Abstract
Abnormal serotonin type 1A (5-HT1A) receptor function and binding have been implicated in the pathophysiology of mood disorders. Preclinical studies have consistently shown that stress decreases the gene expression of 5-HT1A receptors in experimental animals, and that the associated increase in hormone secretion plays a crucial role in mediating this effect. Chronic administration of the mood stabilizers lithium and divalproex (valproate semisodium) reduces glucocorticoid signaling and function in the hippocampus. Lithium has further been shown to enhance 5-HT1A receptor function. To assess whether these effects translate to human subject with bipolar disorder (BD), positron emission tomography (PET) and [18F]trans-4-fluoro-N-(2-[4-(2-methoxyphenyl) piperazino]-ethyl)-N-(2-pyridyl) cyclohexanecarboxamide ([18F]FCWAY) were used to acquire PET images of 5-HT1A receptor binding in 10 subjects with BD, before and after treatment with lithium or divalproex. Mean 5-HT1A binding potential (BPP) significantly increased following mood stabilizer treatment, most prominently in the mesiotemporal cortex (hippocampus plus amygdala). When mood state was also controlled for, treatment was associated with increases in BPP in widespread cortical areas. These preliminary findings are consistent with the hypothesis that these mood stabilizers enhance 5-HT1A receptor expression in BD, which may underscore an important component of these agents' mechanism of action.
Keywords: Positron-emission tomography, lithium, valproic acid, serotonin type 1A receptor, bipolar disorder
Introduction
Serotonin type 1A (5-HT1A) receptor function has been implicated in the pathophysiology of both bipolar disorder (BD) and major depressive disorder (MDD). Furthermore, the effects of chronic lithium and divalproex (valproate semisodium) treatment on neuroendocrine function in patients with BD suggest that mood stabilizing medications enhance post-synaptic 5-HT1A receptor function (reviewed in Drevets et al., 2007; Savitz et al., 2009). In humans, genetic variation in the 5-HT1A receptor gene (HTR1A; rs6295) that putatively reduces post-synaptic 5-HT1A receptor expression (Drevets et al., 2007; Le Francois et al., 2008; Szewczyk et al., 2009) has been associated with increased risk for developing BD, MDD (Lemonde et al., 2003), and major depressive episodes following interferon treatment (Kraus et al., 2007) or hip fracture (Lenze et al., 2008). In addition, the single nucleotide polymorphism (SNP) rs6295 on the 5-HT1A gene has been associated with BD in a meta-analysis of Japanese samples (Kishi et al., 2011). Moreover, epigenetic studies have demonstrated increased peripheral DNA methylation in the promotor region of HTR1A in BD (Carrard et al., 2011).
In unmedicated individuals with BD, post-synaptic 5-HT1A receptor binding (measured as binding potential, BP) reportedly differs with respect to healthy controls. For instance, 5-HT1A receptor density was significantly decreased in the prefrontal cortex in a postmortem study of BD subjects versus controls (Gray et al., 2006). Another post-mortem study found that 5-HT1A mRNA expression was decreased in the dorsolateral prefrontal cortex and hippocampus of MDD subjects, and that BD subjects showed non-significant trends in the same direction versus controls (Lopez-Figueroa et al., 2004). Using positron emission tomography (PET) and [11C]carbonyl-WAY100635, an initial series demonstrated that 5-HT1A binding potential (BPND, the ratio of specifically bound to nondisplaceable radioligand) was reduced in the mesiotemporal cortex (MTC) and other regions in unmedicated depressed subjects with BD or bipolar spectrum disorder who also showed significantly elevated stressed plasma cortisol levels relative to controls (Drevets et al., 1999, 2007; Moses-Kolko et al., 2007). More recently, we assessed 5-HT1A receptor binding in unmedicated BD subjects (n=26) and healthy controls (n=37) using PET and the highly selective 5-HT1A radioligand [18F] trans-4-fluoro-N-(2-[4-(2-methoxyphenyl) piperazino]-ethyl)-N-(2-pyridyl) cyclohexanecarboxamide ([18F]FCWAY); in the MTC the mean BPP (ratio of specifically bound radioligand to plasma radioligand) value was significantly lower in BD subjects versus controls, and individual BPP values were inversely correlated with trough plasma cortisol levels (Nugent et al., in press). In contrast, another study that assessed 5-HT1A receptor binding using PET and [11C] carbonyl-WAY100635 reported that the BPF (ratio of specifically bound radioligand to free radioligand in tissue) was increased in BD subjects versus controls, although this study did not include measures of cortisol secretion (Sullivan et al., 2009). Notably, Sargent and colleagues found no abnormalities in 5-HT1A receptor binding in euthymic BD subjects who were currently medicated with psychotropic medications; most were receiving either lithium or divalproex, suggesting that effective mood stabilizing treatment normalizes 5-HT1A expression (Sargent et al., 2010).
The effects of lithium and divalproex on neuroendocrine function or 5-HT metabolites suggest that they enhance serotonergic neurotransmission (Price et al., 1990). In patients with mood disorders, lithium treatment enhanced plasma prolactin response to L-tryptophan (Cowen et al., 1991) as well as plasma cortisol response to fenfluramine, suggesting increased 5-HT neurotransmission (Mannel et al., 1997; Muhlbauer and Muller-Oerlinghausen, 1985). The addition of lithium to antidepressant drug treatment increased plasma 5-HIAAA (5-hydroxyindoleacetic acid, the main metabolite of serotonin) concentrations in depressed subjects with MDD, suggesting enhanced 5-HT turnover, although platelet and plasma 5-HT did not change (Birkenhager et al., 2007). Subchronic divalproex administration attenuated the hypothermia induced by ipsapirone without affecting the associated increase in ACTH/cortisol release in healthy volunteers (Shiah et al., 1997), but increased the serotonin precurser L-5-hydroxy-tryptophan (L-5-HTP) induced cortisol response in manic subjects, suggesting that divalproex enhances central 5-HT neurotransmission in BD (Maes et al., 1997). These effects may be mediated at the receptor level rather than the post-receptor level, as divalproex did not affect 5-HT-induced calcium mobilization in the platelets of healthy volunteers (Kusumi et al., 1994). Notably, post-synaptic 5-HT1A receptor stimulation plays a role in ACTH and cortisol release (reviewed in Lesch et al., 1990; Li et al., 2004; Savitz et al., 2009); therefore, these data collectively support the hypothesis that post-synaptic 5-HT1A receptor function increases in BD subjects following treatment with lithium or divalproex.
