Abstract
Background
Abnormalities in serum alkaline phosphatase (ALP) and intact parathyroid hormone (PTH) concentrations, as biochemical markers of bone turnover in dialysis patients, correlate with increased mortality in maintenance hemodialysis (MHD) patients. Changes in bone turnover rate vary with age. The mortality predictability of serum ALP and PTH levels in MHD patients may be different across ages.
Methods
We examined differences across four age groups (18 to <45, 45 to <65, 65 to <75 and ≥75 years) in the mortality predictability of serum ALP and PTH in 102 149 MHD patients using Cox models.
Results
Higher serum ALP levels were associated with higher mortality across all ages; however, the ALP–mortality association was much stronger in young patients (<45 years) compared with older patients. The association between higher serum PTH levels and mortality was stronger in older patients compared with the younger groups. Serum PTH levels were incrementally associated with mortality only in middle-aged and elderly patients (≥45 years). Compared with patients with serum PTH 150 to <300pg/mL, the death risks were higher in patients with serum PTH 300 to <600pg/mL [HRs (95% CI): 1.05 (1.01–1.10), 1.15 (1.10–1.21) and 1.25 (1.19–1.31) for patients 45 to <65, 65 to <75 and ≥75 years, respectively], and ≥600pg/mL [HRs(95% CI): 1.07 (1.01–1.14), 1.31(1.21–1.42) and 1.45(1.33–1.59) for age categories 45 to <65, 65 to <75 and ≥75 years, respectively]. However, no significant association between higher serum PTH levels and mortality was observed in patients <45 years.
Conclusions
There are important differences in mortality-predictability of serum ALP and PTH in older MHD patients compared with their younger counterparts. The effect of age needs to be considered when interpreting the prognostic implications of serum ALP and PTH levels.
Keywords: age, alkaline phosphatase, bone turnover markers, hemodialysis, parathyroid hormone
INTRODUCTION
Cardiovascular disease is the leading cause of mortality in dialysis patients [1]. Cardiovascular risk factors among dialysis patients are comprised of traditional risk factors and uremia-related risk factors; the latter includes mineral and bone disorders (MBDs) [2, 3]. Abnormalities of MBD markers including serum calcium, phosphorus and intact parathyroid hormone (PTH) levels have been shown to be associated with increased vascular calcification [4, 5], and higher risks of cardiovascular and all-cause mortality in maintenance hemodialysis (MHD) patients [6–9]. Experimental studies have investigated the role of tissue-nonspecific alkaline phosphatase (TNALP) in the pathogenesis of vascular calcification [10, 11]. TNALP is upregulated under uremic conditions in vessels from rats, which leads to the hydrolysis and inactivation of pyrophosphate, a potent inhibitor of vascular calcification [10, 12]. Furthermore, higher serum total alkaline phosphatase (ALP) levels are independently associated with coronary artery calcification [13], increased hospitalization rates and mortality in MHD patients [14, 15].
The incidence of end-stage renal disease (ESRD) is increasing among the elderly population worldwide [16, 17]. Bone and mineral metabolism in the elderly dialysis population might differ from that of younger patients in response to reduction in dietary protein, phosphorus and calcium intake, reduced physical activity and decreased bone responsiveness to hormonal regulators [18, 19]. A large cohort of MHD patients demonstrated differences in laboratory markers, and treatment of MBD between younger and older patients. The elderly patients tended to have lower levels of serum phosphorus and PTH than the younger patients. Compared with younger MHD patients, there was significantly lower phosphorus binder and cinacalcet usage and a trend towards lower receipt of activated vitamin D compounds in older patients [20]. We recently reported differential associations between serum phosphorus levels and mortality across varying age groups in MHD patients. High serum phosphorus levels correlate with increased mortality across all ages, whereas low serum phosphorus concentrations are associated with higher risks of death only in elderly patients ≥65 years in whom there is a greater likelihood of hypophosphatemia [21]. Serum ALP and PTH levels are biochemical markers of bone turnover in chronic kidney disease (CKD) patients [22, 23], and no survival analysis based on ALP and PTH values in the elderly dialysis population has been done. We hypothesized that serum ALP and PTH levels would show differential associations with mortality across varying age groups in MHD patients.
MATERIALS AND METHODS
Study population
We extracted and analyzed data from a 6-year cohort of all MHD patients from 580 outpatient dialysis facilities of DaVita, a large dialysis organization in the USA. The baseline studied quarter for each patient was the earliest calendar quarter, in which the patient's HD vintage was >90 days. Of the 127 304 patients who underwent HD treatment >90 days during the study period, 580 patients >99 or <18 years or with missing age data and 19 190 patients with missing quarterly baseline data or follow-up periods were excluded. Among the remaining 107 534 patients, we excluded 930 patients for ALP and 19 178 patients for PTH outliers or missing data. For ALP analyses, 4455 patients with serum aspartate transaminase (AST) >40 U/L or missing AST data were excluded. Therefore, the final study population consisted of 102 149 patients for serum ALP and 88 356 patients for serum PTH analyses (Supplementary data, Figure S1). The study was approved by the institutional review committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and DaVita Clinical Research. The requirement for a written consent was exempted due to large sample size, patient anonymity, and the nonintrusive nature of the research.
Clinical and demographic measures
The creation of the DaVita MHD patient cohort has been described previously [24]. Average values were obtained from up to 20 calendar quarters (1 July 2001–30 June 2006) for each laboratory parameter and clinical measure for each patient during the cohort period. Patients were followed for outcomes until 30 June 2007. Dialysis vintage was defined as the duration of time between the first day of HD treatment and the day that the patient entered the cohort study. The demographic data were obtained from the DaVita database. History of preexisting comorbidities and tobacco smoking was obtained by linking the DaVita database to the data from Medical Evidence Form 2728 from the US Renal Data System (USRDS). Available preexisting comorbid conditions were grouped into nine categories: atherosclerotic heart disease, congestive heart failure, other cardiac diseases, hypertension, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer and non-ambulatory state. The causes of death were obtained from the USRDS, and cardiovascular death was defined as death due to myocardial infarction, cardiac arrest, congestive heart failure, cerebrovascular accident or other cardiac diseases.
