Summary
Calmodulin (CaM), a 16 kDa ubiquitous calcium-sensing protein, is known to bind tightly to the calcium release channel/ryanodine receptor (RyR), and modulate RyR function. CaM binding studies using RyR fragments or synthetic peptides have revealed the presence of multiple, potential CaM-binding regions in the primary sequence of RyR. In the present study, we inserted GFP into two of these proposed CaM-binding sequences and mapped them onto the three-dimensional structure of intact cardiac RyR2 by cryo-electron microscopy. Interestingly, we found that the two potential CaM-binding regions encompassing, Arg3595 and Lys4269, respectively, are in close proximity and are adjacent to the previously mapped CaM-binding sites. To monitor the conformational dynamics of these CaM-binding regions, we generated a fluorescence resonance energy transfer (FRET) pair, a dual CFP- and YFP-labeled RyR2 (RyR2R3595-CFP/K4269-YFP) with CFP inserted after Arg3595 and YFP inserted after Lys4269. We transfected HEK293 cells with the RyR2R3595-CFP/K4269-YFP cDNA, and examined their FRET signal in live cells. We detected significant FRET signals in transfected cells that are sensitive to the channel activator caffeine, suggesting that caffeine is able to induce conformational changes in these CaM-binding regions. Importantly, no significant FRET signals were detected in cells co-transfected with cDNAs encoding the single CFP (RyR2R3595-CFP) and single YFP (RyR2K4269-YFP) insertions, indicating that the FRET signal stemmed from the interaction between R3595–CFP and K4269–YFP that are in the same RyR subunit. These observations suggest that multiple regions in the RyR2 sequence may contribute to an intra-subunit CaM-binding pocket that undergoes conformational changes during channel gating.
Key words: Ryanodine receptor/calcium release channel, Calmodulin, Cryo-EM, FRET
Introduction
Calmodulin (CaM) is a 16 kDa ubiquitous calcium-sensing protein. It can bind to more than 30 proteins and enzymes that are involved in growth, apoptosis, motility, mitosis and development (Bähler and Rhoads, 2002). CaM consists of four EF-hand calcium-binding motifs, which give CaM the ability of binding four Ca2+ in total. Two of these EF-hand motifs form the N-terminal lobe of CaM, while the other two form the C-terminal lobe. In muscle excitation–contraction coupling, CaM modulates the major participating transporters, including the ryanodine receptor (RyR) in sarcoplasmic reticulum (SR) membrane and the L-type calcium channel (DHPR) in the transverse tubule (Aracena et al., 2005).
RyR is a calcium release channel that controls calcium flow from the lumen of the SR to the cytosol. It plays a key role in excitation–contraction coupling, during which an electrical stimulus (from a neuron) triggers muscle contraction. The electrical stimulus induces depolarization of the muscle plasma membrane, and activation of the DHPR. RyR is the next target to be activated, resulting in an increase in the concentration of Ca2+ in the cytosol. The increased cytosolic Ca2+ concentration triggers sliding filament movements within the myofibril and results in the contraction of muscle. Mammalian RyRs mainly exist in three isoforms: RyR1 and RyR2 are predominantly distributed in skeletal and cardiac muscle, respectively; RyR3 was first cloned from brain, but is also expressed in the diaphragm, smooth muscle and several abdominal organs (Kushnir et al., 2005).
CaM regulates the RyRs by a direct interaction and by an indirect phosphorylation through CaM-dependent kinase II (Ai et al., 2005). Both apo-CaM (no Ca2+ bound) and Ca2+-CaM (four Ca2+ bound) can bind to all three RyR isoforms with high affinity (Kd value in the nanomolar range) and regulate RyR channel functions. At low (resting) Ca2+ concentration (∼100 nM), CaM exists mainly as apo-CaM, and apo-CaM activates RyR1 (Buratti et al., 1995; Damiani and Margreth, 2000; Tripathy et al., 1995) and RyR3 (Chen et al., 1997; Yamaguchi et al., 2005), but inhibits RyR2 (Balshaw et al., 2001; Xu and Meissner, 2004). At high Ca2+ concentration (∼1 µM), most CaM exists as Ca2+-CaM, and Ca2+-CaM inhibits all three RyR isoforms (Buratti et al., 1995; Chen et al., 1997; Xu and Meissner, 2004; Yamaguchi et al., 2005). Interestingly, apo and Ca2+ forms of CaM bind at distinct but overlapping locations in RyR1 according to three-dimensional (3D) cryo-electron microscopy (cryo-EM) results from our laboratory (Samso and Wagenknecht, 2002; Wagenknecht et al., 1997), implying that CaM moves on the surface of RyR1 by ∼30 Å when it switches between Ca2+-free and Ca2+-bound states. Recently, using a mutant CaM that is incapable of binding Ca2+, we confirmed the distinct binding sites for CaM in RyR1 and demonstrated that the binding location switch is due to binding of Ca2+ to CaM as opposed to effects of Ca2+ itself on the conformation of RyR1 (Huang et al., 2012). Interestingly, apo-CaM binds to RyR2 at a similar binding site to that of Ca2+-CaM on RyR1, in seeming agreement with the inhibitory effects of these two forms of CaM on their respective receptors (Huang et al., 2012).
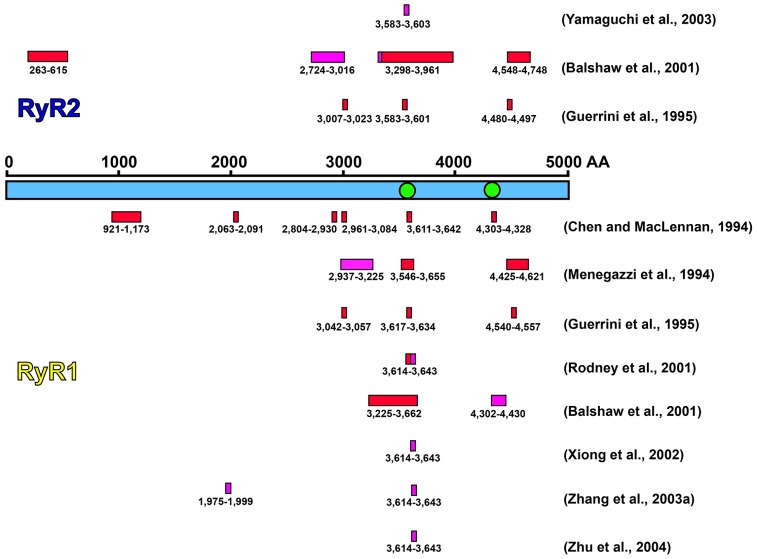
To identify CaM-binding sequences in the primary structure of RyR, CaM overlays on RyR1- and RyR2-derived peptides were used to test the binding capacity of CaM on RyR fragments, which were expressed from RyR cDNA fragments generated from available endonuclease restriction sites (Balshaw et al., 2001; Chen and MacLennan, 1994; Guerrini et al., 1995; Menegazzi et al., 1994). A number of sequences have been reported to be potential CaM-binding sequences (Fig. 1). In RyR1, these include residues 921–1173 (Chen and MacLennan, 1994), 1975–1999 (Zhang et al., 2003a), 2063–2091 (Chen and MacLennan, 1994), 2804–2930 and 2961–3084 (Chen and MacLennan, 1994), 2937–3225 (Menegazzi et al., 1994), 3042–3057 (Guerrini et al., 1995), 3225–3662 (Balshaw et al., 2001), 3546–3655 (Menegazzi et al., 1994), 3611–3642 (Chen and MacLennan, 1994), 3614–3643 (Rodney et al., 2001; Xiong et al., 2002; Zhu et al., 2004), 3617–3634 (Guerrini et al., 1995), 4302–4430 (Balshaw et al., 2001), 4303–4328 (Chen and MacLennan, 1994), 4425–4621 (Menegazzi et al., 1994) and 4540–4557 (Guerrini et al., 1995). In RyR2, these include residues 263–614, 2724–3016, 3007–3023, 3298–3961, 3583–3603, 4480–4497 and 4548–4748 (Balshaw et al., 2001; Guerrini et al., 1995; Yamaguchi et al., 2003). The evidence supporting these hypotheses of CaM regulation is mostly based on studies of synthetic RyR peptides or RyR fragments rather than on the intact, full-length RyR. Although the use of RyR peptides helps simplify the experiments, the results gained from RyR peptide studies may not represent what occurs in the intact RyR, and thus some internal, buried RyR sequences are likely to be detected as potential CaM-binding sequences. CaM, as a soluble protein, however, can only bind to sequences exposed on the surface of RyR.
