Abstract
Introduction:
There is an increase in awareness about the role of nutritional factors in chronic non-communicable diseases. We therefore conducted this study with an aim to assess the relationship between nutritional factor (vitamin B12 and homocysteine [Hcy]) and its association with insulin resistance and inflammatory markers, and differences in traditional and non-traditional risk factors among diabetics and non-diabetics in known cases of coronary artery disease (CAD).
Materials and Methods:
Three hundred consecutive patients with known coronary disease on coronary angiography, who were >25 years old were included in this study. All cases were interviewed using a questionnaire. Blood samples were analyzed for insulin, vitamin B12, Hcy and inflammatory markers (highly sensitive C-reactive protein [hsCRP], interleukin-6 [IL-6], Tumor necrosis factor-alfa [TNF-α]). Insulin resistance was calculated with homeostasis model assessment of insulin resistance (HOMA-IR).
Results:
Mean age of the patients was 60.95 ± 12.3 years. Body mass index and waist hip ratio were comparable in both groups. Triglyceride, very low-density lipoprotein and HbA1C were significantly higher and high-density lipoprotein (HDL) was significantly lower in patients with diabetes. Patients with diabetes had significantly high levels of IL-6, hsCRP and TNF-α compared with non-diabetic patients. Insulin resistance was twofold higher in diabetic patients. Serum vitamin B12 levels were significantly lower and Hcy was significantly higher in the diabetic group compared with the non-diabetic patients. HbA1C, HOMA-IR and Hcy levels were positively correlated with inflammatory markers in the total study population and in the non-diabetic patients; but, in diabetic patients, HbA1C and Hcy showed this relation.
Conclusions:
Vitamin B12 deficiency is common in the diabetic population. Hcy levels were higher in diabetics compared with non-diabetics, and were related to glycemic level and insulin resistance in diabetic patients. Patients with diabetes had higher traditional risk factors than patients without diabetes in known patients with CAD. Glycemic status was associated with insulin resistance and inflammatory markers.
Keywords: Coronary artery disease, homocysteine, inflammatory markers, insulin resistance, type 2 diabetes mellitus, vitamin B12
INTRODUCTION
India has the highest number of patients with diabetes and cardiovascular disease, which will increase in the future.[1] Coronary artery disease (CAD) is a major cause of morbidity and mortality in type 2 diabetic patients.[2] Many prospective studies have evaluated the association of diabetes and cardiovascular disease (CVD) and highlighted the differences in the pattern of CAD among diabetic and non-diabetic patients.[3,4,5] Insulin resistance and a compensatory increase in insulin secretion are associated with a clustering of abnormalities (including impaired glucose tolerance, dyslipidemia and hypertension) that together lead to a significantly increased risk of CVD.[6] Insulin resistance is a proinflammatory condition.[7] Sub-clinical inflammation and associated inflammatory markers have been implicated in the pathogenesis of diabetes and atherosclerosis.[8,9] Increased levels homocysteine (Hcy) have been associated with inflammation and endothelial dysfunction.[10] Vitamin B12 levels are inversely related with Hcy levels.[11] Hence, indirectly, deficiency of vitamin B12 may be associated with insulin resistance and inflammation.[12] Therefore, we hypothesize that nutritional factors, insulin resistance and inflammatory markers should be interrelated in patients with CAD with or without diabetes. We therefore conducted this study with an aim to assess the relationship between nutritional factor (vitamin B12 and Hcy) and its association with insulin resistance and inflammatory markers, and differences in traditional and non-traditional risk factors among diabetics and non-diabetics in known cases of CAD.
MATERIALS AND METHODS
Three hundred patients with known coronary disease were included in this study. Patients who were admitted in the cardiology department for evaluation of chest pain and found to be angiography positive were selected in the study consecutively. Exclusion criteria were presence of chronic kidney disease, hepatic dysfunction, known endocrinal (except diabetes mellitus) or rheumatological diseases or chronic infections. All cases were interviewed using a questionnaire. Height, weight, waist and hip circumference were measured. Body mass index (BMI) was calculated by dividing weight in kilograms by square of height in meters. The waist hip ratio (WHR) was calculated. Obesity was defined by BMI >30 kg/m2. Central obesity was defined by waist >90 cm in male and >80 in female. There were 125 patients with diabetes who were considered as cases and 175 patients without diabetes who served as controls.