Patients who received any activated injectable vitamin D agents (i.e. paricalcitol, calcitriol or doxercalciferol) in the dialysis facility during each calendar quarter were identified, and the administered doses were captured from the DaVita database. Over 90% of DaVita MHD patients who received any activated injectable vitamin D during the study cohort period received paricalcitol; therefore, the administered doses of calcitriol and doxercalciferol were converted to equivalent paricalcitol doses and the total doses of administered vitamin D agents for each patient over the entire cohort were calculated and included in all case-mix adjusted models. The study population was stratified into four age categories (18 to <45, 45 to <65, 65 to <75, and ≥75 years) for data analysis.
Laboratory measures
Blood samples were drawn using standardized techniques in all DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, typically within 24 h. All laboratory values were measured using automated and standardized methods in the DaVita Laboratory. Most laboratory parameters were measured monthly, including serum levels of urea nitrogen, creatinine, albumin, ALP, AST, calcium and phosphorus. Serum PTH concentrations were measured at least quarterly using a first-generation immunoradiometric PTH assay [25]. Serum calcium levels corrected for serum albumin were calculated using the following equation: ‘albumin-corrected calcium (mg/dL) = {0.8 × [4-serum albumin (g/dL)]} + serum calcium (mg/dL).’ Most blood samples were collected prior to HD, except for post-dialysis serum urea nitrogen to calculate urea kinetics. Time-averaged serum ALP and PTH values obtained from up to 20 calendar quarters for each patient during the study cohort period were used in our analyses. We divided time-averaged serum ALP levels a priori into four categories (<80, 80 to <120, 120 to <160 and ≥160 U/L), and time-averaged serum PTH levels a priori into four categories (<150, 150 to <300, 300 to <600 and ≥600pg/mL). The ALP category of 80 to <120 U/L and the PTH category of 150 to <300 pg/mL were designated as the reference groups.
Statistical analysis
Data were summarized using proportions, means ± SD or medians [interquartile ranges (IQR)] as dictated by data type. We evaluated the association of time-averaged serum ALP and PTH as main predictors with all-cause and cardiovascular mortality as outcomes using Cox proportional hazard models and restricted cubic splines within each age category. For each analysis, three models with multivariable adjustments were examined:
Unadjusted models included ALP and PTH categories, and entry calendar quarter (q1–q20).
Case-mix adjusted models included all of the variables in the unadjusted model plus sex, race/ethnicity (Caucasian, African American, Hispanic and Asian), presence of diabetes and nine preexisting comorbidities, dialysis duration (<6 months, 6 to <24 months, 2 to <5 years and ≥5 years), primary insurance (Medicare, Medicaid and others), marital status (married, single, widowed and divorced), history of tobacco smoking, types of vascular access (arteriovenous fistula, arteriovenous graft and catheter), dialysis dose as indicated by single-pool Kt/V, serum AST levels (only for ALP analyses) and the dosage of activated vitamin D agents.
Case-mix plus MBD-adjusted models included all of the covariates in the case-mix model plus serum phosphorus, calcium, ALP (except for the model where serum ALP was the predictor), and PTH (except for the model where serum PTH was the predictor) concentrations.
Patients who received a kidney transplant, switched to peritoneal dialysis or left DaVita dialysis clinics were censored at the time of the event. Missing covariate data (<3% for laboratory and most demographic variables) were imputed by the means or medians of the existing values as appropriate. Two-sided P-values <0.05 were considered to be statistically significant. All statistical analyses were performed using Stata version 11.2 (Stata Corp., College Station, TX).
RESULTS
Cohort description
The baseline demographics, clinical and laboratory characteristics of the 102 149 MHD patients, stratified by age category, are summarized in Table 1. The mean age of the cohort at baseline was 60 ± 16 years, among which there were 35% African Americans and 58% diabetics. Older MHD patients were more likely to be Caucasian, and diabetic, and had lower levels of serum ALP, PTH, phosphorus, albumin and creatinine. They were also less likely to receive activated vitamin D agents, and had a lower mean dose than younger patients (Table 1). The median follow-up time was 2.25 years (IQR 1.21–3.71 years). During the follow-up period (July 2001–June 2007), 53 184 (52%) patients died, with 21 965 (41%) deaths due to cardiovascular causes.
Table 1.