The current preliminary study investigated whether treatment of a small group of BD subjects with lithium and/or divalproex would be associated with increases in post-synaptic 5-HT1A receptor BPND, as measured via PET. This is the first study to use a longitudinal design to measure treatment effects on 5-HT1A receptor binding in BD subjects, and the results may further elucidate the mechanism of action of mood stabilizing drugs.
Methods and materials
Patients and controls
This research was funded and carried out under the National Institute of Mental Health (NIMH) Intramural Research Program (IRP). Subjects were informed regarding the purpose of the study and the risks involved, and gave written consent as approved by the National Institutes of Health (NIH) Combined Neuroscience Institutional Review Board and the NIH Radiation Safety Committee. The participants (n=10; 9 female; mean age 34±11.4 years, range 23–54 years) met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; APA, 2000) criteria for either BD-I, most recent episode depressed, or BD-II, most recent episode depressed. In addition, all BD patients met criteria for a current major depressive episode, and no subject met DSM-IV-TR criteria for a “mixed bipolar” episode based on DSM-IV-TR criteria. Diagnosis was established by both an unstructured diagnostic interview conducted by a psychiatrist (authors PJC, EEB, or WCD) and the Structured Clinical Interview for DSM-IV-TR (First et al., 2002). Volunteers were excluded from participation if they had: exposure to psychotropic medications within the three weeks prior to the first PET scan; a history of substance dependence (excluding nicotine); a history of substance abuse within six months; laboratory evidence of hepatic, thyroid, or renal impairment; a positive urine pregnancy test; or were currently pregnant or nursing. Female subjects were not scanned during a specific menstrual phase so that treatment of acute depressive symptoms was not delayed. Previous studies have not shown significant differences in 5-HT1A binding between menstrual phases (Jovanovic et al., 2006, 2009).
Following the baseline scan, participants were treated with lithium and/or divalproex for at least three months prior to the post-treatment scan; choice of drug was guided by patient history and clinical issues (divalproex, or valproate semisodium, is a compound of sodium valproate and valproic acid). Drug dosage was guided using conventional clinical guidelines and therapeutic blood monitoring (American Psychiatric Association, 2002). One subject also received bupropion to manage depressive symptoms, which was not expected to significantly alter 5-HT1A binding. Clinical depression and mania symptoms were assessed using the Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979) and the Young Mania Rating Scale (YMRS: Young et al., 1978) on both days of PET scanning. Baseline PET data from all subjects were previously published as part of a larger sample of BD patients whose 5-HT1A receptor binding potentials were compared to those of healthy controls (Nugent et al., in press).
PET image acquisition
PET scans were acquired with a GE Advance tomograph (35 contiguous slices, 4.25 mm plane separation; reconstructed resolution=7 mm full-width at half-maximum (FWHM) in all planes) (DeGrado et al., 1994). A transmission scan was performed to enable attenuation correction; a 120-minute dynamic emission scan then was initiated following intravenous bolus administration of approximately 8 mCi of [18F]FCWAY. All scans were performed between 10:00–13:30, and the largest difference in time of day between a patient's pre-treatment and post-treatment scans was 1 h 17 min.
Arterial input function
Whole blood and plasma radioactivity were measured during PET scanning by radial artery sampling. The method of parent compound and metabolite concentrations was performed as previously described (Nugent et al., in press). The fraction of parent radioligand in plasma that was unbound by plasma proteins (fp) was determined from a blood sample drawn from each subject prior to tracer infusion, to which parent radioligand was added.
Magnetic resonance (MR) anatomical imaging
To provide an anatomical framework for the PET data analysis, whole brain anatomical images were obtained with a GE Sigma Scanner (3.0 Tesla) and a 3D MPRAGE sequence (Echo time, TE=2.982 ms, repetition time, TR=7.5 ms, inversion time=725 ms, voxel size=0.9×0.9×1.2 mm). Non-brain tissues were removed from the brain images using either a combination of the brain extraction tool (BET) (Smith, 2002) and manual editing, or the Analysis of Functional NeuroImages (AFNI) tool 3dSkullStrip (NIMH, NIH, Besthesda, Maryland, USA). The resulting whole brain images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) components using the FMRIB automated segmentation tool (FAST) (Zhang et al., 2001), and separate binary mask images were created for each component.
PET image processing and analysis
Individual dynamic PET images were corrected for motion and attenuation and then coregistered with the anatomical MR image. Partial volume correction (PVC) was applied with the Muller-Gartner et al. (1992) method. PVC was applied frame by frame with the mean white matter value estimated as per Giovacchini and colleagues (2005). Images were corrected for intravascular activity (5%), for the partial volume effects of skull activity from [18F]fluoride, and for the [18F]FC metabolite as described by Carson and colleagues (2003). Tissue time-radioactivity curves were then generated on a voxel-wise basis and fitted to a four-parameter, two-tissue compartment model with one parameter fixed for rapid parametric image calculation (Carson et al., 2002). The distribution volume (VT) was calculated as VT = K1/k2·(1 + k3/k4), where K1 is the rate constant for transfer from arterial plasma to tissue, and the remaining k rate constants represent transfer rates between tissue compartments.
Image analysis
Regions of interest (ROI) were defined in structures with abundant post-synaptic 5-HT1A receptor concentrations on a template image, then transferred to a co-registered magnetic resonance image (MRI) and adjusted to accommodate individual anatomy. Regions were defined in the left and right (L and R) mesial temporal cortex (MTC, hippocampus plus amygdala), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), anterior insula (AI), and parieto-occipital cortex (POC), as described by Neumeister and colleagues (2004). The ROI were then transformed from the stereotaxic space of the template back to the original space of the subject's MRI and applied to the VT image. The VT value for each ROI was defined as the mean VT value for all gray matter voxels within that ROI, using the binary gray matter mask derived from the MRI. Regions normally of interest lying close to the skull (e.g. orbital cortex, temporal polar cortex) could not be examined because the [18F]fluoride secondary metabolite of [18F]FCWAY is largely taken up by bone in [18F] FCWAY images.
