Abstract
Cardiovascular disease (CVD) is globally considered as the leading cause of death with 80% of CVD related deaths being reported from low and middle income countries like India. The relatively early onset age of CVD in India in comparison to Western countries also implies that most productive ages of the patient's life are lost fighting the disease. Conventional cardiovascular risk is attributed to lifestyle changes and altered metabolic activity. This forms the basis of a 10-year risk prediction score inspired by the Framingham study. Since South Asians display considerable heterogeneity in risk factors as compared to developed countries, there is a need to identify risk factors which would not only help in primary prevention but also prevent their recurrence. We reviewed published data on novel risk factors and their potential to identify cardiovascular risk at an early stage, with special emphasis on the Indian population. Emerging risk factors were reviewed to identify their potential to prevent CVD progression independently as well as in association with other cardiovascular risk factors. The most commonly studied emerging cardiovascular risk factors included coronary artery calcium score, lipoprotein (a), apolipoproteins, homocysteine, thrombosis markers like fibrinogen, and plasminogen activator inhibitor 1, carotid intima-media thickness, genotypic variations, non-alcoholic fatty liver disease, C-reactive protein, platelets, and birth weight levels. Nonetheless, more studies on large sample size can ascertain the utility of these risk factors in estimation and analysis of cardiovascular risk especially in the Indian context.
Keywords: Cardiovascular disease, emerging risk factors, India epidemiology, carotid intima-media thickness
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death and disability worldwide. It is expected that by 2020, CVD would prevail as the leading cause of death and disability over infectious diseases globally.[1] Cardiovascular disease encompass atherosclerotic vascular diseases like coronary heart disease (CHD), cerebrovascular disease (CBVD), and peripheral arterial diseases. In recent years, demographics and health surveys have reported increasing malaise of CVD among individuals of all socioeconomic strata. According to recent statistics, incidences of CVD-related death and disability in low-income countries have grown at an alarming pace. In 2008, Gupta et al. reported that India alone is burdened with approximately 25% of cardiovascular-related deaths and would serve as a home to more than 50% of the patients with heart ailments worldwide within next 15 years.[2] The seriousness of current scenario could be gauged by the fact that most CVD sufferers in India happens to be in their productive age which may potentially impose huge socioeconomic burden and devastating consequences over the coming years. In 2005, Reddy et al. reported that India has incurred the highest loss in productive years of life worldwide.[3] Presently, the greatest public health challenge to developing countries is to control epidemics of chronic noncommunicable diseases, specifically CVD, CHD, diabetes and stroke which have caused almost doubled mortality rates than other communicable diseases in India.[4]
Over past three decades, the field of medicine has made a drastic progress in diagnosis, prevention and treatment of CVD. The Framingham Heart Study and the Seven Countries’ Study were the two major studies that made significant contribution in identifying major risk factors for CVD.[1,5] Framingham risk score is a widely recognised tool used by clinicians worldwide to calculate 10-year cardiovascular risk in an individual and classify them for risk of coronary death or myocardial infarction (MI).[5] The Framingham risk score has been utilised effectively to portend major CHD events across ethnic groups and races. Substantial body of evidence supported reduction of existing (conventional) risk factors (modifiable or nonmodifiable) leading to the search of new emerging risk factors. In developed countries, predominantly there are five existing modifiable risk factors (high blood pressure, high blood cholesterol, tobacco use (chewing/smoking), diabetes mellitus, and obesity) which constitutes approximately one-third of all CVD cases. In developing countries, in addition to these five existing modifiable risk factors, low vegetable and fruit intake and alcohol abuse ranks first in the list of risk factors. The present review focuses on emerging risk factors for CVD as per Indian context.
SEARCH STRATEGIES
We have identified electronic databases such as MEDLINE, High Wire, Cochrane, and Google Scholar for searching articles from 2001 to June 2012 using the following keywords: “cardiovascular disease”, “cardiovascular disease risk factors”, and “emerging risk factors for cardiovascular disease in India or South Asians.”