Baseline characteristics of 102 149 MHD patients according to the age category
| Age range (years) | All | 18 to <45 | 45 to <65 | 65 to <75 | ≥75 | P |
|---|---|---|---|---|---|---|
| n (%) | 102 149 | 17 649 (17) | 39 321 (39) | 23 896 (23) | 21 283 (21) | NA |
| Age (years) | 60 ± 16 | 35 ± 7 | 55 ± 6 | 69 ± 3 | 80 ± 4 | <0.001 |
| Female (%) | 45 | 40 | 44 | 49 | 47 | <0.001 |
| Race (%) | ||||||
| Caucasian | 46 | 30 | 38 | 52 | 67 | <0.001 |
| African American | 35 | 48 | 40 | 29 | 20 | <0.001 |
| Hispanic | 16 | 19 | 19 | 15 | 10 | <0.001 |
| Asian | 3 | 3 | 3 | 4 | 3 | 0.06 |
| Dialysis duration (%) | ||||||
| <6 month | 58 | 46 | 56 | 60 | 70 | <0.001 |
| 6 to <24 months | 17 | 16 | 17 | 18 | 17 | 0.003 |
| 2 to <5 years | 16 | 18 | 18 | 16 | 11 | <0.001 |
| ≥5 years | 9 | 20 | 9 | 6 | 2 | <0.001 |
| Primary insurance (%) | ||||||
| Medicare | 68 | 60 | 58 | 78 | 81 | <0.001 |
| Medicaid | 6 | 12 | 9 | 2 | 1 | <0.001 |
| Other | 26 | 28 | 33 | 20 | 18 | <0.001 |
| Marital status (%) | ||||||
| Divorced | 8 | 7 | 12 | 8 | 4 | <0.001 |
| Single | 28 | 58 | 30 | 15 | 11 | <0.001 |
| Widowed | 17 | 1 | 8 | 23 | 38 | <0.001 |
| Married | 47 | 34 | 50 | 54 | 47 | <0.001 |
| Vascular access (%) | ||||||
| AVF | 26 | 34 | 26 | 24 | 21 | <0.001 |
| AVG | 30 | 26 | 31 | 33 | 27 | 0.25 |
| Catheter | 44 | 40 | 43 | 43 | 52 | <0.001 |
| Kt/V (single-pool) | 1.52 ± 0.35 | 1.47 ± 0.35 | 1.49 ± 0.34 | 1.56 ± 0.36 | 1.58 ± 0.35 | <0.001 |
| BMI (kg/m2) | 26.9 ± 6.9 | 27.1 ± 7.8 | 28.2 ± 7.4 | 26.6 ± 6.2 | 24.4 ± 5.1 | <0.001 |
| nPNA (g/kg/day) | 0.95 ± 0.25 | 0.97 ± 0.26 | 0.96 ± 0.26 | 0.94 ± 0.25 | 0.91 ± 0.24 | <0.001 |
| Comorbidities (%) | ||||||
| DM | 58 | 34 | 66 | 68 | 51 | <0.001 |
| Atherosclerotic heart disease | 22 | 4.4 | 18 | 29 | 33 | <0.001 |
| Cancer | 4.7 | 1 | 3.1 | 6 | 9 | <0.001 |
| Congestive heart failure | 29 | 12 | 26 | 35 | 38 | <0.001 |
| COPD | 6 | 1.2 | 5 | 9 | 9 | <0.001 |
| Cerebrovascular disease | 8 | 2.5 | 7 | 10 | 11 | <0.001 |
| Hypertension | 80 | 76 | 81 | 81 | 80 | <0.001 |
| Other cardiac diseases | 6 | 2.1 | 4.1 | 7 | 10 | <0.001 |
| Peripheral vascular disease | 12 | 3.4 | 10 | 16 | 16 | <0.001 |
| Non-ambulatory state | 3.2 | 1.2 | 2.9 | 3.7 | 4.6 | <0.001 |
| Current smoking | 5 | 7 | 7 | 4 | 1.8 | <0.001 |
| Laboratory measures (baseline) | ||||||
| ALP (U/L) | 96 (76, 127) | 96 (74, 132) | 100 (78, 133) | 95 (75, 123) | 93 (75, 118) | <0.001 |
| Intact PTH (pg/mL) | 237 (134, 412) | 326 (173, 613) | 252 (144, 434) | 211 (120, 347) | 193 (114, 312) | <0.001 |
| Calcium (mg/dL) | 9.5 ± 0.7 | 9.4 ± 0.8 | 9.5 ± 0.7 | 9.5 ± 0.6 | 9.5 ± 0.6 | <0.001 |
| Phosphorus (mg/dL) | 5.6 ± 1.5 | 6.3 ± 1.6 | 5.7 ± 1.4 | 5.3 ± 1.3 | 5.0 ± 1.2 | <0.001 |
| Albumin (g/dL) | 3.7 ± 0.5 | 3.8 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.4 | 3.6 ± 0.4 | <0.001 |
| Creatinine (mg/dL) | 8.0 ± 3.3 | 10.8 ± 3.8 | 8.3 ± 3.1 | 7.0 ± 2.5 | 6.1 ± 2.2 | <0.001 |
| TIBC (mg/dL) | 209 ± 46 | 209 ± 44 | 212 ± 46 | 208 ± 46 | 204 ± 46 | <0.001 |
| Ferritin (ng/mL) | 373 (177, 698) | 349 (159, 674) | 378 (181, 707) | 391 (187, 722) | 361 (175, 672) | <0.001 |
| Hemoglobin (g/dL) | 12.0 ± 1.4 | 11.9 ± 1.5 | 12.0 ± 1.4 | 12.1 ± 1.3 | 12.1 ± 1.3 | <0.001 |
| WBC (×103/µL) | 7.5 ± 2.5 | 7.2 ± 2.4 | 7.5 ± 2.4 | 7.5 ± 2.4 | 7.6 ± 2.8 | <0.001 |
| % Lymphocytes | 20.5 ± 7.8 | 22.7 ± 8.1 | 20.8 ± 7.7 | 19.6 ± 7.6 | 19.0 ± 7.5 | <0.001 |
| AST (U/L) | 18 ± 8 | 18 ± 9 | 18 ± 9 | 18 ± 7 | 19 ± 7 | <0.001 |
| Activated vitamin Da (%) | 82 | 84 | 85 | 80 | 77 | <0.001 |
| Paricalcitol doseb (µg/week) | 10 (7, 15) | 13 (8, 19) | 11 (8, 16) | 9 (7, 14) | 8 (6, 12) | <0.001 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; nPNA, normalized protein nitrogen appearance; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; TIBC, total iron-binding capacity; WBC, white blood cells; AST, aspartate transaminase.
Continuous variables expressed as mean ± SD or median (interquartile range); categorical variables expressed as number (percent); P-for trend shows the differences in each age category.
aPercentages of patients who received activated vitamin D agents including paricalcitol, calcitriol and doxercalciferol.
bWeekly average dose in any patients who received paricalcitol during follow-up.
