Summary
Primary cilia are ubiquitous sensory organelles that concentrate transmembrane signaling proteins essential for sensing environmental cues. Mislocalization of crucial ciliary signaling proteins, such as the tetrameric cyclic nucleotide-gated (CNG) channels, can lead to cellular dysfunction and disease. Although several cis- and trans-acting factors required for ciliary protein trafficking and localization have been identified, whether these mechanisms act in a protein- and cell-specific manner is largely unknown. Here, we show that CNG channel subunits can be localized to discrete ciliary compartments in individual sensory neurons in C. elegans, suggesting that channel composition is heterogeneous across the cilium. We demonstrate that ciliary localization of CNG channel subunits is interdependent on different channel subunits in specific cells, and identify sequences required for efficient ciliary targeting and localization of the TAX-2 CNGB and TAX-4 CNGA subunits. Using a candidate gene approach, we show that Inversin, transition zone proteins, intraflagellar transport motors and a MYND-domain protein are required to traffic and/or localize CNG channel subunits in both a cell- and channel subunit-specific manner. We further find that TAX-2 and TAX-4 are relatively immobile in specific sensory cilia subcompartments, suggesting that these proteins undergo minimal turnover in these domains in mature cilia. Our results uncover unexpected diversity in the mechanisms that traffic and localize CNG channel subunits to cilia both within and across cell types, highlighting the essential contribution of this process to cellular functions.
Key words: C. elegans, CNG channels, Cilia
Introduction
Primary cilia are sensory organelles that are present on the surface of most cell types in metazoans. Cilia contain a microtubule-based axoneme surrounded by a specialized membrane that houses transmembrane signaling proteins essential for the ability of cells to sense and respond to their external environment (Drummond, 2012; Green and Mykytyn, 2010; Lancaster and Gleeson, 2009; Singla and Reiter, 2006). In particular, localization of the tetrameric cyclic nucleotide-gated (CNG) cation channels to the ciliary membrane is crucial for the sensory functions of diverse cell types, including photoreceptors and olfactory sensory neurons (Fesenko et al., 1985; Menco, 1997; Nakamura and Gold, 1987). Although several components of the trafficking mechanisms that target CNG channels to sensory cilia have now been identified (Jenkins et al., 2006; Jenkins et al., 2009; Kizhatil et al., 2009), it is unclear whether similar mechanisms operate across all cell types, or whether distinct pathways contribute to the ciliary localization of CNG channels in a cell-specific manner.
CNG channels comprise A and B subunits whose exact stoichiometries are cell-type specific. For instance, whereas cone photoreceptor CNG channels contain two CNGA3 and two CNGB3 subunits, olfactory sensory neuron CNG channels contain two CNGA2, one CNGA4 and one CNGB1b subunit (Kaupp and Seifert, 2002; Pifferi et al., 2006). The subunit stoichiometry regulates multiple aspects of channel function including cNMP specificity and affinity, ion permeation and gating kinetics, and must, therefore, be precisely regulated (Bönigk et al., 1999; Dzeja et al., 1999; Frings et al., 1995; Kaupp and Seifert, 2002). However, although individual sensory neurons can express multiple A or B subunits, whether channel composition in their cilia is homogeneous, or varies as a function of location, has not been explored systematically.
Cilia are also essential for the functions of sensory neurons in the nematode C. elegans, and are formed by remarkably conserved mechanisms (Inglis et al., 2007; Vincensini et al., 2011). As in other organisms, sensory cilia in C. elegans concentrate transmembrane signaling molecules including receptors and channels, and are essential for sensory signal transduction. Eight of the twelve sensory neuron types in the bilateral amphid organs of the head rely on cGMP-mediated signaling for chemo- and thermosensation (Bargmann and Horvitz, 1991a; Bargmann and Horvitz, 1991b; Bargmann and Mori, 1997; Bargmann et al., 1990). These neurons express the tax-4 and tax-2 encoded CNGA and CNGB channel subunits, respectively; loss of function of either gene abolishes their sensory properties (Coburn and Bargmann, 1996; Dusenbery et al., 1975; Komatsu et al., 1996). Both proteins localize to sensory cilia (Coburn and Bargmann, 1996; Komatsu et al., 1996) and are thought to play a role in primary sensory signal transduction. Heterologous expression experiments have shown that the cGMP-gated channel comprises TAX-2 and TAX-4 subunits, although the TAX-4 CNGA subunit can also homotetramerize to form a functional channel (Komatsu et al., 1999; Komatsu et al., 1996). The C. elegans genome is also predicted to encode four less well-characterized members of the CNG channel family (CNG-1–CNG-4) (Cho et al., 2005; Cho et al., 2004; Smith et al., 2013).
Here, we show that CNG channel subunits can be localized to different ciliary subcompartments in C. elegans, indicating that the subunit composition of CNG channels can vary across the cilium in a cell-type-specific manner. We demonstrate that ciliary localization of the CNGA and CNGB channel subunits is interdependent on other CNG subunits in individual sensory neuron types, and describe remarkable cell-type-specific diversity in the mechanisms that traffic and/or localize individual CNG channel subunits to cilia. We find little evidence of CNG channel turnover in specific ciliary subdomains, implying that CNG channels are not trafficked continuously throughout the cilium. Our results describe a striking complexity of CNG channel ciliary targeting and localization mechanisms in C. elegans that could contribute to specialized sensory transduction processes.
Results
Phylogenetic analyses of CNG channel subunit genes in Caenorhabditis nematodes
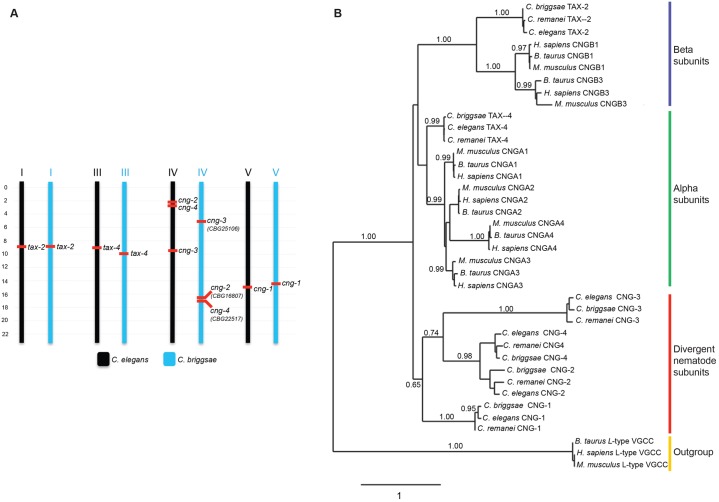
We first performed a detailed sequence and phylogenetic analysis of the predicted C. elegans CNG channel subunit genes, including the CNG-1–CNG-4 subunits. Orthologs of each of the six C. elegans CNG channel genes are encoded by the genomes of the closely related C. briggsae and C. remanei nematodes (Fig. 1). The identities of the orthologs were further confirmed by determining that the chromosomal locations of the orthologous gene pairs are conserved between C. elegans and C. briggsae (Fig. 1A). This is consistent with previous demonstrations of synteny conservation between 1∶1 orthologs with high sequence identity in these two nematode species (Hillier et al., 2007; Vergara and Chen, 2010). Although orthologs of tax-2, tax-4 and cng-1 are also present in other nematode genera, clear orthologs of cng-2, cng-3 and cng-4 appear to be absent in some nematodes such as Pristionchus pacificus and Brugia malayi, suggesting gene loss.
Fig. 1.
Analysis of synteny and phylogeny for CNG channel subunits. (A) Chromosomal location of CNG channel subunit genes in C. elegans and C. briggsae. The C. briggsae orthologs of cng-2-4 were identified by mining the orthology database InParanoid (Ostlund et al., 2010), and the orthology confirmed by reconstructing their phylogenetic relationship. (B) Phylogenetic analysis of CNG channel subunits from C. elegans, C. briggsae, C. remanei, Bos taurus, Mus musculus and Homo sapiens. Inferred phylogeny was constructed using PhyML (Guindon et al., 2009) and derived from amino acid alignments using MUSCLE (Edgar, 2004). The L-type voltage-gated Ca2+ channels were used as an outgroup in the analysis. Reconstruction of Bayesian trees using MrBayes version 3.2 (Ronquist et al., 2012) generated the same tree topology (not shown).
Phylogenetic analyses indicate that the nematode CNG-1–CNG-4 proteins are clearly divergent from previously identified CNGA and CNGB channel subunits (Fig. 1B). We tentatively assigned CNG-1 and CNG-3 to the CNGA, and CNG-2 and CNG-4 to the CNGB subfamilies on the basis of two sequence criteria. First, a C-terminal leucine zipper-like coiled-coil domain is found in mammalian CNGA but not in CNGB subunits and shown to play a role in correct subunit assembly (Shuart et al., 2011; Zhong et al., 2002). Only TAX-4, CNG-1 and CNG-3 are predicted to contain a C-terminal coiled coil domain with high probability (supplementary material Fig. S1). Second, a glutamic acid or aspartic acid residue required to confer ion selectivity is present in the predicted pore domains of CNGA but not CNGB channel subunits (Eismann et al., 1994; Root and MacKinnon, 1993; Seifert et al., 1999); this residue appears to be present only in TAX-4, CNG-1 and CNG-3 (supplementary material Fig. S2). In addition, heterologous expression experiments indicate that CNG-1 and CNG-3 can modulate the properties of TAX-2–TAX-4 heterotetrameric channels (D.M.O'H., X. D. Zhang, N. D. L'Etoile and T.-Y. Chen, unpublished results). These observations indicate that Caenorhabditis nematodes contain multiple conserved and divergent CNG channel subunits that contribute to the modulation and diversification of CNG channel properties.
Subsets of CNG channel subunit genes are co-expressed in sensory neurons
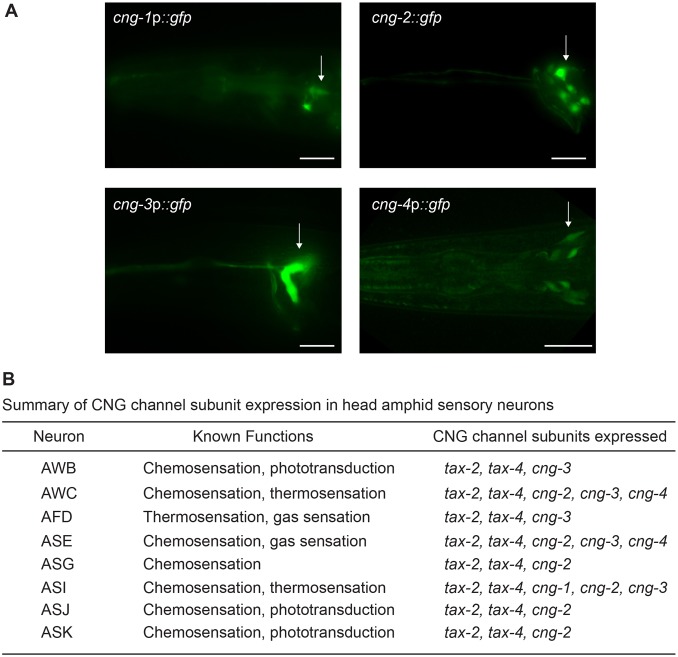
tax-2, tax-4, cng-1, cng-3 and cng-4 are expressed in subsets of sensory neurons in C. elegans (Cho et al., 2005; Cho et al., 2004; Coburn and Bargmann, 1996; Komatsu et al., 1996; Smith et al., 2013) (Fig. 2A). Upstream regulatory sequences of cng-2 also drove gfp expression exclusively in six pairs of head sensory neurons in the adult (Fig. 2A). Expressing cells were identified by cell body position and overlap with cells that take up lipophilic dyes (Herman and Hedgecock, 1990; Perkins et al., 1986). A cng-4p::gfp transcriptional fusion gene was also weakly expressed in neurons located around the posterior pharyngeal bulb (Fig. 2A); two of the expressing neuronal cell types have been identified (Smith et al., 2013). Although expression driven by these transgenes probably does not fully recapitulate their endogenous expression patterns, combined analyses of these expression patterns indicate that CNG channel subunit genes are co-expressed in multiple neuron types, with individual cells expressing several members of the CNGA and CNGB gene subfamilies (Fig. 2B). Thus, CNG channel subunits might multimerize in different configurations to mediate signal transduction in specific neurons.