Fasting blood samples were collected after 14-h fasting. Lipids were measured by using Cholesterol oxidase 4-aminoantipyrine (CHOD PAP), Lipase/Glycerol kinase (LIP/GK) and enzymatic reaction, and low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) were calculated by the Freidwald formula. The interassay precision was 3.84% and the intra assay precision was 2% for all biochemical parameters. HbA1C was measured by boronate affinity assay. Tumor necrosis factor-alfa (TNF-α), interleukin-6 (IL-6), highly sensitive C-reactive protein (hsCRP) and Hcy were measured by enzyme-linked immunosorbent assay method with kits manufactured by Gen-probe Diaclone, France, Biochek, CA, USA, and Axis-shield Diagnostic Ltd, UK. Insulin, vitamin B12 and folic acid assays were performed by the micro-particle enzyme immunoassay (MEIA) and Ion Capture MEIA methods, with commercial kits supplied by Abbott Laboratory, USA. Intra-assay precision and interassay precision were <5% and <10% for the above parameters. Insulin resistance was calculated using the HOMA model (HOMA-IR = fasting insulin [μIU/ml] *fasting glucose [mmol/l]/22.5). Atherogenic dyslipidemia was defined as triglyceride level ≥150 mg/dl and high-density lipoprotein (HDL) cholesterol level <40 mg/dl. Vitamin B12 deficiency was defined by <200 pg/ml, folate deficiency by <3 ng/ml and hyperhomocysteinemia by >15 μmol/L. The study was approved by the Institutional Ethics Committee. Informed consent was obtained from all patients.
Statistical analysis was carried out using EPI Info, version 3.5.3 (CDC; Atlanta; USA) and SPSS Version 20. Data were presented as mean ± SD, median (range) or number (%) unless specified. All non-parametric data like hypertension, dyslipidemia, smoking and number of vessels involved were analyzed by the Chi-square test. All parametric data like BMI, WHR, lipid parameters, HbA1C, HOMA-IR, vitamin B12, Hcy and inflammatory markers were analyzed by the Student's t-test. If Bartlett's Chi-square test for equality of population variances was <0.05, then the Kruskal–Wallis test was applied. Pearson correlation was used to evaluate the correlation between nutritional factors like vitamin B12 and Hcy with inflammatory markers (hsCRP, IL-6 and TNF-α) and HOMA-IR. Multiple regression analysis was performed after adjustment for age, sex, BMI and presence of hypertension. A P < 0.05 was considered statistically significant.
RESULTS
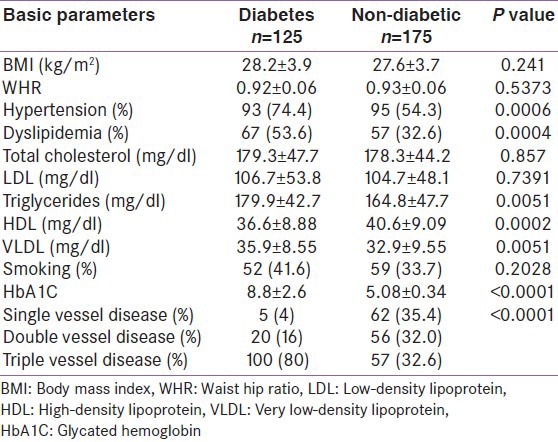
Three hundred patients with known cardiovascular disease (M: 216; F: 84; age: 25-92 years) were studied. Mean age of the patients was 60.9 ± 12.4 years (range 25-92). There was no age difference between males and females (M: 60.95 ± 12.3 years; F: 61.03 ± 12.9; P = 0.10). The basic characteristics of the study population are given in Table 1. BMI and WHR were comparable in both groups. Obesity was present in 28% of the patients with diabetes and 26.3% of the patients without diabetes (P = 0.84). Central obesity was observed in 84% of the cases and in 81.1% the controls (P = 0.62). Number of patients with hypertension and dyslipidemia was significantly higher in the diabetic patients compared with the non-diabetic patients. Triglyceride, VLDL and HbA1C were significantly higher and HDL was significantly lower in patients with diabetes compared with those without diabetes. There was no significant difference in serum total cholesterol and LDL cholesterol between the two groups. Serum LDL levels (>100 mg/dl) were comparable in both groups (40% vs. 38.9%; P = 0.93). Hypertriglyceridemia (69.6% vs. 58.3%; P = 0.02) and low HDL (68% vs. 45.1%; P = 0.0001) were more common in diabetics than in non-diabetics. Diabetic patients had more severe CAD than non-diabetics [Table 1].
Table 1.