Effect modification by age on serum alkaline phosphatase and mortality association
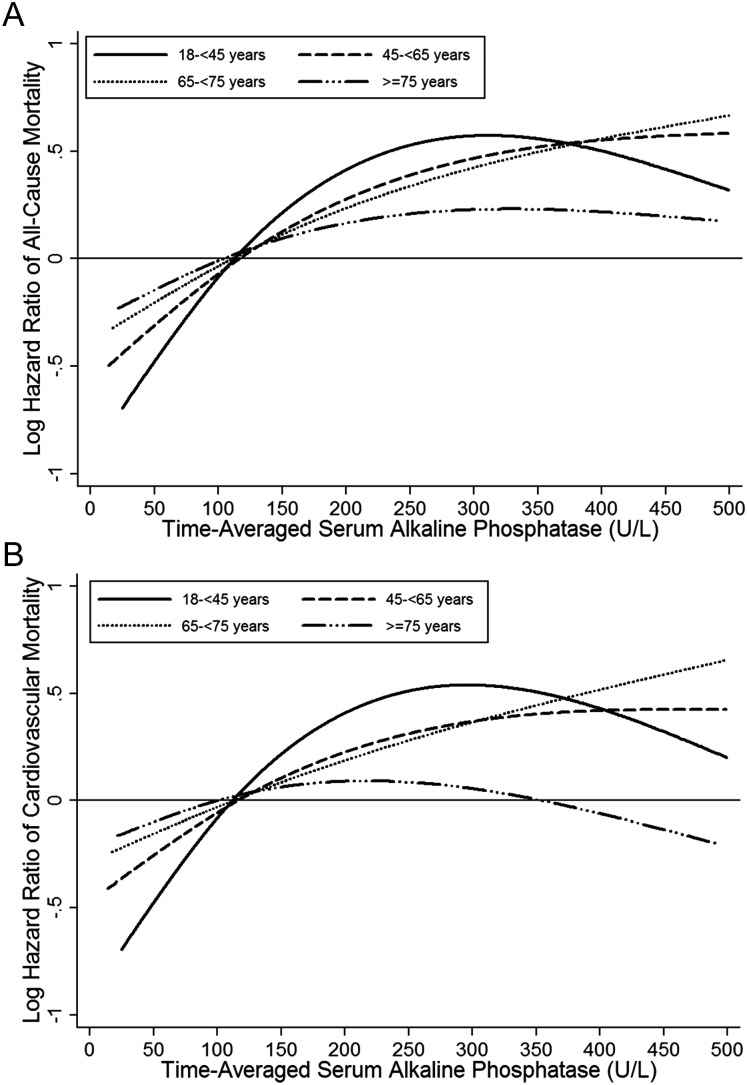
Serum ALP levels were incrementally associated with all-cause and cardiovascular mortality risks across all ages. However, in patients <45 years, there was a higher mortality risk associated with serum ALP elevation up to 300 U/L than in the other age groups. The ALP-mortality association appeared to be weaker in patients ≥75 years compared with the younger groups (Figure 1, Supplementary data, Figures S2 and S3). Compared with a serum ALP level of 80 to <120 U/L, serum ALP levels of 120 to <160 and ≥160 U/L showed a 23% [hazard ratio (HR) 1.23, 95% confidence interval (CI) 1.13–1.32], and 59% (HR 1.59, 95% CI 1.47–1.71) higher mortality risk in patients <45 years, whereas death HRs associated with serum levels of ALP 120 to <160 and ≥160 U/L were progressively lower in older age categories [HRs (95% CI): 1.16 (1.11–1.20) and 1.41 (1.35–1.47) for patients 45 to <65 years old, 1.10 (1.06–1.15) and 1.32 (1.25–1.38) for patients 65 to <75 years old, and 1.12 (1.07–1.16) and 1.21 (1.14–1.28) for patients ≥75 years, respectively] (Table 2). A similar pattern was observed with cardiovascular mortality (Supplementary data, Table S1).
FIGURE 1:
Cubic splines of HRs of all-cause mortality (A) and cardiovascular mortality (B) for time-averaged serum ALP levels using Cox regression analyses comparing four different age groups of 102 144 HD patients (n = 17 645 for patients 18 to <45 years old, n = 39 320 for patients 45 to <65 years old, n = 23 896 for patients 65 to <75 years old and n = 21 283 for patients ≥75 years old). Model adjusted for sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, marital status, primary insurance, types of vascular access, dialysis dose as indicated by single-pool Kt/V, the dosage of activated vitamin D agents, serum aspartate transaminase, albumin corrected calcium, phosphorus and intact PTH concentrations.
Table 2.
HRs (95% CIs) of all-cause mortality comparing time-averaged serum ALP categories (reference: 80 to <120 U/L) using Cox regression analyses in 102 149 HD patients stratified by age category
| Serum ALP (U/L) | All-cause death HRs (95% CIs) |
||
|---|---|---|---|
| Unadjusted | Case-mix adjusted | Case-mix + MBD adjusted | |
| Age 18 to <45 years, n = 17 649 (unadjusted), 17 645 (case-mix), 17 645 (case-mix + MBD) | |||
| <80 | 0.73 (0.66–0.81) | 0.71 (0.65–0.79) | 0.71 (0.65–0.79) |
| 80 to <120 | Reference | Reference | Reference |
| 120 to <160 | 1.32 (1.22–1.42) | 1.22 (1.13–1.32) | 1.23 (1.13–1.32) |
| ≥160 | 1.80 (1.67–1.93) | 1.60 (1.48–1.72) | 1.59 (1.47–1.71) |
| Age 45 to <65 years, n = 39 321 (unadjusted), 39 320 (case-mix), 39 320 (case-mix + MBD) | |||
| <80 | 0.89 (0.85–0.93) | 0.83 (0.80–0.87) | 0.83 (0.80–0.87) |
| 80 to <120 | Reference | Reference | Reference |
| 120 to <160 | 1.14 (1.10–1.18) | 1.16 (1.12–1.21) | 1.16 (1.11–1.20) |
| ≥160 | 1.46 (1.41–1.52) | 1.42 (1.37–1.48) | 1.41 (1.35–1.47) |
| Age 65 to <75 years, n = 23 896 (unadjusted), 23 896 (case-mix), 23 896 (case-mix + MBD) | |||
| <80 | 0.96 (0.92–1.00) | 0.87 (0.83–0.91) | 0.88 (0.85–0.92) |
| 80 to <120 | Reference | Reference | Reference |
| 120 to <160 | 1.08 (1.04–1.13) | 1.12 (1.07–1.17) | 1.10 (1.06–1.15) |
| ≥160 | 1.37 (1.31–1.44) | 1.36 (1.30–1.43) | 1.32 (1.25–1.38) |
| Age ≥75 years, n = 21 283 (unadjusted), 21 283(case-mix), 21 283 (case-mix + MBD) | |||
| <80 | 1.01 (0.97–1.05) | 0.92 (0.88–0.96) | 0.93 (0.89–0.97) |
| 80 to <120 | Reference | Reference | Reference |
| 120 to <160 | 1.08 (1.04–1.13) | 1.13 (1.08–1.18) | 1.12 (1.07–1.16) |
| ≥160 | 1.28 (1.22–1.35) | 1.27 (1.20–1.34) | 1.21 (1.14–1.28) |
Case-mix model is adjusted for sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, marital status, primary insurance, types of vascular access, dialysis dose as indicated by single-pool Kt/V, serum aspartate transaminase levels and the dosage of activated vitamin D agents.