Fig. 2.
CNG channel subunits are expressed in subsets of ciliated sensory neurons. (A) Expression of cng fusion genes in the heads of adult hermaphrodites. Sensory neuron cell bodies are indicated by an arrow. Extent of cng sequences present in the fusion constructs: cng-1, 1.2 kb upstream regulatory sequences of F14H8.6a; cng-2, 2.1 kb upstream regulatory and first ten exons of Y76B12C.1; cng-3, 764 bp upstream regulatory sequences of F38E11.12; cng-4, 772 bp upstream regulatory sequences of C23H5.7. Note that the expression pattern of cng-1 reported here differs from a previous report (Cho et al., 2005) probably due to differences in the extent of regulatory sequences used to drive gfp expression. cng-4p::gfp expression (shown here as a maximum projection confocal image) was too weak to reliably identify all expressing cells. Anterior is left. Scale bars: 20 µm. (B) Summary of CNG channel subunits known to be expressed in the indicated sensory neurons in the head amphid organ. Four sensory neurons (AWA, ADF, ASH and ADL) do not express any CNG channel subunits.
CNG channel subunits localize to distinct ciliary subdomains in a cell-specific manner
TAX-2 and TAX-4 are localized to sensory cilia in multiple sensory neuron types, and TAX-2 expression has also been observed in sensory neuron axons (Coburn and Bargmann, 1996; Komatsu et al., 1996). We previously showed that TAX-2 is localized to a discrete proximal domain (termed the middle segments) of the AWB sensory cilia (Mukhopadhyay et al., 2008), although localization in other sensory cilia has not been examined. To determine whether other C. elegans CNG channel subunits are also targeted to cilia and are localized to specific ciliary subdomains, we expressed functional GFP-tagged channel subunits in specific cell types (supplementary material Fig. S3). We selected the bilateral AWB and ASK chemosensory neurons in the head amphid organs for these analyses, because each expresses different sets of CNG channels (Fig. 2B), exhibits markedly distinct cilia morphologies (Perkins et al., 1986; Ward et al., 1975), and requires different mechanisms of cilia biogenesis and ciliary protein transport (Kaplan et al., 2012; Mukhopadhyay et al., 2007; Olivier-Mason et al., 2013; Snow et al., 2004). For instance, whereas the heterotrimeric Kinesin-II and homodimeric OSM-3 anterograde motors function redundantly to build the middle segments of both AWB and ASK-like cilia, OSM-3 is required to generate the distal segments of only ASK-like cilia, not AWB cilia (Mukhopadhyay et al., 2007; Snow et al., 2004). In addition, mutations in proteins required for ciliary protein trafficking appear to affect ciliary transmembrane protein localization differently in AWB and ASK, or ASK-like cilia (Kaplan et al., 2012; Olivier-Mason et al., 2013).
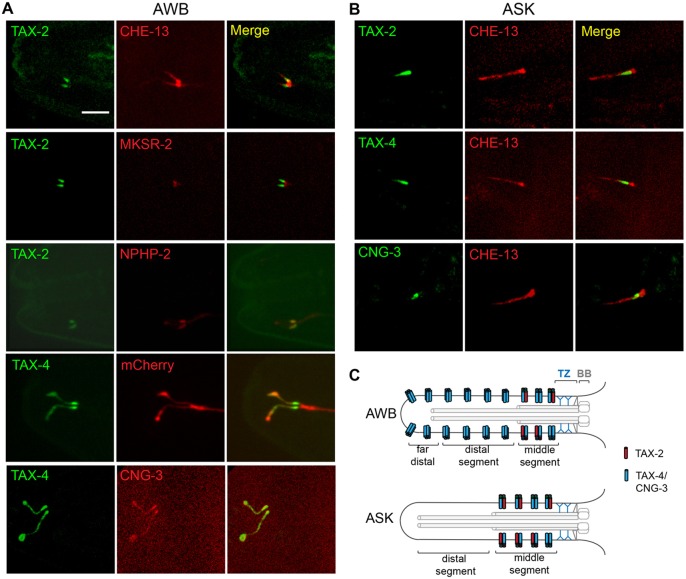
We verified that TAX-2::GFP expressed under an AWB-specific promoter was localized to a proximal region of each of the AWB neuron ciliary branches and excluded from the distal regions (Fig. 3A). The region of TAX-2 localization was distal to the basal body and transition fiber zone marked by CHE-13::TagRFP IFT protein accumulation, as well as the transition zone marked by localization of an MKSR-2::TagRFP fusion protein (Bialas et al., 2009; Reiter et al., 2012; Williams et al., 2011) (Fig. 3A). The region of TAX-2::GFP localization was instead reminiscent of the Inversin compartment observed in several cilia types in vertebrates (Shiba et al., 2009; Zhao and Malicki, 2011). As in mammalian cells, the Inversin homolog NPHP-2 is localized to the middle segment in C. elegans sensory cilia and has a role in placement of the transition zone (Warburton-Pitt et al., 2012). We found that localization of TAX-2::GFP in AWB cilia overlapped almost fully with that of NPHP-2::mCherry (Fig. 3A, also see supplementary material Table S1). We did not observe TAX-2::GFP localization in AWB axons. Similar to TAX-2::GFP, TAX-4::GFP expressed under an AWB-specific promoter was also enriched at the middle segments and largely excluded from the basal body and transition fiber regions; however, this fusion protein was also present throughout the AWB ciliary branches (Fig. 3A). Consistent with classification of CNG-3 as a CNGA subunit, localization of CNG-3::mCherry overlapped with that of TAX-4 in AWB cilia (Fig. 3A). When expressed under an AWB-specific promoter, CNG-1::GFP and CNG-2::GFP were present only in the AWB cell bodies, whereas CNG-4::GFP expression was not detected (data not shown).
Fig. 3.
CNG channel subunits are localized to distinct ciliary subdomains in a cell-specific manner. Colocalization of TAX-2::GFP, TAX-4::GFP and CNG-3::mCherry protein fusions in AWB (A) and TAX-2::GFP, TAX-4::GFP and CNG-3::GFP in ASK (B) cilia of adult hermaphrodites with the indicated fusion proteins marking ciliary subcompartments. Anterior is left. Scale bar: 5 µm. (C) Cartoons summarize localization patterns of examined CNG proteins in AWB and ASK cilia. Depicted channel subunit stoichiometry is hypothetical but based on previous observations showing that TAX-4, but not TAX-2, can form functional homomeric channels, and that TAX-2 and TAX-4 can heterotetramerize (Komatsu et al., 1999; Komatsu et al., 1996). TZ, transition zone; BB, basal body (Reiter et al., 2012). The middle and distal segments are defined as ciliary domains that contain doublet or singlet microtubules, respectively; the far-distal segment in AWB cilia was defined as a region that did not appear to contain an axoneme (Mukhopadhyay et al., 2007; Perkins et al., 1986; Ward et al., 1975).
The localization of a subset of CNG channel subunits was distinct in the ASK cilia. When expressed under an ASK-specific promoter, TAX-2 and TAX-4 fusion proteins both localized to the middle segment, distal to the basal body and transition fiber region (Fig. 3B). CNG-3::GFP exhibited similar localization (Fig. 3B). CNG-1 expression was not detected, and both CNG-2 and CNG-4 fusion proteins were restricted to the ASK cell bodies (data not shown). Together, these observations show that whereas TAX-2 is localized to the middle segments of both AWB and ASK cilia, TAX-4 and CNG-3 are localized to the middle segments of ASK cilia, but are present throughout the branches of AWB cilia (summarized in Fig. 3C).
Ciliary localization of CNG channel subunits is dependent on other CNG subunits in AWB and ASK sensory neurons
In mammals, CNGB1 is required for ciliary targeting of CNGA2 in several examined cell types (Hüttl et al., 2005; Jenkins et al., 2006; Michalakis et al., 2006). Interestingly, although ciliary targeting of CNGA2 also requires CNGA4 in olfactory neurons (Michalakis et al., 2006), CNGA4 is dispensable for this targeting in MDCK cells (Jenkins et al., 2006) suggesting possible cell-specific mechanisms of CNG ciliary targeting. We asked whether CNG channel subunits are interdependent for ciliary targeting in AWB and ASK. We found that ciliary localization of TAX-2 was fully dependent on TAX-4 in both AWB and ASK (Table 1). Thus, in tax-4 mutants, we either did not observe any TAX-2::GFP expression, or observed expression only in the cell body (Table 1). TAX-2::GFP might be degraded owing to mislocalization or failure to associate with TAX-4, as observed previously for other mislocalized CNG channels (Hüttl et al., 2005; Kizhatil et al., 2009; Michalakis et al., 2006). The TAX-2::GFP expression defect in AWB in tax-4 mutants was rescued upon AWB-specific expression of tax-4 (Table 2). Mutations in cng-3 affected TAX-2 ciliary localization only in ASK; this defect was rescued upon expression of cng-3 specifically in ASK (Table 2). By contrast, ciliary localization of TAX-4 was independent of both TAX-2 and CNG-3 in AWB, but dependent on only TAX-2 in ASK (Table 1). Similarly, the effects of mutations in tax-2 and tax-4 on localization of CNG-3 was different in AWB and ASK (Table 1). We conclude that association of different channel subunits in a cell-specific manner is required for their efficient ciliary localization.
Table 1. Ciliary localization of CNG channel subunits is dependent on other subunits in a cell-specific manner.