INDIAN PERSPECTIVE OF CARDIOVASCULAR DISEASE
Asian Indian pedigree constitute over one-fifth of the world population. Asian Indian phenotype is marked by combination of clinical (larger waist-to-hip and waist-to-height ratios signalling excess visceral adiposity), biochemical [insulin resistance, lower adiponectin and higher C-reactive protein (CRP) levels], and metabolic aberrations (raised triglycerides, low high-density lipoprotein cholesterol [HDL-C]).[6] In 2006, Gaziano et al. predicted that individuals of Asian Indian ethnicity would account for 40-60% of global CVD burden within the next 10-15 years.[7] Over last 30 years, the rate of CHD-related incidence has increased from 2 to 6% in rural population and from 4 to 12% in urban population.[8] The prevalence of so called Asian Indian phenotype in South Asians has led to their excessive vulnerability to diabetes and premature CHD.[9] Presence of high lipoprotein (Lp) (a), environmental and lifestyle risk factors explain the growing prevalence of heart diseases in India.[8,9] Higher predisposition to metabolic syndrome characterized by insulin resistance, hyperinsulinemia, type 2 diabetes, impaired glucose tolerance, central obesity, hypertension and dyslipidemia (high triglyceride and low high density lipoprotein levels) is another important reason for increased CVD incidence in Indian population. The rural-urban differences, public-private health care, low awareness across the region, long-term and asymptomatic nature of noncommunicable risk factors and disease delays diagnosis of CVD and serve as a roadblock to seek care and self-management of risk factors. The genetic and environmental factors act as the important etiological clues to diversity in terms of disease presentation, therapeutic needs and responses to treatment. Hence ethnicity is regarded as a probable independent risk factor that could help in recognising treatment goals and the choice of therapy in particular population or race.[10,11]
Existing (conventional) risk factors and why there is a need for emerging risk factors
Over 300 existing (conventional) risk factors for CVDs have been discovered which mostly fall into two broad categories: 1) modifiable and 2) non-modifiable [Table 1]. Modifiable risk factors are the factors which if treated and controlled would reduce the CVD risk while nonmodifiable risk factors could not be modified to reduce the CVD burden.[11,12] The existing risk factors were based on three primary criteria: 1) high prevalence in different populations; 2) significant independent impact on the risk of CHD and stroke; and 3) reduced cardiovascular risk with treatment and control. Though existing risk factors such as smoking, dyslipidaemia, hypertension, diabetes and obesity have been considered while calculating the risk among various ethnic groups, they could not fully explain excess risk in Indians and other ethnic groups, indicating a search for alternative risk factors. About 30-50% of coronary disease patients have been reported asymptomatic with absence of any conventional risk factor. In United States, adults with no history of CVD have been identified to possess intermediate risk and 10-year CVD risk of 10-20%. In the wake of increasing incidences of cardiovascular events in people who were apparently healthy and asymptomatic, both the American Heart Association and National Cholesterol Education Program Adult Treatment Panel-III (NCEP-ATP III) issued directives to identify these individuals who may be sufficiently at high risk of coronary event in the future and justified efforts directed at aggressive risk reduction. It was also reported that even the modest elevation in blood pressure, cholesterol and glucose levels would predispose an individual at a CVD risk.[12]
Table 1.
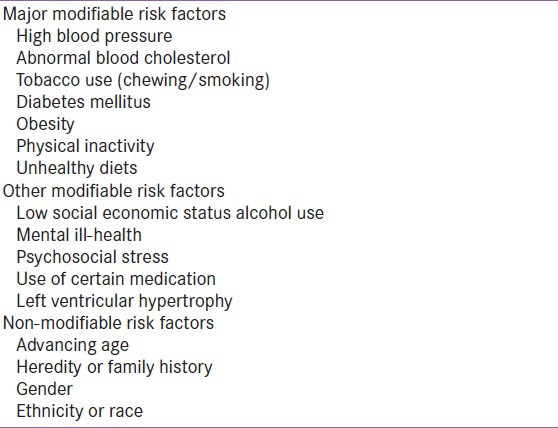
In 2005, Assmann et al., reported limitations of cardiovascular risk prediction based on NCEP guidelines.[13] It was indicated that Framingham risk score could not be applied globally in cardiovascular risk prediction. This data was supported by another study where the risk of CHD was overestimated and the overall cardiovascular risk calculation based on Framingham risk score was almost double the actual risk; criticizing the use of conventional risk factors in identifying patients with high risk as patients with very high risk of CVD.[14] Another drawback in NCEP-based risk prediction was that it automatically classified all diabetics into “high risk category” and this automatic classification of diabetes mellitus as “coronary risk equivalent” did not apply to all population specifically those with a low baseline risk of CHD. The Prospective Cardiovascular Münstery (PROCAM) study reported the similar finding where only 26% of the diabetes patients were included in the high-risk category.[14] This false overestimation of risk calculated by Framingham or PROCAM score was well addressed by new emerging risk factors like total cholesterol, low-density lipoprotein (LDL) cholesterol and HDL-C which constituted the basis of Framingham risk score. The impetus to explore new risk factors for CVD risk prediction was derived from the predictive models, based on conventional risk factors, which were reported as underutilized and possessed lower accuracy than desired.[15]
Emerging risk factors
More than 100 new/emerging risk factors have been discovered for their ability to improve global risk assessment.[15] Most of the emerging risk factors were actually the existing risk factors which had their independent risk predictive potential recently confirmed, despite being discovered long back. Any prospective biomarker or new risk factor was referred as emerging risk factor if able to address the following questions.[16]
Is the biomarker readily measured?