Basic parameters of study population
Nutritional factors
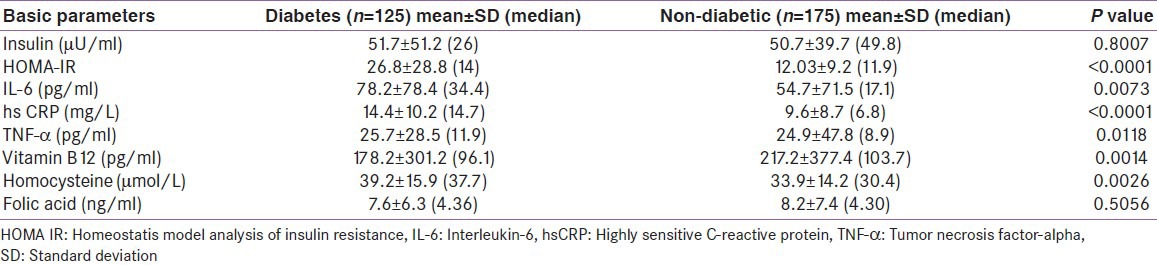
Serum vitamin B12 levels were significantly lower and Hcy was significantly higher in the diabetic group compared with the non-diabetic patients [Table 2]. Folic acid was comparable in both groups. Vitamin B12 deficiency was detected in 86.7% of the patients (cases 90.4% vs. control 84%; P = 0.15). Hyperhomocysteinemia was observed in 95.3% of the patients, with no significant difference between cases and controls (97.6% vs. 93.7%; P = 0.19). Only 2.5% of the patients had folic acid deficiency (cases 3.2% vs. control 2.3%; P = 0.90).
Table 2.
Insulin resistance, infl ammatory and nutritional markers in diabetic and non-diabetic patients
Vitamin B12 levels were negatively correlated with Hcy levels in the total study population and in both groups separately. It was also negatively correlated with HOMA-IR in diabetic patients only. There was no direct correlation between vitamin B12 levels and inflammatory markers. Hcy levels were positively correlated with inflammatory markers in the total study population and in non-diabetic patients, and there was no correlation in the diabetic patients [Table 3]. This correlation persisted even after adjustment for age, sex, and BMI in multiple regression analysis [Table 4].
Table 3.
Correlation of vitamin B12 and homocysteine, homeostatis model analysis of insulin resistance and infl ammatory markers*
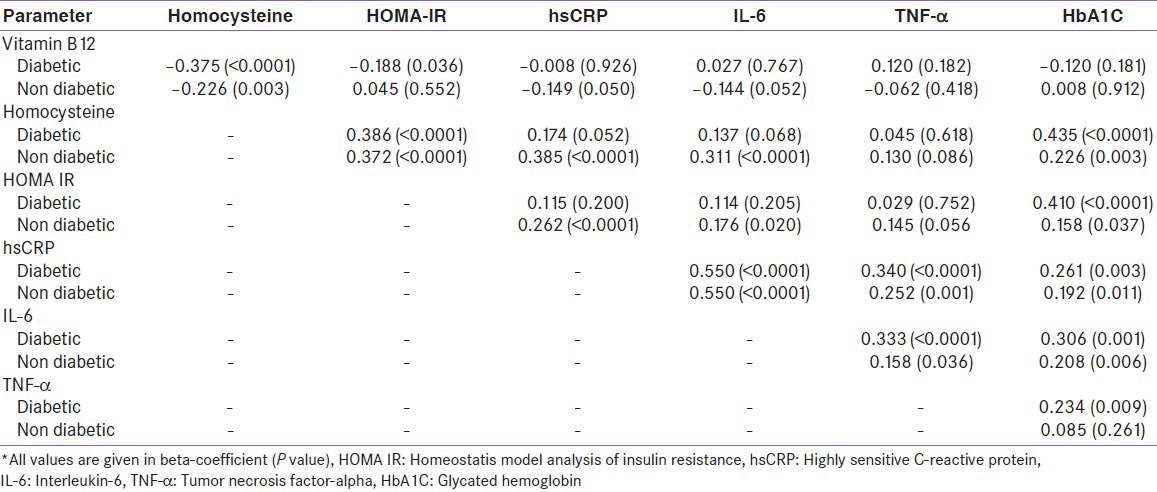
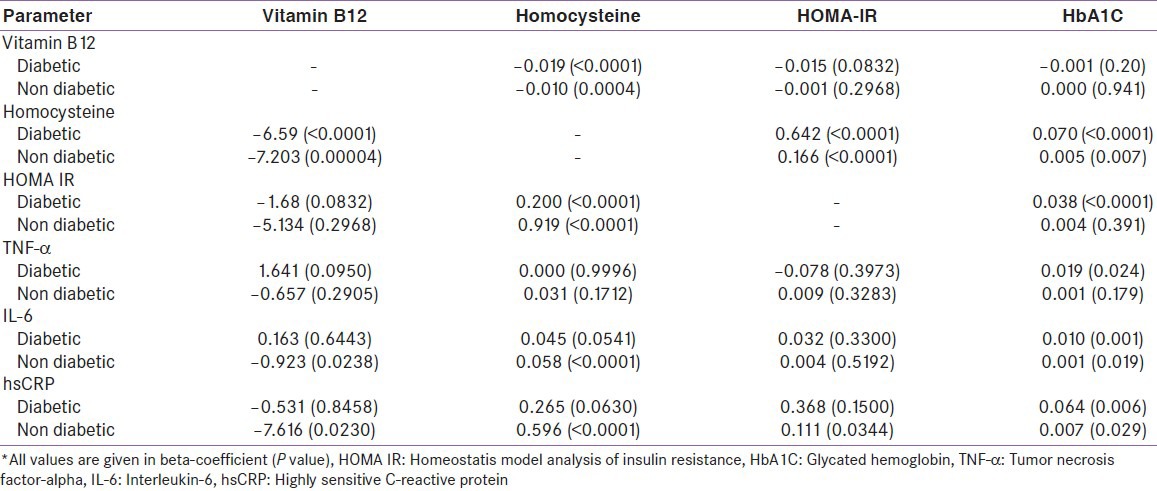
Table 4.
Interrelation of vitamin B12 and homocysteine; homeostatis model analysis of insulin resistance and infl ammatory markers after adjustment with age, sex, body mass index and hypertension in multiple regression analysis
Insulin resistance and inflammation
Diabetic patients were more insulin resistant (HOMA-IR) than non-diabetics, although the insulin levels were comparable. Patients with diabetes had significantly high levels of inflammatory markers; IL-6, hsCRP and TNF-α, compared with non-diabetic patients. hsCRP was more significantly elevated compared with IL-6 and TNF-α [Table 2]. HOMA-IR showed a positive correlation with Hcy levels [Figure 1] and inflammatory markers (IL-6 and hsCRP) in the total study population [Figure 1] and non-diabetic population, but this relation was only evident with Hcy in diabetic patients [Table 3]. This association persisted even after adjustment for age, sex and BMI in multiple regression analysis [Table 4]. HbA1C had a significant positive correlation with Hcy, HOMA-IR [Figure 1] and inflammatory markers (TNF-α, IL-6 and hsCRP) [Tables 3 and 4] [Figure 1].
Figure 1.
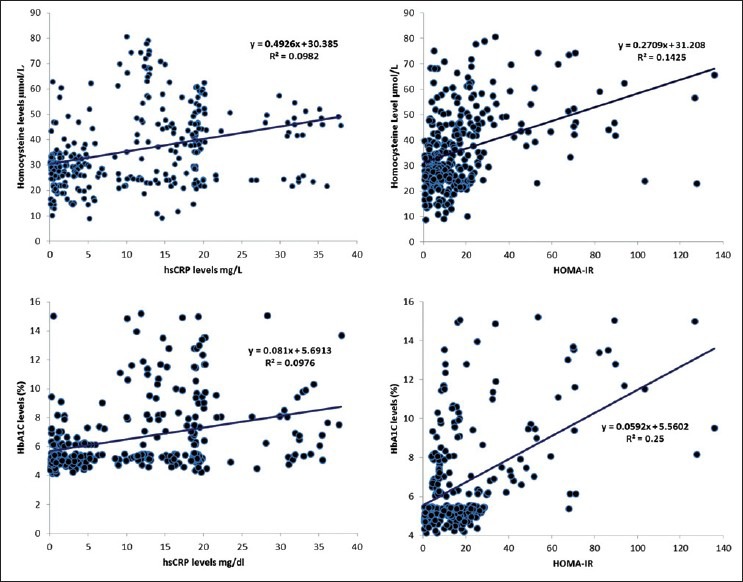
Scatter plot with trendline showing correlation between HbA1C, homeostasis model assessment of insulin resistance, highly sensitive C-reactive protein, and homocysteine levels
DISCUSSION
This study revealed significant differences between diabetics and non-diabetics in known cases of CVD compared with population-based observational and prospective studies.[3,4] Among traditional risk factors, there were no difference in BMI, WHR and number of patients with obesity and central obesity between the two groups. A similar observation was made by a recently published study.[5] Obesity and central obesity are strong risk factors for T2DM and CVD;[13,14] hence, we expected this difference to persist even after the onset of CVD. It may due to the similar risk conferred by obesity and central obesity to CVD in patients with or without diabetes. Hypertension was more common in diabetic patients as compared with non-diabetic subjects. There is a strong relationship between diabetes and hypertension.