Fig. 1.
Proposed CaM-binding sites in the primary sequences of RyR1 and RyR2. Regions proposed for Ca2+-CaM binding are shown as red boxes, whereas regions proposed for both apo-CaM and Ca2+-CaM binding are shown as pink boxes. [Figure adapted from Balshaw et al. (Balshaw et al., 2001) with modification.] The two green circles indicate positions where GFP was inserted (see Materials and Methods) into the sequence of RyR2 in two of the proposed CaM-binding regions, specifically, after residues Arg3595 and Lys4269.
To further test these potential CaM-binding sequences, in this study we used site-specific GFP insertion coupled with 3D cryo-EM, to map two of the proposed CaM-binding sequences. 3D localization of inserted GFP is based on structural differences between 3D reconstructions of GFP-inserted RyR2 and no-insertion RyR2 control. The two binding sequences that we investigated were: sequence 3581–3612 in RyR2 (corresponding to sequence 3614–3643 in RyR1) and sequence 4261–4286 in RyR2 (4303–4328 in RyR1). Fig. 1 illustrates schematically the two GFP insertion sites in the primary sequence and their correlation to the reported CaM-binding sequences. The DNA sequence of GFP was inserted after Arg3595 for mapping sequence 3581–3612 (henceforth named RyR2R3595-GFP), and after Lys4269 for the sequence 4261–4286 (henceforth named RyR2K4269-GFP), respectively. Extensive experimental evidence supports the view that amino-acid residues within the sequence 3614–3643 in RyR1 and the corresponding sequence 3581–3610 in RyR2 are involved in binding CaM (Balshaw et al., 2001; Chen and MacLennan, 1994; Guerrini et al., 1995; Menegazzi et al., 1994; Rodney et al., 2001; Xiong et al., 2002; Zhang et al., 2003a; Zhu et al., 2004). Sequence 4261–4286 in RyR1 (4303–4328 in RyR2) was first detected as a CaM-binding sequence in our 125I-labeled CaM binding study (Chen and MacLennan, 1994), and later confirmed in a 35S-labeled CaM binding study (Balshaw et al., 2001). This sequence is also close to several other proposed CaM-binding sequences (Balshaw et al., 2001; Guerrini et al., 1995) (see Fig. 1). Our 3D localization of GFP in RyR2R3595-GFP and RyR2K4269-GFP demonstrated that both sequence 3581–3612 and 4261–4286 could be CaM-binding sequences, since both of them are adjacent to the CaM-binding sites determined previously (Huang et al., 2012; Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997). Interestingly, the two GFP locations are also close to one another, allowing us to generate a fluorescence resonance energy transfer (FRET) probe to characterize the conformational changes around the CaM-binding site when the RyR switches between the closed and open states. Furthermore, our FRET study has also established that there are interactions between CaM-binding sequences 3581–3612 and 4261–4286 from the same subunit within the tetrameric RyR molecule. Thus, our data suggest that CaM-binding sequences 3581–3612 and 4261–4286 form an intra-subunit binding pocket for CaM.
Results
Construction of a GFP structural marker in the CaM-binding sequences
In previous cryo-EM analyses of RyR1/RyR2–CaM interactions, the physical locations of CaM binding were determined (Huang et al., 2012; Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997). To gain insight into which regions of the RyR amino-acid sequence are potentially involved in CaM binding, we used site-specific GFP insertion coupled with 3D localization of inserted GFP by cryo-EM techniques to map the CaM-binding sequences onto the 3D architecture of RyR2. Briefly, we genetically tagged the residue of interest with GFP, and identified the GFP location as an extra mass in the density map that was derived from cryo-EM. To minimize potential disruptive effects of the insertion on folding of both GFP and RyR2, two Gly-rich spacers (ten residues and nine residues) flanked the inserted GFP (Kratz et al., 1999). Most of our cryo-EM mappings using this method are consistent with other studies. For instance, 3D localization of Tyr2801 in the phosphorylation domain (Meng et al., 2007) is consistent with the docking models from two groups in which a crystal structure of RyR fragments containing the phosphorylated reside was fitted into a RyR cryo-EM structure (Sharma et al., 2012; Yuchi et al., 2012); 3D localization of Tyr846 (Wang et al., 2011) is consistent with a docking model based on a pseudo-atomic structure of the N-terminal fragment (Zhu et al., 2013); and 3D localization of Arg626 in the FK506-binding protein (FKBP)-adjoining domain by combining FRET and cryo-EM studies (Wang et al., 2011) is consistent with binding assays that implicate this region in the RyR–FKBP interaction (Girgenrath et al., 2013). However, a shortcoming of the long linkers is that they may allow GFP to move away from the target sites. In our previous mapping of Ser437 in the N-terminal region, the inserted GFP containing long linkers was located between domain 5 and 9 (Wang et al., 2007), roughly 60 Å from the insertion site that is predicted from the docking model of N-terminal ABC domains (Tung et al., 2010). The offset may come from the flexibility of linkers, from the flexibility of the loop structure of RyR2 where the Ser437 located, and from the cylindrical shape of GFP with an overall length of nearly 50 Å.
In this study, GFP mappings were done with our refined insertion protocol, in which both linkers are truncated by five or six residues, such that the entire GFP sequence, with a short Gly-rich linker (four residues) on each side, can be inserted into two of the proposed CaM-binding regions in RyR2: after Arg3595 for mapping the sequence 3581–3612, and after Lys4269 for the sequence 4261–4286. With the short linkers, we will be able to map the surface-exposed residues of RyR2 more precisely because of the reduced extendibility and flexibility from the linkers. If the GFP-labeled sequence is involved in CaM binding to RyR1, the GFP in the 3D reconstruction should be at or adjacent to one of the previously reported CaM-binding sites. The recombinant RyR2-GFP cDNAs were then expressed in HEK293 cells. The RyR2 function was characterized by measuring Ca2+ release induced by sequential additions of increasing concentrations of caffeine in HEK293 cells transfected with wild-type RyR2 (RyR2WT), RyR2R3595-GFP or RyR2K4269-GFP, using the fluorescent Ca2+ indicator dye fluo-3 AM. Fig. 2 shows that both RyR2R3595-GFP and RyR2K4269-GFP form functional Ca2+-release channel in HEK293 cells (Fig. 2B,C), with significantly reduced response to caffeine (Fig. 2H).
Fig. 2.