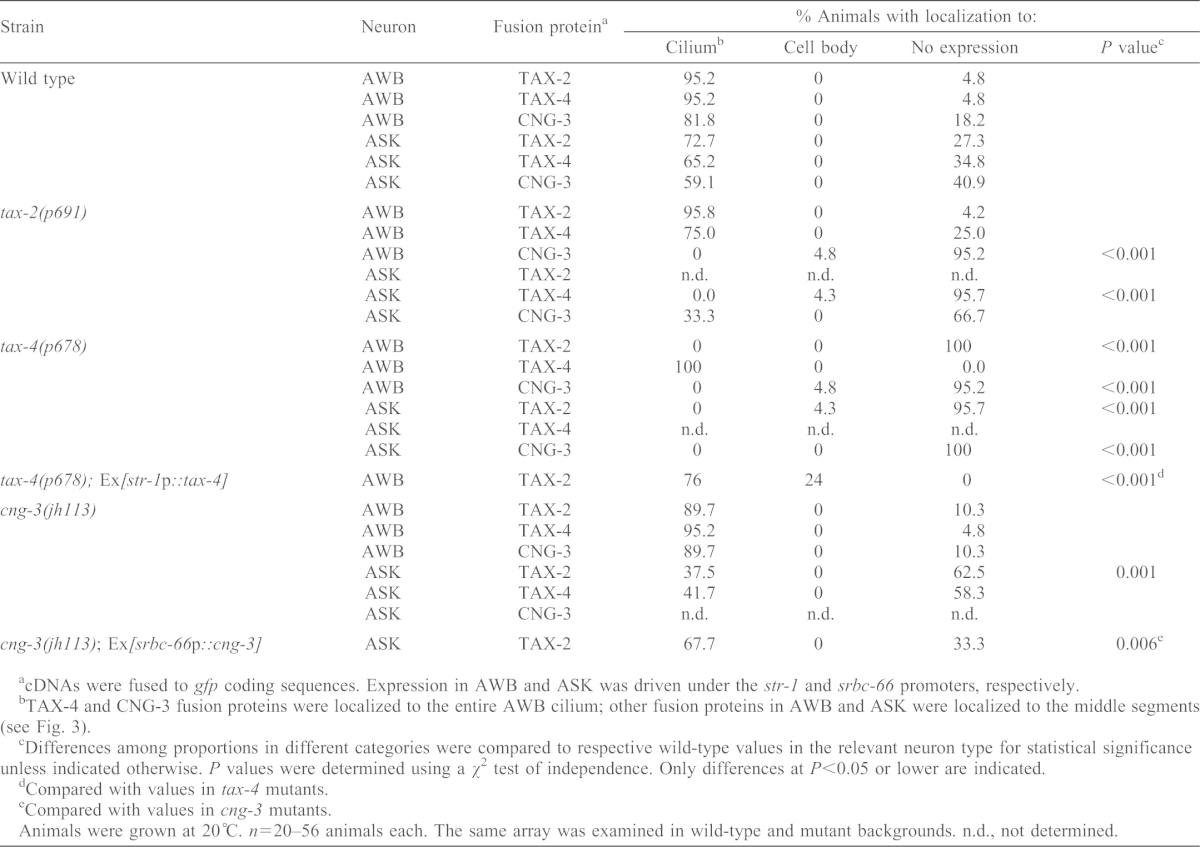
cDNAs were fused to gfp coding sequences. Expression in AWB and ASK was driven under the str-1 and srbc-66 promoters, respectively.
TAX-4 and CNG-3 fusion proteins were localized to the entire AWB cilium; other fusion proteins in AWB and ASK were localized to the middle segments (see Fig. 3).
Differences among proportions in different categories were compared to respective wild-type values in the relevant neuron type for statistical significance unless indicated otherwise. P values were determined using a χ2 test of independence. Only differences at P<0.05 or lower are indicated.
Compared with values in tax-4 mutants.
Compared with values in cng-3 mutants.
Animals were grown at 20°C. n = 20–56 animals each. The same array was examined in wild-type and mutant backgrounds. n.d., not determined.
Table 2. Targeting and localization of TAX-2 and TAX-4 to AWB and ASK cilia require distinct trans-acting factors.
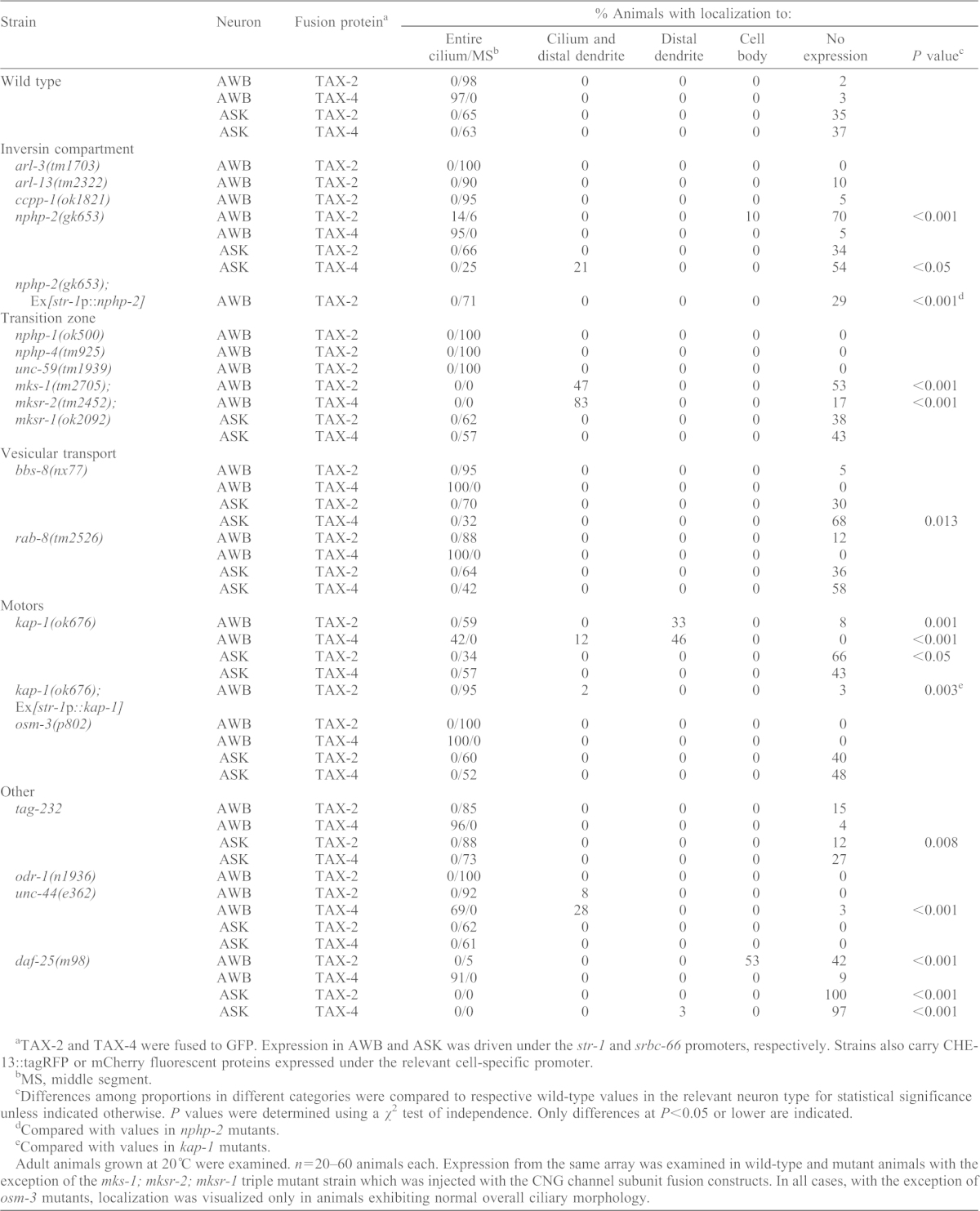
TAX-2 and TAX-4 were fused to GFP. Expression in AWB and ASK was driven under the str-1 and srbc-66 promoters, respectively. Strains also carry CHE-13::tagRFP or mCherry fluorescent proteins expressed under the relevant cell-specific promoter.
MS, middle segment.
Differences among proportions in different categories were compared to respective wild-type values in the relevant neuron type for statistical significance unless indicated otherwise. P values were determined using a χ2 test of independence. Only differences at P<0.05 or lower are indicated.
Compared with values in nphp-2 mutants.
Compared with values in kap-1 mutants.
Adult animals grown at 20°C were examined. n = 20–60 animals each. Expression from the same array was examined in wild-type and mutant animals with the exception of the mks-1; mksr-2; mksr-1 triple mutant strain which was injected with the CNG channel subunit fusion constructs. In all cases, with the exception of osm-3 mutants, localization was visualized only in animals exhibiting normal overall ciliary morphology.
Ciliary localization of CNG channel proteins requires trans-acting mechanisms that function in a neuron- and subunit-specific manner
In addition to requiring specific CNG channel subunits, CNG channel trafficking to cilia has also been shown to depend on the KIF17 homodimeric kinesin-2 motor, the PACS-1 intracellular sorting protein and the Ankyrin-G membrane adaptor (Jenkins et al., 2006; Jenkins et al., 2009; Kizhatil et al., 2009). However, whether these represent general or cell-specific mechanisms of ciliary protein targeting is unclear. To identify molecules that play a role in ciliary targeting and localization of CNG channels in C. elegans, we analyzed CNG channel localization in animals mutant for genes implicated in multiple ciliary protein transport pathways (Emmer et al., 2010; Lim et al., 2011; Nachury et al., 2010; Pazour and Bloodgood, 2008). In particular, we focused on the possible mechanisms restricting localization of TAX-2, but not TAX-4, to the middle segments of AWB cilia.
Inversin compartment components
In addition to being marked by the presence of NPHP-2/Inversin (Warburton-Pitt et al., 2012), the middle segments of cilia in C. elegans are also enriched for the ADP ribosylation factor small GTPase ARL-13. ARL-13 has a role in ciliary transmembrane protein targeting and localization, and in the regulation of IFT (Cevik et al., 2010; Li et al., 2010; Li et al., 2012). This domain is also enriched for polyglutamylated tubulin; alterations of levels of this post-translational modification, as in ccpp-1 deglutamylase mutants, result in altered ciliary protein transport and localization, and progressive ciliary degeneration defects (O'Hagan et al., 2011). We asked whether proteins localized to the middle segments play a role in targeting or localization of CNG channels.
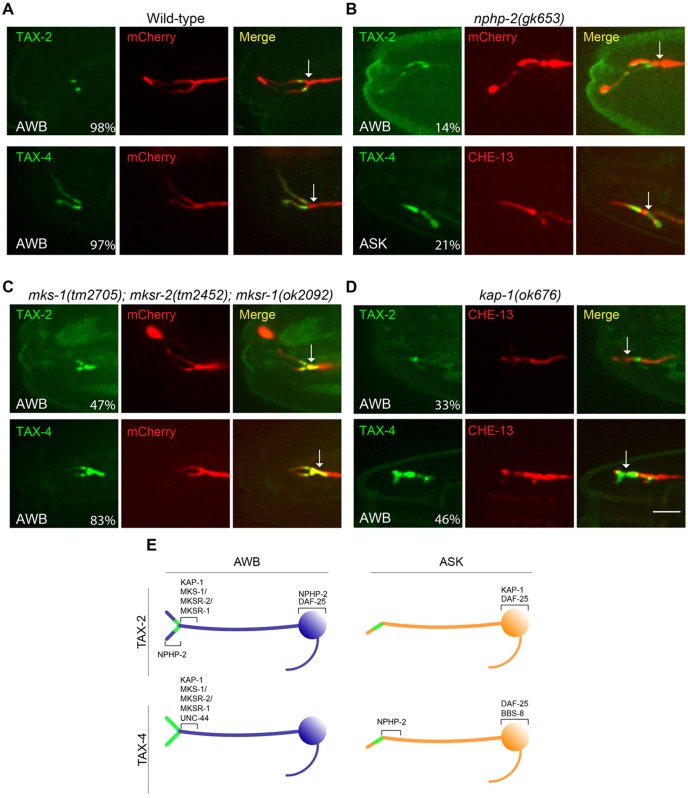
No effects were observed on TAX-2 localization in AWB cilia in arl-13, the related small GTPase arl-3 (Li et al., 2010) or ccpp-1 mutants (Table 2). However, we found that the effects of NPHP-2/Inversin were both CNG channel subunit and cell-type specific. ∼70% of nphp-2 mutants did not exhibit any TAX-2::GFP expression in AWB, suggesting that the protein is mislocalized and degraded (Table 2). However, in animals in which expression could be observed, TAX-2::GFP was mislocalized throughout the cilium in a small number of animals (compare Fig. 4A and 4B; Table 2), suggesting that NPHP-2 also plays a role in restricting TAX-2::GFP to the AWB ciliary middle segment. TAX-2::GFP ciliary localization defects were rescued upon AWB-specific expression of nphp-2 (Table 2). No effects were observed on TAX-4::GFP localization in AWB (Table 2). In ASK, TAX-4::GFP, but not TAX-2::GFP, accumulated at the distal dendritic regions (Fig. 4B; Table 2). Interestingly, TAX-4 continued to be excluded from the basal body and transition fiber region of ASK cilia in these animals (Fig. 4B).