[15] Several studies showed that the prevalence of hypertensives in type 2 diabetes is between 50% and 73%.[3,16] This high prevalence of hypertension in type 2 diabetes mellitus is associated with the presence of insulin resistance syndrome due to the activation of sympathoadrenal system, rennin angiotensin system and sodium retention.[15] Among lipid abnormalities, a significant difference was only observed for serum triglyceride and HDL levels between the two groups, which reflects the underlying higher insulin resistance in diabetic patients and its effect. There was no difference in the total cholesterol and LDL cholesterol levels between the two groups. Hence, it may be inferred that LDL cholesterol confers a similar risk in diabetics and non-diabetics as also reported by the Strong Heart Study, where the hazard ratio 1.07 was equal in both groups.[5]
In the present study, a high percentage of patients had vitamin B12 deficiency and hyperhomocysteinemia, which was more marked in patients with diabetes. Dietary vitamin B12 deficiency is a severe problem in India due to vegetarianism, and causes hyperhomocysteinemia.[17,18] Similarly, a high prevalence of hyperhomocysteinemia has been reported in the Indian population even without CAD[19,20] and also with CAD.[21] Yajnik et al.[20] reported vitamin B12 deficiency and hyperhomocysteinemia in 81% and 79% of the urban middle class population without CAD. Vitamin B12 deficiency is associated with CAD in the Indian population.[22] In this study, all patients had underlying CAD, which can explain the high percentage of vitamin B12 deficiency. Serum vitamin B12 levels were significantly lower and Hcy levels were significantly higher in patients with diabetes than those without diabetes in the present study. Several studies have reported a similar finding.[23,24,25] However, another study from India did not find any difference in the serum vitamin B12 and Hcy levels in patients with CAD with or without diabetes.[26] Serum vitamin B12 levels had a significant negative association with Hcy levels in this study, which has also been reported by others from India.[20,21,22] Vitamin B12 acts as a coenzyme while folic acid provides the methyl essential for the reactions to take place. Therefore, folic acid and vitamin B12 deficiency can cause reduction in methylene tetrahydrofolate reductase activity, leading to a decrease in methionine synthesis and Hcy accumulation.[27] A meta-analysis of 27 observational studies[28] and population-based prospective studies[29] have shown Hcy to be an independent risk factor for CAD, independent of other cardiovascular risk factors. Although supplementation of vitamin B12 has shown to reduce Hcy levels,[30,31] a meta-analysis of several trials did not reveal any cardiovascular benefit.[32]
In this study, although Hcy was positively correlated with insulin resistance and inflammatory markers in the study population, serum vitamin B12 levels were not. This suggests that vitamin B12 does not have a direct association with non-traditional cardiovascular risk factors, but may play a role, indirectly, through increasing the Hcy levels. This is also supported by a cross-sectional study in Asian Indians, which reported no correlation between serum vitamin B12 and HOMA-IR.[33] The Framingham offspring study demonstrated a modest association between hyperinsulinemia and fasting Hcy levels.[34] Some studies have observed a positive association between Hcy and insulin resistance,[34,35] whereas others were unable to document such an association.[36,37] Similar findings have been noted for association of Hcy and inflammatory markers.[38] These differences can be explained by a different subset of population studies, differences in anthropometric parameters and associated comorbidities. Several mechanisms have been proposed to explain the atherogenic actions of Hcy, which include vascular endothelial dysfunction, direct cytotoxic effects to vascular endothelial cells, proliferation of vascular smooth muscle cells, lipid peroxidation, platelet activation and induction of inflammation.[39]