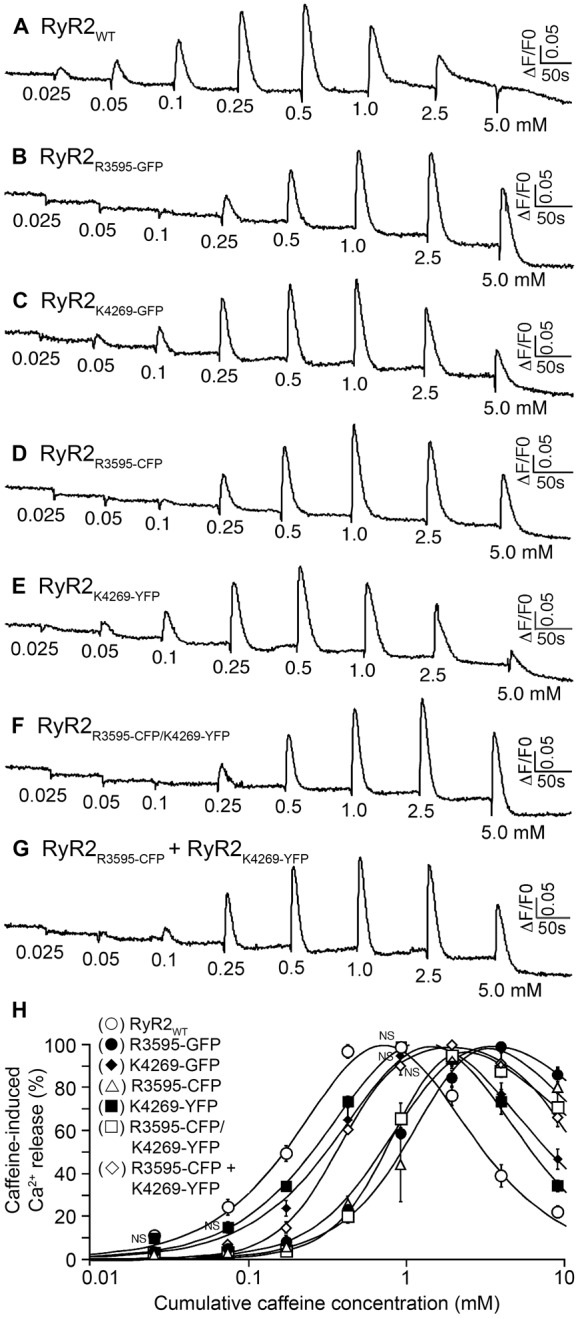
Functional characterization of RyR2s labeled with GFP, CFP or YFP. HEK293 cells were transfected with RyR2WT (A), RyR2R3595-GFP (B), RyR2K4269-GFP (C), RyR2R3595-CFP (D), RyR2K4269-YFP (E), or RyR2R3595-CFP/K4269-YFP (F), or co-transfected with both RyR2R3595-CFP and RyR2K4269-YFP cDNAs (G). Fluorescence intensity of fluo-3-AM-loaded transfected cells was monitored before and after the additions of various concentrations of caffeine. The numbers (under the traces) indicate caffeine concentrations. Traces shown are from representative experiments. (H) Ca2+ release in relation to cumulative caffeine concentration in transfected HEK293 cells. Data shown are means ± s.e.m. (n = 3). Except for those labeled ‘NS’ (not significant), all other data points of GFP-, CFP- or YFP-labeled RyR2 are significantly different from that of RyR2WT for a given caffeine concentration (P<0.05).
Two-dimensional and three-dimensional localization of R3595-GFP in RyR2
Purified RyR2R3595-GFP was imaged by cryo-EM (Fig. 3A). In the images taken without tilting the specimen, RyR2R3595-GFP showed a typical tetrameric RyR shape, which is a square with a central cross and four protruding corners (Fig. 3A). Images of untilted RyR2R3595-GFP particles were aligned and averaged to increase the signal-to-noise ratio (Fig. 3B), and compared with the averaged images of RyR2WT control (no-GFP-insertion; Fig. 3C). Four symmetrically related strong positive regions were apparent after subtracting the averaged images of the RyR2WT control from that of RyR2R3595-GFP (Fig. 3D). Statistical analysis (t-tests) showed these positive regions to be significant at more than 99.9% confidence levels. We ascribe these regions to the inserted GFP locations on RyR2R3595-GFP. Since the two-dimensional (2D) analysis described above was only done with untilted particles, all in the fourfold symmetric orientation, the localization provided XY coordinates for the GFP but the coordinate along the Z-axis (defined as parallel to the fourfold symmetry axis of RyR) was indeterminate (Fig. 3E), hence the need for 3D reconstructions.
Fig. 3.
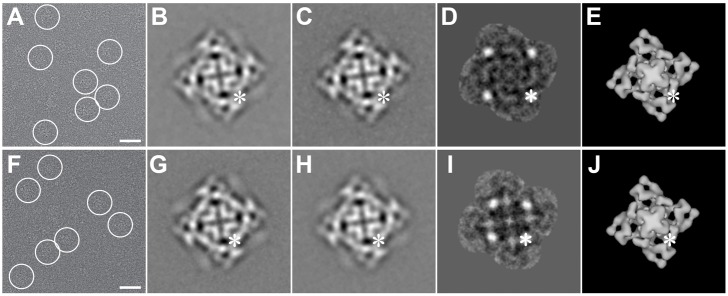
Two-dimensional analysis of RyR2R3595-GFP and RyR2K4269-GFP. (A,F) Cryo-EM CCD image of RyR2R3595-GFP and RyR2K4269-GFP, respectively, showing protein particles embedded in a thin layer of vitreous ice. The tetrameric structure of RyR2 is well preserved, and several individual particles are marked with circles. Scale bars: 500 Å. (B,G), 2D average of RyR2R3595-GFP and RyR2K4269-GFP, respectively, determined from 321 and 300 selected individual particles with defocus value ranges between −2.8 and −3.2 µm and between −2.8 and −3.6 µm, respectively. (C,H), 2D average of RyR2WT control from 380 and 333 particles in the same defocus range as were used for B and G, respectively. (D,I) The difference map obtained by subtracting the image in C from the image in B and the image in H from G, respectively. Bright white spots represent positive differences (one spot is highlighted by an asterisk), which correspond to the four extra masses contributed by GFP insertion. The images shown in B–D and G–H represent the projection of RyR as seen from the lumen side of the sarcoplasmic reticulum, which is shown for 3D models in E and J. The asterisk highlights the location of GFP within the plane of projection. The width of frames in B–J is 544 Å.
3D reconstruction of RyR2R3595-GFP identified a square prism shape, the standard RyR2 appearance (Liu et al., 2002; Sharma et al., 1998) composed of 14 cytoplasmic domains connecting to a transmembrane region (Fig. 4A,B). Compared with the no-GFP-insertion RyR2WT control, RyR2R3595-GFP contained four major excess density regions, each located between domains 3 and 8a (highlighted with red circles in Fig. 4A,B). Subtraction of the 3D volume of RyR2WT from RyR2R3595-GFP provided a clear picture of where the excess density regions were located (Fig. 4C,D). Because these regions were within the areas detected by 2D analysis and there were no other substantial excess densities nearby, we attribute them to be the locations of inserted GFP in RyR2R3595-GFP. This location is close to the previously mapped locations of both apo-CaM- and Ca2+-CaM-binding sites on RyR1, and of apo-CaM on RyR2 (Huang et al., 2012; Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997), and is therefore consistent with evidence for the RyR2 sequence 3581–3612 being involved in binding CaM.
Fig. 4.