The anatomical boundaries of the raphe nucleus are not clearly evident in MR images, so the ROI for measuring radioactivity in this structure was defined directly on PET images (Drevets et al., 1999; Toczek et al., 2003). A cylindrical ROI (9 mm diameter, 4.8 mm height) was positioned over the raphe in the VT image with the inferior-most aspect situated at the midbrain/pontine junction. The raphe VT values were measured by applying the ROI to VT images calculated without PVC, because the raphe binding estimates that use gray matter-based PVC would be highly sensitive to small errors in delineation of the raphe border. Where low binding made localization difficult on the PET images, the raphe ROI was placed on the MRI image based upon the spatial relationship between the raphe and the cerebral aqueduct.
A reference tissue ROI was defined in the cerebellar white matter (Parsey et al., 2005). directly on the MRI image using 12 mm diameter circular regions in the left and right cerebellar peduncles, in a slice roughly centered superiorly/inferiorly in the peduncles. The mean of the left and right distribution volumes was used as the reference VT.
Several choices of dependent variable are available to describe radiotracer binding in PET studies. Binding potential (BP) estimates specific binding to target receptors with reference to some reference concentration. Following consensus definitions and nomenclature (Innis et al., 2007), we chose as our primary outcome measure the most reliable measure, BPP, a measure of BP relative to total plasma concentration (VT ROI–VND = K1k3/k2k4 = fp Bavail/KD). As a secondary measure, we also calculated BPF, calculated as (VT ROI–VND)/fp =(1/fp)·K1k3/k2k4; this measure gives specific binding relative to free plasma concentration of tracer. We treated this parameter as a secondary measure because of its relatively greater sensitivity to measurement error in fp.
Statistical analysis
Pre- and post-treatment clinical ratings were compared using paired-sample t-tests. To investigate possible sources of bias in the BP measures, paired t-tests also were performed to detect differences before and after treatment in fp and VT in the cerebellar reference region.
Because we expected that all regional values would be correlated, in addition to correlations between measures over time, we carried out a linear mixed model with both time (pre-/post-treatment) and region as repeated measures and a compound symmetry covariance matrix. Diagnosis, time, gender, age, and MADRS score were fixed effects. Only main effects were included in the model, with the exception of the region by time interaction, to assess regional specificity of any significant treatment effects. Eleven regions were examined: left and right MTC, ACC, PCC, AI, POC, and raphe nucleus. False discovery rate (FDR) correction for multiple comparisons was applied, and we also reported whether p-values remained significant after applying the more conservative Bonferroni correction. Post-hoc tests of estimated un-weighted means were carried out to assess the difference between diagnostic groups in each region. We examined BP (BPP, BPF) in this manner. To determine if changes in binding correlated with clinical improvement, we repeated our mixed models adding the main effect of MADRS score.
Results
Demographic and clinical characteristic of the subject sample appear in Table 1. Three subjects had BD-I. Seven subjects (two with BD-I) were treated with lithium monotherapy, and one (BD-I) was treated with a combination of lithium and divalproex; the remainder were taking divalproex monotherapy. At the time of baseline scanning, the mean depression severity was in the moderate range, with three individuals exhibiting mild depression, and seven subjects exhibiting moderate depressive symptoms. At the post-treatment scan, the mean change in MADRS scores showed a non-significant trend towards improvement (t=2.164, p=0.062). Following treatment, three subjects had MADRS scores in the moderate range (20–33), four subjects had mild residual depressive symptoms (pre-treatment mean=22, post-treatment mean=9), two subjects had scores in the remitted range (1–4), and the post-treatment MADRS score was missing for one patient. YMRS scores significantly decreased (t=3.010, p=0.020) following treatment (pre-scan YMRS unavailable for one subject, and post-scan YMRS unavailable for another subject).
Table 1.
Demographic information for included subjects, means (standard deviations) are given.
| n | Age | Gender | Onset age | Time off meds (wks) n=8a | MADRS | YMRS | Injected dose (mCi) | Free fraction | Ref VT | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | 10 | 34 (11.4) | 9 F (90%) | 16 (4.9) | 54 (54.0) range: 7–145 | 23 (5.8) range: 12–32 | 9 (5.1) (n=9) range: 0–10 | 7.91 (0.67) | 0.117 (0.047) | 0.486 (0.292) |
| Post-treatment | - | - | - | - | - | 13 (10.6) (n=9) range: 1–25 | 5 (3.6) (n=9) range 3–18 | 7.98 (0.10) | 0.113 (0.045) | 0.482 (0.255) |
We were unable to establish time off medications for one subject; however, the subject met the minimum time off medications allowable by the study (three weeks).
MADRS: Montgomery-Asberg Depression Rating Scale; VT: distribution volume; YMRS: Young Mania Rating Scale.
The free fraction of [18F]FCWAY in plasma and in the reference tissue VT did not change significantly between pre- and post-treatment scans (Table 1, p=0.85 and p=0.97, respectively).
Test of the a priori hypothesis that regional BPP increases following treatment
In the linear mixed model, BPP showed a significant main effect of time (F1,196.9=26. 9, p<0.001), with mean BPP increasing following treatment. A significant main effect of region was also observed (F10,189.0=48.5, p<0.001). Age had no significant effect (F1,9.505=0.001, p=0.978). Although the time×region interaction was not significant (F10,189.0=0.775, p=0.653), post-hoc tests were used to evaluate regional specificity in the main effect of time finding (Table 2 and Figure 1). After applying either the FDR or the Bonferroni correction for multiple comparisons, a significant increase in binding was noted in the LMTC. The main effect of time remained significant after removing the subject exposed to bupropion (F=32.35, p<0.001). The significant increase in BPP in the LMTC was also retained when this subject was removed from the analysis (p<0.001).
Table 2.
Unweighted means from the mixed model for binding potential (BPP) pre- and post-treatment. Means are given for the mixed model both with and without using Montgomery-Asberg Depression Rating Scale (MADRS) as a covariate.
| BPP | Without MADRS covariate |
With MADRS covariate |
||
|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| LACC | 4.213 | 4.922 | 3.893 | 5.415a |
| RACC | 4.333 | 4.948 | 4.012 | 5.339a |
| LAI | 4.653 | 5.391 | 4.332 | 5.752a |
| RAI | 4.684 | 5.483 | 4.363 | 5.869a |
| LMTC | 7.456 | 9.121a | 7.136 | 9.455a |
| RMTC | 7.286 | 8.219b | 6.966 | 8.678a |
| LPCC | 3.918 | 4.485 | 3.597 | 4.944a |
| RPCC | 3.710 | 4.312 | 3.390 | 4.823a |
| LPOC | 3.624 | 4.424 | 3.304 | 4.756a |
| RPOC | 3.702 | 4.333 | 3.382 | 4.730a |
| Raphe | 3.314 | 3.257 | 2.993 | 3.580 |
Indicates that post-treatment value is significantly different from pre-treatment value at p<0.05 after both FDR and Bonferroni correction for multiple comparisons.
