Abstract
Background:
Onset of puberty in boys usually occurs by 14 years of age. Some boys may exhibit delayed sexual maturation till about 17-18 years of age. However, pubertal onset beyond 18 years of age is exceedingly rare.
Materials and Methods:
Patients diagnosed as idiopathic hypogonadotropic hypogonadism (IHH) who had onset of puberty (increase in testicular volume >10 ml) while on androgen therapy were studied. These patients were evaluated prospectively.
Results:
There were nine subjects that were included in the study. The pre-therapy testicular volumes ranged from 3 to 6 ml. Luteinizing hormone (LH) levels increased from 1.2 ± 0.96 to 2.8 ± 1.0 IU/L, follicular stimulating hormone (FSH) levels increased from 1.5 ± 0.79 to 3.5 ± 1.9 IU/L, and testosterone increased from 0.36 ± 0.16 to 3.4 ± 2.1 ng/ml. Three out of nine patients had testosterone levels below 3 ng/ml.
Conclusion:
Our present study indicates that pubertal development can occur in patients presenting with hypogonadotropic hypogonadism after 18 years of age. However, acquired pubertal status may be subnormal
Keywords: Delayed puberty, reversal of hypogonadotropic hypogonadism, progression of puberty
INTRODUCTION
Normal male pubertal development requires a timed activation of the pituitary gonadal axes and normal progression of sexual maturation through different stages. The two sexual maturation spurts characterized by sertoli and Leydig cell proliferation during the antenatal and pubertal periods are considered important for normal pubertal development.[1] Clinical aberrations from this normal pattern of pubertal development are well described, i.e., precocious or delayed pubertal development and hypogonadism. However, factors governing these aberrations are poorly understood.
Delay in pubertal onset and subsequent normal sexual maturation as in constitutional delay of growth and puberty (CDGP) is well known. The various factors attributed for pubertal delay include nutritional and hormonal factors, systemic diseases, familial, and unknown genetic and environmental factors.[2] Available literature from books and reviews on pediatric endocrinology indicates that delay in pubertal onset in the absence of systemic diseases beyond the age of 18 years is exceedingly rare.[3,4] Till 1991, only one case of Kallmann syndrome that had pubertal onset after 20 years age was described. Recently, Raivio et al. described 15 males diagnosed as hypogonadotropic hypogonadism after 18 years of age, who demonstrated increasing testicular volumes and improving sperm counts while on treatment.[5] They showed sustained improvement in these parameters even after stopping of therapy. These authors believed that exposure to androgens may have in an unknown manner activated the hypothalamopituitary gonadal axis that was responsible for a late onset of puberty in these patients. They termed this phenomenon as a reversible form of hypogonadotropic hypogonadism. Here, we describe nine males who presented at or after 18 years of age with lack of secondary sexual characters, and had onset of pubertal development while on androgen therapy.
MATERIALS AND METHODS
Design
Case observational study.
Methods
We studied patients of idiopathic hypogonadotropic hypogonadism (IHH), who had increase in testicular volume (to at least 10 ml bilaterally) while on androgen therapy. These patients were then followed up prospectively. Diagnosis of IHH was made on the basis of clinical features (testicular volume less than 6 ml), initial presentation after 18 years of age, low to normal levels of gonadotropins, low testosterone levels (<1 ng/ml), and normal sellar imaging. Patients with evidence of systemic diseases, hypothyroidism, growth hormone (GH) deficiency, hyperprolactinemia, hypocortisolism, and history of treatment [with human chorionic gonadotropin (HCG) or luteinizing hormone-releasing hormone (LHRH) therapy] for hypogonadism were excluded from the study.
The presenting complaints, anthropometry, pubertal status, and sex hormones at the first visit were noted from the medical records. These patients had been on testosterone therapy (intramuscular testoviron injections containing testosterone undecanoate and testosterone succinate 100 mg intramuscularly every 21 days for 2 months and then 250 mg every 21 days). Testosterone therapy was stopped when they demonstrated increase in testicular volumes to at least 10 ml bilaterally. Reassessments were made after stopping androgen therapy for at least 5 months. At this visit, pubertal status was recorded, sex hormones were estimated, and semen examination was performed. Testicular volume was estimated by Prader's orchidometer. After the re-evaluation visit, these patients were evaluated at a 3-6 monthly interval.
Hormonal analysis
Luteinizing hormone (LH), follicular stimulating hormone (FSH), and testosterone levels were measured with radioimmunoassay (kit supplied by Diagnostics Systems Laboratories, Inc. Webster, TX, USA).