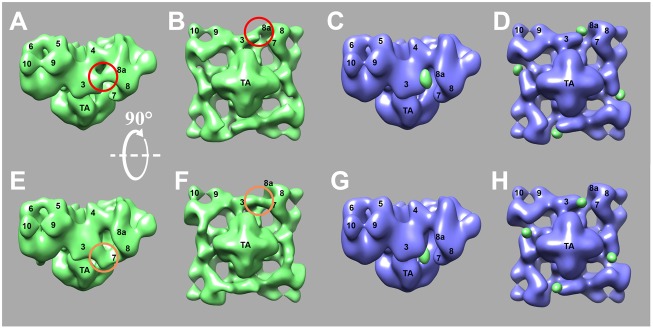
Three-dimensional reconstruction of RyR2R3595-GFP and RyR2K4269-GFP. (A,B,E,F) Surface representations of the 3D reconstruction of RyR2R3595-GFP (A,B) and RyR2K4269-GFP (E,F), shown in a side view (A,E) and in bottom view (B,F). The side view shows the surface that would be perpendicular to the plane of the sarcoplasmic reticulum membrane, and the bottom view shows the surface parallel to the sarcoplasmic reticulum membrane, which in situ would face the sarcoplasmic reticulum lumen. Red and orange circles highlight the locations of the extra masses representing GFP. (C,D,G,H) Surface representations of the 3D reconstruction of RyR2 control shown in blue, and the difference map [subtraction of 3D volume of RyR2 control from that of RyR2R3595-GFP (C and D) or RyR2K4269-GFP (G and H)] shown in green and superimposed on the 3D reconstruction of RyR2 control.
Two-dimensional and three-dimensional localization of K4269-GFP in RyR2
We next investigated the GFP location in the RyR2K4269-GFP. Purified RyR2K4269-GFP was imaged and analyzed using identical methods to those used for RyR2R3595-GFP. Cryo-EM showed the normal, standard RyR shape (Fig. 3F). 2D analysis (Fig. 3G–I) revealed four significant, symmetrically related positive peaks in the difference map, which represented the GFP location. Comparing the 3D reconstruction of RyR2K4269-GFP with RyR2WT control, we found that the two reconstructions are similar except for the extra masses representing the inserted GFP, which were clearly present on the side of domain 3 facing domain 7 (Fig. 4E–H). This location is also close to both the previously mapped Ca2+-CaM-binding site in RyR1 and the apo-CaM-binding site in RyR2 (Huang et al., 2012; Wagenknecht et al., 1997).
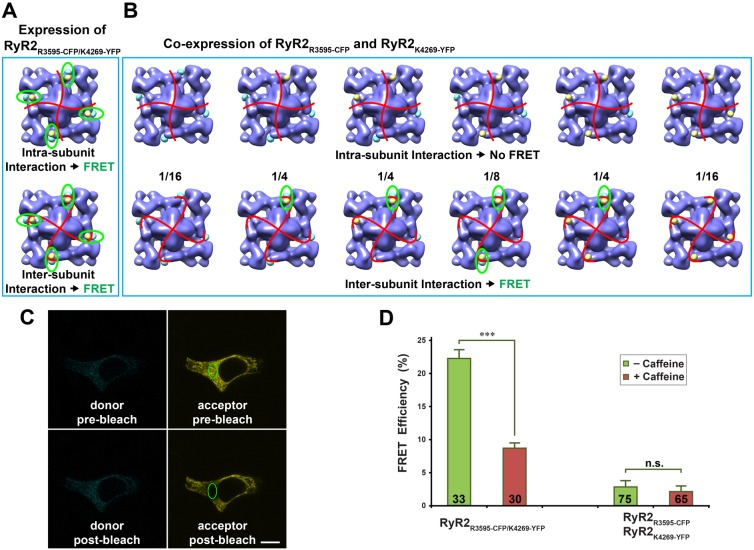
FRET characterization of dynamic conformational changes around the CaM-binding site
Based on the structural information from cryo-EM, we demonstrated that both sequences, 3581–3612 and 4261–4286 in RyR2 (3614–3643 and 4303–4328 in RyR1), are adjacent to the CaM-binding sites. Fortuitously, the two GFP reporters are located in close proximity, the distance between them being ∼40 Å, a spatial arrangement that is favorable for characterizing conformational dynamics by FRET. We first replaced the GFP insertion in the RyR2R3595-GFP and RyR2K4269-GFP with a cyan fluorescent protein (CFP) and a yellow fluorescent protein (YFP), respectively, to generate two single-insertion FRET probes, RyR2R3595-CFP and RyR2K4269-YFP. Subsequently, we constructed a cDNA with dual fluorescent protein insertions in the RyR2 primary sequence, RyR2R3595-CFP/K4269-YFP (Fig. 5A,B). We next carried out FRET analyses in HEK293 cells that were transfected with the cDNAs to probe for conformational changes around the CaM-binding site. Note that HEK293 cells expressing RyR2R3593-CFP, RyR2K4269-YFP, RyR2R3595-CFP/K4269-YFP, or co-expressing RyR2R3593-CFP and RyR2K4269-YFP form functional Ca2+ release channels (Fig. 2D–G). However, similar to GFP insertions, the CFP and YFP insertions also significantly suppressed the response of the RyR2 channel to caffeine (Fig. 2H).
Fig. 5.
Dynamic interaction between two CaM-binding sequences characterized by FRET analysis. (A) Structural model of the dual insertion RyR2R3595-CFP/K4269-YFP. The cyan and yellow spheres represent CFP and YFP, respectively. Each RyR2 molecule contains four FRET pairs (highlighted by green ellipses). Red curves represent a possible boundary between the four subunits. FRET signals will be detected regardless of whether two structural domains bearing R3595-CFP and K4269-YFP are contained within one RyR2 subunit (i.e. an intra-subunit interaction, top panel) or belong to two different subunits (i.e. an inter-subunit interaction, bottom panel). (B) Models of six possible hybrid RyR2 tetramers when two cDNAs encoding RyR2R3595-CFP and RyR2K4269-YFP are co-expressed. The top row shows six RyR2 tetramer structures for the situation in which two structural domains bearing R3595-CFP and K4269-YFP are contained within one RyR subunit. In this case, no FRET signal will be detected, since the distance between CFP and YFP in the two neighboring subunits is over 180 Å. The bottom row shows six RyR2 tetramer structures for the case in which the two structural domains bearing R3595-CFP and K4269-YFP belong to two different subunits. In this case, four out of six structures have at least one FRET pair (highlighted by green ellipses). Numbers between two rows are the mathematical probability ratios for the six possible structures, assuming that RyR2 tetramers formed by random assembly of the two differently labeled subunits. (C) Confocal images of a HEK293 cell expressing RyR2R3595-CFP/K4269-YFP cDNA, showing cyan and yellow fluorescence before and after photobleaching. The green ellipse demarcates the area selected for photobleaching. Scale bar: 10 µm. (D) FRET efficiency determined by photobleaching of acceptor. Data are means ± s.e.m., with the number of the cells indicated on the bars. ***P<0.001; n.s., not significant.
Fig. 5C shows the results of acceptor photobleaching experiments to measure FRET efficiency in the HEK293 cells that expressed RyR2R3595-CFP/K4269-YFP. Cyan (donor) and yellow (acceptor) fluorescence images were recorded before and after photobleaching of the acceptor and used to calculate the FRET efficiency. As shown in Fig. 5D, the average FRET efficiency in the cells that expressed RyR2R3595-CFP/K4269-YFP in the absence of any treatment was 22.1±1.1% (mean ± s.e.m., n = 33 cells). The addition of 5 mM caffeine, a pharmacological activator of RyR channel activity, significantly decreased the FRET efficiency to 8.7±0.5% (n = 30, P<0.001). The strong FRET that we observed supports the cryo-EM results that place residues R3595 and K4269 in close proximity, and the decrease in FRET efficiency upon caffeine treatment indicates that the two putative CaM-binding sequences separate from one another when the RyR channel switches from the closed to the open conformation (see model in Fig. 6).
Fig. 6.