Indicates that post-treatment value trended towards a significant difference from pre-treatment value at 0.05<p<0.1 after FDR correction for multiple comparisons.
LACC and RACC: left and right anterior cingulate cortex; LAI and RAI: left and right anterior insula; LMTC and RMTC: left and right mesiotemporal cortex; LPCC and RPCC: left and right posterior cingulate cortex, LPOC and RPOC: left and right parieto-occipital cortex. MADRS: Montgomery-Asberg Depression Rating Scale.
Figure 1.
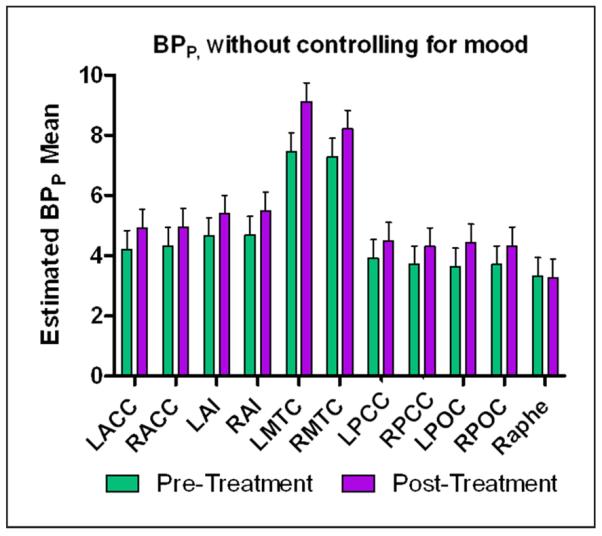
Graph of means derived from the mixed model of binding potential (BPP) pre- and post-treatment without controlling for mood scores. The significant increase in BPP before and after treatment in the left mesiotemporal cortex (MTC) survived Bonferroni and false discovery rate (FDR) correction for multiple comparisons. Error bars represent standard error.
LACC: left anterior cingulate cortex; LAI: left anterior insula; LMTC: left mesiotemporal cortex; LPOC: left parieto-occipital cortex; RACC: right anterior cingulate cortex; RAI: right anterior insula; RMTC: right mesiotemporal cortex; RPOC: right parieto-occipital cortex.
Results for secondary outcome measure: BPF
When the mixed model was repeated for BPF, the main effects of time (F10,190.3=26.7, p<0.001), region (F10,189.0=43.9, p<0.001), and age (F10,8.1=5.7, p=0.044) were significant. No significant interactions were observed for time by region (F10,189.0=0.62, p=0.793). The increase in binding in the bilateral left MTC remained significant after applying either the FDR or the more conservative Bonferroni correction (Table 3).
Table 3.
Unweighted means from the mixed model for distribution volume (VT) and binding potential (BPF) pre- and post-treatment. Means are given for the mixed model both with and without using Montgomery-Asberg Depression Rating Scale (MADRS) as a covariate.
| BPF | Without MADRS covariate |
With MADRS covariate |
||
|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| LACC | 38.463 | 44.729 | 40.576 | 44.896 |
| RACC | 38.743 | 45.457 | 40.855 | 45.031 |
| LAI | 41.855 | 48.937 | 43.967 | 48.388 |
| RAI | 42.406 | 49.085 | 44.519 | 48.647 |
| LMTC | 67.957 | 83.475a | 70.070 | 83.920a |
| RMTC | 67.280 | 77.953 | 69.393 | 79.239 |
| LPCC | 36.146 | 40.747 | 38.259 | 40.571 |
| RPCC | 33.925 | 38.885 | 36.038 | 38.964 |
| LPOC | 31.926 | 39.852 | 34.039 | 38.801 |
| RPOC | 32.747 | 38.653 | 34.860 | 37.952 |
| Raphe | 28.583 | 30.163 | 30.695 | 28.816 |
Indicates that post-treatment value is significantly different from pre-treatment value at p<0.05 after both false discovery rate (FDR) and Bonferroni correction for multiple comparisons.
LACC: left anterior cingulate cortex; LAI: left anterior insula; LMTC: left mesiotemporal cortex; LPCC: left posterior cingulate cortex; LPOC: left parieto-occipital cortex; MADRS: Montgomery-Asberg Depression Rating Scale; RACC: right anterior cingulate cortex; RAI: right anterior insula; RMTC: right mesiotemporal cortex; RPCC: right posterior cingulate cortex; RPOC: right parieto-occipital cortex.
Effect of MADRS scores on BPP and BPF
When the linear mixed model was repeated with MADRS as a covariate, change in BPP showed significant effects of time (F1,183.4=66.4, p<0.001), MADRS score (F1,180.3=29.1, p<0.001), and region (F10,175.3=53.1, p<0.001). Age had no significant effect. After controlling for MADRS scores and applying the FDR or the Bonferroni correction for multiple comparisons, a significant effect of time was seen in all regions examined, with the exception of the raphe (see Table 2, Figure 2).
Figure 2.
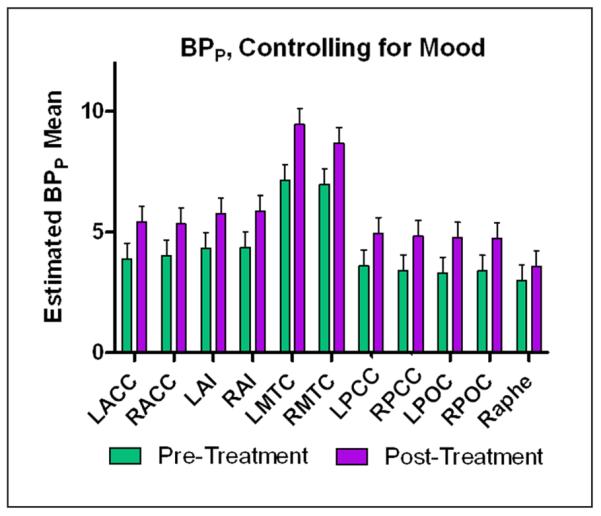
Graph of means derived from the mixed model of binding potential (BPP) pre- and post-treatment controlling for mood scores. All regions except for the raphe showed significant differences before and after treatment, Bonferroni corrected for multiple comparisons. Error bars represent standard error.