Does the biomarker add value to existing tests and improve its risk predictive ability?
Will the biomarker enhance clinician's decision making ability and improve patient management?
These biomarkers were referred as emerging risk factors since they were directly associated with increased risk of CVD but their causative, quantitative and independent contributions to CVD were not fully elucidated with respect to conventional risk factors.[17] These emerging risk factors helped to reclassify intermediate patients’ risk for major CHD events, requiring more aggressive risk reduction.[17,18] American Association for Clinical Chemistry's scientific academy had developed National Academy of Clinical Biology Laboratory Medicine and Practice Guidelines for utilization of emerging laboratory biomarkers of cardiovascular and stroke risk in a primary prevention setting. These guidelines assist clinical and laboratory practice decisions concerning patients at increased risk for specific diseases. Emerging risk factors are broadly categorized as either early markers of risk due to genetic polymorphisms or markers of existing disease [Table 2].[13,18]
Table 2.
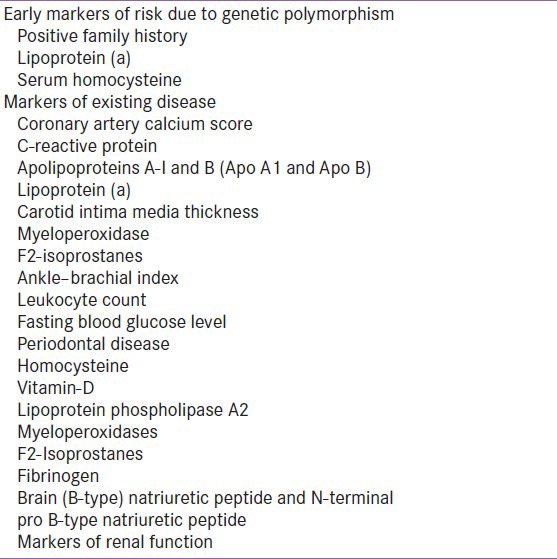
Early markers of risk due to genetic polymorphism
Genetic polymorphism is defined as change in DNA sequence due to single nucleotide polymorphism (SNP), sequence repeats, insertion/deletion and recombination. This genetic polymorphism increases the risk of CVD and hence needs to be considered for cardiovascular risk prediction. In recent times, risk prediction algorithms based on genetic information have reported to improve prognosis of CVD.[19] Till date, more than 100 genetic variants have been discovered with the help of Genome Wide Association Studies. Several SNP have been identified in genes encoding HDL, LDL, triglycerides CRP and body mass index which lead to an increased risk of MI.[19] Genuine associations of SNP with CHD provided an early glimpse into the underlying genetic risk of coronary diseases.[20]
Positive family history of premature CVD is a known genetic risk factor for incident CVD.[21] It has significantly improved risk reclassification and has been added to risk prediction models. In 2005, Murabito et al. reported doubled risk of MI, even after adjustment of conventional risk factors, in patients with family history of CVD in comparison to patient without family history of CVD.[22] The heritability of MI provided 40-60% estimate of genetic variance in MI risk, indicating genetic factors to be critically involved in the pathogenesis of CVD and could be used for risk prediction for CVD.[19] The American Heart Association and Centers for Disease Control and Prevention also recognized positive family history as a risk factor and provided elaborate tools to help individuals assess their own family history of disease.[23]
Homocysteine is an emerging new risk factor for cardiovascular disease. Hyperhomocysteinemia is associated with an increased risk of several complex diseases, including CVDs. The level of plasma homocysteine depends on the combined effects of genetic and environmental factors. Polymorphisms of genes encoding homocysteine metabolism-related enzymes (methylenetetrahydrofolate reductase, methionine synthase, methionine synthase reductase, and cystathionine beta-synthase) influence plasma homocysteine concentration and thereby cardiovascular health. A common polymorphism in the gene coding for 5,10-methylene tetrahydrofolate reductase (C677T, Ala --> Val) is associated with decreased activity of the enzyme due to thermolability. Homozygosity for the Val allele leads to a mild-to-moderate hyperhomocysteinemia which is recognized as an independent risk factor for atherosclerosis.[24]
Lipoprotein (a) is known as an independent risk factor for CVD. It is a unique lipoprotein composed of LDL-P to which apolipoprotein (Apo) (a) is attached by a disulfide bond to Apo B. It stimulates clotting or plaque rupture leading to thrombotic events, arterial blockade and finally leading to acute clinical events. Lp (a) is a very stable parameter and remains unaffected by diet and drugs. Smaller isoforms of apo (a) with fewer kringle-4 repeats has been associated with elevated plasma concentration of Lp (a) causing heightened risk for CVD.[25]
Emerging risk factors of existing disease
Coronary artery calcium score
Coronary artery calcification (CAC) has been directly correlated with increased risk of future cardiac events, establishing its prognostic value in CVD risk prediction. A zero CAC score was interpreted as following in different CAD risk populations: (1) asymptomatic adults: Low CAD risk and low risk of near-term coronary events; (2) older asymptomatic patients with risk factors: A moderate increased risk of events; (3) patients with intermediate-to-high risk of CAD: A presence of MI (on provocative testing) with high risk of near-term coronary events.[26] CAC score unfailingly provides better risk prediction than Framingham risk score.[27]