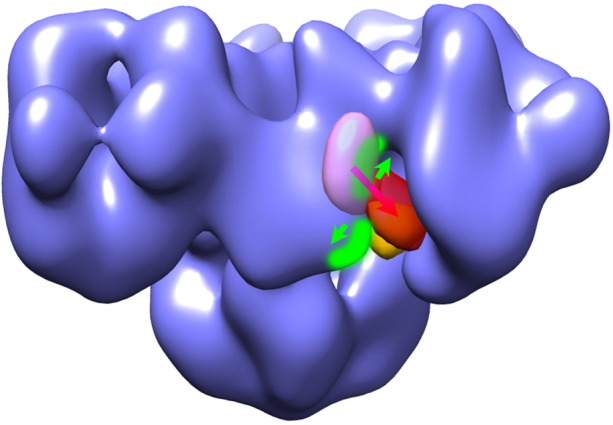
Hypothetical model of RyR conformation changes in the CaM-binding region and translocation of CaM when the RyR channel switches from closed to open. The transparent purple mass represents the apo-CaM-binding site, and the transparent red mass is the Ca2+-CaM-binding site in RyR1. The orange mass indicates the apo-CaM-binding site in RyR2. Two structural domains bearing CaM-binding sequences 3615–3644 and 4303–4328 in RyR1 are highlighted in green. Green arrows demonstrate that the two domains move apart when the RyR channel is activated, and the purple-red arrow indicates the shift of CaM from the apo-binding site to the Ca2+ binding site that occurs in RyR1 but not in RyR2. Figure adapted from Samsó and Wagenknecht and Huang et al., with modifications (Samsó and Wagenknecht, 2002; Huang et al., 2012).
When the two cDNAs (RyR2R3595-CFP and RyR2K4269-YFP) are co-expressed in HEK293 cells, various hybrid tetrameric RyR2s could form (see models in Fig. 5B). FRET pairs will be formed only when the following two criteria are both satisfied: (1) the donor (CFP) and acceptor (YFP) are within a certain distance of each other (normally below 100 Å); (2) the two domains containing CFP and YFP must belong to two neighboring RyR2 subunits. The models in the lower panel in Fig. 5B highlight FRET pairs that occur only when the two domains form an inter-subunit interaction. No FRET will be detected in the case of an intra-subunit interaction (top panel in Fig. 5B), because the distance between CFP and YFP in the two neighboring subunits is over 180 Å, which is far beyond the distance limitation between donor and acceptor for energy transfer. Little or no significant FRET (2–3%) or caffeine-induced reduction in FRET was observed in the cells co-expressing cDNAs of RyR2R3595-CFP and RyR2K4269-YFP (Fig. 5D). These residual, non-specific FRET signals most probably arise from the diffusion of nearby RyR2R3595-CFP into the photobleached region, because FRET was measured in live cells instead of fixed cells to maintain near physiological conditions. Using a fluorescence recovery after photobleaching (FRAP) experiment, we showed that the diffusion of RyR2 in the live cells would not substantially interfere with the FRET efficiency determined by the photobleaching approach, but a 3–5% RyR2 diffusion indeed exists in live cells (Liu et al., 2010). The observation of little or no FRET in the cells co-transfected with RyR2R3595-CFP and RyR2K4269-YFP indicates that these two closely located CaM-binding sequences are from the same subunit within the homo-tetrameric RyR.
Discussion
Sequences not directly involved in CaM binding
As indicated in Fig. 1, numerous regions in the sequences of RyR1 and RyR2 have been implicated in binding CaM. The physical locations of Ca2+-CaM and apo-CaM binding on the surface of RyR1 and of apo-CaM on RyR2 have previously been established by 3D cryo-EM (Huang et al., 2012; Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997). For this study we focused on the two CaM-binding sequences that contain residues Arg3595 and Lys4269 (RyR2 sequence numbers) because the proposed sites that are located N-terminally to residues 3581–3610 in RyR2 (3614–3643 in RyR1) are considered unlikely on the basis of subsequent experimental findings, particularly several cryo-EM analyses of GFP-insertions and docking models of crystal structures of RyR fragments. The proposed RyR2 CaM-binding sequence 263–615 (Balshaw et al., 2001) is incompatible with the EM data of Wang et al. and the X-ray crystallography/molecular docking results from Tung et al. (Wang et al., 2007; Tung et al., 2010). The predicted CaM-binding sequence 921–1173 in RyR1 (Chen and MacLennan, 1994) is also incompatible with experimental and modeling studies on a fragment that overlaps this sequence (Zhu et al., 2013). Proposed CaM-binding sequences in the vicinity of 2724–3016 in RyR2 (Balshaw et al., 2001) and 2804–2930 in RyR1 (Chen and MacLennan, 1994) contain a phosphorylation site that, according to cryo-EM (Meng et al., 2007) and X-ray crystallography and molecular docking results (Yuchi et al., 2012) is not near the known CaM-binding locations.
One of the proposed CaM-binding sequences located N-terminally to the sequence 3614–3643 in RyR1 deserves further consideration. Zhang et al. found that a peptide corresponding to RyR1 residues 1975–1999 binds apo-CaM and that this same region was protected from tryptic digestion in the presence of apo-CaM (Zhang et al., 2003a). Several attempts have been made by our laboratory to insert GFP into the corresponding region of RyR2, but these were unsuccessful, apparently because they interfered with binding the accessory protein FKBP12.6 to RyR2, which we make use of to purify the GFP-modified RyR2. However, cryo-EM of RyR2 containing GFP insertions that lie on either side of 1975–1999 (1942–1966 in RyR2), specifically at Thr1874 and Thr2023, showing that these insertions are not in the vicinity of known CaM-binding sites (Jones et al., 2008; Zhang et al., 2003b), but further study of this region is needed to ascertain whether or not the sequence 1975–1999 is involved in direct interactions with CaM.
Sequence 4261–4286
Several proposed CaM-binding sequences are on the C-terminal side of the well-established CaM-binding sequence, 3614–3643 in RyR1 (3581–3610 in RyR2). These sequences fall within the range 4480–4748 in RyR2 and within 4302–4621 in RyR1 (Fig. 1). Previously, we used GFP insertion and cryo-EM to map residue Asp4365 in RyR2 (corresponding to Asp4413 in RyR1) to a region in domain 3 that was within a few nanometers of the apo-CaM-binding location (Liu et al., 2002). Here we have inserted GFP at Lys4269 in RyR2, which corresponds to RyR1 residue 4311, which lies within the proposed CaM-binding sequences proposed by Chen and MacLennan (4303–4328) and by Balshaw et al. (4302–4430) in RyR1 (Balshaw et al., 2001; Chen and MacLennan, 1994).