LACC: left anterior cingulate cortex; LAI: left anterior insula; LMTC: left mesiotemporal cortex; LPOC: left parieto-occipital cortex; RACC: right anterior cingulate cortex; RAI: right anterior insula; RMTC: right mesiotemporal cortex; RPOC: right parieto-occipital cortex.
When the mixed model was repeated for BPF, the main effects of time (F10,181.8=7.5, p=0.007), region (F10,176.468=49.7, p<0.001), MADRS (F1,182.7=14.4, p<0.001), and age (F10,8.4=5.4, p=0.047) were significant. The time by region interaction was not significant (F10,176.5=0.88, p=0.554). When examining individual regions, the increase in binding in the left MTC following treatment remained significant after applying the FDR or Bonferroni corrections (Table 3).
Discussion
These preliminary results demonstrate that post-synaptic 5-HT1A receptor BPP increases significantly in the bilateral MTC in BD subjects following treatment with lithium and/or divalproex. Moreover, when depression ratings were included in the statistical model as a covariate, the increase in the BPP following treatment became significant in every cortical region examined, suggesting that the effects of mood stabilizer treatment on post-synaptic 5-HT1A binding are widespread. The observation that the MTC emerged as an area of primary importance irrespective of clinical response (i.e. whether or not mood effects were not controlled for) suggests it is a site where alterations in 5-HT1A receptor function may prove particularly relevant for the serotonergic mechanisms of mood stabilizer treatment. Nevertheless, the hippocampus also has the highest post-synaptic 5-HT1A receptor concentration, so our comparisons may simply have been more sensitive to detecting BPP changes in this region.
Notably BPP did not change significantly in the raphe, where [18F]FCWAY uptake is predominantly attributable to pre-synaptic 5-HT1A receptor binding. This negative finding is noteworthy in light of the results of our previous study comparing 5-HT1A receptor binding between unmedicated BD patients (of which the subjects in the current study are a subset) versus healthy controls (Nugent et al., in press); that study found significantly decreased BPP in BD subjects in the MTC and some other cortical regions where [18F]FCWAY uptake is predominantly attributable to post-synaptic 5-HT1A receptor binding, but no group difference in the raphe. When the current group of BD subjects was compared to a gender- and age-matched subset of healthy controls from that study, they also showed reduced binding, although the result did not reach statistical significance (adjusted mean BPP in healthy subjects was 8.239 and 7.995 for left and right MTC, respectively, while mean BPP in BD subjects was 7.367 and 7.197, for left and right MTC, respectively). In addition, these subjects showed very little difference in mean raphe BPP (adjusted means of 3.391 and 3.225 in healthy and BD subjects, respectively). Taken together, these data suggest that the effects of the pathophysiology of BD and of mood stabilizer treatment on 5-HT1A receptor expression may selectively involve the post-synaptic 5-HT1A receptor system.
The reduced 5-HT1A receptor binding seen in BD could conceivably be due to the diathesis toward cortisol hypersecretion in some individuals with mood disorders (Drevets, 2001; Lopez et al., 1998), and to the fact that mood stabilizing treatments affect glucocorticoid signaling that also may influence 5-HT1A receptor expression. In rodents, post-synaptic 5-HT1A receptor gene expression is down-regulated by glucocorticoid receptor (GR) stimulation; for example, in rats, hippocampal 5-HT1A mRNA expression was found to be increased by adrenalectomy and decreased by corticosterone administration, chronic stress, or elevated trough corticosterone levels (Drevets et al., 2007; Hesen and Joels, 1996; Lopez et al., 1998; Meijer and de Kloet, 1994, 1995; Meijer et al., 1997; Mendelson and McEwen, 1991; Watanabe et al., 1993; Zhong and Ciaranello, 1995). Glucocorticoid hormone effects are of particular interest in the pathophysiology of BD, given that glucocorticoids are among only a few agents capable of triggering both depressive and manic episodes in BD patients (Wei et al., 2004). Notably, chronic administration of the mood stabilizers lithium and divalproex robustly up-regulated the GR chaperone protein Bcl-2-associated athanogene (BAG1), which interacts with GRs and attenuates their nuclear trafficking and function (Liman et al., 2005; Schneikert et al., 1999). In experimental animals, neuronal BAG1 overexpression plays a role in regulating recovery from or conferring resilience against the development of putative rodent behavioral analogues of anxiety, depression, and mania, particularly under conditions of elevated glucocorticoid concentrations (Maeng et al., 2008; Zhou et al., 2005). Thus, by counteracting the effects of cortisol hypersecretion, lithium and divalproex may conceivably increase post-synaptic 5-HT1A receptor expression in BD.
Notably, the effects of stress and GR stimulation on 5-HT1A receptor expression show a similar pattern to our results; both selectively involve post-synaptic 5-HT1A receptor expression. For example, Flugge and colleagues showed that in tree shrews, chronic psychosocial stress reduced 5-HT1A receptor density in the PCC, parietal cortex, prefrontal cortex, and hippocampus, but did not significantly alter receptor density in the raphe (Flugge, 1995). Moreover, Fairchild and colleagues showed that in rats, 5-HT1A autoreceptor function in the raphe was attenuated following chronic exposure to elevated corticosterone levels, but this effect was not associated with reduced 5-HT1A receptor mRNA expression (and instead appeared to involve changes in receptor-effector coupling) (Fairchild et al., 2003). The differential effects of lithium and divalproex observed herein on pre-synaptic versus post-synaptic 5-HT1A receptor binding thus appear compatible with the hypotheses that 5-HT1A receptor expression is reduced in BD subjects who hypersecrete cortisol, and that lithium and divalproex treatment increase post-synaptic 5-HT1A receptor expression by attenuating glucocorticoid signaling by upregulating BAG1 activity. Moreover, because BAG1 up-regulation, lithium, and valproate all attenuate GR nuclear translocation and inhibit GR activity specifically under elevated (pathophysiologic) glucocorticoid concentrations—but not under lower glucocorticoid levels (Zhou et al 2005)—this model may accommodate evidence that lithium does not increase 5-HT1A receptor expression in healthy, non-stressed rats (McQuade et al., 2004), and that divaproex enhances the cortisol response to serotonergic challenge in BD subjects (Maes et al., 1997) but not in healthy volunteers (Shiah et al., 1997). Alternative mechanisms through which lithium and divalproex may enhance 5-HT1A receptor expression include their neurotrophic/neuroprotective effects, which could increase the neuronal processes expressing 5-HT1A receptor protein (Manji et al., 2001). In addition, the elevation in 5-HT1A receptor binding could reflect enhanced 5-HT1A receptor gene promoter activity, given that high affinity activator protein 1 (AP-1) sites exist in this promoter and that both lithium and divalproex robustly increase AP-1 DNA binding activity (Chen et al., 1999; Yuan et al., 1998). Nevertheless, it is unclear whether the latter mechanisms would account for effects limited to 5-HT1A receptor expression in the post-synaptic versus the pre-synaptic system or in the depressed/stressed state versus the healthy/resting state.