The Multi-Ethnic Study of Atherosclerosis reported CAC score as an excellent risk predictor of CAD events.[28] In an Indian study involving 500 asymptomatic subjects, Wasnic and coworkers provided reference percentile of CAC score to screen and stratify risk of coronary events in Indian population using noninvasive computed tomography.[29] In 2010, Scheuner et al., reported advanced CAC as an emerging indication for aggressive cardiovascular risk factor modification.[30] They also reported positive correlation of family history with CAC and emphasized addition of familial risk stratification (presence or absence of premature CHD in first degree relatives) in the prognosis of CVD. According to the American Heart Association, the use of CAC quantification falls in class IIb recommendation in patients with intermediate risk for improving risk assessment[31] and the recent American College of Cardiology Foundation/American Heart Association Task Force on Practice guidelines in 2010 used class IIa recommendation i.e., CAC scanning had usefulness in intermediate-risk patients.[27] In a systematic review of imaging guidelines for asymptomatic CADs, 11 out of 14 guidelines supported the use of CAC score in intermediate-risk patients.[32] It was strongly recommended that CAC scoring must be conducted only by certified physicians who had competence not only in the interpretation of CAC scans but also in clinical application and patient counseling. Although Grayburn mentioned that there was no randomiszed controlled trials available to support treatment decisions or outcome based on an abnormal CAC score,[33] two clinical trials strongly favored the use of CAC score in prevention of relative risk of coronary events which would help in restoring the use of CAC score for treatment decisions.[34,35] The first study, St. Francis Heart Study (n = 1005), concluded atorvastatin dose of 20 mg per day when administered to patients with a CAC score >400 caused 42% decrement in relative risk and 6.3% reduction in the absolute risk of coronary events.[34] The other study, Early Identification of Sub-clinical Atherosclerosis by Noninvasive Imaging Research (n = 2137) randomly assigned participants to either undergo or not undergo CAC scanning. Patients who underwent CAC scanning reported significant improvements in blood pressure, cholesterol levels, waist circumference, and Framingham risk score than patients who did not undergo CAC scanning.[35]
C-reactive protein
Elevated levels of serum CRP serve as a strong independent predictor of risk of MI, stroke, peripheral arterial diseases and cardiovascular mortality, hence termed as high sensitivity CRP (hsCRP).[36] The normal baseline CRP value was 2 mg/L for cardiovascular risk prediction and 10 mg/L for ACS patients. In 2002, Ridker et al., reported the role of abnormal CRP values in the development of atherosclerotic CVD. In addition, healthy individuals who were free from overt infection or inflammation were also reported to be associated with the risk of cardiovascular event.[37] Further in 2008, Ridker et al., confirmed hsCRP as a strong, independent predictor of future heart disease.[38] The Cardiovascular Health Study evaluated hsCRP levels in men and women aged 65 years or more without a history of vascular disease.[39] The study reported strong association between elevated levels of hsCRP with increased 10-year risk of CHD beyond traditional risk factors. As per initial clinical assessment, CRP measurement was not recommended for routine use in patients with low risk (10-year CHD event risk <5% as per Framingham score) but may be of value in patients with multiple mild disturbances; recommended for routine measurements in patients (males aged >50 years and females >60 years) with intermediate risk (10-year risk of 5-20%); hsCRP measurements may be considered in certain patients with CHD and risk equivalents; and was considered reasonable for patients with premature family history of CHD or cases of established CHD with history of recurrent events despite appropriate therapy. As per on-treatment management decisions, CRP was considered reasonable in patients with intermediate risk, CHD or CHD risk equivalent or a history of recurrent coronary events and may be used to determine intensity of therapy and was considered for patients with family history of premature CHD history.[40] Further investigation are undergoing to ascertain possible clinical utility of hsCRP in therapeutic decision making in patients with family history of premature CHD.