The localization of 4261–4286 was quite unambiguous. Both 2D analysis and 3D reconstruction of RyR2K4269-GFP clearly resolved the inserted GFP, which localized to the bottom of domain 3 and facing domain 7, a location that is within a few nanometers of the known apo-CaM- and Ca2+-CaM-binding locations. However, biochemical evidence supporting the involvement of 4261–4286 of RyR2 (4303–4328 of RyR1) in binding of CaM is insufficient. Only two [125I]CaM and [35S]CaM overlay experiments (Balshaw et al., 2001; Chen and MacLennan, 1994), which detected the binding of RyR1 immobilized fragments to radio-labeled CaMs, showed that 4303–4328 and 4302–4430 of RyR1 were able to bind CaM. The binding of a RyR fragment to CaM does not necessarily indicate that CaM binds to that specific sequence within the intact RyR, because RyR folding might restrict exposure of the sequence and/or its conformation. Here we have shown for an intact RyR, rather than a RyR fragment, that GFP inserted within this region is probably at least partly surface-exposed as opposed to being internal (buried). If the site is internal, the insertion would probably disrupt the structure of RyR2, at least locally, and prevent GFP localization. Additionally, the fact that the GFP in RyR2K4269-GFP is located close to the actual CaM-binding site (Huang et al., 2012; Wagenknecht et al., 1997), supports the involvement of 4261–4286 of RyR2 in CaM binding. However, strong evidence against the involvement of this sequence is the lack of an effect on CaM binding to RyR1 when a region containing residues 4274–4535 is deleted by site-directed mutagenesis (Yamaguchi et al., 2001). Also, mutations within the sequence 3581–3612 are known to eliminate CaM binding to RyR (discussed below), suggesting that the sequence 4274–4535 is not capable of independent CaM binding. It is possible that the sequence 4274–4535 is capable of binding CaM with a much lower affinity than the 3581–3612 region, but at present there is no evidence for this, or for any physiologically relevant reason for such a secondary CaM-binding site. Nevertheless, we cannot exclude this region of RyR from playing some role in CaM binding.
Sequence 3581–3612
The interpretation of the results for GFP inserted within the sequence 3581–3612 was not straightforward because of its location in a cleft bordered by several RyR domains. GFP was found to locate between domains 3 and 8a (Fig. 4C,D), which is close to all three of the CaM-binding positions that have been determined (apo-CaM- and Ca2+-CaM-binding sites on RyR1 and apo-CaM-binding site on RyR2) (Huang et al., 2012; Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997). This location is consistent with 3581–3612 of RyR2 (and by inference the homologous sequence 3615–3644 in RyR1) as an authentic CaM-binding sequence, but there was still uncertainty as to whether R3595-GFP was linked to domain 3 or domain 8a. Considering the reported locations of apo-CaM and Ca2+-CaM on RyR1 as well as apo-CaM on RyR2 (Huang et al., 2012; Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997), we favor the domain 3 location for the sequence 3581–3612 because it is more compatible with both the Ca2+-CaM and apo-CaM physical binding locations. In addition, our FRET result indicates sequences 3581–3612 and 4261–4286 from the same subunit are located in close proximity in the tetrameric RyR2 molecule. This finding increases the possibility that both sequences occur on the same domain (domain 3). Future studies to determine the location of 3581–3612 more precisely could be crucial for understanding the regulatory mechanism of CaM.
Unlike sequence 4302–4328 of RyR1 (4261–4286 of RyR2), the sequence 3614–3643 of RyR1 (3581–3612 of RyR2) as a CaM-binding sequence is well supported by several types of studies, including amino-acid sequence prediction (Takeshima et al., 1989), CaM overlays (Balshaw et al., 2001; Chen and MacLennan, 1994; Guerrini et al., 1995; Menegazzi et al., 1994), point mutation and deletion mutation (Yamaguchi et al., 2001; Yamaguchi et al., 2003) as well as tryptic digestion protection (Moore et al., 1999). Sequence 3614–3643 of RyR1 (3581–3612 of RyR2) was also shown to be involved in binding both apo-CaM and Ca2+-CaM (Moore et al., 1999). We identified this sequence at the region of overlap between the apo-CaM-binding site on RyR1 and the apo-CaM-binding site on RyR2 (similar to the Ca2+-CaM-binding site on RyR1) as determined by cryo-EM (Huang et al., 2012; Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997), and this is fully consistent with all available evidence that supports this sequence as being essential for CaM binding to RyRs.
Conformational changes in the CaM-binding region of RyR and translocation of CaM
Previous functional studies showed that apo-CaM activates RyR1 but inhibits RyR2, whereas Ca2+-CaM inhibits both isoforms (Balshaw et al., 2001; Buratti et al., 1995; Damiani and Margreth, 2000; Rodney et al., 2000; Tripathy et al., 1995; Yamaguchi et al., 2003). Our previous cryo-EM studies showed distinctly different binding locations of apo-CaM and Ca2+-CaM on RyR1 (Samsó and Wagenknecht, 2002; Wagenknecht et al., 1997). Interestingly, apo-CaM binds to RyR2 at a similar location to that of Ca2+-CaM on RyR1, in seeming agreement with the inhibitory effects of these two forms of CaM on their respective receptors (Fig. 6) (Huang et al., 2012). Using a mutant CaM that is incapable of binding calcium, we have determined that the mutant CaM binds to RyR1 at the apo-CaM site, regardless of the calcium concentration. The existence of the two overlapping but distinct binding sites for apo-CaM and Ca2+-CaM on RyR1 imply that the binding location switch is due to Ca2+ binding to CaM, as opposed to direct effects of Ca2+ on RyR1, and that the switch to the alternative apo-CaM site on RyR1 (but not on RyR2) is involved in the activation effect of apo-CaM on RyR1 (Huang et al., 2012).
In this study, we demonstrated by cryo-EM that two hypothesized CaM-binding sequences in RyR2, 3581–3612 and 4261–4286 (corresponding to 3614–3643 and 4302–4328 in RyR1) are both in close proximity to the previously found locations of CaM. In addition, we found that when the RyR2 channel is activated, the structural domains bearing these two sequences move apart, indicating that conformational changes associated with channel activation occur in the vicinity of receptor-bound CaM. We suggest that these conformational changes also affect CaM binding, and vice versa, which would be consistent with the known influences CaM on channel gating. Clearly, more detailed studies of conformational changes in this region of RyR are needed. RyR activation is induced either by direct interaction with DHPR (for RyR1), by a small amount of Ca2+ influx from DHPR (for RyR2) or by Ca2+ released from SR stores through nearby RyRs (RyR1 and RyR2). Global conformational changes underlie RyR activation, including changes in the clamp region (Tian et al., 2013), in the transmembrane region that result in ion channel activation (Samsó et al., 2009), and, as we show here for the first time, in the CaM-binding region of RyR1. Upon RyR activation the two fluorophore-labeled sequences move apart, a structural change that is likely to be influenced by CaM. We hypothesize that for RyR2, the presence of either apo-CaM or Ca2+-CaM inhibits the conformational change, whereas for RyR1 apo-CaM facilitates and Ca2+-CaM inhibits it, thereby accounting for the regulatory effects of CaM on the activity of RyR2 and RyR1. Additional biophysical characterization, by FRET and other techniques, of this conformational change on both RyR2 and RyR1 and in the presence of CaM and calcium should allow us to refine or reject this hypothesis. It is also possible that the conformational change we have discovered plays a role in the termination of Ca2+ release, particularly for RyR2 for which the mechanism by which Ca2+ release is terminated during excitation–contraction coupling in the heart is not understood (Cannell and Kong, 2012; Stern and Cheng, 2004).
Materials and Methods
Materials and chemicals
The detergent CHAPS, the redox reagent DTT, and the protease inhibitor leupeptin were purchased from CalBiochem (La Jolla, CA, USA). All the other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). GFP-inserted mouse RyR2s were constructed, expressed in HEK293 cells and purified as described previously (Liu et al., 2002).
Construction of GFP-tagged RyR2s
The cloning and construction of the 15-kb full-length cDNA encoding the mouse RyR2 has been described previously (NCBI reference sequence NP_076357.2, GI:124430578) (Zhao et al., 1999). The cDNA encoding GFP with Gly-rich linkers (four residues) on each side and an AscI site was obtained by PCR. The AscI site was introduced into a cDNA fragment of RyR2 after a specific residue (Arg3595 or Lys4269) by overlap extension using PCR. The fragment with the AscI site was subcloned into the full-length RyR2 cDNA. The AscI–AscI fragment containing GFP and the linkers were then subcloned into the full-length RyR2 at the introduced AscI site. The sequences of all PCR fragments and the orientation of the inserted GFP cDNA were verified by DNA sequencing analysis.