Fig. 4.
Different trans-acting factors are required for ciliary targeting and localization of TAX-2 and TAX-4 in AWB and ASK. (A–D) Localization of TAX-2 and TAX-4 fusion proteins in AWB and ASK cilia in the indicated genetic backgrounds. Fusion proteins or mCherry alone were expressed under the str-1 or srbc-66 promoters in AWB and ASK, respectively. Note that in D, protein localization was examined only in animals exhibiting normal AWB ciliary morphology. Numbers indicate the percentage of animals exhibiting the shown phenotype (see Table 2). Scale bar: 5 µm. Arrows indicate base of cilium in all images. (E) Summary of hypothesized requirement of proteins in trafficking and localization of TAX-2 and TAX-4 fusion proteins to the cilia of AWB and ASK. Proteins depicted as acting in the cell body might play a role in sorting or trafficking; proteins at the ciliary base might regulate trafficking into or out of the cilium; proteins shown as acting within the cilium might modulate anchoring or trafficking within the cilium.
NPHP-2/Inversin contains an ankyrin repeat domain implicated in protein–protein interactions (Li et al., 2006; Mosavi et al., 2004; Warburton-Pitt et al., 2012). The ankyrin repeat domain of Inversin has been shown to be sufficient for ciliary localization of Inversin in nodal but not kidney cilia in mice, and rescues the left–right asymmetry defects of Inversin mutants (Watanabe et al., 2003). We asked whether the ankyrin repeats of NPHP-2 (NPHP-2-AKR) are sufficient to target and/or restrict TAX-2 localization to the AWB ciliary middle segments. We found that a NPHP-2-AKR::mCherry fusion protein was localized to AWB cilia, but unlike full-length NPHP-2 protein, was present throughout the ciliary branches (supplementary material Table S1). However, expression of this domain did not restore TAX-2 localization to the AWB cilia in nphp-2 mutants (supplementary material Table S1), suggesting that the ankyrin repeats of NPHP-2 are not sufficient to target TAX-2 to cilia.
Transition zone components
Because NPHP-2 plays a role in regulating correct transition zone placement (Warburton-Pitt et al., 2012), and mutations in transition zone proteins have been shown to result in defects in ciliary protein sorting and composition (Chih et al., 2012; Craige et al., 2010; Czarnecki and Shah, 2012; McEwen et al., 2007; Shiba and Yokoyama, 2012; Williams et al., 2011), we next determined whether mutations in transition zone proteins alter localization of CNG channel subunits. Proteins in the transition zone region include septin, as well as a large complex of proteins such as NPHP and MKS, which are implicated in nephronophthisis and Meckel–Gruber syndromes; many of these proteins interact with each other genetically and physically, and comprise a ciliary diffusion barrier or ciliary gate (Bialas et al., 2009; Chih et al., 2012; Czarnecki and Shah, 2012; Hu et al., 2010; Hu and Nelson, 2011; Jauregui and Barr, 2005; Sang et al., 2011; Shiba and Yokoyama, 2012; Williams et al., 2011). We saw no effects on TAX-2 localization in nphp-1, nphp-4 or unc-59 septin single mutants in AWB cilia (Table 2). However, both TAX-2 and TAX-4 were mislocalized in mks-1; mksr-2; mksr-1 triple mutants (Bialas et al., 2009) in AWB such that both fusion proteins accumulated at the ciliary base or distal dendritic regions in a subset of examined AWB cilia (Fig. 4C; Table 2). Surprisingly, TAX-2 and TAX-4 localization was unaltered in ASK cilia in mks-1; mksr-2; mksr-1 animals (Table 2). Consistent with previous observations that MKS-1, MKSR-1 and MKSR-2 are not essential for cilia formation, AWB or ASK cilia morphologies were not grossly altered in mks-1; mksr-2; mksr-1 triple mutants (Fig. 4C and not shown).
Vesicular transport pathways
To determine whether proteins involved in exocytic vesicular transport of ciliary proteins are required for CNG channel localization, we examined animals mutant for the BBSome complex component bbs-8 and the rab-8 small GTPase. Although mutations in rab-8 did not affect CNG channel subunit localization in either AWB or ASK, a large fraction of bbs-8 mutant animals failed to exhibit TAX-4::GFP expression in ASK (Table 2).
Molecular motors
Ciliary targeting of olfactory CNG channels requires active transport through the KIF17/OSM-3 homodimeric kinesin-2 motor (Jenkins et al., 2006). As mentioned above, both heterotrimeric Kinesin-II and OSM-3 anterograde motors are required for ciliogenesis of AWB and ASK, albeit by distinct mechanisms (Mukhopadhyay et al., 2007; Snow et al., 2004). We asked whether motor-driven transport is required to target CNG channels to AWB or ASK cilia. Mutations in the Kinesin-II motor accessory subunit kap-1 resulted in altered localization of both TAX-2 and TAX-4 fusion proteins in AWB cilia in ∼30–45% of examined animals (Fig. 4D; Table 2). In these animals, both TAX-2 and TAX-4 fusion proteins accumulated at the distal dendritic regions, with little or no localization in the AWB ciliary axonemes, despite the presence of normal ciliary morphology (Fig. 4D; Table 2). The localization defect of TAX-2 in AWB was rescued upon cell-specific expression of kap-1 (Table 2). However, in ASK, although a large fraction of animals failed to express TAX-2::GFP, both fusion proteins were localized normally in expressing animals (Table 2). We did not observe effects of osm-3 mutations on CNG channel localization in either AWB or ASK cilia (Table 2), although ASK cilia were truncated, as noted previously (Perkins et al., 1986).
Other candidate proteins
We also examined animals mutant for genes previously implicated in targeting of CNG channel subunits in other organisms. Ciliary targeting of CNGB1b requires interaction with PACS-1, and CK2-mediated phosphorylation of both PACS-1 and CNGB1b (Jenkins et al., 2009). However, localization of TAX-2 and TAX-4 was largely unaffected in tag-232 PACS-1 mutants in AWB or ASK cilia (Table 2). We could not easily examine kin-3/kin-10 CK2 mutants because mutations in these genes result in lethality (Hu et al., 2006). Ankyrin-G is required to traffic the CNGB1 subunit to photoreceptor cilia in mammals (Kizhatil et al., 2009). We observed mislocalization of TAX-4::GFP to the distal dendrites of AWB neurons in animals mutant for unc-44, the sole ankyrin gene encoded by the C. elegans genome (Hedgecock et al., 1985; Otsuka et al., 1995) (Table 2). No effects were observed on TAX-2 localization in AWB or ASK, or TAX-4 localization in ASK in unc-44 mutants (Table 2). We also asked whether sensory activity is required for correct CNG channel trafficking and localization. However, mutations in the odr-1 receptor guanylyl cyclase, which is required for AWB sensory functions (L'Etoile and Bargmann, 2000), did not affect TAX-2 localization in AWB (Table 2).
Finally, we examined a role for the DAF-25/Ankmy2 MYND domain protein in CNG channel localization. DAF-25 was recently shown to be required to target receptor guanylyl cyclases to sensory cilia in C. elegans (Fujiwara et al., 2010; Jensen et al., 2010), suggesting a possible role in localizing cGMP signaling cascade components. We found that in AWB, TAX-4::GFP localization was unaffected, whereas TAX-2::GFP expression was either lost or restricted to the cell bodies (Table 2). Expression of both CNG channel subunits was nearly fully abolished in ASK in daf-25 mutants (Table 2). Although the effects of loss of daf-25 and nphp-2 functions on CNG channel subunit localization are not identical, we investigated whether DAF-25 mediates TAX-2 ciliary localization in AWB in part by regulation of localization of NPHP-2. Indeed, we found that >70% of examined daf-25 mutants failed to express NPHP-2 in AWB (supplementary material Table S1), suggesting that DAF-25 regulates ciliary localization of TAX-2 in part by regulation of localization or trafficking of NPHP-2 in AWB. Thus, DAF-25 appears to play a crucial role in trafficking and/or sorting of multiple, but not all, proteins implicated in cGMP signaling in a cell-specific manner. Together, these results indicate that CNG channels are targeted and localized to AWB and ASK cilia through diverse cell- and protein-specific mechanisms (summarized in Fig. 4E).
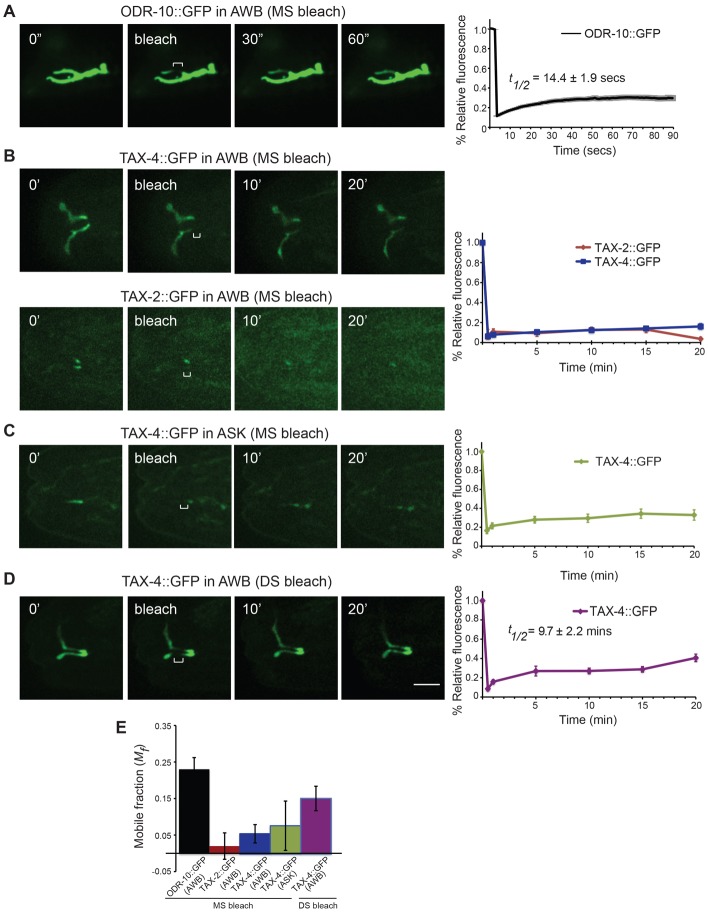
TAX-2 and TAX-4 fusion proteins are immobile in ASK and AWB cilia middle segments
Ciliary transmembrane proteins, including mammalian CNG channels, are mobile within the ciliary membrane, although diffusion into the cilium proper is restricted by the ciliary diffusion barrier (Chih et al., 2012; Hu et al., 2010; Jenkins et al., 2006). We asked whether TAX-2 and TAX-4 fusion proteins are also mobile within the ASK and AWB cilium by quantifying fluorescence recovery after photobleaching (FRAP).