RESULTS
Nine patients fulfilled the inclusion criteria. Details of pubertal status and sex hormones at the initial visit and reassessments made after stopping androgen therapy for 5 months are given in Table 1. One patient had a brother with similar problem, but complete clinical details were not available. There was no history of delayed puberty in the parents or siblings.
Table 1.
Clinical details of patients showing reversibility of idiopathic hypogonadotropic hypogonadism
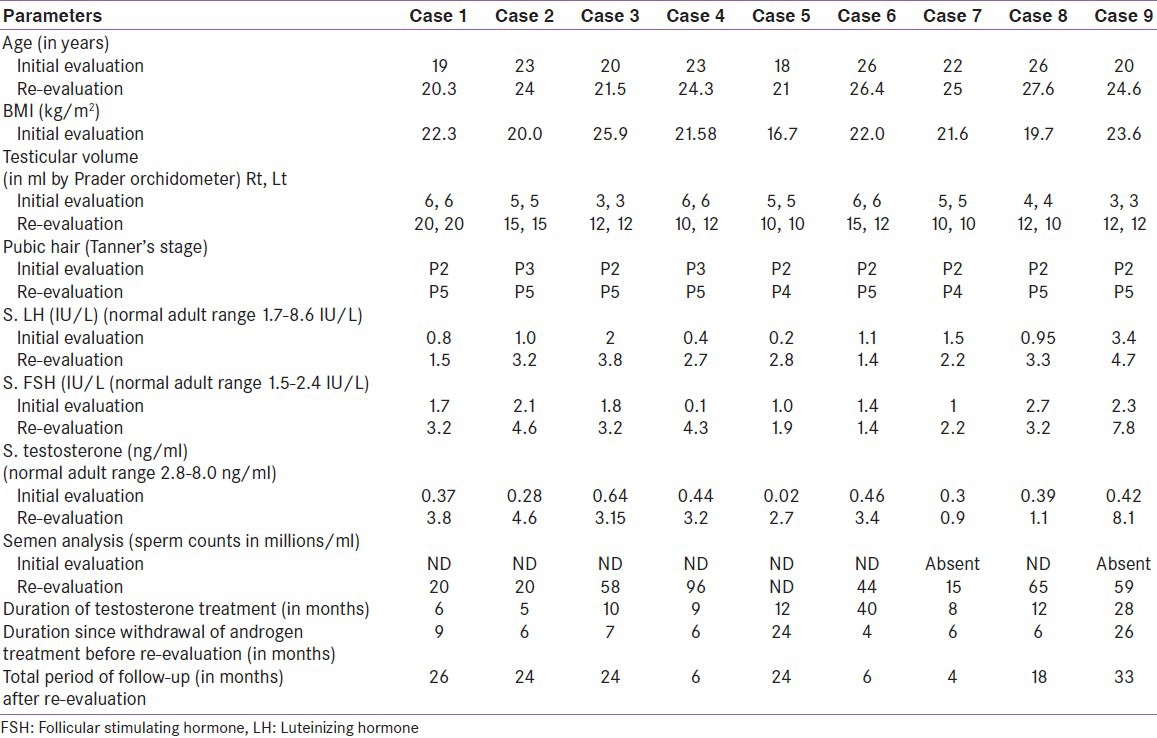
The pre-therapy testicular volumes ranged from 3 to 6 ml and testosterone levels ranged from 0.02 to 0.39 ng/ml. All patients had been on at least 5 months of testosterone therapy. Post-testosterone testicular volumes ranged from 10 to 20 ml and testosterone levels ranged from 0.9 to 8.1 ng/ml. Initial semen examination was not available for most patients. Two patients were unable to produce semen. Semen examination done post-testosterone therapy demonstrated sperm counts varying from 15 to 96 millions/ml. Two patients (case 6 and case 8) fathered a child each. Case 8 required 3 months of HCG therapy for fathering a child. Sperm counts rose from 15 to 44 million with HCG therapy in this patient. All these patients have been followed further for an average period of 18 (4-33) months after discontinuation of testosterone therapy.
DISCUSSION
IHH is a clinical entity characterized by lack of pubertal development, inappropriately low levels of gonadotropins, and absence of pituitary lesion on imaging.[6] The characteristic male phenotype described is a tall male with eunuchoidal proportions, small testis, and absence of sperm counts. Partial forms of hypogonadotropic hypogonadism presenting with incomplete and/or arrested pubertal development have also been described.[7,8,9] Some males with hypogonadism diagnosed during adolescence may develop puberty albeit delayed (constitutional delay of puberty). However, onset of puberty/reversal of the hypogonadotropic state beyond 18 years of age is exceedingly rare.
