 7 to 8 weeks of age (Jackson Laboratory) were use in functional overloading experiment. Functional overloading of mouse soleus muscle was produced by the bilateral surgical ablation of the synergistic gastrocnemius muscle as previously described (Liu et al., 2010). At the indicated time points, mice were killed and soleus was collected for analyses.
Western blot analysis
Muscle homogenate and myoblast lysate were prepared in 4°C RIPA buffer [50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 2 mM sodium fluoride, 2 mM EDTA, and protease/phosphatase inhibitor cocktails (Sigma-Aldrich)]. Muscle homogenate was then sonicated for 5 seconds. Debris was removed by centrifugation at 12,000 g for 10 minutes at 4°C. Protein concentration of the supernatant was determined by using the BioRad protein assay kit. The supernatant was used for western blot analysis or immunoprecipitation. Western blot analysis was performed as previously described (Chen et al., 2005). Antibodies against Src, pY416-Src, p38, and phosphorylated p38 were from Cell Signaling and antibody for TACE was from QED. Antibody against phosphorylated tyrosine was from Zymed Laboratories. Antibody against myogenin (F5D) was purchased from the Developmental Studies Hybridoma Bank at the University of Iowa. Antibody against p21 was obtained from Santa Cruz Biotechnology. Rabbit antibody against pY702-TACE was raised utilizing a peptide of the potential tyrosine phosphorylation site in TACE with phosphorylated Y702 (K696KLDKQYESL705) at New England Peptide Inc.
Transfection of siRNA
The on-target smart pool siRNA specific for Src and control siRNA were purchased from Dharmacon (Denver, CO) and Ambion (Austin, TX), respectively, and were introduced into C2C12 myoblasts by electroporation (5 µg) with the Nucleofector system (Lonza, Walkersville, MD, USA) according to manufacturer's protocol.
Immunoprecipitation
Cell lysate containing 500 µg protein in 0.5 ml RIPA buffer was mixed with 4 µg of an antibody against TACE (QED) and rocked for 3 hours at 4°C. Protein G/A agarose beads (30 µl, Thermo Scientific) were then added to the mixture and rocked overnight at 4°C. Pelleted protein G/A agarose beads were washed three times in RIPA buffer and once in PBS, denatured in Laemmli buffer for 5 minutes at 95°C, and separated by SDS-PAGE. Western blot analysis was performed to evaluate the co-precipitation of TACE and Src.
Determination of TACE activity
TACE activity was assessed by determining either the rate of the cleavage a 12-residue peptide spanning the Ala76 to Val77 residues in pro-TNFα in cell lysate as previously described (Zhan et al., 2007) or by measuring the increase in TNFα concentration in the culture medium by using ELISA as previously described (Chen et al., 2007).
Generation of recombinant adenovirus
To create the recombinant adenovirus encoding the TACE mutant TACE-Y702A and the wild-type TACE, the mutation of Tyr702 to alanine was introduced using the overlap extension PCR method (Ho et al., 1989) using the Tace cDNA in the pCDNA3.1 vector as the template. The sequences of the four primers used are: Primer 1F, 5′-GTGCCGTAC-GTCGATGCAGAGC-3′; Primer 2R, 5′-GAAACAGAGACAGGGATTCAGCCTGCTG-TCCAGT-3′; Primer 3F, 5′-TAAGAAACTGGACAAGCAGGCTGAATCCCTG-3′; and Primer 4R, 5′-CTAGAAGGCACAGTCGAGGCTGATC-3′. The resultant TACE-Y702A or wild-type Tace cDNA was inserted into a shuttle vector pAdTrack-CMV at the XhoI and XbaI sites. The plasmids were linearized at PmeI, and then transformed into BJ5183 E. coli that contains the adenoviral backbone plasmid pAdEasy-1. Selected recombinants were linearized and transfected into HEK293 packaging cells, and purified using the Adeno-X™ virus purification kit from Clontech.
Immunofluorescence
Myoblasts grown in Bioflex® plates were fixed in the plates and subjected to immunofluorescence staining as previously described (Liu et al., 2011) using antibodies against pY416-Src (Cell Signaling) and pY702-TACE. The silicon membrane at the bottom of Bioflex® plates on which cells were attached was then cut out, covered with antifade mounting medium with DAPI (Vector Laboratories) and mounted onto a glass slide. Immunofluorescence labeling of frozen soleus sections was carried out as previously described (Liu et al., 2011). The antibody for Pax7 was from the Development Studies Hybridoma Bank at the University of Iowa, and that for MyoD (5.8A) was from BD Biosciences. Images were acquired using a Nikon A1 confocal microscope (Nikon, Tokyo, Japan). Adjustment of brightness, contrast, color balance and final image size was achieved using Adobe Photoshop CS (Adobe Systems, San Jose, CA, USA). Colocalization of activated Src and TACE was quantified by using the NIS-Elements software (Nikon) in terms of Pearson coefficient.