Caffeine-induced Ca2+ release measurements
Free cytosolic Ca2+ concentration in transfected HEK293 cells was measured using the fluorescent Ca2+ indicator dye fluo-3 AM as described previously (Li and Chen, 2001).
Cryo-grid preparation
The expression and purification of RyR2R3595-GFP and RyR2K4269-GFP was carried out as described previously (Liu et al., 2002). Cryo-grids prepared for 2D and 3D localization of GFP-inserted RyR2s were prepared as follows. Purified GFP-inserted RyR2 (0.8 µl at a concentration about 0.2 mg/ml in 20 mM NaMOPS, pH 7.4, 1 M NaCl, 1% CHAPS, 2 mM DTT, 2 µg/ml leupeptin) was diluted with 4 µl buffer (20 mM NaMOPS, pH 7.4, 400 mM KCl, 3.0 mM EGTA, 0.5% CHAPS, 2 mM DTT, 2 µg/ml leupeptin). A 4-µl volume was applied to 300 mesh grids coated with a thin continuous carbon film suspended over a thick holey carbon film for 30 seconds. The grids were blotted with Whatman no. 1 filter paper for about 3 seconds before plunging into liquid ethane.
Cryo-electron microscopy and image processing
Cryo-EM data collection and image processing were performed in a similar manner to that described previously (Huang et al., 2012). The final 3D reconstructions of RyR2R3595-GFP (resolution 30 Å) and RyR2K4269-GFP (resolution 31 Å) were computed from 8002 and 6934 particles, respectively.
FRET measurement
For FRET analysis, the GFP insertion in the RyR2R3595-GFP and RyR2K4269-GFP were replaced by CFP and YFP, respectively. In addition to the single insertions, a dual insertion RyR2R3595-GFP/K4269-YFP was constructed as described previously (Liu et al., 2010). FRET measurements in the HEK293 cells expressing RyR2R3595-CFP/K4269-YFP, or co-expressing RyR2R3595-CFP and RyR2K4269-YFP were performed as described previously (Liu et al., 2010).
Acknowledgments
We gratefully acknowledge the 3D-EM and Advanced Light Microscopy and Image Analysis Core Facilities at the Wadsworth Center.
Footnotes
Author contributions
Z.L., T.W. and S.R.W.C. designed the research; X.H., Ying L., R.W., X.Z., Yingjie L., A.K. and Z.L. performed experiments; X.H., Yingjie L., S.R.W.C. and Z.L. analyzed data; X.H., S.R.W.C., T.W. and Z.L. wrote the manuscript.
Funding
This work was supported by the National Institutes of Health [grant numbers R01HL095541 to Z.L and R01AR040615 to T.W.]; and by the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada to S.R.W.C. Deposited in PMC for release after 12 months.
References
- Ai X., Curran J. W., Shannon T. R., Bers D. M., Pogwizd S. M. (2005). Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 97, 1314–1322 10.1161/01.RES.0000194329.41863.89 [DOI] [PubMed] [Google Scholar]
- Aracena P., Hidalgo C., Hamilton S. L. (2005). RyR1 modulation by calmodulin. Ryanodine Receptors: Structure, Function and Dysfunction in Clinical Disease Wehrens X H T, Marks A R, ed163–168New York, NY: Springer [Google Scholar]
- Bähler M., Rhoads A. (2002). Calmodulin signaling via the IQ motif. FEBS Lett. 513, 107–113 10.1016/S0014-5793(01)03239-2 [DOI] [PubMed] [Google Scholar]
- Balshaw D. M., Xu L., Yamaguchi N., Pasek D. A., Meissner G. (2001). Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J. Biol. Chem. 276, 20144–20153 10.1074/jbc.M010771200 [DOI] [PubMed] [Google Scholar]
- Buratti R., Prestipino G., Menegazzi P., Treves S., Zorzato F. (1995). Calcium dependent activation of skeletal muscle Ca2+ release channel (ryanodine receptor) by calmodulin. Biochem. Biophys. Res. Commun. 213, 1082–1090 10.1006/bbrc.1995.2238 [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Kong C. H. T. (2012). Local control in cardiac E-C coupling. J. Mol. Cell. Cardiol. 52, 298–303 10.1016/j.yjmcc.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Chen S. R., MacLennan D. H. (1994). Identification of calmodulin-, Ca(2+)-, and ruthenium red-binding domains in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 269, 22698–22704 [PubMed] [Google Scholar]
- Chen S. R. W., Li X., Ebisawa K., Zhang L. (1997). Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J. Biol. Chem. 272, 24234–24246 10.1074/jbc.272.39.24234 [DOI] [PubMed] [Google Scholar]
- Damiani E., Margreth A. (2000). Pharmacological clues to calmodulin-mediated activation of skeletal ryanodine receptor using [3H]-ryanodine binding. J. Muscle Res. Cell Motil. 21, 1–8 10.1023/A:1005635330773 [DOI] [PubMed] [Google Scholar]
- Girgenrath T., Mahalingam M., Svensson B., Nitu F. R., Cornea R. L., Fessenden J. D. (2013). N-terminal and central segments of the type 1 ryanodine receptor mediate its interaction with FK506-binding proteins. J. Biol. Chem. 288, 16073–16084 10.1074/jbc.M113.463299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R., Menegazzi P., Anacardio R., Marastoni M., Tomatis R., Zorzato F., Treves S. (1995). Calmodulin binding sites of the skeletal, cardiac, and brain ryanodine receptor Ca2+ channels: modulation by the catalytic subunit of cAMP-dependent protein kinase? Biochemistry 34, 5120–5129 10.1021/bi00015a024 [DOI] [PubMed] [Google Scholar]
- Huang X., Fruen B., Farrington D. T., Wagenknecht T., Liu Z. (2012). Calmodulin-binding locations on the skeletal and cardiac ryanodine receptors. J. Biol. Chem. 287, 30328–30335 10.1074/jbc.M112.383109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Meng X., Xiao B., Cai S., Bolstad J., Wagenknecht T., Liu Z., Chen S. R. W. (2008). Localization of PKA phosphorylation site, Ser(2030), in the three-dimensional structure of cardiac ryanodine receptor. Biochem. J. 410, 261–270 10.1042/BJ20071257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz P. A., Böttcher B., Nassal M. (1999). Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc. Natl. Acad. Sci. USA 96, 1915–1920 10.1073/pnas.96.5.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir A., Mollah A. K. M. M., Wehrens X. H. T. (2005). Evolution of the ryanodine receptor gene family. Ryanodine Receptors: Structure, Function and Dysfunction in Clinical Disease Wehrens X H T, Marks A R, ed1–8New York, NY: Springer [Google Scholar]
- Li P., Chen S. R. W. (2001). Molecular basis of Ca(2)+ activation of the mouse cardiac Ca(2)+ release channel (ryanodine receptor). J. Gen. Physiol. 118, 33–44 10.1085/jgp.118.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhang J., Li P., Chen S. R. W., Wagenknecht T. (2002). Three-dimensional reconstruction of the recombinant type 2 ryanodine receptor and localization of its divergent region 1. J. Biol. Chem. 277, 46712–46719 10.1074/jbc.M208124200 [DOI] [PubMed] [Google Scholar]
- Liu Z., Wang R., Tian X., Zhong X., Gangopadhyay J., Cole R., Ikemoto N., Chen S. R. W., Wagenknecht T. (2010). Dynamic, inter-subunit interactions between the N-terminal and central mutation regions of cardiac ryanodine receptor. J. Cell Sci. 123, 1775–1784 10.1242/jcs.064071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegazzi P., Larini F., Treves S., Guerrini R., Quadroni M., Zorzato F. (1994). Identification and characterization of three calmodulin binding sites of the skeletal muscle ryanodine receptor. Biochemistry 33, 9078–9084 10.1021/bi00197a008 [DOI] [PubMed] [Google Scholar]