Carotid intima-media thickness
Carotid intima-media thickness (CIMT) is a widely recognised imaging marker of generalized atherosclerosis. It is represented as the double line pattern (lumen-intima and the media-adventitia interfaces) on the near and the far wall of the carotid artery. CIMT has been characterized as early atherosclerosis and as nonatherosclerotic compensatory enlargement; both these characteristics differently influence risk prediction of cardiovascular events in large epidemiological studies.[41]
Various trials supported CIMT in CVD prediction.[42,43,44,45] A meta-analysis of eight observational population based studies reported significant association of CIMT and cardiovascular risk.[42] Analysis of 9 lipid-lowering trials reported strong correlation between CIMT and LDL reduction; reduction of CIMT by 0.73% was accounted for 10% reduction in LDL-C per year. Cardiovascular risk factors together with CIMT and plaque have shown a small but increasing effect for CVD prediction.[43] Incorporation of information on CIMT and carotid plaque has led to reclassification of approximately 23% individuals to a different category in a recent meta-analysis. Further, addition of CIMT to traditional risk factors increased area under the receiver-operating characteristic curve from 0.74 to 0.765.[44] In the Northern Manhattan Study, 10-year Framingham vascular risk calculation was considerably improved in the presence of carotid plaque and >50% individuals in low and moderate Framingham risk categories were reclassified into higher risk category.[45] Therefore, risk prediction based on traditional risk factors may be reconsidered and CIMT and carotid plaque would help in further refining cardiovascular risk prediction.
Lipoprotein (a)
In a recent review, Lp (a) was specifically correlated with enhanced risk of CHD in a continuous nonthreshold manner. Its normal limits are below 25 mg/dL and some laboratories used ≥30 mg/dL as a cut off point for elevated Lp (a) levels.[40] It was found to have positive risk predictive potential additive to other measures of lipoprotein risk factors and Framingham risk score. Remarkably, the risk prediction by Lp (a) was found to be independent of LDL-C, non-HDL-C and presence of other risk factors.[46] Since elevated plasma Lp (a) concentration are regulated by Lp (a) gene, a very strong family history of vascular events would warrant its assessment. Lp (a) measurement was considered for assistance with on-treatment management decisions in patients with an intermediate-risk, CHD/CHD risk equivalent, premature family history or history of recurrent cardiovascular events of CHD or established CHD, despite appropriate therapy.
Apolipoprotein B
Apolipoprotein B concentration has been regarded as the direct measurement of total number of circulating atherogenic particles. LDL Apo B particle promotes initiation, development and progression of atherosclerosis and is considered far more important than VLDL Apo B particle in driving atherogenesis. Due to small size and higher concentration of LDL, LDL Apo B complex gets entrapped within subintimal space of arterial wall, leading to atherogenesis. Apo B concentrations more accurately depicts the number of LDL particles and LDL-related CVD risk in patients where LDL particles on an average possess less or more cholesterol than normal conditions, including diabetes, metabolic syndrome, abdominal obesity, hypertriglyceridema, familial dyslipoproteinemia etc. Apo B measurement was considered reasonable for patients with intermediate-risk with CHD or CHD risk equivalents, recurrent events, and family history of premature CHD. Few studies indicated Apo B to be more closely related to the risk of vascular disease than LDL-C.[47,48] A recent analysis of risk estimates for non-HDL-C and Apo B suggested Apo B to be the best predictor, LDL-C as the worst and non-HDL-C as the intermediate risk predictor.[48] Apo B has shown considerable advantage over LDL-C in terms of its accurate and standardized measurements, was found to be inexpensive and reliable in clinical laboratories and did not require fasting conditions before measurement.[49]
Homocysteine
Homocysteine is a sulfur amino acid and a normal by-product in methionine metabolic pathway and excess homocysteine is removed via liver and kidney. More than two decades back, homocysteine was reported as an independent risk factor for cardiovascular disease. Each 5 μmol/L rise in homocysteine levels conferred ~9% increase in the risk of CHD events, independent of other conventional CHD risk factors.[50] Various studies reported elevated levels of homocysteine with increased risk of CVD.[50,51,52,53] In 1992, acute MI or death due to coronary disease was significantly associated with increased homocysteine levels, after adjusting for other risk factors.[50] In 2002, Wald et al. conducted meta-analysis where lowering homocysteine by 3 μmol/L was reported to reduce the risk of ischemic heart disease by 16%, deep vein thrombosis by 25% and stroke by 24%.[51] Another recent Indian study involving 250 subjects found a positive correlation between homocysteine levels and cardiovascular risk even after multivariate adjustments.[52] Homocysteine was also recognized as a significant independent risk factor for young MI patients, indicating the need to evaluate homocysteine in all young patients with MI, especially in the absence of traditional risk factors.[53]