We first photobleached the middle segments of AWB cilia expressing TAX-2::GFP, TAX-4::GFP or the ODR-10::GFP GPCR fusion proteins. Although we observed partial recovery of ODR-10::GFP with a t1/2 of ∼14.4±1.9 seconds (Fig. 5A,E), we observed little to no fluorescence recovery of TAX-2::GFP or TAX-4::GFP even after 20 minutes (Fig. 5B,E). In particular, no fluorescence recovery was observed when one ciliary branch expressing TAX-2::GFP was photobleached while sparing the other branch (Fig. 5B), indicating that TAX-2::GFP does not exchange between the two branches. We also observed no significant recovery of TAX-4::GFP fluorescence upon photobleaching an area in the middle segment of ASK cilia (Fig. 5C,E). Photobleaching the distal segment of AWB cilia expressing TAX-4::GFP resulted in partial recovery of fluorescence (Fig. 5D,E; t1/2 = 9.7±2.2 minutes). The timescale, although not the extent, of TAX-4::GFP fluorescence recovery in the AWB cilia distal segment is similar to that observed upon photobleaching fluorescent reporter-tagged CNGA2 subunits in the more distal regions of primary cilia in MDCK cells; ∼75% of CNGA2 subunits were shown to be mobile in these cilia with a recovery time constant of ∼10 minutes (Jenkins et al., 2006). These results suggest that TAX-4 and TAX-2 are relatively immobile within AWB or ASK cilia middle segments.
Fig. 5.
TAX-2::GFP and TAX-4::GFP are relatively immobile in AWB and ASK cilia middle segments. (A–D) Still images of AWB or ASK cilia expressing the indicated fusion proteins pre-bleach, at bleach and at different times post-bleach. The percentage recovery of fluorescence is shown on the right. Bleached regions are indicated by brackets. MS, middle segments; DS, distal segments. (E) The mobile fraction of the indicated fusion proteins in AWB or ASK cilia. n = 10 cilia for each. Scale bar: 5 µm.
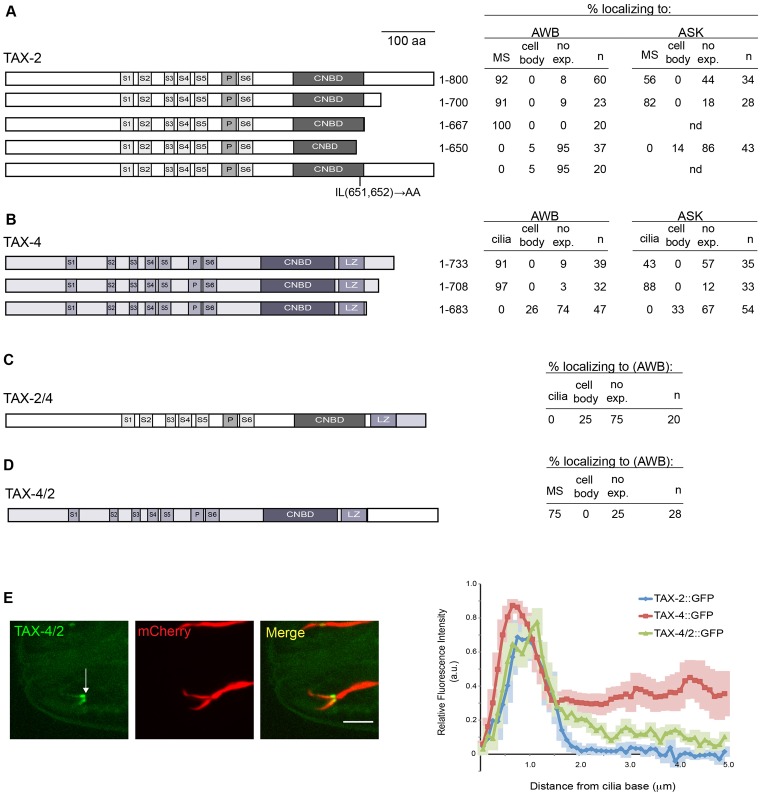
C-terminal sequences in TAX-2 and TAX-4 are necessary and partly sufficient for ciliary subdomain localization
Sequences in the CNGB1 subunit required for ciliary targeting have been identified. These include a C-terminal RVxP motif (Jenkins et al., 2006), a seven amino acid sequence in the C-terminus that is required for interaction with Ankyrin-G (Kizhatil et al., 2009), and N-terminal sequences that are phosphorylated by CK2 and are required for interaction with the PACS-1 protein (Jenkins et al., 2009). We identified an RVRP motif in the C-terminus of TAX-4 (not shown), but did not detect other known CNG channel ciliary targeting sequences in either TAX-2 or TAX-4, suggesting that these proteins are targeted to cilia through alternative cis-acting sequences.
To identify residues required for ciliary localization, we generated GFP-tagged fusion TAX-2 and TAX-4 fusion constructs lacking defined amino acid sequences and examined expression of GFP in AWB and ASK. We found that a truncated TAX-2 protein lacking the C-terminal 100 amino acids [TAX-2(1–700)] continued to be localized to the AWB and ASK middle segments similar to full-length TAX-2 (Fig. 6A). Further deletion of all C-terminal residues up to but not including the cNMP binding domain (CNBD) [TAX-2(1–667)] also remained localized to the AWB cilia middle segments (Fig. 6A). However, truncation of an additional 17 amino acids [TAX-2(1–650)] including 14 amino acids comprising the highly conserved cNMP binding domain resulted in loss of expression with a small fraction of animals exhibiting weak expression only in the cell bodies (Fig. 6A). Substitution of the conserved isoleucine and leucine (651,652) residues within the required sequences in the CNBD with alanines in the context of the full-length TAX-2 fusion protein also resulted in loss of expression (Fig. 6A). Taken together, these results suggest that the C-terminal domain of TAX-2 is dispensable for ciliary localization but that residues in the cNMP binding domain might mediate TAX-2 membrane targeting, ciliary trafficking or protein stability either directly or indirectly through association with TAX-4.
Fig. 6.
Sequences in TAX-2 and TAX-4 required for ciliary localization. (A,B) Expression of GFP-tagged full-length or truncated TAX-2 (A) or TAX-4 (B) proteins in AWB and ASK. Proteins were expressed in AWB and ASK under the str-1 or srbc-66 promoters, respectively. S1–S6, transmembrane regions; P, P loop; CNBD, cNMP binding domain; LZ, leucine zipper; no exp., no expression; MS, middle segments. Lack of expression of mutated proteins could result from altered protein trafficking, stability or membrane targeting. (C,D) Expression of indicated chimeric fusion proteins in AWB. (E) Localization pattern of TAX-4/2::GFP in AWB cilia. The TAX-4/2::GFP fusion protein and mCherry were driven under the str-1 promoter. Quantification of average relative fluorescence intensities of full-length TAX-2::GFP, full-length TAX-4:GFP and TAX-4/2::GFP across AWB cilia are shown on the right. Arrow indicates point of initiation of fluorescence intensity measurements. Distribution of TAX-4/2 is different from that of TAX-4 at P<0.001, but statistically indistinguishable from that of TAX-2 (ANOVA and post-hoc correction for multiple comparisons). n = 10 cilia each. Scale bar: 5 µm.
In the case of TAX-4, we found that truncation of the C-terminal 50 amino acids [TAX-4(1–708)] including the RVRP motif had little or no effect on ciliary localization of the GFP-tagged protein in either AWB or ASK (Fig. 6B). However, deletion of an additional 25 amino acids [TAX-4(1–683)] resulted in loss of GFP expression in both AWB and ASK with only ∼25–30% of examined animals expressing GFP in cell bodies (Fig. 6B). Thus, C-terminal residues in TAX-4 might be important for correct ciliary trafficking and/or protein stability.
We next asked whether the C-terminal sequences of TAX-2 and TAX-4 are sufficient to confer ciliary targeting and subdomain localization. To address this issue, we generated TAX-2–TAX-4 (TAX-2/4) chimeras fused to GFP and examined localization in AWB. In the TAX-2/4 chimera, C-terminal sequences downstream of the cyclic nucleotide-binding domain of TAX-2 were replaced with the C-terminal 106 amino acids of TAX-4 including the leucine zipper-like coiled coil domain (Fig. 6C). In the TAX-4/2 chimera, all sequences C-terminal to the leucine zipper domain in TAX-4 were replaced with the C-terminal 100 amino acids of TAX-2 (Fig. 6D). Although the TAX-2/4 chimeric fusion protein was either not expressed or remained in the AWB cell bodies (Fig. 6C), the ciliary localization pattern of the TAX-4/2::GFP chimeric protein partly resembled that of TAX-2 (Fig. 6E). The chimeric TAX-4/2 protein weakly rescued AWB-mediated sensory behaviors in tax-4 mutants (supplementary material Fig. S3), suggesting that appropriate ciliary subdomain localization is important for channel function. However, it is also possible that the TAX-4/2 chimeric channel has altered channel properties. Our observations suggest that ciliary targeting or localization signals are distributed in TAX-2, such that although the C-terminal domain is not necessary, it is partly sufficient for localization of CNG channels to the AWB cilia middle segments.
Discussion
We found that the subunit composition of CNG channels varies across the cilium in a cell-type-specific manner. Moreover, the targeting and localization of these subunits to distinct ciliary subcompartments is regulated by multiple mechanisms whose roles are also distinct in individual sensory neuron types. Our results indicate that the localization of CNG channel subunits in cilia is highly regulated, and suggest that diversity in ciliary targeting and localization mechanisms contributes to the sculpting of ciliary signaling microdomains and precision in cell-specific sensory transduction.
Differential localization of CNG channel subunits could define ciliary signaling subcompartments
We and others have previously shown that candidate G-protein-coupled chemosensory receptors are also localized to sensory neuron cilia in C. elegans (e.g. Colosimo et al., 2004; Kim et al., 2009; McGrath et al., 2011; Sengupta et al., 1996; Troemel et al., 1995). However, these receptors are not restricted to a specific ciliary subdomain, but are distributed throughout the cilia in the examined cell types. By contrast, TAX-2, CNG-3 and TAX-4 are all localized to the middle segment in ASK cilia. In AWB cilia, whereas TAX-2 is similarly restricted in localization, TAX-4 and CNG-3 are also present in the distal segments. Thus, although the middle segments of ASK and AWB cilia might contain CNG channels that include TAX-2, channels in the AWB cilia distal segments do not contain this subunit. Given the role of CNG channel stoichiometry in regulating channel properties (Bönigk et al., 1999; Dzeja et al., 1999; Frings et al., 1995; Kaupp and Seifert, 2002), these observations suggest that the distal and middle ciliary domains in AWB mediate distinct signal transduction processes.
What purpose does heterogeneity in CNG channel composition across the AWB cilia serve? It is likely that TAX-2 and TAX-4 are the primary CNG channel subunits in C. elegans because mutations in either of these genes severely affects neuronal functions, whereas mutations in other examined CNG subunit genes lead to more minor defects (Cho et al., 2005; Cho et al., 2004; Coburn and Bargmann, 1996; Dusenbery et al., 1975; Komatsu et al., 1996; Smith et al., 2013). TAX-4 can homotetramerize in the AWB cilia distal segments to mediate signal transduction. The single channel properties of TAX-4 homotetrameric channels are distinct from TAX-4–TAX-2 heterotetrameric channels upon misexpression in heterologous cells (Komatsu et al., 1999; Komatsu et al., 1996). For instance, the EC50 values for cGMP are higher for the TAX-2–TAX-4 channel than for TAX-4 channels alone (Komatsu et al., 1999). The ASK neurons respond to water-soluble attractive chemicals (Bargmann and Horvitz, 1991a; Kim et al., 2009; Macosko et al., 2009), whereas AWB generally responds to volatile repellent chemicals (Ha et al., 2010; Pradel et al., 2007; Troemel et al., 1997). We speculate that heterogeneity in channel composition across the cilium increases the dynamic range of the response, thereby allowing the AWB neurons to respond to toxic chemicals over a wider concentration range.