Immunohistochemistry and histology
Excised soleus was fixed with 3.7% formaldehyde and embedded in paraffin. Cross sections were prepared and subjected to immunochemical staining using the MOM Immunodetection Kit (Vector Laboratories) according to manufacturer's protocol. Antibodies against myogenin (F5D) and embryonic MHC (eMHC, F1.652) were purchased from the Developmental Studies Hybridoma Bank at the University of Iowa. Hematoxylin-and-eosin (H&E)-stained soleus sections were prepared by the Breast Center Pathology Core (Baylor College of Medicine, Houston, TX, USA).
Acknowledgments
The authors declare that they have no conflict of interest.
Footnotes
Author contributions
A.N., Y.W., H.L., M.Z. and B.J. conducted experiments, analyzed data and generated the figures. Y.-P.L. designed experiments and wrote the manuscript.
Funding
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases [grant number R01 AR049022 to Y.-P.L.]. Deposited in PMC for release after 12 months.
References
- Aikawa R., Nagai T., Kudoh S., Zou Y., Tanaka M., Tamura M., Akazawa H., Takano H., Nagai R., Komuro I. (2002). Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension 39, 233–238 10.1161/hy0202.102699 [DOI] [PubMed] [Google Scholar]
- Alexandropoulos K., Baltimore D. (1996). Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 10, 1341–1355 10.1101/gad.10.11.1341 [DOI] [PubMed] [Google Scholar]
- Bidwell J. P., Pavalko F. M. (2010). The load-bearing mechanosome revisited. Clin. Rev. Bone Miner Metab 8, 213–223 10.1007/s12018-010-9075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A. (2002). Tumor necrosis factor-alpha converting enzyme. Int. J. Biochem. Cell Biol. 34, 1–5 10.1016/S1357-2725(01)00097-8 [DOI] [PubMed] [Google Scholar]
- Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S. et al. (1997). A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–733 10.1038/385729a0 [DOI] [PubMed] [Google Scholar]
- Blair H. C., Robinson L. J., Zaidi M. (2005). Osteoclast signalling pathways. Biochem. Biophys. Res. Commun. 328, 728–738 10.1016/j.bbrc.2004.11.077 [DOI] [PubMed] [Google Scholar]
- Boppart M. D., Hirshman M. F., Sakamoto K., Fielding R. A., Goodyear L. J. (2001). Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. Am. J. Physiol. 280, C352–C358 [DOI] [PubMed] [Google Scholar]
- Chen S. E., Gerken E., Zhang Y., Zhan M., Mohan R. K., Li A. S., Reid M. B., Li Y. P. (2005). Role of TNF-alpha signaling in regeneration of cardiotoxin-injured muscle. Am. J. Physiol. 289, C1179–C1187 10.1152/ajpcell.00062.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. E., Jin B., Li Y. P. (2007). TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. 292, C1660–C1671 10.1152/ajpcell.00486.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Rodríguez E., Montero J. C., Esparís-Ogando A., Yuste L., Pandiella A. (2002). Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol. Biol. Cell 13, 2031–2044 10.1091/mbc.01-11-0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone G., Alemà S., Tatò F. (1991). Transcription of muscle-specific genes is repressed by reactivation of pp60v-src in postmitotic quail myotubes. Mol. Cell. Biol. 11, 3331–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone G., Ciuffini L., Gauzzi M. C., Provenzano C., Strano S., Gallo R., Castellani L., Alemà S. (2003). v-Src inhibits myogenic differentiation by interfering with the regulatory network of muscle-specific transcriptional activators at multiple levels. Oncogene 22, 8302–8315 10.1038/sj.onc.1206915 [DOI] [PubMed] [Google Scholar]
- Fan H., Turck C. W., Derynck R. (2003). Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-alpha converting enzyme and of an alternatively translated polypeptide. J. Biol. Chem. 278, 18617–18627 10.1074/jbc.M300331200 [DOI] [PubMed] [Google Scholar]
- Flück M., Carson J. A., Gordon S. E., Ziemiecki A., Booth F. W. (1999). Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am. J. Physiol. 277, C152–C162 [DOI] [PubMed] [Google Scholar]
- Guasconi V., Puri P. L. (2009). Chromatin: the interface between extrinsic cues and the epigenetic regulation of muscle regeneration. Trends Cell Biol. 19, 286–294 10.1016/j.tcb.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Bai X. H., Lodyga M., Xu J., Yang B. B., Keshavjee S., Post M., Liu M. (2004). Conversion of mechanical force into biochemical signaling. J. Biol. Chem. 279, 54793–54801 10.1074/jbc.M406880200 [DOI] [PubMed] [Google Scholar]
- Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. (1996). Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 10.1074/jbc.271.2.695 [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 10.1016/0378-1119(89)90358-2 [DOI] [PubMed] [Google Scholar]