Anecdotal cases of reversal of hypogonadotropic hypogonadism in patients with Kallmann syndrome have been reported. Pitteloud et al. described a family with mutation in fibroblast growth factor receptor 1 gene, presenting as reversible Kallmann syndrome, delayed puberty, and isolated anosmia.[10] Ribeiro et al. reported a KAL1 gene mutation in a patient with reversible Kallmann syndrome.[11] Reversal of hypogonadotropic hypogonadism was also reported in a patient with fertile eunuch syndrome.[12] Recently, Raivio et al. described 15 males who developed pubertal features while receiving treatment for hypogonadotropic hypogonadism.[5] These males presented at or after 18 years of age, with absent (n = 6) to partial (n = 9) puberty. Testicular volumes increased from 8 ml to 16 ml, testosterone levels increased, and spermatogenesis was documented while on hormonal therapy. The authors also showed sustained reversal of hypogonadotropic state after discontinuation of hormonal treatment. They proposed that exposure to sex steroids in an unknown manner may have activated the hypothalamopituitary gonadal axis.
Some subjects in the present cohort had had testicular volumes ranging from 4 to 6 ml, thereby indicating a partial activation of the hypothalamic gonadal axis. There was development of secondary sexual characters after 18 years of age, while on androgen therapy. Our present cohort was homogeneous in terms of the therapy used, since all patients were on testosterone therapy. In the study by Raivio et al., of 15 patients, 3 patients received GnRH and 7 had been on a mixed regimen of gonadotropins or GnRH – agents known to increase the testicular volume. Testicular enlargement with androgens in patients with hypogonadotropic hypogonadism is rare. Testosterone is known to suppress the hypothalamopituitary gonadal axis and thereby inhibit the sertoli cell/tubular proliferation. In a group of 59 patients with hypogonadotropic hypogonadism, testicular volumes remained significantly low (4.31 ± 1.8 ml) after 4 years of testosterone therapy and testicular volumes increased to 14 ml in the subgroup receiving HCG therapy (14 ± 2.0 ml).[13] In the present study, androgens seem to have a stimulatory effect on the maturation of the hypothalamic gonadal axis. This situation is analogous to XY males with CAH (congenital adrenal hyperplasia) where true precocious puberty occurs possibly due to sex steroid priming of the hypothalamus.[14,15] A short course of androgens is also known to stimulate the latent pituitary gonadal axis in patients with CDGP.[2,16,17] It is possible that hypothalamic neurons behave differently to exposure to sex steroids.
The progression of sexual maturation after stopping androgens in the present group of patients was heterogeneous. Two patients had adult testicular volumes (15-20 ml) and seven patients had pubertal testicular volumes though subnormal (10-12 ml).[18] Post-therapy testosterone levels were normal in one patient (8.1 ng/ml), low normal in five patients (3.2-4.6 ng/ml), and low (0.9, 1.1, and 2.7 ng/ml) in three patients. Three patients demonstrated low sperm counts (15-20 millions/ml). Such a heterogeneous pubertal development with testicular volumes ranging from 7.5 to 25 ml, testosterone levels ranging from 2.16 to 5.7 ng/ml, and sperm counts ranging from 0.3 to 152 × 10−6 millions/cc was also reported by Raivio et al.[5]
The present cohort had onset/progression of pubertal development while on androgen therapy. Whether androgen therapy was causal for sexual maturation is not clear. Sexual maturation was heterogeneous with normal, low normal, to low androgen levels 6 months after stopping androgen therapy. Exposure to sex steroids at a timed appearance is required for normal sexual maturation A delayed androgen exposure may be able to initiate a pubertal spurt in some hypogonad patients after 18 years of age; however, GnRH activity is either suboptimal or may decay with passage of time. A similar postulation for the premature termination of gonadal function has been described in patients with acquired hypogonadotropic hypogonadism.[19] This may have implications on future bone health and fertility prospects. Need for HCG therapy for fertility by case 8 support this view?