Clustering of signaling molecules including channels in specific ciliary subdomains has been noted previously. In vertebrate olfactory cilia, both CNG channels and Ca2+-gated chloride channels required for signal amplification are suggested to be clustered at the proximal end of the distal ciliary segment (Badamdorj et al., 2008; Flannery et al., 2006; Matsuzaki et al., 1999), although there might be organism-specific differences (Lowe and Gold, 1991; Takeuchi and Kurahashi, 2008). In Drosophila mechanosensory organs, a TRPN channel required for primary mechanotransduction is localized to the distal ciliary region, whereas TRPV channels that regulate signal amplification are instead localized to a proximal zone (Cheng et al., 2010; Lee et al., 2010; Liang et al., 2011). Similarly, the PKD-2 polycystin 2 channel is enriched in the proximal regions of male sensory neuron cilia in C. elegans (Bae et al., 2006; Barr et al., 2001; Barr and Sternberg, 1999), and signaling proteins are also localized non-uniformly in Chlamydomonas flagella (Fujiu et al., 2009; Iomini et al., 2006). Thus, differential clustering of signaling molecules to specific sensory cilia subdomains could be a general mechanism to increase response sensitivity and fidelity.
A diversity of targeting and localization mechanisms regulates CNG channel subunit localization to AWB and ASK cilia
Ciliary trafficking of CNG channels has been examined in only a limited number of cell types, including photoreceptors. We found that the trafficking and ciliary localization of individual CNG channel subunits appears to be regulated in a distinct manner in different cell types. For instance, the role of the Kinesin-II motor in ciliary trafficking of TAX-2 and TAX-4 is distinct in AWB and ASK. Similarly, ciliary trafficking and localization of TAX-2 and TAX-4 are affected differently in AWB and ASK in mks-1; mksr-2; mksr-1 triple mutants. This diversity of trafficking and localization mechanisms might be related in part to the diversity of AWB and ASK cilia structures. The middle segment of the AWB cilia is much shorter than that in the ASK cilia, and is built by distinct IFT motor mechanisms (Mukhopadhyay et al., 2007). Similarly, the transition zone composition and structures might be partly distinct in the two neuron types. Diversity in trafficking and localization mechanisms has also been suggested to play a role in targeting polycystin channels to the sensory cilia of C. elegans males (Bae et al., 2008; Bae et al., 2006).
A surprising observation was our finding that CNG channel subunits are relatively immobile within either the AWB or ASK middle segment ciliary membrane, although they exhibit limited mobility in the distal segments. This is in contrast to other transmembrane signaling molecules, such as GPCRs and TRPV channels, which appear to move throughout C. elegans cilia (Kaplan et al., 2012; Olivier-Mason et al., 2013; Qin et al., 2005). However, the Kinesin-II motor subunit appears to play a role in regulating correct TAX-2 and TAX-4 localization to the AWB cilia because in kap-1 mutants, we observed accumulation of these fusion proteins at the dendritic tip in a subset of cells. This accumulation is similar to the accumulation of the PKD-2::GFP polycystin fusion protein observed at the ciliary base of the CEM male sensory neurons in Kinesin-II and the klp-6 kinesin-3 mutant backgrounds (Bae et al., 2006; Peden and Barr, 2005). It has been suggested and/or shown that IFT plays a role in regulating ciliary abundance of membrane proteins such as PKD-2 in C. elegans cilia, and PKD2 and the CAV2 Ca2+ channel in Chlamydomonas flagella (Bae et al., 2006; Fujiu et al., 2009; Huang et al., 2007; Qin et al., 2001; Qin et al., 2005), although similar to observations reported here, no movement of PKD-2 has been observed in C. elegans sensory cilia (Qin et al., 2005). The absence of significant recovery of CNG channel fusion protein fluorescence in AWB and ASK cilia upon photobleaching the middle segments argues against a requirement for IFT in maintaining ciliary localization of these channels in these domains under the examined conditions. One possibility is that these proteins are trafficked through IFT or other mechanisms to cilia middle segments early in development, but are irreversibly anchored once localized, perhaps in association with lipid rafts (Brady et al., 2004; Ding et al., 2008). An alternative hypothesis is that CNG channel turnover in the middle segments is initiated only under defined conditions. For instance, we previously showed that sensory cilia morphologies are modified by sensory activity, and that this modification requires Kinesin-II (Mukhopadhyay et al., 2008). Thus, specific sensory signals might trigger IFT-mediated CNG channel trafficking in the middle ciliary segments. The ciliary diffusion barrier could also restrict movement of CNG channels into the cilium consistent with cell-specific defects in CNG channel ciliary localization in transition zone mutants. Complexity in CNG channel composition and ciliary targeting and localization mechanisms might provide a template for the action of multiple regulatory mechanisms. These mechanisms ensure that the sensory functions of the cell are optimized for the prevailing conditions and must, therefore, necessarily be distinct in different cell types. It will be interesting to determine whether similar complexity regulates the ciliary localization of CNG channel and other signaling proteins across cell types in other organisms.
Materials and Methods
Strains
Animals were grown on OP50 bacteria at 20°C using standard procedures. Transgenic strains were generated by microinjecting plasmids expressing fusion proteins or fluorescent reporters under the str-1 and srbc-66 promoters at 5 and 10 ng/µl, respectively. unc-122p::gfp (50 ng/µl) was used as a co-injection marker. The presence of mutations in strains was confirmed by PCR-based genotyping, sequencing or visible phenotypes.
Phylogenetic analyses
Cyclic nucleotide-gated channel subunit sequences were sourced from WormBase (www.wormbase.org) and GenBank. In the case of the C. elegans CNG channel subunits CNG-2, CNG-3 and CNG-4, orthologs were identified in C. briggsae through reciprocal BlastP searches and by inferring phylogenetic relationships. Orthologs in other nematodes were detected using the orthology database InParanoid (Ostlund et al., 2010), and orthologs from mammals were sourced using InParanoid and BlastO (Zhou and Landweber, 2007). Chromosomal locations of C. briggsae CNG channel subunits were mined from WormBase.
Orthologs were aligned using the multiple sequence alignment software MUSCLE v3.8.31 (Edgar, 2004), and gaps were systematically stripped after alignment. Phylogenetic relationships were inferred by reconstructing trees by maximum likelihood using the PhyML command-line interface (Guindon and Gascuel, 2003). The appropriate model was selected using ProtTest v.3 (Darriba et al., 2011), and determined from 120 models to be the LG+G model (Le and Gascuel, 2008) with gamma distribution parameter = 1.2, and four substitution rate categories. Bayesian trees were constructed using MrBayes version 3.2 (Ronquist et al., 2012) and the related WAG model (Whelan and Goldman, 2001). The analysis used a Markov Chain Monte Carlo (MCMC) method that ran for 3 million generations, sampled every tenth generation.
Molecular biology
str-1p::tax-2::gfp and str-1p::tax-4::gfp constructs were generated as described (Mukhopadhyay et al., 2008). srbc-66p::tax-2::gfp and srbc-66p::tax-4::gfp constructs were generated by fusing tax-2 and tax-4 cDNAs in frame to gfp in an expression vector containing 2.1 kb of srbc-66 upstream regulatory sequences (Kim et al., 2009). Truncated tax-2 and tax-4 sequences were generated by PCR using iProof polymerase (Bio-Rad) and replaced full-length tax-2 or tax-4 coding sequences in str-1p::tax-2::gfp or srbc-66p::tax-2::gfp expression vectors. Point mutations were made using the QuikChange Lightning Site-Directed Mutagenesis kit (Agilent). The NPHP-2-AKR construct contains aa 1–408 of NPHP-2 that includes the 12 ankyrin repeat domains. For cell-specific rescue of protein localization, gfp coding sequences in the above constructs were deleted and replaced with a stop codon. All constructs were confirmed by sequencing.
Chemotaxis assays
2-nonanone repulsion assays were carried out essentially as described (Troemel et al., 1997).
Microscopy
Animals were anesthetized with 10 mM tetramisole and mounted on agarose pads set on microscope slides. Images were captured on an inverted spinning-disk microscope (Zeiss Axiovert with a Yokogawa CSU-22 spinning-disk confocal head). SlideBook 5.0 software (Intelligent Imaging Innovations, 3i) was used to capture optical sections of cilia at 0.25 µm intervals. Images were z-projected at maximum intensity values. Intensity values were measured using ImageJ software (National Institutes of Health).
FRAP
Photobleaching of TAX-2::GFP, TAX-4::GFP, and ODR-10::GFP ciliary signals was performed using a MicroPoint laser system (Photonic Instruments) equipped with an NL100 nitrogen laser (Stanford Research Systems). Equivalent laser intensity (35%), repetitions (1), exposure time (100 mseconds), and gain/offset were applied to all FRAP experiments. Fluorescence intensity for TAX-2::GFP and TAX-4::GFP was recorded prior to photobleaching and post-photobleaching at timepoints: 0, 1, 5, 10, 15 and 20 minutes. Fluorescence intensity when photobleaching ODR-10::GFP was recorded every 1 second. For TAX-2::GFP and TAX-4::GFP, maximum projection images, consisting of three z-planes at 0.25 µm intervals, were captured using Slidebook 5.0 software. Absolute intensity values were calculated for the photobleached region by subtracting the background. Calculated intensity ratios of the photobleached region were normalized against mock photobleached animals over an identical time course. Percentage recovery of signal following photobleaching was then plotted against time post-photobleach. The mobile fraction (Mf) and the half-time of equilibration (t1/2) were calculated as described (Bancaud et al., 2010).
Supplementary Material
Acknowledgments
We are grateful to Astrid Cornils for help with cell identification, John Satterlee for the cng-2::gfp fusion gene, the Caenorhabditis Genetics Center for strains, members of the Sengupta lab cilia squad for reagents and advice, and Oliver Blacque, Max Heiman, Oliver Hobert and the Sengupta lab cilia squad for comments on the manuscript.
Footnotes
Author contributions
M.W., A.G.B. and D.M.O. performed experiments; M.W. and D.M.O. analyzed data; M.W., D.M.O., and P.S. wrote the manuscript.