In the present study, diabetic patients had higher insulin resistance, which is in accordance with the current understanding of the pathogenesis of diabetes.[40] However, if we consider a cut-off of 2.6 for HOMA-IR as an indicator of insulin resistance in patients with normoglycemia[41] and 3.8 in patients with diabetes,[42] both groups had evidence of underlying severe insulin resistance. Insulin resistance and its associated abnormalities may have an important role in the development and progression of atherosclerosis and cardiovascular disease in the Indian population.[43] The exact cause of insulin resistance is not known. Genetic, nutritional and environmental factors have been implicated to explain insulin resistance. In the present study, HOMA-IR was positively correlated with Hcy levels and inflammatory markers in patients without diabetes only. In patients with diabetes, HOMA-IR was positively correlated with glycemic control. It is postulated that inflammation is mediated by two different mechanisms, i.e., through activation of nuclear factor-κβ or oxidative stress. In diabetic patients, hyperglycemia and relative insulin deficiency activate both mechanisms while Hcy only operates through the oxidative mechanism.[44] This suggests that in diabetic patients, glycemic status overrides the oxidative stress induced by Hcy; hence, there is no correlation between Hcy and inflammatory markers in diabetic patients. A similar observation has been made by other studies in diabetic patients with manifest atherosclerotic heart disease.[45]
In the present study, inflammatory markers were significantly higher in patients with diabetes compared with those without diabetes. Several studies have reported higher levels of inflammatory markers in diabetics compared with non-diabetics in patients without underlying CVD[7,46] or with CVD.[47] Inflammatory markers (hsCRP and IL-6) were positively associated with insulin resistance in patients without diabetes and with glycemic control in patients with diabetes in this study. In a study among US adults aged 17 years and over, elevated CRP concentrations increased with increasing HbA1c levels, and suggested an association between glycemic control and systemic inflammation in people with established diabetes.[48] Increase in inflammatory markers has been associated with progression of CAD[49] and diabetes[50] in prospective, population-based studies. However, in the present study, although the TNF-α level was higher in diabetic patients than in non-diabetic patients, it did not show any association with insulin resistance. TNF-α levels were positively related with glycemic control. Larger studies had shown a positive correlation of TNF-α with insulin resistance.[49,50] TNF-α is also associated with the amount of adipose tissue.[46] There was no difference in BMI in both groups in this study, which may explain our finding. Inflammation and inflammatory markers increase the expression of adhesion molecules in vascular endothelium and may play an active role in initiation, progression and ultimately, thrombotic complications of atherosclerosis.[8]