Myeloperoxidase
Myeloperoxidase (MPO), a leucocyte-derived enzyme belongs to heme-peroxidase superfamily. It generates reactive intermediates leading to oxidative damage of host lipids and proteins.[54] The MPOs are present within atherosclerotic plaque in human arteries and contributes to atherogenesis by catalysing oxidative reactions in the vascular wall. MPO strongly predicted future coronary events in healthy individuals independent of other traditional risk factors, indicating MPO as a potential clinical useful marker of CHD risk.[55]
F2-Isoprostanes
Over the past few years, F2-isoprostanes (IsoPs) has emerged as the most sensitive and reliable biomarker of lipid peroxidation in vivo.[56] Two to three times higher IsoP levels have been observed in people who smoke and nearly 2-fold higher levels in adults with high LDL-C and low HDL-C levels. In diabetics too, 2-3 times higher IsoP levels have been reported. In a case-control study (93 subjects with confirmed CHD and 93 age- and sex-matched healthy controls) independent predictive value of IsoP in CHD was determined. The study found patients with ≥2 risk factors to have higher levels of IsoP, confirming it as a potential independent risk factor for CHD.[57] In another study, nine different lipid peroxidation products (IsoPs and eight different hydroxy fatty acids) were measured in patients who underwent diagnostic coronary angiography. Of nine lipid peroxidation products, IsoPs and 9-HETE levels were significantly higher in patients with CHD than those without CHD. Furthermore, adding IsoP (or 9-HETE) values to Framingham risk score significantly improved the ability to predict angiographic CHD, indicating its potential clinical utility. In 2005, Gross et al., compared IsoP values and the extent of CAC score in a biracial cohort of 2850 young healthy men and women.[58] Approximately 23% of men in the highest IsoP quartile manifested calcification compared to only 12% of the men in the lowest IsoP quartile. High IsoP levels have shown not only an increased risk for disease but also provides information about disease severity.
Vitamin D
Limited sun exposure served as a means of increasing vitamin D. In 2008, Michael et al., reported strong association between low vitamin D status and adverse cardiovascular outcomes, including high blood pressure, diabetes, obesity and hyperthyroidism. The team also suggested 50,000 IU vitamin D2 or D3 weekly for 8-12 weeks followed by maintenance with 1,000 to 2,000 IU daily for restoring vitamin D to optimal levels in patients with CVD which may improve their heart health and prognosis.[59] Insufficient levels of vitamin D have shown to activate the renin-angiotensin-aldosterone system which lead to hypertension and thickening of heart and blood vessel walls. In the Framingham Heart Study, participants with vitamin D levels lower than15 ng/mL when enrolled had twice higher risk of cardiovascular events than participants with higher levels of vitamin D.[60] In NHANES III national cohort registry (n = 15088 subjects), higher vitamin D levels were associated with lower risk of diabetes, hypertension, high triglycerides, and obesity (http://www.lef.org/).
Ankle-brachial index
Ankle-brachial index (ABI) is the ratio of systolic pressure at the ankle to that in the arm. It is a non-invasive, sensitive, quick, easy to measure and a cost-effective marker to diagnose and assess severity of peripheral arterial disease and generalized atherosclerosis. In 1995, Kuller et al., reported ABI score ≤0.90 as an indicator of subclinical atherosclerosis and led to an increased incidence of cardiovascular mortality, MI, and stroke.[61] The relative risks were independent of baseline CVD and traditional risk factors, indicating its independent role in prediction of cardiovascular events and may improve cardiovascular risk prediction beyond Framingham risk score.[62] In another study, ABI score ≤0.90 had a specificity of >98% for the diagnosis of PAD and 92% specificity for prediction of CHD and stroke.[63] However, sufficient data is lacking which could assess ABI for cardiac risk assessment in asymptomatic intermediate-risk patients.[18]
CONCLUSIONS
Traditional/existing risk factors have been the major cause of all major cardiovascular events. Despite adjustment of these risk factors, cardiovascular related death and disability is still progressing in the developed and developing nations, indicating absence of reliable and effective risk predicting biomarkers. Cardiovascular risk estimation with Framingham risk score also faced severe criticism for applicability across the globe. Diverse ethnic group and variation in their susceptibility to cardiovascular risk factors has led to identification of new emerging risk factors which helped to reclassify intermediate patient's risk for major CHD events, demanding more aggressive risk reduction. These emerging risk factors are measurable, improve cardiovascular risk prediction, and also assist clinician in making decisions concerning patients at increased risk for specific diseases.