This study is the first to measure 5-HT1A receptor binding potential in BD subjects before and after mood stabilizer treatment. Nevertheless, the observation that mood stabilizer treatment may normalize 5-HT1A receptor binding is potentially compatible with the results of the above-mentioned study of euthymic BD subjects medicated with lithium and/or divalproex, which showed no difference in BPND relative to healthy controls (Sargent et al., 2010).
Although the PET methodology does not directly address the functional significance of changes in 5-HT1A receptor BPP, within the context of the clinical and preclinical data reviewed in the Introduction, our data suggest that the mood stabilizer-induced increase in post-synaptic 5-HT1A receptor BPP contributes to the increases in serotonergic neurotransmission associated with chronic lithium and divalproex administration. The reported increases in plasma cortisol response to fenfluramine following lithium treatment (Mannel et al., 1997; Muhlbauer and Muller-Oerlinghausen, 1985) and in the L-5-HTTP-induced cortisol response following divalproex treatment (Maes et al., 1997) in BD patients appear particularly compatible with enhanced post-synaptic 5-HT1A receptor function. In the case of lithium, the increase in post-synaptic 5-HT1A receptor BPP fits within the context of increased serotonin release more generally, implying that these changes would interact to enhance post-synaptic 5-HT1A receptor neurotransmission. For example, an in vitro study of primary serotonergic neurons from the rat raphe nuclei found that lithium increased 5-HT release following both acute and chronic lithium exposure, and that while expression of tryptophan hydroxylase 2 (TPH2; rate-limiting enzyme in cerebral 5-HT synthesis) decreased under acute exposure, this enzyme's expression returned to normal under chronic exposure, suggesting the maintenance of enhanced 5-HT release (Scheuch et al., 2010). Nevertheless, lithium's effects on serotonergic function are complex, and also involve altered activation of protein kinase C (PKC) and the coupling of serotonin receptors to G proteins (e.g. Hahn et al., 2005). Furthermore, genetic factors associated with the risk of developing BD, such as the HTR1A variants described above and variations in the TPH2 gene (Campos et al., 2011), may conceivably influence the effects of mood stabilizers on serotonergic neurotransmission. The effect of mood stabilizers on serotonergic function may also be regionally-dependent; for example, one study found that chronic lithium administration increased serotonin release and decreased 5-HT type 2 receptor binding in the hippocampus but not in the cortex (Treiser et al., 1981), potentially compatible with our identification of the MTC as an area of particular importance to the serotonergic mechanisms of mood stabilizers.
Finally, our results conceivably relate to the neurobiological mechanisms underlying lithium's usefulness in the treatment of depression, given that antidepressant drugs from multiple classes have been shown to increase post-synaptic 5-HT1A receptor neurotransmission, and that this effect appears to play a role in antidepressant mechanisms (Chaput et al., 1991; Haddjeri et al., 1998; Savitz et al., 2009). In randomized, controlled clinical trials of bipolar depression, subjects receiving lithium plus placebo showed rates of improvement and durable recovery that did not differ significantly from those seen in subjects receiving lithium plus conventional antidepressant agents (Nemeroff et al., 2001; Sachs et al., 2007). The evidence reviewed above that lithium and divalproex enhance post-synaptic 5-HT1A receptor neurotransmission along with our finding that treatment with these mood stabilizers is associated with increased post-synaptic 5-HT1A receptor BPP thus appear compatible with the effects of other agents that exert antidepressant effects. Furthermore, it is noteworthy that in rats, the addition of lithium to the chronic administration of a variety of antidepressant drugs resulted in increased tonic activation of hippocampal 5-HT1A receptors (Haddjeri et al., 1998). This finding was hypothesized to underlie the clinical observation that, in MDD, lithium augments the antidepressant response to conventional antidepressant drugs from multiple pharmacological classes (Bauer et al., 2010).
Several limitations of our study merit comment. A primary limitation was the small sample size. Nevertheless, based upon the magnitude of the effect sizes observed, even this small sample provided adequate power to detect changes in BPP associated with mood stabilizer treatment. Our sample size was insufficient, however, to support direct comparisons between treatment responders and non-responders. A further limitation is that our cohort was almost entirely female. This limits the generalizability of the results to the BD population as a whole, and further studies in more balanced samples are warranted. A related limitation is that we chose not to fix the time of scanning with the menstrual phase of female subjects so that treatment of acutely depressed subjects was not delayed. Studies have previously investigated the effect of the menstrual phase on 5-HT1A receptor binding, and although there is evidence for increased BP in the dorsal raphe in the luteal as compared to the follicular phase, no significant differences were found across menstrual phase in areas where 5-HT1A receptor expression is post-synaptic (Jovanovic et al., 2006, 2009). Thus, it is unlikely that a systematic difference in menstrual phase between scan sessions would have influenced our finding in the MTC. Another limitation is that subjects were treated with two different agents, or a combination of agents. Unfortunately, there were not enough subjects in each group to co-vary for specific drug regimen. Further studies are needed to evaluate differential effects between mood stabilizers. A limitation of the PET methodology is that [18F]FC, the primary metabolite of the tracer [18F] FCWAY, is predominantly taken up by bone. Thus, due to spatial resolution limitations, we could not accurately measure BPP in cortical regions situated near the skull that otherwise would have been of interest in BD, such as the orbitofrontal cortex and ventrolateral prefrontal cortex. Nevertheless, this study provides compelling evidence for increased 5-HT1A BP following mood stabilizer treatment in deep regions such as the MTC and cingulate cortex, and future studies with larger sample sizes are warranted.