There were some limitations of our study. Firstly, the study population consisted of confirmed cases of CAD without a control group, because they are more likely to reveal alterations in inflammatory markers, insulin resistance and dietary factors, being at the extreme end of the disease spectrum. Secondly, being a cross-sectional study, long-term follow-up data were not available.
CONCLUSIONS
Vitamin B12 deficiency was common and patients with diabetes had lower levels of vitamin B12. Diabetic patients had higher levels of insulin resistance, inflammatory markers and Hcy compared with non-diabetics. Nutritional factors were inter-related with insulin resistance and inflammation, and may play an important role in the pathogenesis of diabetes and cardiovascular disease. Patients with diabetes had more traditional risk factors (hypertension, dyslipidemia) than patients without diabetes in known patients with CAD. However, there was no difference in BMI and WHR. Although prospective trials were unable to show beneficial effects of vitamin supplementation,[42] long-term prospective studies are required in our population with an underlying high prevalence of nutritional deficiency to show the beneficial effect of nutritional supplementation on non-communicable diseases.
ACKNOWLEDGMENT
We thank the Deenanath Mangeshkar Hospital and Research Centre, Pune, for providing the necessary facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Mercer BN, Morais S, Cubbon RM, Kearney MT. Diabetes mellitus and the heart. Int J Clin Pract. 2012;66:640–7. doi: 10.1111/j.1742-1241.2012.02924.x. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc Med. 2010;20:90–5. doi: 10.1016/j.tcm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Lee ET, Peterson LE, Devereux RB, Rhoades ER, Umans JG, et al. Differences in risk factors for coronary heart disease among diabetic and nondiabetic individuals from a population with high rates of diabetes: The strong heart study. J Clin Endocrinol Metab. 2012;97:3766–74. doi: 10.1210/jc.2012-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laakso M. Insulin resistance and cardiovascular disease. British J Diabetes and Vascular Disease. 2002;2:S9–11. [Google Scholar]
- 7.Garg MK, Dutta MK, Brar KS. Inflammatory markers in metabolic syndrome. Int J Diab Dev Ctries. 2012;32:131–7. [Google Scholar]
- 8.Maiti R, Agrawal NK. Atherosclerosis in diabetes mellitus: Role of inflammation. Indian J Med Sci. 2007;61:292–306. [PubMed] [Google Scholar]
- 9.Tousoulis D, Kampoli AM, Papageorgiou N, Androulakis E, Antoniades C, Toutouzas K, et al. Pathophysiology of atherosclerosis: The role of inflammation. Curr Pharm Des. 2011;17:4089–110. doi: 10.2174/138161211798764843. [DOI] [PubMed] [Google Scholar]
- 10.Oudi ME, Aouni Z, Mazigh C, Khochkar R, Gazoueni E, Haouela H, et al. Homocysteine and markers of inflammation in acute coronary syndrome. Exp Clin Cardiol. 2010;15:e25–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–8. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS, Heo YR. Plasma total homocysteine, folate, and vitamin B12 status in Korean adults. J Nutr Sci Vitaminol (Tokyo) 2002;48:290–7. doi: 10.3177/jnsv.48.290. [DOI] [PubMed] [Google Scholar]
- 13.Siren R, Eriksson JG, Vanhanen H. Waist circumference a good indicator of future risk for type 2 diabetes and cardiovascular disease. BMC Public Health. 2012;12:631. doi: 10.1186/1471-2458-12-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 15.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities: The role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–81. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 16.Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: A prospective observational study (UKPDS 75) Diabetologia. 2006;49:1761–9. doi: 10.1007/s00125-006-0297-1. [DOI] [PubMed] [Google Scholar]