Funding
This work was supported in part by the National Institutes of Health [grant numbers R37 GM56223 to P.S. and T32 GM007122 to M.W. and A.G.B.]. D.M.O'H. was supported by internal funding from George Washington University. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.127274/-/DC1
References
- Badamdorj D., Edwards D. A., French D. A., Kleene S. J. (2008). Identification of Cl(Ca) channel distributions in olfactory cilia. Math. Methods Appl. Sci. 31, 1860–1873 10.1002/mma.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y. K., Qin H., Knobel K. M., Hu J., Rosenbaum J. L., Barr M. M. (2006). General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development 133, 3859–3870 10.1242/dev.02555 [DOI] [PubMed] [Google Scholar]
- Bae Y. K., Lyman-Gingerich J., Barr M. M., Knobel K. M. (2008). Identification of genes involved in the ciliary trafficking of C. elegans PKD-2. Dev. Dyn. 237, 2021–2029 10.1002/dvdy.21531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud A., Huet S., Rabut G., Ellenberg J. (2010). Fluorescence perturbation techniques to study mobility and molecular dynamics of proteins in live cells: FRAP, photoactivation, photoconversion, and FLIP. Cold Spring Harb. Protoc. 2010, top90 10.1101/pdb.top90 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R. (1991a). Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7, 729–742 10.1016/0896-6273(91)90276-6 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R. (1991b). Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251, 1243–1246 10.1126/science.2006412 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Mori I. (1997). Chemotaxis and thermotaxis. C. elegans II Riddle D S, Blumenthal T, Meyer B J, Priess J R, edCold Spring Harbor, NY: Cold Spring Harbor Press; [PubMed] [Google Scholar]
- Bargmann C. I., Thomas J. H., Horvitz H. R. (1990). Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 55, 529–538 10.1101/SQB.1990.055.01.051 [DOI] [PubMed] [Google Scholar]
- Barr M. M., Sternberg P. W. (1999). A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401, 386–389 10.1038/43913 [DOI] [PubMed] [Google Scholar]
- Barr M. M., DeModena J., Braun D., Nguyen C. Q., Hall D. H., Sternberg P. W. (2001). The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11, 1341–1346 10.1016/S0960-9822(01)00423-7 [DOI] [PubMed] [Google Scholar]
- Bialas N. J., Inglis P. N., Li C., Robinson J. F., Parker J. D., Healey M. P., Davis E. E., Inglis C. D., Toivonen T., Cottell D. C. et al. (2009). Functional interactions between the ciliopathy-associated Meckel syndrome 1 (MKS1) protein and two novel MKS1-related (MKSR) proteins. J. Cell Sci. 122, 611–624 10.1242/jcs.028621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönigk W., Bradley J., Müller F., Sesti F., Boekhoff I., Ronnett G. V., Kaupp U. B., Frings S. (1999). The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 19, 5332–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. D., Rich T. C., Le X., Stafford K., Fowler C. J., Lynch L., Karpen J. W., Brown R. L., Martens J. R. (2004). Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol. Pharmacol. 65, 503–511 10.1124/mol.65.3.503 [DOI] [PubMed] [Google Scholar]
- Cevik S., Hori Y., Kaplan O. I., Kida K., Toivenon T., Foley-Fisher C., Cottell D., Katada T., Kontani K., Blacque O. E. (2010). Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J. Cell Biol. 188, 953–969 10.1083/jcb.200908133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L. E., Song W., Looger L. L., Jan L. Y., Jan Y. N. (2010). The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67, 373–380 10.1016/j.neuron.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B., Liu P., Chinn Y., Chalouni C., Komuves L. G., Hass P. E., Sandoval W., Peterson A. S. (2012). A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 14, 61–72 10.1038/ncb2410 [DOI] [PubMed] [Google Scholar]
- Cho S. W., Choi K. Y., Park C. S. (2004). A new putative cyclic nucleotide-gated channel gene, cng-3, is critical for thermotolerance in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 325, 525–531 10.1016/j.bbrc.2004.10.060 [DOI] [PubMed] [Google Scholar]
- Cho S. W., Cho J. H., Song H. O., Park C. S. (2005). Identification and characterization of a putative cyclic nucleotide-gated channel, CNG-1, in C. elegans. Mol. Cells 19, 149–154 [PubMed] [Google Scholar]
- Coburn C. M., Bargmann C. I. (1996). A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17, 695–706 10.1016/S0896-6273(00)80201-9 [DOI] [PubMed] [Google Scholar]
- Colosimo M. E., Brown A., Mukhopadhyay S., Gabel C., Lanjuin A. E., Samuel A. D., Sengupta P. (2004). Identification of thermosensory and olfactory neuron-specific genes via expression profiling of single neuron types. Curr. Biol. 14, 2245–2251 10.1016/j.cub.2004.12.030 [DOI] [PubMed] [Google Scholar]
- Contreras J. E., Srikumar D., Holmgren M. (2008). Gating at the selectivity filter in cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA 105, 3310–3314 10.1073/pnas.0709809105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige B., Tsao C. C., Diener D. R., Hou Y., Lechtreck K. F., Rosenbaum J. L., Witman G. B. (2010). CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927–940 10.1083/jcb.201006105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki P. G., Shah J. V. (2012). The ciliary transition zone: from morphology and molecules to medicine. Trends Cell Biol. 22, 201–210 10.1016/j.tcb.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D. (2011). ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27, 1164–1165 10.1093/bioinformatics/btr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. Q., Fitzgerald J. B., Matveev A. V., McClellan M. E., Elliott M. H. (2008). Functional activity of photoreceptor cyclic nucleotide-gated channels is dependent on the integrity of cholesterol- and sphingolipid-enriched membrane domains. Biochemistry 47, 3677–3687 10.1021/bi7019645 [DOI] [PubMed] [Google Scholar]
- Drummond I. A. (2012). Cilia functions in development. Curr. Opin. Cell Biol. 24, 24–30 10.1016/j.ceb.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery D. B., Sheridan R. E., Russell R. L. (1975). Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics 80, 297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja C., Hagen V., Kaupp U. B., Frings S. (1999). Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J. 18, 131–144 10.1093/emboj/18.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113 10.1186/1471-2105-5-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eismann E., Müller F., Heinemann S. H., Kaupp U. B. (1994). A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc. Natl. Acad. Sci. USA 91, 1109–1113 10.1073/pnas.91.3.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer B. T., Maric D., Engman D. M. (2010). Molecular mechanisms of protein and lipid targeting to ciliary membranes. J. Cell Sci. 123, 529–536 10.1242/jcs.062968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. (1985). Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313, 310–313 10.1038/313310a0 [DOI] [PubMed] [Google Scholar]
- Flannery R. J., French D. A., Kleene S. J. (2006). Clustering of cyclic-nucleotide-gated channels in olfactory cilia. Biophys. J. 91, 179–188 10.1529/biophysj.105.079046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S., Seifert R., Godde M., Kaupp U. B. (1995). Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron 15, 169–179 10.1016/0896-6273(95)90074-8 [DOI] [PubMed] [Google Scholar]
- Fujiu K., Nakayama Y., Yanagisawa A., Sokabe M., Yoshimura K. (2009). Chlamydomonas CAV2 encodes a voltage- dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 19, 133–139 10.1016/j.cub.2008.11.068 [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Teramoto T., Ishihara T., Ohshima Y., McIntire S. L. (2010). A novel zf-MYND protein, CHB-3, mediates guanylyl cyclase localization to sensory cilia and controls body size of Caenorhabditis elegans. PLoS Genet. 6, e1001211 10.1371/journal.pgen.1001211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. A., Mykytyn K. (2010). Neuronal ciliary signaling in homeostasis and disease. Cell. Mol. Life Sci. 67, 3287–3297 10.1007/s00018-010-0425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Guindon S., Delsuc F., Dufayard J. F., Gascuel O. (2009). Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 537, 113–137 10.1007/978-1-59745-251-9_6 [DOI] [PubMed] [Google Scholar]
- Ha H. I., Hendricks M., Shen Y., Gabel C. V., Fang-Yen C., Qin Y., Colón-Ramos D., Shen K., Samuel A. D., Zhang Y. (2010). Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 68, 1173–1186 10.1016/j.neuron.2010.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Thomson J. N., Perkins L. A. (1985). Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111, 158–170 10.1016/0012-1606(85)90443-9 [DOI] [PubMed] [Google Scholar]
- Herman R. K., Hedgecock E. M. (1990). Limitation of the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature 348, 169–171 10.1038/348169a0 [DOI] [PubMed] [Google Scholar]
- Hillier L. W., Miller R. D., Baird S. E., Chinwalla A., Fulton L. A., Koboldt D. C., Waterston R. H. (2007). Comparison of C. elegans and C. briggsae genome sequences reveals extensive conservation of chromosome organization and synteny. PLoS Biol. 5, e167 10.1371/journal.pbio.0050167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Nelson W. J. (2011). Ciliary diffusion barrier: the gatekeeper for the primary cilium compartment. Cytoskeleton (Hoboken) 68, 313–324 10.1002/cm.20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Bae Y. K., Knobel K. M., Barr M. M. (2006). Casein kinase II and calcineurin modulate TRPP function and ciliary localization. Mol. Biol. Cell 17, 2200–2211 10.1091/mbc.E05-10-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Milenkovic L., Jin H., Scott M. P., Nachury M. V., Spiliotis E. T., Nelson W. J. (2010). A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 10.1126/science.1191054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Diener D. R., Mitchell A., Pazour G. J., Witman G. B., Rosenbaum J. L. (2007). Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179, 501–514 10.1083/jcb.200704069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttl S., Michalakis S., Seeliger M., Luo D. G., Acar N., Geiger H., Hudl K., Mader R., Haverkamp S., Moser M. et al. (2005). Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J. Neurosci. 25, 130–138 10.1523/JNEUROSCI.3764-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis P. N., Ou G., Leroux M. R., Scholey J. M. (2007). The sensory cilia of Caenorhabditis elegans. Wormbook The C. elegans Research Community 10.1895/wormbook.1.126.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C., Li L., Mo W., Dutcher S. K., Piperno G. (2006). Two flagellar genes, AGG2 and AGG3, mediate orientation to light in Chlamydomonas. Curr. Biol. 16, 1147–1153 10.1016/j.cub.2006.04.035 [DOI] [PubMed] [Google Scholar]
- Jauregui A. R., Barr M. M. (2005). Functional characterization of the C. elegans nephrocystins NPHP-1 and NPHP-4 and their role in cilia and male sensory behaviors. Exp. Cell Res. 305, 333–342 10.1016/j.yexcr.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Jenkins P. M., Hurd T. W., Zhang L., McEwen D. P., Brown R. L., Margolis B., Verhey K. J., Martens J. R. (2006). Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 16, 1211–1216 10.1016/j.cub.2006.04.034 [DOI] [PubMed] [Google Scholar]
- Jenkins P. M., Zhang L., Thomas G., Martens J. R. (2009). PACS-1 mediates phosphorylation-dependent ciliary trafficking of the cyclic-nucleotide-gated channel in olfactory sensory neurons. J. Neurosci. 29, 10541–10551 10.1523/JNEUROSCI.1590-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen V. L., Bialas N. J., Bishop-Hurley S. L., Molday L. L., Kida K., Nguyen P. A., Blacque O. E., Molday R. S., Leroux M. R., Riddle D. L. (2010). Localization of a guanylyl cyclase to chemosensory cilia requires the novel ciliary MYND domain protein DAF-25. PLoS Genet. 6, e1001199 10.1371/journal.pgen.1001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan O. I., Doroquez D. B., Cevik S., Bowie R. V., Clarke L., Sanders A. A., Kida K., Rappoport J. Z., Sengupta P., Blacque O. E. (2012). Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr. Biol. 22, 451–460 10.1016/j.cub.2012.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Seifert R. (2002). Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769–824 [DOI] [PubMed] [Google Scholar]