In summary, although the results should be considered preliminary due to the small sample size, this study provides evidence that 12 weeks of treatment with lithium and/or divalproex in subjects with BD was associated with increased post-synaptic 5-HT1A receptor BPP in the MTC and, when controlling for mood state, in other areas of the cortex as well. Without controlling for mood state, the MTC emerged as the site where the significant increase in BPP remained significant after applying corrections for multiple comparisons, potentially indicating that increased 5-HT1A receptor function in this region contributes to the serotonergic mechanisms of mood stabilizer treatment. In addition, confirmatory results were obtained in the secondary analysis examining BPF, indicating that our finding was robust across multiple modeling approaches. This study is the first to provide in vivo evidence that treatment with mood stabilizers increases post-synaptic 5-HT1A receptor binding, and provides new evidence for the neurobiological effects of these agents in BD.
Acknowledgements
The authors are grateful to Joan Williams and Michele Drevets for assistance with subject recruitment and evaluation, and to the PET technicians for technical support during the PET studies. The authors also appreciate the assistance of the NIH Clinical Center Department of Anesthesiology for placement of arterial catheters. Ioline Henter provided excellent editorial assistance.
Funding This work was supported in part by the IRP of the National Institute of the NIMH and NIH.
HM and CAZ are listed as co-inventors on a patent for the use of ketamine in major depression and have assigned their patent rights on ketamine to the US government. HM and WCD are currently employees of Johnson & Johnson Pharmaceuticals. WCD has consulted for Pfizer Pharmaceuticals, Johnson and Johnson Pharmaceuticals, Eisai, Inc., and Myriad/ Rules Based Medicine, Inc.
Footnotes
Conflicts of interest All other authors report no biomedical financial interests or potential conflicts of interest.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition, Text Revision DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;159:1–50. [PubMed] [Google Scholar]
- Bauer M, Adli M, Bschor T, et al. Lithium's emerging role in the treatment of refractory major depressive episodes: Augmentation of antidepressants. Neuropsychobiology. 2010;62:36–42. doi: 10.1159/000314308. [DOI] [PubMed] [Google Scholar]
- Birkenhager TK, van den Broek WW, Fekkes D, et al. Lithium addition in antidepressant-resistant depression: Effects on platelet 5-HT, plasma 5-HT and plasma 5-HIAA concentration. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1084–1088. doi: 10.1016/j.pnpbp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Campos SB, Miranda DM, Souza BR, et al. Association study of tryptophan hydroxylase 2 gene polymorphisms in bipolar disorder patients with panic disorder comorbidity. Psychiatr Genet. 2011;21:106–111. doi: 10.1097/YPG.0b013e328341a3a8. [DOI] [PubMed] [Google Scholar]
- Carrard A, Salzmann A, Malafosse A, et al. Increased DNA methylation status of the serotonin receptor 5HTR1A gene promoter in schizophrenia and bipolar disorder. J Affect Disord. 2011;132:450–453. doi: 10.1016/j.jad.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Carson RE, Toczek MT, Lang LX, et al. Human functional imaging with the 5-HT1A ligand F-18-FCWAY. J Nucl Med. 2002;43(Suppl):55. [Google Scholar]
- Carson RE, Wu Y, Lang L, et al. Brain uptake of the acid metabolites of F-18-labeled WAY 100635 analogs. J Cereb Blood Flow Metab. 2003;23:249–260. doi: 10.1097/01.WCB.0000046145.31247.7A. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology. 1991;5:219–229. [PubMed] [Google Scholar]
- Chen G, Yuan PX, Jiang YM, et al. Valproate robustly enhances AP-1 mediated gene expression. Brain Res Mol Brain Res. 1999;64:52–58. doi: 10.1016/s0169-328x(98)00303-9. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, McCance SL, Ware CJ, et al. Lithium in tricyclic-resistant depression. Correlation of increased brain 5-HT function with clinical outcome. Br J Psychiatry. 1991;159:341–346. doi: 10.1192/bjp.159.3.341. [DOI] [PubMed] [Google Scholar]
- DeGrado TR, Turkington TG, Williams JJ, et al. Performance characteristics of a whole-body PET scanner. J Nucl Med. 1994;35:1398–1406. [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, et al. Serotonin-1A receptor imaging in recurrent depression: Replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Leitch MM, Ingram CD. Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology. 2003;45:925–934. doi: 10.1016/s0028-3908(03)00269-7. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychaitric Institute, Biometrics Research; New York, NY: 2002. [Google Scholar]
- Flugge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovacchini G, Toczek MT, Lang L, et al. 5-HT1A receptors are reduced in temporal lobe epilepsy after partial-volume correction. J Nucl Med. 2005;44:1128–1135. [PMC free article] [PubMed] [Google Scholar]
- Gray L, Scarr E, Dean B. Serotonin 1a receptor and associated G-protein activation in schizophrenia and bipolar disorder. Psychiatry Res. 2006;143:111–120. doi: 10.1016/j.psychres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Umapathy Wang HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39:355–363. doi: 10.1016/j.jpsychires.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Hesen W, Joels M. Modulation of 5HT1A responsiveness in CA1 pyramidal neurons by in vivo activation of corticosteroid receptors. J Neuroendocrinol. 1996;8:433–438. doi: 10.1046/j.1365-2826.1996.04724.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Cerin A, Karlsson P, et al. A PET study of 5-HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res. 2006;148:185–193. doi: 10.1016/j.pscychresns.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Karlsson P, Cerin A, et al. 5-HT(1A) receptor and 5-HTT binding during the menstrual cycle in healthy women examined with [(11)C] WAY100635 and [(11)C] MADAM PET. Psychiatry Res. 2009;172:31–37. doi: 10.1016/j.pscychresns.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Kishi T, Okochi T, Tsunoka T, et al. Serotonin 1A receptor gene, schizophrenia and bipolar disorder: An association study and meta-analysis. Psychiatry Res. 2011;185:20–26. doi: 10.1016/j.psychres.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Al-Taie O, Schafer A, et al. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- Kusumi I, Koyama T, Yamashita I. Effect of mood stabilizing agents on agonist-induced calcium mobilization in human platelets. J Psychiatry Neurosci. 1994;19:222–225. [PMC free article] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, et al. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Shardell M, Ferrell RE, et al. Association of serotonin-1A and 2A receptor promoter polymorphisms with depressive symptoms and functional recovery in elderly persons after hip fracture. J Affect Disord. 2008;111:61–66. doi: 10.1016/j.jad.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Mayer S, Disselkamp-Tietze J, et al. 5-HT1A receptor responsivity in unipolar depression. Evaluation of ipsapirone-induced ACTH and cortisol secretion in patients and controls. Biol Psychiatry. 1990;28:620–628. doi: 10.1016/0006-3223(90)90400-v. [DOI] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, et al. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: Application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 2004;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman J, Ganesan S, Dohm CP, et al. Interaction of BAG1 and Hsp70 mediates neuroprotectivity and increases chaperone activity. Mol Cell Biol. 2005;25:3715–3725. doi: 10.1128/MCB.25.9.3715-3725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, et al. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- McQuade R, Leitch MM, Gartside SE, et al. Effect of chronic lithium treatment on glucocorticoid and 5-HT1A receptor messenger RNA in hippocampal and dorsal raphe nucleus regions of the rat brain. J Psychopharmacol. 2004;18:496–501. doi: 10.1177/026988110401800406. [DOI] [PubMed] [Google Scholar]
- Maeng S, Hunsberger JG, Pearson B, et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci U S A. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Calabrese J, Jayathilake K, et al. Effects of subchronic treatment with valproate on L-5-HTP-induced cortisol responses in mania: Evidence for increased central serotonergic neurotransmission. Psychiatry Res. 1997;71:67–76. doi: 10.1016/s0165-1781(97)00046-2. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Mannel M, Muller-Oerlinghausen B, Czernik A, et al. 5-HT brain function in affective disorder: d,l-Fenfluramine-induced hormone release and clinical outcome in long-term lithium/carbamazepine prophylaxis. J Affect Disord. 1997;46:101–113. doi: 10.1016/s0165-0327(97)00093-1. [DOI] [PubMed] [Google Scholar]
- Meijer OC, de Kloet ER. Corticosterone suppresses the expression of 5-HT1A receptor mRNA in rat dentate gyrus. Eur J Pharmacol. 1994;266:255–261. doi: 10.1016/0922-4106(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Meijer OC, de Kloet ER. A role for the mineralocorticoid receptor in a rapid and transient suppression of hippocampal 5-HT1A receptor mRNA by corticosterone. J Neuroendocrinol. 1995;7:653–657. doi: 10.1111/j.1365-2826.1995.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Van Oosten RV, De Kloet ER. Elevated basal trough levels of corticosterone suppress hippocampal 5-hydroxytryptamine(1A) receptor expression in adrenally intact rats: Implication for the pathogenesis of depression. Neuroscience. 1997;80:419–426. doi: 10.1016/s0306-4522(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, McEwen BS. Autoradiographic analyses of the effects of restraint-induced stress on 5-HT1A, 5-HT1C and 5-HT2 receptors in the dorsal hippocampus of male and female rats. Neuroendocrinology. 1991;54:454–461. doi: 10.1159/000125951. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Thase ME, et al. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [C-11]WAY-100635. Synapse. 2007;61:523–530. doi: 10.1002/syn.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlbauer HD, Muller-Oerlinghausen B. Fenfluramine stimulation of serum cortisol in patients with major affective disorders and healthy controls: Further evidence for a central serotonergic action of lithium in man. J Neural Transm. 1985;61:81–94. doi: 10.1007/BF01253053. [DOI] [PubMed] [Google Scholar]
- Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Evans DL, Gyulai L, et al. Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry. 2001;158:906–912. doi: 10.1176/appi.ajp.158.6.906. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Bain EE, Carlson PJ, et al. Reduced post-synaptic serotonin type 1A receptor binding in bipolar depression. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2012.11.005. in press. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, et al. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Price LH, Charney DS, Delgado PL, et al. Lithium and serotonin function: Implications for the serotonin hypothesis of depression. Psychopharmacology (Berl) 1990;100:3–12. doi: 10.1007/BF02245781. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Rabiner EA, Bhagwagar Z, et al. 5-HT(1A) receptor binding in euthymic bipolar patients using positron emission tomography with [carbonyl-(11)C]WAY-100635. J Affect Disord. 2010;123:77–80. doi: 10.1016/j.jad.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuch K, Holtje M, Budde H, et al. Lithium modulates tryptophan hydroxylase 2 gene expression and serotonin release in primary cultures of serotonergic raphe neurons. Brain Res. 2010;1307:14–21. doi: 10.1016/j.brainres.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Schneikert J, Hubner S, Martin E, et al. A nuclear action of the eukaryotic cochaperone RAP46 in downregulation of glucocorticoid receptor activity. J Cell Biol. 1999;146:929–940. doi: 10.1083/jcb.146.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiah IS, Yatham LN, Lam RW, et al. Effects of divalproex sodium on 5-HT1A receptor function in healthy human males: Hypothermic, hormonal, and behavioral responses to ipsapirone. Neuropsychopharmacology. 1997;17:382–390. doi: 10.1016/S0893-133X(97)00087-0. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12:155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczek MT, Carson RE, Lang L, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- Treiser SL, Cascio CS, O'Donohue TL, et al. Lithium increases serotonin release and decreases serotonin receptors in the hippocampus. Science. 1981;213:1529–1531. doi: 10.1126/science.6269180. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Sakai RR, McEwen BS, et al. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, et al. Glucocorticoid receptor overexpression in forebrain: A mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yuan PX, Chen G, Huang LD, et al. Lithium stimulates gene expression through the AP-1 transcription factor pathway. Brain Res Mol Brain Res. 1998;58:225–230. doi: 10.1016/s0169-328x(98)00114-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhong P, Ciaranello RD. Transcriptional regulation of hippocampal 5-HT1a receptors by corticosteroid hormones. Brain Res Mol Brain Res. 1995;29:23–34. doi: 10.1016/0169-328x(94)00225-4. [DOI] [PubMed] [Google Scholar]
- Zhou R, Gray NA, Yuan P, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