Case-mix + MBD adjusted model includes all of the case-mix covariates plus serum albumin corrected calcium, phosphorus and intact PTH concentrations.
MBDs, mineral and bone disorders.
Effect modification by age on serum intact parathyroid hormone and mortality association
Higher serum PTH levels showed a significant and linear association with higher all-cause and cardiovascular mortality risks only in MHD patients ≥45 years and the PTH–mortality association was much stronger in older patients compared with the younger groups. In contrast, there was no significant association between higher serum PTH levels and mortality in patients <45 years (Figure 2, Supplementary data, Figures S4 and S5). In a case-mix and MBD-adjusted model, young patients (<45 years) with serum PTH levels of <150 pg/mL had a 15% [HRs (95% CI): 0.85 (0.76–0.95)] lower risk of all-cause mortality compared with those with serum PTH levels of 150 to <300 pg/mL; however, higher serum PTH levels (≥300 pg/mL) were not associated with increased all-cause mortality in young patients. In older age categories (≥45 years), the death risks were significantly higher in patients with serum PTH levels of 300 to <600 pg/mL [Fully adjusted HRs (95% CI): 1.05 (1.01–1.10), 1.15 (1.10–1.21) and 1.25 (1.19–1.31) for age categories 45 to <65, 65 to <75 and ≥75 years, respectively], and ≥600 pg/mL [fully adjusted HRs (95% CI): 1.07 (1.01–1.14), 1.31 (1.21–1.42) and 1.45 (1.33–1.59) for patients 45 to <65, 65 to <75 and ≥75 years old, respectively] (Table 3). Similar results were found with cardiovascular mortality (Supplementary data, Table S2). Similar patterns were observed after additional adjustment for nutritional markers including body mass index (BMI), serum levels of albumin, creatinine and normalized protein nitrogen appearance (nPNA); however, the association seemed to be stronger across all ages (Supplementary data Table S3 and Figure S6). In models containing interaction terms for serum PTH levels and age (<45 versus ≥45 years), interaction terms were statistically significant, confirming that there was a differential association between serum PTH levels and mortality in different age strata (P-interaction = 0.02 for all-cause mortality and P-interaction <0.001 for cardiovascular mortality).
FIGURE 2.
Cubic splines of HRs of all-cause mortality (A) and cardiovascular mortality (B) for time-averaged serum intact PTH levels using Cox regression analyses comparing four different age groups of 88 354 HD patients (n = 14 654 for patients 18 to <45 years old, n = 34 997 for patients 45 to <65 years old, n = 20 908 for patients 65 to <75 years old and n = 17 795 for patients ≥75 years old). Model adjusted for sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, marital status, primary insurance, types of vascular access, dialysis dose as indicated by single-pool Kt/V, the dosage of activated vitamin D agents, serum albumin corrected calcium, phosphorus and ALP concentrations.
Table 3.
HRs (95% CIs) of all-cause mortality comparing time-averaged serum intact PTH categories (Reference: 150 to <300 pg/mL) using Cox regression analyses in 88 356 HD patients stratified by age category
| Serum intact PTH (pg/mL) | All-cause death HRs (95% CIs) |
||
|---|---|---|---|
| Unadjusted | Case-mix adjusted | Case-mix + MBD adjusted | |
| Age 18 to <45 years, n = 14 656 (unadjusted), 14 654 (case-mix), 14 654 (case-mix + MBD) | |||
| <150 | 1.66 (1.50–1.83) | 0.85 (0.76–0.95) | 0.85 (0.76-0.95) |
| 150 to <300 | Reference | Reference | Reference |
| 300 to <600 | 0.88 (0.81–0.95) | 1.01 (0.94–1.10) | 0.95 (0.88–1.03) |
| ≥600 | 0.93 (0.85–1.02) | 1.14 (1.04–1.25) | 0.92 (0.84–1.02) |
| Age 45 to <65 years, n = 34 997 (unadjusted), 34 997 (case-mix), 34 997 (case-mix + MBD) | |||
| <150 | 1.55 (1.49–1.63) | 0.86 (0.82–0.90) | 0.86 (0.82–0.90) |
| 150 to <300 | Reference | Reference | Reference |
| 300 to <600 | 0.99 (0.95–1.02) | 1.11 (1.07–1.16) | 1.05 (1.01–1.10) |
| ≥600 | 1.09 (1.03–1.15) | 1.22 (1.15–1.29) | 1.07 (1.01–1.14) |
| Age 65 to <75 years, n = 20 908 (unadjusted), 20 908 (case-mix), 20 908 (case-mix + MBD) | |||
| <150 | 1.47 (1.40–1.53) | 0.79 (0.75–0.83) | 0.79 (0.75–0.84) |
| 150 to <300 | Reference | Reference | Reference |
| 300 to <600 | 1.07 (1.02–1.11) | 1.22 (1.17–1.28) | 1.15 (1.10–1.21) |
| ≥600 | 1.32 (1.22–1.42) | 1.50 (1.39–1.62) | 1.31 (1.21–1.42) |
| Age ≥75 years, n = 17 795 (unadjusted), 17 795 (case-mix), 17 795 (case-mix + MBD) | |||
| <150 | 1.40 (1.34–1.46) | 0.84 (0.80–0.88) | 0.83 (0.79–0.88) |
| 150 to <300 | Reference | Reference | Reference |
| 300 to <600 | 1.14 (1.09–1.19) | 1.29 (1.23–1.35) | 1.25 (1.19–1.31) |
| ≥600 | 1.35 (1.24–1.47) | 1.59 (1.45–1.74) | 1.45 (1.33–1.59) |
Case-mix model is adjusted for sex, race/ethnicity, presence of diabetes mellitus, nine preexisting comorbidities, history of tobacco smoking, dialysis duration categories, marital status, primary insurance, types of vascular access, dialysis dose as indicated by single-pool Kt/V and the dosage of activated vitamin D agents.