ACKNOWLEDGMENT
Max Neeman International, New Delhi, was responsible for preparation of this review article
Footnotes
Source of Support: AstraZeneca Pharma India Ltd
Conflict of Interest: None declared
REFERENCES
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 3.Reddy KS, Shah B, Varghese C, Ramadoss A. Responding to the challenge of chronic diseases in India. Lancet. 2005;366:1744–9. doi: 10.1016/S0140-6736(05)67343-6. [DOI] [PubMed] [Google Scholar]
- 4.Prasad DS, Kabir Z, Dash AK, Das BC. Cadiovascular risk factors in developing countries: A review of clinico-epidemiological evidence. CVD Prev Control. 2010;5:115–23. [Google Scholar]
- 5.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 6.Ali MK, Narayan KM, Tandon N. Diabetes and coronary heart disease: Current perspectives. Indian J Med Res. 2010;132:587–97. [PMC free article] [PubMed] [Google Scholar]
- 7.Gaziano TA, Reddy KS, Paccaud F, Horton S, Chaturvedi V. Cardiovascular Disease. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease control priorities in developing countries. 2nd ed. New York: Oxford University Press; 2006. pp. 645–62. [Google Scholar]
- 8.Enas EA, Singh V, Munjal YP, Gupta R, Patel KC, Bhandari S, et al. Recommendations of the second Indo-US health summit on prevention and control of cardiovascular disease among Asian Indians. Indian Heart J. 2009;61:165–274. [PubMed] [Google Scholar]
- 9.Enas EA, Chacko V, Senthilkumar A, Puthumana N, Mohan V. Elevated lipoprotein (a) - a genetic risk factor for premature vascular disease in people with and without standard risk factors: A review. Dis Mon. 2006;52:5–50. doi: 10.1016/j.disamonth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Forouhi NG, Sattar N. CVD risk factors and ethnicity: A homogeneous relationship? Atheroscler Suppl. 2006;7:11–9. doi: 10.1016/j.atherosclerosissup.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Smith SC, Greenland P, Grundy SM. AHA Conference Proceedings: Prevention Conference V: beyond secondary prevention: Identifying the high-risk patient for primary prevention: Executive summary: American Heart Association. Circulation. 2000;101:111–6. doi: 10.1161/01.cir.101.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The 3rd Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Assmann G, Cullen P, Fruchart JC, Greten H, Naruszewicz M, Olsson A, et al. International Task Force for Prevention of Coronary Heart Disease. Implications of emerging risk factors for therapeutic intervention. Nutr Metab Cardiovasc Dis. 2005;15:373–81. doi: 10.1016/j.numecd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Hense HW, Schulte H, Löwel H, Assmann G, Keil U. Framingham risk function overestimates risk of coronary heart disease in men and women from Germany-results from the MONICA Augsburg and the PROCAM cohorts. Eur Heart J. 2003;24:937–45. doi: 10.1016/s0195-668x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 15.Brotman DJ, Walker E, Lauer MS, O’Brien RG. In search of fewer independent risk factors. Arch Intern Med. 2005;165:138–45. doi: 10.1001/archinte.165.2.138. [DOI] [PubMed] [Google Scholar]
- 16.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115:949–52. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 17.Hackman DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: A critical review of the evidence. JAMA. 2003;290:932–40. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 18.Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, et al. Emerging Risk Factors for Coronary Heart Disease: A Summary of Systematic Reviews Conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496–7. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 19.Thanassoulis G, Vasan RS. Genetic cardiovascular risk prediction: Will we get there? Circulation. 2010;122:2323–34. doi: 10.1161/CIRCULATIONAHA.109.909309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan TM, Krumholz HM, Lifton RP, Spertus JA. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. JAMA. 2007;297:1551–61. doi: 10.1001/jama.297.14.1551. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: A prospective study of parents and offspring. JAMA. 2004;291:2204–11. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 22.Murabito JM, Pencina MJ, Nam BH, D’Agostino RB, Sr, Wang TJ, Lloyd-Jones D, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–23. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 23.Bare LA, Morrison AC, Rowland CM, Shiffman D, Luke MM, Iakoubova OA, et al. Five common gene variants identify elevated genetic risk for coronary heart disease. Genet Med. 2007;9:682–9. doi: 10.1097/gim.0b013e318156fb62. [DOI] [PubMed] [Google Scholar]
- 24.Malinowska A, Chmurzynska A. Polymorphism of genes encoding homocysteine metabolism-related enzymes and risk for cardiovascular disease. Nutr Res. 2009;29:685–95. doi: 10.1016/j.nutres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Kyutoku M, Nakagami H, Nakagami F, Koriyama H, Kiomy OM, Shimamura M, et al. Lipoprotein (a) is a Therapeutic Target for Cardiovascular Disease. Immunol Endocr Metab Agents Med Chem. 2012;12:118–21. [Google Scholar]
- 26.Greenland P, Bonow RO. How low risk is a coronary calcium score of zero? The importance of conditional probability. Circulation. 2008;117:1627–9. doi: 10.1161/CIRCULATIONAHA.108.767665. [DOI] [PubMed] [Google Scholar]
- 27.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Rosen BD, Fernandes V, McClelland RL, Carr JJ, Detrano R, Bluemke DA, et al. Relationship between Baseline Coronary Calcium Score and Demonstration of Coronary Artery Stenoses During Follow up in the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2009;2:1175–83. doi: 10.1016/j.jcmg.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasnik A, Raut A, Morani A. Coronary calcium scoring in asymptomatic Indian population: Correlation with age, gender and risk factors-a prospective study on 500 subjects. Indian Heart J. 2007;59:232–8. [PubMed] [Google Scholar]