- Kim K., Sato K., Shibuya M., Zeiger D. M., Butcher R. A., Ragains J. R., Clardy J., Touhara K., Sengupta P. (2009). Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 326, 994–998 10.1126/science.1176331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K., Baker S. A., Arshavsky V. Y., Bennett V. (2009). Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science 323, 1614–1617 10.1126/science.1169789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H., Mori I., Rhee J. S., Akaike N., Ohshima Y. (1996). Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17, 707–718 10.1016/S0896-6273(00)80202-0 [DOI] [PubMed] [Google Scholar]
- Komatsu H., Jin Y. H., L'Etoile N., Mori I., Bargmann C. I., Akaike N., Ohshima Y. (1999). Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res. 821, 160–168 10.1016/S0006-8993(99)01111-7 [DOI] [PubMed] [Google Scholar]
- L'Etoile N. D., Bargmann C. I. (2000). Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron 25, 575–586 10.1016/S0896-6273(00)81061-2 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Gleeson J. G. (2009). The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 19, 220–229 10.1016/j.gde.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Q., Gascuel O. (2008). An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320 10.1093/molbev/msn067 [DOI] [PubMed] [Google Scholar]
- Lee J., Moon S., Cha Y., Chung Y. D. (2010). Drosophila TRPN( = NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE 5, e11012 10.1371/journal.pone.0011012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Mahajan A., Tsai M. D. (2006). Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry 45, 15168–15178 10.1021/bi062188q [DOI] [PubMed] [Google Scholar]
- Li Y., Wei Q., Zhang Y., Ling K., Hu J. (2010). The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J. Cell Biol. 189, 1039–1051 10.1083/jcb.200912001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang Q., Wei Q., Zhang Y., Ling K., Hu J. (2012). SUMOylation of the small GTPase ARL-13 promotes ciliary targeting of sensory receptors. J. Cell Biol. 199, 589–598 10.1083/jcb.201203150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Madrid J., Saleh H. S., Howard J. (2011). NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton (Hoboken) 68, 1–7 10.1002/cm.20493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. S., Chua C. E., Tang B. L. (2011). Rabs and other small GTPases in ciliary transport. Biol. Cell 103, 209–221 10.1042/BC20100150 [DOI] [PubMed] [Google Scholar]
- Lowe G., Gold G. H. (1991). The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J. Physiol. 442, 147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. (1991). Predicting coiled coils from protein sequences. Science 252, 1162–1164 10.1126/science.252.5009.1162 [DOI] [PubMed] [Google Scholar]
- Macosko E. Z., Pokala N., Feinberg E. H., Chalasani S. H., Butcher R. A., Clardy J., Bargmann C. I. (2009). A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171–1175 10.1038/nature07886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki O., Bakin R. E., Cai X., Menco B. P., Ronnett G. V. (1999). Localization of the olfactory cyclic nucleotide-gated channel subunit 1 in normal, embryonic and regenerating olfactory epithelium. Neuroscience 94, 131–140 10.1016/S0306-4522(99)00228-6 [DOI] [PubMed] [Google Scholar]
- McEwen D. P., Koenekoop R. K., Khanna H., Jenkins P. M., Lopez I., Swaroop A., Martens J. R. (2007). Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc. Natl. Acad. Sci. USA 104, 15917–15922 10.1073/pnas.0704140104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath P. T., Xu Y., Ailion M., Garrison J. L., Butcher R. A., Bargmann C. I. (2011). Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477, 321–325 10.1038/nature10378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menco B. P. (1997). Ultrastructural aspects of olfactory signaling. Chem. Senses 22, 295–311 10.1093/chemse/22.3.295 [DOI] [PubMed] [Google Scholar]
- Michalakis S., Reisert J., Geiger H., Wetzel C., Zong X., Bradley J., Spehr M., Hüttl S., Gerstner A., Pfeifer A. et al. (2006). Loss of CNGB1 protein leads to olfactory dysfunction and subciliary cyclic nucleotide-gated channel trapping. J. Biol. Chem. 281, 35156–35166 10.1074/jbc.M606409200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosavi L. K., Cammett T. J., Desrosiers D. C., Peng Z. Y. (2004). The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 13, 1435–1448 10.1110/ps.03554604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S., Lu Y., Qin H., Lanjuin A., Shaham S., Sengupta P. (2007). Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 26, 2966–2980 10.1038/sj.emboj.7601717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S., Lu Y., Shaham S., Sengupta P. (2008). Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev. Cell 14, 762–774 10.1016/j.devcel.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M. V., Seeley E. S., Jin H. (2010). Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26, 59–87 10.1146/annurev.cellbio.042308.113337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. (1987). A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature 325, 442–444 10.1038/325442a0 [DOI] [PubMed] [Google Scholar]
- O'Hagan R., Piasecki B. P., Silva M., Phirke P., Nguyen K. C., Hall D. H., Swoboda P., Barr M. M. (2011). The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans. Curr. Biol. 21, 1685–1694 10.1016/j.cub.2011.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier-Mason A., Wojtyniak M., Bowie R. V., Nechipurenko I. V., Blacque O. E., Sengupta P. (2013). Transmembrane protein OSTA-1 shapes sensory cilia morphology via regulation of intracellular membrane trafficking in C. elegans. Development 140, 1560–1572 10.1242/dev.086249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund G., Schmitt T., Forslund K., Köstler T., Messina D. N., Roopra S., Frings O., Sonnhammer E. L. (2010). InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Res. 38, D196–D203 10.1093/nar/gkp931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A. J., Franco R., Yang B., Shim K. H., Tang L. Z., Zhang Y. Y., Boontrakulpoontawee P., Jeyaprakash A., Hedgecock E., Wheaton V. I. et al. (1995). An ankyrin-related gene (unc-44) is necessary for proper axonal guidance in Caenorhabditis elegans. J. Cell Biol. 129, 1081–1092 10.1083/jcb.129.4.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Bloodgood R. A. (2008). Targeting proteins to the ciliary membrane. Curr. Top. Dev. Biol. 85, 115–149 10.1016/S0070-2153(08)00805-3 [DOI] [PubMed] [Google Scholar]
- Peden E. M., Barr M. M. (2005). The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr. Biol. 15, 394–404 10.1016/j.cub.2004.12.073 [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Hedgecock E. M., Thomson J. N., Culotti J. G. (1986). Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487 10.1016/0012-1606(86)90314-3 [DOI] [PubMed] [Google Scholar]
- Pifferi S., Boccaccio A., Menini A. (2006). Cyclic nucleotide-gated ion channels in sensory transduction. FEBS Lett. 580, 2853–2859 10.1016/j.febslet.2006.03.086 [DOI] [PubMed] [Google Scholar]
- Pradel E., Zhang Y., Pujol N., Matsuyama T., Bargmann C. I., Ewbank J. J. (2007). Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104, 2295–2300 10.1073/pnas.0610281104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Rosenbaum J. L., Barr M. M. (2001). An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr. Biol. 11, 457–461 10.1016/S0960-9822(01)00122-1 [DOI] [PubMed] [Google Scholar]
- Qin H., Burnette D. T., Bae Y. K., Forscher P., Barr M. M., Rosenbaum J. L. (2005). Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15, 1695–1699 10.1016/j.cub.2005.08.047 [DOI] [PubMed] [Google Scholar]
- Reiter J. F., Blacque O. E., Leroux M. R. (2012). The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 13, 608–618 10.1038/embor.2012.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., Huelsenbeck J. P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M. J., MacKinnon R. (1993). Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron 11, 459–466 10.1016/0896-6273(93)90150-P [DOI] [PubMed] [Google Scholar]
- Sang L., Miller J. J., Corbit K. C., Giles R. H., Brauer M. J., Otto E. A., Baye L. M., Wen X., Scales S. J., Kwong M. et al. (2011). Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145, 513–528 10.1016/j.cell.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Eismann E., Ludwig J., Baumann A., Kaupp U. B. (1999). Molecular determinants of a Ca2+-binding site in the pore of cyclic nucleotide-gated channels: S5/S6 segments control affinity of intrapore glutamates. EMBO J. 18, 119–130 10.1093/emboj/18.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P., Chou J. H., Bargmann C. I. (1996). odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell 84, 899–909 10.1016/S0092-8674(00)81068-5 [DOI] [PubMed] [Google Scholar]
- Shiba D., Yokoyama T. (2012). The ciliary transitional zone and nephrocystins. Differentiation 83, S91–S96 10.1016/j.diff.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Shiba D., Yamaoka Y., Hagiwara H., Takamatsu T., Hamada H., Yokoyama T. (2009). Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J. Cell Sci. 122, 44–54 10.1242/jcs.037408 [DOI] [PubMed] [Google Scholar]
- Shuart N. G., Haitin Y., Camp S. S., Black K. D., Zagotta W. N. (2011). Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat. Commun. 2, 457 10.1038/ncomms1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V., Reiter J. F. (2006). The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313, 629–633 10.1126/science.1124534 [DOI] [PubMed] [Google Scholar]
- Smith H. K., Luo L., O'Halloran D., Guo D., Huang X. Y., Samuel A. D., Hobert O. (2013). Defining specificity determinants of cyclic GMP-mediated gustatory sensory transduction in Caenorhabditis elegans. Genetics [Epub ahead of print] 10.1534/genetics.113.152660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow J. J., Ou G., Gunnarson A. L., Walker M. R., Zhou H. M., Brust-Mascher I., Scholey J. M. (2004). Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6, 1109–1113 10.1038/ncb1186 [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Kurahashi T. (2008). Distribution, amplification, and summation of cyclic nucleotide sensitivities within single olfactory sensory cilia. J. Neurosci. 28, 766–775 10.1523/JNEUROSCI.3531-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel E. R., Chou J. H., Dwyer N. D., Colbert H. A., Bargmann C. I. (1995). Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83, 207–218 10.1016/0092-8674(95)90162-0 [DOI] [PubMed] [Google Scholar]
- Troemel E. R., Kimmel B. E., Bargmann C. I. (1997). Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169 10.1016/S0092-8674(00)80399-2 [DOI] [PubMed] [Google Scholar]
- Vergara I. A., Chen N. (2010). Large synteny blocks revealed between Caenorhabditis elegans and Caenorhabditis briggsae genomes using OrthoCluster. BMC Genomics 11, 516 10.1186/1471-2164-11-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincensini L., Blisnick T., Bastin P. (2011). 1001 model organisms to study cilia and flagella. Biol. Cell 103, 109–130 10.1042/BC20100104 [DOI] [PubMed] [Google Scholar]
- Warburton-Pitt S. R., Jauregui A. R., Li C., Wang J., Leroux M. R., Barr M. M. (2012). Ciliogenesis in Caenorhabditis elegans requires genetic interactions between ciliary middle segment localized NPHP-2 (inversin) and transition zone-associated proteins. J. Cell Sci. 125, 2592–2603 10.1242/jcs.095539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Thomson N., White J. G., Brenner S. (1975). Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J. Comp. Neurol. 160, 313–337 10.1002/cne.901600305 [DOI] [PubMed] [Google Scholar]
- Watanabe D., Saijoh Y., Nonaka S., Sasaki G., Ikawa Y., Yokoyama T., Hamada H. (2003). The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development 130, 1725–1734 10.1242/dev.00407 [DOI] [PubMed] [Google Scholar]
- Whelan S., Goldman N. (2001). A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18, 691–699 10.1093/oxfordjournals.molbev.a003851 [DOI] [PubMed] [Google Scholar]
- Williams C. L., Li C., Kida K., Inglis P. N., Mohan S., Semenec L., Bialas N. J., Stupay R. M., Chen N., Blacque O. E. et al. (2011). MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192, 1023–1041 10.1083/jcb.201012116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Malicki J. (2011). Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 30, 2532–2544 10.1038/emboj.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H., Molday L. L., Molday R. S., Yau K. W. (2002). The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 420, 193–198 10.1038/nature01201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Landweber L. F. (2007). BLASTO: a tool for searching orthologous groups. Nucleic Acids Res. 35, W678–W682 10.1093/nar/gkm278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.