Case-mix + MBD adjusted model includes all of the case-mix covariates plus serum albumin corrected calcium, phosphorus and alkaline phosphatase concentrations.
Intact PTH, intact parathyroid hormone; MBDs, mineral and bone disorders.
DISCUSSION
In this retrospective analysis of over 100 000 MHD patients, we found differential associations of mortality with serum levels of ALP and PTH across varying age categories. Although higher serum ALP levels were associated with higher risks of mortality across all ages, the strength of association was stronger in young patients (<45 years) compared with older patients. Serum PTH levels showed a linear and robust association with mortality only in middle-aged and elderly patients (≥45 years), whereas there was a null association between higher serum PTH levels and mortality in patients <45 years. The PTH-mortality association appeared to be stronger in the older age categories compared with the younger groups. Similar patterns of association were observed with all-cause and cardiovascular mortality.
Our study is the first to compare mortality predictability of serum ALP and PTH levels across varying age categories in MHD patients. Serum ALP levels showed a monotonic and incremental association with mortality across the entire continuum of ALP levels in all age categories of MHD patients, independent of the parameters of MBDs and liver enzymes. However, the ALP–mortality association was stronger in young patients (<45 years) compared with older patients. The associations between elevated serum ALP levels and increased mortality have been observed not only in MHD [14, 15] and pre-dialysis CKD patients [26–28], but also in the general population [29]. Drechsler et al. [30] found a strong association between both serum bone ALP (BAP) and total ALP levels with short-term (6 month) mortality in incident dialysis patients. However, the ALP–mortality association was attenuated to the null when long-term (4-year) mortality was examined [30]. However, this study [30] used baseline serum levels of bone-specific and total ALP (measured at a single time-point) to study the association between serum ALP levels and long-term mortality. In contrast, our study examined the ALP–mortality association using time-averaged serum ALP levels, which may better reflect the long-term associations between cumulative exposure of higher ALP levels and mortality. Similarly, a higher serum BAP level was observed to be a predictor of mortality in male HD patients [31]. Our findings of the linear ALP–mortality association even at low serum ALP levels could possibly be related to vascular calcification through its pyrophosphate link. Vascular calcification has been independently associated with adverse cardiovascular outcomes and mortality in HD patients [32–34]. Smooth muscle cells in medial vascular calcification express higher levels of ALP [35], which may promote vascular calcification by lowering inorganic pyrophosphate [36], which is a potent inhibitor of extracellular mineralization [37, 38]. Thus, increased serum ALP levels may explain the prominent ALP–mortality association in younger patients, in whom vascular calcification is usually less.
In our study, we found a robust association between higher serum PTH levels and increased mortality risks only in MHD patients ≥45 years, and the PTH–mortality association was stronger in older patients compared with the younger groups. This finding was consistent with the results from previous publications that reported an association between high serum PTH levels (but not low serum PTH levels) and increased mortality in MHD patients [6, 9, 24]. In the general population, higher serum PTH levels were associated with higher risks of all-cause and cardiovascular mortality in the elderly population (≥65 years) [39, 40]. Serum PTH levels positively correlated with BMI, fat mass and lean mass in MHD patients [41, 42]. Elderly dialysis patients tended to have worse nutritional parameters [43, 44] that strongly correlated with increased mortality in dialysis patients [45, 46]. Nutritional factors likely did not confound the more prominent PTH–mortality association in elderly patients observed in the current study, as this association remained significant after additional adjustment for nutritional surrogates. Possible mechanisms that may explain the link between high serum PTH levels and mortality include vascular calcification, vascular remodeling [47–49] and detrimental effects on the myocardium such as left-ventricular hypertrophy and cardiac fibrosis [50–53]. However, we found that the PTH–mortality association was less prominent in young MHD patients (<45 years) compared with older patients. This finding may in part be due to differences in traditional risk factors associated with vascular calcification such as age, diabetes and hypertension [54] across age groups.
Our study found that the ALP–mortality association was stronger in younger patients, while the PTH–mortality association was more prominent in older patients (≥45 years). Several reasons could explain the discrepancy in mortality between serum ALP and PTH levels across ages. Serum BAP levels were not measured in our study. ALP is produced by different organs, including bone, liver, kidney and intestines [22] and sources of ALP could be different between age groups. Besides PTH, BAP production is influenced by other hormones including growth hormone and 1,25(OH)2vitamin D3, and bone responsiveness to these hormones also changes with age [18, 55–57]. We did not have information on serum levels of growth hormone or 1,25(OH)2vitamin D3 to examine this association. Additionally, an in vitro study investigating PTH signaling in human marrow stromal cells demonstrated that bone responsiveness to PTH varied with age [19]. It is important to stress that the PTH–survival interaction is likely more complex than its effect on bone, since the PTH has direct effects on other tissues, including vascular smooth muscle cells and myocardium [48, 52, 58].