- Meng X., Xiao B., Cai S., Huang X., Li F., Bolstad J., Trujillo R., Airey J., Chen S. R. W., Wagenknecht T. et al. (2007). Three-dimensional localization of serine 2808, a phosphorylation site in cardiac ryanodine receptor. J. Biol. Chem. 282, 25929–25939 10.1074/jbc.M704474200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. P., Rodney G., Zhang J-Z., Santacruz-Toloza L., Strasburg G., Hamilton S. L. (1999). Apocalmodulin and Ca2+ calmodulin bind to the same region on the skeletal muscle Ca2+ release channel. Biochemistry 38, 8532–8537 10.1021/bi9907431 [DOI] [PubMed] [Google Scholar]
- Rodney G. G., Williams B. Y., Strasburg G. M., Beckingham K., Hamilton S. L. (2000). Regulation of RYR1 activity by Ca(2+) and calmodulin. Biochemistry 39, 7807–7812 10.1021/bi0005660 [DOI] [PubMed] [Google Scholar]
- Rodney G. G., Moore C. P., Williams B. Y., Zhang J-Z., Krol J., Pedersen S. E., Hamilton S. L. (2001). Calcium binding to calmodulin leads to an N-terminal shift in its binding site on the ryanodine Receptor. J. Biol. Chem. 276, 2069–2074 [DOI] [PubMed] [Google Scholar]
- Samsó M., Wagenknecht T. (2002). Apocalmodulin and Ca2+-calmodulin bind to neighboring locations on the ryanodine receptor. J. Biol. Chem. 277, 1349–1353 10.1074/jbc.M109196200 [DOI] [PubMed] [Google Scholar]
- Samsó M., Feng W., Pessah I. N., Allen P. D. (2009). Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 7, e85 10.1371/journal.pbio.1000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M. R., Penczek P., Grassucci R., Xin H-B., Fleischer S., Wagenknecht T. (1998). Cryoelectron microscopy and image analysis of the cardiac ryanodine receptor. J. Biol. Chem. 273, 18429–18434 10.1074/jbc.273.29.18429 [DOI] [PubMed] [Google Scholar]
- Sharma P., Ishiyama N., Nair U., Li W., Dong A., Miyake T., Wilson A., Ryan T., MacLennan D. H., Kislinger T. et al. (2012). Structural determination of the phosphorylation domain of the ryanodine receptor. FEBS J. 279, 3952–3964 10.1111/j.1742-4658.2012.08755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D., Cheng H. (2004). Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium 35, 591–601 10.1016/j.ceca.2004.01.013 [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. et al. (1989). Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339, 439–445 10.1038/339439a0 [DOI] [PubMed] [Google Scholar]
- Tian X., Liu Y., Liu Y., Wang R., Wagenknecht T., Liu Z., Chen S. R. W. (2013). Ligand-dependent conformational changes in the clamp region of the cardiac ryanodine receptor. J. Biol. Chem. 288, 4066–4075 10.1074/jbc.M112.427864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A., Xu L., Mann G., Meissner G. (1995). Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys. J. 69, 106–119 10.1016/S0006-3495(95)79880-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung C. C., Lobo P. A., Kimlicka L., Van Petegem F. (2010). The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature 468, 585–588 10.1038/nature09471 [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Radermacher M., Grassucci R., Berkowitz J., Xin H-B., Fleischer S. (1997). Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J. Biol. Chem. 272, 32463–32471 10.1074/jbc.272.51.32463 [DOI] [PubMed] [Google Scholar]
- Wang R., Chen W., Cai S., Zhang J., Bolstad J., Wagenknecht T., Liu Z., Chen S. R. W. (2007). Localization of an NH(2)-terminal disease-causing mutation hot spot to the “clamp” region in the three-dimensional structure of the cardiac ryanodine receptor. J. Biol. Chem. 282, 17785–17793 10.1074/jbc.M700660200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhong X., Meng X., Koop A., Tian X., Jones P. P., Fruen B. R., Wagenknecht T., Liu Z., Chen S. R. W. (2011). Localization of the dantrolene-binding sequence near the FK506-binding protein-binding site in the three-dimensional structure of the ryanodine receptor. J. Biol. Chem. 286, 12202–12212 10.1074/jbc.M110.194316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L-W., Newman R. A., Rodney G. G., Thomas O., Zhang J-Z., Persechini A., Shea M. A., Hamilton S. L. (2002). Lobe-dependent regulation of ryanodine receptor type 1 by calmodulin. J. Biol. Chem. 277, 40862–40870 10.1074/jbc.M206763200 [DOI] [PubMed] [Google Scholar]
- Xu L., Meissner G. (2004). Mechanism of calmodulin inhibition of cardiac sarcoplasmic reticulum Ca2+ release channel (ryanodine receptor). Biophys. J. 86, 797–804 10.1016/S0006-3495(04)74155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Xin C., Meissner G. (2001). Identification of apocalmodulin and Ca2+-calmodulin regulatory domain in skeletal muscle Ca2+ release channel, ryanodine receptor. J. Biol. Chem. 276, 22579–22585 10.1074/jbc.M102729200 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Xu L., Pasek D. A., Evans K. E., Meissner G. (2003). Molecular basis of calmodulin binding to cardiac muscle Ca(2+) release channel (ryanodine receptor). J. Biol. Chem. 278, 23480–23486 10.1074/jbc.M301125200 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Xu L., Pasek D. A., Evans K. E., Chen S. R. W., Meissner G. (2005). Calmodulin regulation and identification of calmodulin binding region of type-3 ryanodine receptor calcium release channel. Biochemistry 44, 15074–15081 10.1021/bi051251t [DOI] [PubMed] [Google Scholar]
- Yuchi Z., Lau K., Van Petegem F. (2012). Disease mutations in the ryanodine receptor central region: crystal structures of a phosphorylation hot spot domain. Structure 20, 1201–1211 10.1016/j.str.2012.04.015 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang J-Z., Danila C. I., Hamilton S. L. (2003a). A noncontiguous, intersubunit binding site for calmodulin on the skeletal muscle Ca2+ release channel. J. Biol. Chem. 278, 8348–8355 10.1074/jbc.M209565200 [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Z., Masumiya H., Wang R., Jiang D., Li F., Wagenknecht T., Chen S. R. W. (2003b). Three-dimensional localization of divergent region 3 of the ryanodine receptor to the clamp-shaped structures adjacent to the FKBP binding sites. J. Biol. Chem. 278, 14211–14218 10.1074/jbc.M213164200 [DOI] [PubMed] [Google Scholar]
- Zhao M., Li P., Li X., Zhang L., Winkfein R. J., Chen S. R. W. (1999). Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 274, 25971–25974 10.1074/jbc.274.37.25971 [DOI] [PubMed] [Google Scholar]
- Zhu X., Ghanta J., Walker J. W., Allen P. D., Valdivia H. H. (2004). The calmodulin binding region of the skeletal ryanodine receptor acts as a self-modulatory domain. Cell Calcium 35, 165–177 10.1016/j.ceca.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhong X., Chen S. R. W., Banavali N., Liu Z. (2013). Modeling a ryanodine receptor N-terminal domain connecting the central vestibule and the corner clamp region. J. Biol. Chem. 288, 903–914 10.1074/jbc.M112.429670 [DOI] [PMC free article] [PubMed] [Google Scholar]