- 17.Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr. 2004;24:299–326. doi: 10.1146/annurev.nutr.24.012003.132440. [DOI] [PubMed] [Google Scholar]
- 18.Elmadfa I, Singer I. Vitamin B12 and homocysteine status among vegetarians: A global perspective. Am J Clin Nutr. 2009;89:1693–1698S. doi: 10.3945/ajcn.2009.26736Y. [DOI] [PubMed] [Google Scholar]
- 19.Misra A, Vikram NK, Pandey RM, Dwivedi M, Ahmad FU, Luthra K, et al. Hyperhomocysteinemia, and low intakes of folic acid and vitamin B12 in urban North India. Eur J Nutr. 2002;41:68–77. doi: 10.1007/s003940200010. [DOI] [PubMed] [Google Scholar]
- 20.Yajnik CS, Deshpande SS, Lubree HG, Naik SS, Bhat DS, Uradey BS, et al. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775–82. [PubMed] [Google Scholar]
- 21.Bhagwat VR, Yadav AS, Rathod IM. Homocysteine, lipid indices and antioxidants in patients with ischaemic heart disease from Maharashtra, India. Singapore Med J. 2009;50:418–24. [PubMed] [Google Scholar]
- 22.Kumar J, Garg G, Sundaramoorthy E, Prasad PV, Karthikeyan G, Ramakrishnan L, et al. Vitamin B12 deficiency is associated with coronary artery disease in an Indian population. Clin Chem Lab Med. 2009;47:334–8. doi: 10.1515/CCLM.2009.074. [DOI] [PubMed] [Google Scholar]
- 23.Agulló-Ortuño MT, Albaladejo MD, Parra S, Rodríguez-Manotas M, Fenollar M, Ruíz-Espejo F, et al. Plasmatic homocysteine concentration and its relationship with complications associated to diabetes mellitus. Clin Chim Acta. 2002;326:105–12. doi: 10.1016/s0009-8981(02)00287-5. [DOI] [PubMed] [Google Scholar]
- 24.Diakoumopoulou E, Tentolouris N, Kirlaki E, Perrea D, Kitsou E, Psallas M, et al. Plasma homocysteine levels in patients with type 2 diabetes in a Mediterranean population: Relation with nutritional and other factors. Nutr Metab Cardiovasc Dis. 2005;15:109–17. doi: 10.1016/j.numecd.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Al-Maskari MY, Waly MI, Ali A, Al-Shuaibi YS, Ouhtit A. Folate and vitamin B12 deficiency and hyperhomocysteinemia promote oxidative stress in adult type 2 diabetes. Nutrition. 2012;28:e23–6. doi: 10.1016/j.nut.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–41. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 27.Gambhir JK. Homocysteine metabolism in health and disease. Indian Heart J. 2000;52:S9–15. [PubMed] [Google Scholar]
- 28.Cleophas TJ, Hornstra N, van Hoogstraten B, van der Meulen J. Homocysteine, a risk factor for coronary artery disease or not. A meta-analysis? Am J Cardiol. 2000;86:1005–9. doi: 10.1016/s0002-9149(00)01137-1. [DOI] [PubMed] [Google Scholar]
- 29.Verhoef P, Stampfer MJ. Prospective studies of homocysteine and cardiovascular disease. Nutr Rev. 1995;53:283–8. doi: 10.1111/j.1753-4887.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 30.Deshmukh US, Joglekar CV, Lubree HG, Ramdas LV, Bhat DS, Naik SS, et al. Effect of physiological doses of oral vitamin B12 on plasma homocysteine: A randomized, placebo-controlled, double-blind trial in India. Eur J Clin Nutr. 2010;64:495–502. doi: 10.1038/ejcn.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yajnik CS, Lubree HG, Thuse NV, Ramdas LV, Deshpande SS, Deshpande VU, et al. Oral vitamin B12 supplementation reduces plasma total homocysteine concentration in women in India. Asia Pac J Clin Nutr. 2007;16:103–9. [PubMed] [Google Scholar]
- 32.Huang T, Chen Y, Yang B, Yang J, Wahlqvist ML, Li D. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clin Nutr. 2012;31:448–54. doi: 10.1016/j.clnu.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Gammon CS, von Hurst PR, Coad J, Kruger R, Stonehouse W. Vegetarianism, vitamin B12 status, and insulin resistance in a group of predominantly overweight/obese South Asian women. Nutrition. 2012;28:20–4. doi: 10.1016/j.nut.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: The Framingham offspring study. Diabetes Care. 2001;24:1403–10. doi: 10.2337/diacare.24.8.1403. [DOI] [PubMed] [Google Scholar]