Several limitations of this study need to be considered. First, our analyses were based on observational data, and no causal relationships can be established from the study results. Second, information on serum BAP levels was not available; therefore, elevated levels of total serum ALP could possibly stem from liver disease or other sources. However, we excluded patients with serum AST>40 U/L or missing AST data from our analyses, and we accounted for differences in serum levels of AST across exposure groups in case-mix adjusted analyses, indicating that the ALP–mortality association is likely to be independent of liver pathology. Third, we lacked data on oral medications related to treatment of MBDs including phosphorus binders and calcimimetics. However, it is noteworthy that calcimimetics became available in the USA during the last few months of this study cohort; hence, our cohort belongs to the pre-calcimimetic era. Fourth, serum 25(OH) vitamin D, 1,25(OH)2vitamin D3 and fibroblast growth factor-23 levels were not available in our study population and may have resulted in residual confounding. Fifth, we lacked data on inflammatory markers such as C-reactive protein. However, correction for malnutrition inflammation complex syndrome (MICS) surrogates might lead to over-adjustment which could introduce sources of error/bias particularly if mortality effects of serum ALP and PTH levels are exerted through modulation of MICS-related pathways; therefore, the case-mix adjusted models should be considered more appropriate.
The strengths of our study include its large sample size with relatively long follow-up periods. Patient data were obtained from DaVita dialysis facilities which were under uniform administrative care, and all laboratory values were measured in a single laboratory with optimal quality assurance. We adjusted for multiple potential confounders including baseline comorbidities, dialysis duration, receipt of activated injectable vitamin D treatment and doses in multivariable models. We used time-averaged measures with all laboratory data, rather than a single baseline measure in our analyses. The use of time-averaged survival models in this study reduces the risk of exposure misclassification and may better reflect the association between long-term cumulative exposure to higher levels of serum ALP and PTH and mortality over time.
In conclusion, our study revealed differential associations of serum ALP and PTH with death risks among MHD patients from various age groups. The ALP–mortality association was much stronger in young patients (<45 years) compared with older patients. Serum PTH levels showed a linear and significant association with mortality only in patients ≥45 years, whereas there was no significant association between higher serum PTH levels and mortality in young patients (<45 years). Our findings suggest that age is an effect modifier of the association of serum ALP and PTH levels with mortality in MHD patients. Further prospective studies are warranted to confirm these findings.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research® (DCR) for providing the clinical data, analysis and review for this research project and for advancing the knowledge and practice of kidney care. The study was supported by a research grant from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health R01 DK078106, K24 DK091419, a philanthropist grant from Mr. Harold Simmons and a research grant from DaVita Clinical Research. K.K.Z. has received honoraria from Genzyme/Sanofi and Shire, manufacturers of phosphorus binders. W.L.L. is supported by a Sanofi fellowship award. C.M.R. is supported by a research grant from the National Institutes of Diabetes, Digestive and Kidney Disease of the National Institutes of Health F32 DK093201.
REFERENCES
- 1.Collins AJ, Foley RN, Herzog C, et al. Excerpts from the US renal data system 2009 annual data report. Am J Kidney Dis. 2010;55:S1–S420. doi: 10.1053/j.ajkd.2009.10.009. A426–A427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13:1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 3.Sarnak MJ. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis. 2003;41:11–17. doi: 10.1016/s0272-6386(03)00372-x. [DOI] [PubMed] [Google Scholar]
- 4.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 5.Qunibi WY, Nolan CA, Ayus JC. Cardiovascular calcification in patients with end-stage renal disease: a century-old phenomenon. Kidney Int Suppl. 2002;62:S73–S80. doi: 10.1046/j.1523-1755.62.s82.15.x. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 7.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26:1938–1947. doi: 10.1093/ndt/gfq304. [DOI] [PubMed] [Google Scholar]
- 9.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Lomashvili KA, Garg P, Narisawa S, et al. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narisawa S, Harmey D, Yadav MC, et al. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res. 2007;22:1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 12.Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008;73:989–991. doi: 10.1038/ki.2008.104. [DOI] [PubMed] [Google Scholar]
- 13.Shantouf R, Kovesdy CP, Kim Y, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blayney MJ, Pisoni RL, Bragg-Gresham JL, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–663. doi: 10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]
- 15.Regidor DL, Kovesdy CP, Mehrotra R, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. USRDS 2012 Annual Data Report; pp. 216–228. [Google Scholar]
- 17.Jager KJ, van Dijk PC, Dekker FW, et al. The epidemic of aging in renal replacement therapy: an update on elderly patients and their outcomes. Clin Nephrol. 2003;60:352–360. doi: 10.5414/cnp60352. [DOI] [PubMed] [Google Scholar]
- 18.Horst RL, Goff JP, Reinhardt TA. Advancing age results in reduction of intestinal and bone 1,25-dihydroxyvitamin D receptor. Endocrinology. 1990;126:1053–1057. doi: 10.1210/endo-126-2-1053. [DOI] [PubMed] [Google Scholar]
- 19.Zhou S, Bueno EM, Kim SW, et al. Effects of age on parathyroid hormone signaling in human marrow stromal cells. Aging cell. 2011;10:780–788. doi: 10.1111/j.1474-9726.2011.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelletier S, Roth H, Bouchet JL, et al. Mineral and bone disease pattern in elderly haemodialysis patients. Nephrol Dial Transplant. 2010;25:3062–3070. doi: 10.1093/ndt/gfq128. [DOI] [PubMed] [Google Scholar]