- 30.Scheuner MT, Setodji CM, Pankow JS, Blumenthal RS, Keeler E. General Cardiovascular Risk Profile identifies advanced coronary artery calcium and is improved by family history: The multiethnic study of atherosclerosis. Circ Cardiovasc Genet. 2010;3:97–5. doi: 10.1161/CIRCGENETICS.109.894527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, et al. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol. 2000;36:326–40. doi: 10.1016/s0735-1097(00)00831-7. [DOI] [PubMed] [Google Scholar]
- 32.Ferket BS, Genders TS, Colkesen EB, Visser JJ, Spronk S, Steyerberg EW, et al. Systematic review of guidelines on imaging of symptomatic coronary artery disease. J Am Coll Cardiol. 2011;57:1591–600. doi: 10.1016/j.jacc.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 33.Grayburn PA. Interpreting the coronary-artery calcium score. N Engl J Med. 2012;366:294–6. doi: 10.1056/NEJMp1110647. [DOI] [PubMed] [Google Scholar]
- 34.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: The St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–72. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 35.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: The EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–32. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM. High-sensitivity C-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–8. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–7. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 39.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: The cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 40.Davidson MH, Ballantyne CM, Jacobson TA, Bittner VA, Braun LT, Brown AS, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: Advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;5:338–67. doi: 10.1016/j.jacl.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima—media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 43.Bartel S, Franzo AR, Rundek T. Carotid intima media thickness and plaque from risk assessment and clinical use to genetic discoveries. Respect Med. 2012 In Press. [Google Scholar]
- 44.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–7. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rundek T, Elkind MS, Di Tullio MR, Carrera E, Jin Z, Sacco RL, et al. Patent foramen ovale and migraine: A cross-sectional study from the Northern Manhattan Study (NOMAS) Circulation. 2008;118:1419–24. doi: 10.1161/CIRCULATIONAHA.108.771303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordestgaard BG, Chapman MJ, Ray K, Bore’n J, Andreotti F, Watts GF, et al. Lipoprotein (a) as a cardiovascular risk factor-current status. Eur Heart J. 2010;31:2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–85. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 48.Contois JH, McConnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, et al. Apolipoprotein B and cardiovascular disease risk: Position statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin Chem. 2009;55:407–19. doi: 10.1373/clinchem.2008.118356. [DOI] [PubMed] [Google Scholar]
- 49.Ganji V, Kafai MR. Population reference values for plasma total homocysteine homocysteine concentrations in US adults after the fortification of cereals with folic acid. Am J Clin Nutr. 2006;84:989–94. doi: 10.1093/ajcn/84.5.989. [DOI] [PubMed] [Google Scholar]
- 50.Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, Ullmann D, et al. A prospective study of plasma homocyst (e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–81. [PubMed] [Google Scholar]
- 51.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhagwat VR, Yadav AS, Rathod IM. Homocysteine, lipid indices and antioxidants in patients with ischaemic heart disease from Maharashtra, India. Singapore Med J. 2009;50:418–24. [PubMed] [Google Scholar]
- 53.Kumar A, Khan SA, Parvez A, Zaheer MS, Rabbani MU, Zafar L. The prevalence of hyperhomocysteinemia and its correlation with conventional risk factors in young patients with myocardial infarction in a tertiary care centre of India. Biomed Res. 2011;22:225–9. [Google Scholar]
- 54.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–91. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karakas M, Koeing W, Zierer A, Herder C, Rottbauer W, Baumert J, et al. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: Results from the MONICA/KORA Augsburg study. J Intern Med. 2012;271:43–50. doi: 10.1111/j.1365-2796.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 56.Davies SS, Roberts LJ., 2nd F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50:559–66. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brümmer J, et al. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: A matched case-control study. Circulation. 2004;109:843–8. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 58.Gross M, Steffes M, Jacobs DR, Jr, Yu X, Lewis L, Lewis CE, et al. Plasma F2-Isoprostanes and Coronary Artery Calcification: The CARDIA Study. Clin Chem. 2005;51:125–31. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 60.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;29(117):503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuller LH, Shemanski L, Psaty BM, Borhani NO, Gardin J, Haan MN, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–6. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- 62.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enoch A, Ijeoma A. The role of ankle-brachial index as a screening test for coronary artery disease in the Hispanic population. South Med J. 2008;101:1117–20. doi: 10.1097/SMJ.0b013e318189aabc. [DOI] [PubMed] [Google Scholar]