- Jin Z. G., Wong C., Wu J., Berk B. C. (2005). Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J. Biol. Chem. 280, 12305–12309 10.1074/jbc.M500294200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Chen S. E., Jin B., Carson J. A., Niu A., Durham W., Lai J. Y., Li Y. P. (2010). TIMP3: a physiological regulator of adult myogenesis. J. Cell Sci. 123, 2914–2921 10.1242/jcs.057620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Niu A., Chen S. E., Li Y. P. (2011). Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 25, 1914–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluís F., Perdiguero E., Nebreda A. R., Muñoz-Cánoves P. (2006). Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 16, 36–44 10.1016/j.tcb.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Lu H., Shah P., Ennis D., Shinder G., Sap J., Le-Tien H., Fantus I. G. (2002). The differentiation of skeletal muscle cells involves a protein-tyrosine phosphatase-alpha-mediated C-Src signaling pathway. J. Biol. Chem. 277, 46687–46695 10.1074/jbc.M209643200 [DOI] [PubMed] [Google Scholar]
- Mann D. L. (2003). Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu. Rev. Physiol. 65, 81–101 10.1146/annurev.physiol.65.092101.142249 [DOI] [PubMed] [Google Scholar]
- Martineau L. C., Gardiner P. F. (2001). Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J. Appl. Physiol. 91, 693–702 [DOI] [PubMed] [Google Scholar]
- Moss M. L., Jin S. L., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W. J., Clay W. C., Didsbury J. R., Hassler D. et al. (1997). Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385, 733–736 10.1038/385733a0 [DOI] [PubMed] [Google Scholar]
- Nadruz W., Jr, Corat M. A., Marin T. M., Guimarães Pereira G. A., Franchini K. G. (2005). Focal adhesion kinase mediates MEF2 and c-Jun activation by stretch: role in the activation of the cardiac hypertrophic genetic program. Cardiovasc. Res. 68, 87–97 10.1016/j.cardiores.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Palacios D., Mozzetta C., Consalvi S., Caretti G., Saccone V., Proserpio V., Marquez V. E., Valente S., Mai A., Forcales S. V. et al. (2010). TNF/p38α/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 7, 455–469 10.1016/j.stem.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan J. N., Gonyea W. J. (1997). Effect of radiation on satellite cell activity and protein expression in overloaded mammalian skeletal muscle. Anat. Rec. 247, 179–188 [DOI] [PubMed] [Google Scholar]
- Plotkin L. I., Mathov I., Aguirre J. I., Parfitt A. M., Manolagas S. C., Bellido T. (2005). Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. 289, C633–C643 10.1152/ajpcell.00278.2004 [DOI] [PubMed] [Google Scholar]
- Rangaswami H., Schwappacher R., Marathe N., Zhuang S., Casteel D. E., Haas B., Chen Y., Pfeifer A., Kato H., Shattil S. et al. (2010). Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci. Signal. 3, ra91 10.1126/scisignal.2001423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. D., Yong D., Parry D. J. (1994). Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 17, 608–613 10.1002/mus.880170607 [DOI] [PubMed] [Google Scholar]
- Sai X., Naruse K., Sokabe M. (1999). Activation of pp60(src) is critical for stretch-induced orienting response in fibroblasts. J. Cell Sci. 112, 1365–1373 [DOI] [PubMed] [Google Scholar]
- Serrano A. L., Baeza-Raja B., Perdiguero E., Jardí M., Muñoz-Cánoves P. (2008). Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 7, 33–44 10.1016/j.cmet.2007.11.011 [DOI] [PubMed] [Google Scholar]
- Soond S. M., Everson B., Riches D. W., Murphy G. (2005). ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J. Cell Sci. 118, 2371–2380 10.1242/jcs.02357 [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. (1997). Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 10.1146/annurev.cellbio.13.1.513 [DOI] [PubMed] [Google Scholar]
- Tidball J. G. (2005). Mechanical signal transduction in skeletal muscle growth and adaptation. J. Appl. Physiol. 98, 1900–1908 10.1152/japplphysiol.01178.2004 [DOI] [PubMed] [Google Scholar]
- Ungvari Z., Wolin M. S., Csiszar A. (2006). Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxid. Redox Signal. 8, 1121–1129 10.1089/ars.2006.8.1121 [DOI] [PubMed] [Google Scholar]
- Wang J. H., Thampatty B. P. (2006). An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol. 5, 1–16 10.1007/s10237-005-0012-z [DOI] [PubMed] [Google Scholar]
- Wang Y., Botvinick E. L., Zhao Y., Berns M. W., Usami S., Tsien R. Y., Chien S. (2005). Visualizing the mechanical activation of Src. Nature 434, 1040–1045 10.1038/nature03469 [DOI] [PubMed] [Google Scholar]
- Wozniak A. C., Kong J., Bock E., Pilipowicz O., Anderson J. E. (2005). Signaling satellite-cell activation in skeletal muscle: markers, models, stretch, and potential alternate pathways. Muscle Nerve 31, 283–300 10.1002/mus.20263 [DOI] [PubMed] [Google Scholar]
- Yoon H., Boettiger D. (1994). Expression of v-src alters the expression of myogenic regulatory factor genes. Oncogene 9, 801–807 [PubMed] [Google Scholar]
- Zhan M., Jin B., Chen S. E., Reecy J. M., Li Y. P. (2007). TACE release of TNF-alpha mediates mechanotransduction-induced activation of p38 MAPK and myogenesis. J. Cell Sci. 120, 692–701 10.1242/jcs.03372 [DOI] [PMC free article] [PubMed] [Google Scholar]