Delay in pubertal onset in the absence of pituitary masses or systemic diseases after 18 years of age is rare.[3,4] Our present study indicates that pubertal development can occur in patients presenting with hypogonadotropic hypogonadism with normal sellar imaging, normal body mass index (BMI), and absence of systemic diseases after 18 years of age. However, the attained pubertal status may be subnormal. Reversibility of the hypogonadotropic state is partial.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, et al. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:152–60. doi: 10.1210/jcem.87.1.8131. [DOI] [PubMed] [Google Scholar]
- 2.De Luca F, Argente J, Cavallo L, Crowne E, Delemarre-Van de Waal HA, De Sanctis C, et al. International Workshop on Management of Puberty for Optimum Auxological Results. Management of puberty in constitutional delay of growth and puberty. J Pediatr Endocrinol Metab. 2001;14(Suppl 2):953–7. doi: 10.1515/jpem-2001-s207. [DOI] [PubMed] [Google Scholar]
- 3.Styne DM. In: Puberty and its disorders. Vol. 20. Philadelphia: W.B. Suanders; 1991. Puberty and its disorder in Boys; p. 4371. [Google Scholar]
- 4.Wilkins L, editor. The Diagnosis and Treatment of Endocrine Disorders in Childhood and Adolescence. 2nd ed. chapter 10. Springfield, Illinois: Thomas; 1957. Variations in the pattern of adolescent development. [Google Scholar]
- 5.Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–73. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman AR, Crowley WF., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307:1237–41. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- 7.Boyar RM, Wu RH, Kapen S, Hellman L, Weitzman ED, Finkelstein JW. Clinical and laboratory heterogeneity in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1976;43:1268–75. doi: 10.1210/jcem-43-6-1268. [DOI] [PubMed] [Google Scholar]
- 8.Hornstein O. Klin Wochenschr. Vol. 37. German: 1959. Puberal FSH deficiency (spermiogenetic infantilism), a special form of partial anterior pituitary gland insufficiency; pp. 105–6. [DOI] [PubMed] [Google Scholar]
- 9.Albert A, Underdahl LO, Greene LF, Lorenz N. Male hypogonadism. VII. The testis in partial gonadotropic failure during puberty (lack of luteinizing hormone only) Proc Staff Meet Mayo Clin. 1955;30:31–43. [PubMed] [Google Scholar]
- 10.Pitteloud N, Acierno JS, Jr, Meysing AU, Dwyer AA, Hayes FJ, Crowley WF., Jr Reversible kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab. 2005;90:1317–22. doi: 10.1210/jc.2004-1361. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro RS, Vieira TC, Abucham J. Reversible Kallmann syndrome: Report of the first case with a KAL1 mutation and literature review. Eur J Endocrinol. 2007;156:285–90. doi: 10.1530/eje.1.02342. Review. Erratum in: Eur J Endocrinol 2007;156:703. [DOI] [PubMed] [Google Scholar]
- 12.Pitteloud N, Boepple PA, DeCruz S, Valkenburgh SB, Crowley WF, Jr, Hayes FJ. The fertile eunuch variant of idiopathic hypogonadotropic hypogonadism: Spontaneous reversal associated with a homozygous mutation in the gonadotropin-releasing hormone receptor. J Clin Endocrinol Metab. 2001;86:2470–5. doi: 10.1210/jcem.86.6.7542. [DOI] [PubMed] [Google Scholar]
- 13.Bistritzer T, Lunenfeld B, Passwell JH, Theodor R. Hormonal therapy and pubertal development in boys with selective hypogonadotropic hypogonadism. Fertil Steril. 1989;52:302–6. doi: 10.1016/s0015-0282(16)60859-2. [DOI] [PubMed] [Google Scholar]
- 14.Pescovitz OH, Comite F, Cassorla F, Dwyer AJ, Poth MA, Sperling MA, et al. True precocious puberty complicating congenital adrenal hyperplasia: Treatment with a luteinizing hormone-releasing hormone analog. J Clin Endocrinol Metab. 1984;58:857–61. doi: 10.1210/jcem-58-5-857. [DOI] [PubMed] [Google Scholar]
- 15.Desai M, Colaco MP, Choksi CS, Ambadkar MC, Vaz FE, Gupte C. Isosexual precocity: The clinical and etiologic profile. Indian Pediatr. 1993;30:607–23. [PubMed] [Google Scholar]
- 16.Rogol AD. Pubertal androgen therapy in boys. Pediatr Endocrinol Rev. 2005;2:383–90. [PubMed] [Google Scholar]
- 17.Pozo J, Argente J. Ascertainment and treatment of delayed puberty. Horm Res. 2003;60(Suppl 3):35–48. doi: 10.1159/000074498. [DOI] [PubMed] [Google Scholar]
- 18.Zachmann M, Prader A, Kind HP, et al. Testicular volume during adolescence. Helv Paediatr Acta. 1974;29:61–72. [PubMed] [Google Scholar]
- 19.Nachtigall LB, Boepple PA, Pralong FP, Crowley WF., Jr Adult-onset idiopathic hypogonadotropic hypogonadism: A treatable form of male infertility. N Engl J Med. 1997;336:410–5. doi: 10.1056/NEJM199702063360604. [DOI] [PubMed] [Google Scholar]