- 35.Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Insulin resistance is associated with elevated plasma total homocysteine levels in healthy, non-obese subjects. Atherosclerosis. 1998;139:197–8. doi: 10.1016/s0021-9150(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 36.Anan F, Masaki T, Umeno Y, Yonemochi H, Eshima N, Saikawa T, et al. Correlations between homocysteine levels and atherosclerosis in Japanese type 2 diabetic patients. Metabolism. 2007;56:1390–5. doi: 10.1016/j.metabol.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Godsland IF, Rosankiewicz JR, Proudler AJ, Johnston DG. Plasma total homocysteine concentrations are unrelated to insulin sensitivity and components of the metabolic syndrome in healthy men. J Clin Endocrinol Metab. 2001;86:719–23. doi: 10.1210/jcem.86.2.7213. [DOI] [PubMed] [Google Scholar]
- 38.Shai I, Stampfer MJ, Ma J, Manson JE, Hankinson SE, Cannuscio C, et al. Homocysteine as a risk factor for coronary heart diseases and its association with inflammatory biomarkers, lipids and dietary factors. Atherosclerosis. 2004;177:375–81. doi: 10.1016/j.atherosclerosis.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 40.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Pathogenesis and treatment. Lancet. 2008;371:2153–6. doi: 10.1016/S0140-6736(08)60932-0. [DOI] [PubMed] [Google Scholar]
- 41.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–5. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 42.Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: Third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007) Nutr Metab (Lond) 2010;7:26. doi: 10.1186/1743-7075-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huffman MD, Prabhakaran D, Osmond C, Fall CH, Tandon N, Lakshmy R, et al. Incidence of cardiovascular risk factors in an Indian urban cohort: results from the New Delhi birth cohort. J Am Coll Cardiol. 2011;57:1765–74. doi: 10.1016/j.jacc.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu JT. Circulating homocysteine is an inflammation marker and a risk factor of life-threatening inflammatory diseases. J Biomed Lab Sci. 2007;19:107–11. [Google Scholar]
- 45.Akalin A, Alatas O, Colak O. Relation of plasma homocysteine levels to atherosclerotic vascular disease and inflammation markers in type 2 diabetic patients. Eur J Endocrinol. 2008;158:47–52. doi: 10.1530/EJE-07-0470. [DOI] [PubMed] [Google Scholar]
- 46.Shankar A, Li J. Positive association between high-sensitivity C-reactive protein level and diabetes mellitus among US non-Hispanic black adults. Exp Clin Endocrinol Diabetes. 2008;116:455–60. doi: 10.1055/s-2007-1004563. [DOI] [PubMed] [Google Scholar]
- 47.Souza JR, Oliveira RT, Blotta MH, Coelho OR. Serum levels of interleukin-6 (Il-6), interleukin-18 (Il-18) and C-reactive protein (CRP) in patients with type-2 diabetes and acute coronary syndrome without ST-segment elevation. Arq Bras Cardiol. 2008;90:86–90. doi: 10.1590/s0066-782x2008000200004. [DOI] [PubMed] [Google Scholar]
- 48.King DE, Mainous AG, 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26:1535–9. doi: 10.2337/diacare.26.5.1535. [DOI] [PubMed] [Google Scholar]
- 49.Iso H, Noda H, Ikeda A, Yamagishi K, Inoue M, Iwasaki M, et al. The impact of C-reactive protein on risk of stroke, stroke subtypes, and ischemic heart disease in middle-aged Japanese: The Japan public health center-based study. J Atheroscler Thromb. 2012;19:756–66. [PubMed] [Google Scholar]
- 50.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]