- 21.Lertdumrongluk P, Rhee CM, Park J, et al. Association of serum phosphorus concentration with mortality in elderly and nonelderly hemodialysis patients. J Ren Nutr. 2013 doi: 10.1053/j.jrn.2013.01.018. April 29 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD) Kidney Int Suppl. 2009;76:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson P, Sharp CA, Magnusson M, et al. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 2001;60:257–265. doi: 10.1046/j.1523-1755.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25:2724–2734. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nussbaum SR, Zahradnik RJ, Lavigne JR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1987;33:1364–1367. [PubMed] [Google Scholar]
- 26.Abramowitz M, Muntner P, Coco M, et al. Serum alkaline phosphatase and phosphate and risk of mortality and hospitalization. Clin J Am Soc Nephrol. 2010;5:1064–1071. doi: 10.2215/CJN.08621209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beddhu S, Ma X, Baird B, et al. Serum alkaline phosphatase and mortality in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1805–1810. doi: 10.2215/CJN.01560309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Ureche V, Lu JL, et al. Outcome predictability of serum alkaline phosphatase in men with pre-dialysis CKD. Nephrol Dial Transplant. 2010;25:3003–3011. doi: 10.1093/ndt/gfq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonelli M, Curhan G, Pfeffer M, et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. 2009;120:1784–1792. doi: 10.1161/CIRCULATIONAHA.109.851873. [DOI] [PubMed] [Google Scholar]
- 30.Drechsler C, Verduijn M, Pilz S, et al. Bone alkaline phosphatase and mortality in dialysis patients. Clin J Am Soc Nephrol. 2011;6:1752–1759. doi: 10.2215/CJN.10091110. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi I, Shidara K, Okuno S, et al. Higher serum bone alkaline phosphatase as a predictor of mortality in male hemodialysis patients. Life Sci. 2012;90:212–218. doi: 10.1016/j.lfs.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Okuno S, Ishimura E, Kitatani K, et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417–425. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Rennenberg RJ, Kessels AG, Schurgers LJ, et al. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–197. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shantouf RS, Budoff MJ, Ahmadi N, et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanahan CM, Cary NR, Salisbury JR, et al. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 36.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 37.Lomashvili KA, Cobbs S, Hennigar RA, et al. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 38.Schibler D, Russell RG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci. 1968;35:363–372. [PubMed] [Google Scholar]
- 39.Hagstrom E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin D status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab. 2004;89:5477–5481. doi: 10.1210/jc.2004-0307. [DOI] [PubMed] [Google Scholar]
- 41.Drechsler C, Grootendorst DC, Boeschoten EW, et al. Changes in parathyroid hormone, body mass index and the association with mortality in dialysis patients. Nephrol Dial Transplant. 2011;26:1340–1346. doi: 10.1093/ndt/gfq541. [DOI] [PubMed] [Google Scholar]
- 42.Ishimura E, Okuno S, Tsuboniwa N, et al. Significant positive association between parathyroid hormone and fat mass and lean mass in chronic hemodialysis patients. J Clin Endocrinol Metab. 2013;98:1264–1270. doi: 10.1210/jc.2012-3883. [DOI] [PubMed] [Google Scholar]
- 43.Canaud B, Tong L, Tentori F, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2011;6:1651–1662. doi: 10.2215/CJN.03530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celik G, Oc B, Kara I, et al. Comparison of nutritional parameters among adult and elderly hemodialysis patients. Int J Med Sci. 2011;8:628–634. doi: 10.7150/ijms.8.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dwyer JT, Larive B, Leung J, et al. Are nutritional status indicators associated with mortality in the hemodialysis (HEMO) study? Kidney Int. 2005;68:1766–1776. doi: 10.1111/j.1523-1755.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 46.Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 47.Amann K, Tornig J, Flechtenmacher C, et al. Blood-pressure-independent wall thickening of intramyocardial arterioles in experimental uraemia: evidence for a permissive action of PTH. Nephrol Dial Transplant. 1995;10:2043–2048. [PubMed] [Google Scholar]
- 48.Perkovic V, Hewitson TD, Kelynack KJ, et al. Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press Res. 2003;26:27–33. doi: 10.1159/000069761. [DOI] [PubMed] [Google Scholar]
- 49.Rashid G, Bernheim J, Green J, et al. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. Am J Physiol Renal Physiol. 2007;292:F1215–F1218. doi: 10.1152/ajprenal.00406.2006. [DOI] [PubMed] [Google Scholar]
- 50.Baczynski R, Massry SG, Kohan R, et al. Effect of parathyroid hormone on myocardial energy metabolism in the rat. Kidney Int. 1985;27:718–725. doi: 10.1038/ki.1985.71. [DOI] [PubMed] [Google Scholar]
- 51.Bogin E, Massry SG, Harary I. Effect of parathyroid hormone on rat heart cells. J Clin Invest. 1981;67:1215–1227. doi: 10.1172/JCI110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saleh FN, Schirmer H, Sundsfjord J, et al. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003;24:2054–2060. doi: 10.1016/j.ehj.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Smogorzewski M, Perna AF, Borum PR, et al. Fatty acid oxidation in the myocardium: effects of parathyroid hormone and CRF. Kidney Int. 1988;34:797–803. doi: 10.1038/ki.1988.252. [DOI] [PubMed] [Google Scholar]
- 54.Moe SM. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur J Clin Invest. 2006;36:51–62. doi: 10.1111/j.1365-2362.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 55.Crippa GE, Beloti MM, Cardoso CR, et al. Effect of growth hormone on in vitro osteogenesis and gene expression of human osteoblastic cells is donor-age-dependent. J Cell Biochem. 2008;104:369–376. doi: 10.1002/jcb.21628. [DOI] [PubMed] [Google Scholar]
- 56.Duque G, Macoritto M, Dion N, et al. 1,25(OH)2D3 acts as a bone-forming agent in the hormone-independent senescence-accelerated mouse (SAM-P/6) Am J Physiol Endocrinol Metab. 2005;288:E723–E730. doi: 10.1152/ajpendo.00180.2004. [DOI] [PubMed] [Google Scholar]
- 57.Manolagas SC, Burton DW, Deftos LJ. 1,25-Dihydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J Biol Chem. 1981;256:7115–7117. [PubMed] [Google Scholar]
- 58.Wang R, Wu L, Karpinski E, et al. The changes in contractile status of single vascular smooth muscle cells and ventricular cells induced by bPTH-(1-34) Life Sci. 1993;52:793–801. doi: 10.1016/0024-3205(93)90077-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.