Abstract
Introduction:
Endocrine complications are common after hematopoietic stem cell transplant (HSCT). Although HSCT is performed at various centers in India, no study is available for endocrine dysfunctions among them. This study was carried out with the objective to evaluate endocrine dysfunction among patients undergone HSCT in the past.
Materials and Methods:
We carried out a cross-sectional study in a 50 post-HSCT recipients (39 allogenic, 11 autologous). All relevant data were collected from patient's records. Samples for hormonal estimation were collected and stimulation tests for cortisol and growth hormone were interpreted based on peak values achieved during insulin tolerance test.
Results:
The mean age of patients was 26.3 ± 16.9 years (range 4-74). Adrenal insufficiency (AI) was present in 60%, hypergonadotropic hypogonadism (HH) in 60%, growth hormone deficiency (GHD) in 54%, hypothyroidism in 4%, hyperprolactinemia in 4%, new onset diabetes after transplant in 4%, and impaired fasting glucose in 6%. Multiple endocrine complications were common. GHD was present in 77% of children (n = 22) although height standard deviation score was not statistically different compared to those who didn’t have GHD. HH was present in 36% of children. In adults (n = 28), 36% had GHD, all females had HH, and 89% of males had HH. Germ cell dysfunction with compensated Leydig cell dysfunction was the most common pattern of HH in males. Fifteen patients had graft versus host disease (GVHD). GVHD had no bearing on development of endocrine deficiencies. AI was related to duration after and type of transplant, but was unrelated to steroid intake.
Conclusions:
Endocrine manifestations are common after HSCT; they can occur as early or late complications. All HSCT recipients should have endocrine evaluation as per prevailing guidelines.
Keywords: Adrenal insufficiency, diabetes, growth hormone deficiency, hematopoietic stem cell transplant, hypogonadism, hypothyroidism
INTRODUCTION
Gatti and colleagues reported the first successful allogenic marrow graft in a patient with severe combined immunological deficiency using a sibling donor in 1968.[1] With the refinement of HLA testing, anti-cancer drugs, intensive care units, the spectrum of hematopoietic stem cell transplant (HSCT) has widened to include malignant and non-malignant hematological conditions, and connective tissue disorders. With increasing availability and affordability, HSCT is being performed with refinement of the procedure and post-transplant care, the quantum of long-term survivors in ever increasing. With increased survival after HSCT, more long-term complications are being observed.[2]
Endocrine manifestations are seen both after autologous and allogenic procedures. These can arise as a consequence of the underlying disease, chemotherapy and radiotherapy used in preparative regimens, the HSCT itself, immunosuppressive drugs, graft versus host disease (GVHD), induction of autoimmunity. The recipient might develop endocrine organ dysfunction in the acute period after transplant or at a later stage.[3]
The endocrine concerns after HSCT are quite different in the pediatric and adult recipients. In the pediatric population, the growth and puberty are the main concerns, while in adults fertility is the main concern. Although HSCT has been performed in India at many centers,[4] there is no data on endocrine dysfunction among patients receiving HSCT. Hence, this study was carried out with the aim to evaluate endocrine dysfunction among patients undergone HSCT in the past.
MATERIALS AND METHODS
This study was conducted as a cross-sectional study on HSCT recipients. Patients who had received steroids in the preceding 6 months prior to endocrine testing were excluded. All patients had received busulphan (16 mg/kg in 4 days) and cyclophosphamide (120 mg/kg in 2 days). GVHD prophylaxis with cyclosporine A (5 mg/kg). Patients with acute lymphoblastic leukemia received prophylactic cranial irradiation (12 Gy). The HSCT carried out at our center used a steroid free protocol and none of the patient received total body irradiation (TBI). The study group was divided into two groups: Group-I comprised of children and adolescents (<18 years, n = 22) and group-II comprised of adults (>18 years, n = 28). Group-I comprised of 44% of patients with equal male and female distribution. This group was divided into two subgroups in order to see the effect of puberty age ≤10 years (45%) and >10 years (55%). All subjects were analyzed according to duration post HSCT: ≤1 year (46%) and >1 year (54%), type of HSCT– allogenic (78%) and autologous (22%), presence (30%) or absence (70%) of GVHD. Each patient underwent clinical and biochemical evaluations. Samples for hormonal estimation were collected in fasting state at 0800 hours and kept at –80°C. Stimulation tests for cortisol and growth hormone (GH) were interpreted based on peak values achieved during insulin tolerance test (ITT), which is the gold standard.[5,6,7] This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Human Ethics Committee. Written informed consent was obtained from all subjects/patients.
Hormone levels were measured using commercial kit provided by Immunotech, Beckman Coulter Company, France. Primary and subclinical hypothyroidism was defined as low free thyroxin (FT4) (normal 0.8-2.1 ng/mL) with raised thyroid-stimulating hormone (TSH) (normal 0.5-6.5 μIU/mL) and normal FT4 with raised TSH, respectively. A peak cortisol <18 μg/dL[5] after ITT constituted adrenal insufficiency and peak GH level <10 ng/mL in children and adolescent (≤18 years) and <5 ng/mL[6,7] in adults defined GH deficiency (GHD). Gonadal failure was defined as elevated luteinizing hormone (LH) and follicular-stimulating hormone (FSH) (>15 IU/L and >20 IU/L, respectively). In males, gonadal failure was further classified as germ cell (GC) failure (elevated FSH, normal LH) or Leydig cell (LC) failure (elevated LH) which was called compensated if testosterone levels were normal (3-10 ng/mL) or decompensated if testosterone levels were <3 ng/mL. Serum prolactin levels >25 ng/mL constituted hyperprolactinemia. Diabetes mellitus and dysglycemia were defined as per prevailing American Diabetes Association guidelines.[8] Insulin resistance and secretion were calculated with homeostatic model assessment (HOMA-IR = Fasting glucose in mmol/L*Fasting insulin in μIU/L divided by 22.5; HOMA-β =20*Fasting insulin in μIU/L divided by Fasting glucose in mmol/L – 3.5).[9,10]
Statistical analysis was carried out using EPI INFO 3.5.3 (CDC, Atlanta, GA, USA). Data were presented as mean ± SD or number (%) unless specified. All parametric data were analyzed by Student's t-test. If Barlett's Chi-square test for equality of population variances was <0.05, then the Kruska–Wallis test was applied. All non-parametric data were analyzed by the Chi-square test. If value in any cell was <5 then the Fisher exact test was used. A P < 0.05 was considered statistically significant.
RESULTS
This study consisted of 50 patients who have undergone HSCT. Basic characteristic of study population is given in Table 1. Twenty-seven (54%) patients had malignant hematopoietic diseases and 23 (46%) had non-malignant hematopoietic diseases [Table 1]. Endocrinal abnormalities according to age group are given in Table 2. Adrenal insufficiency (AI) was present in 60%, hypergonadotropic hypogonadism (HH) in 60%, GHD in 54%, hypothyroidism in 4%, hyperprolactinemia in 4%, new onset diabetes after transplant (NODAT) in 4%, and impaired fasting glucose (IFG) in 6% in the total study group. All patients had one or more hormone deficiency in various combinations. Twenty-eight percent had single hormone deficiency, 66% had two, and 16% had three hormonal deficiencies [Figure 1].
Table 1.
Basic characteristics of study population
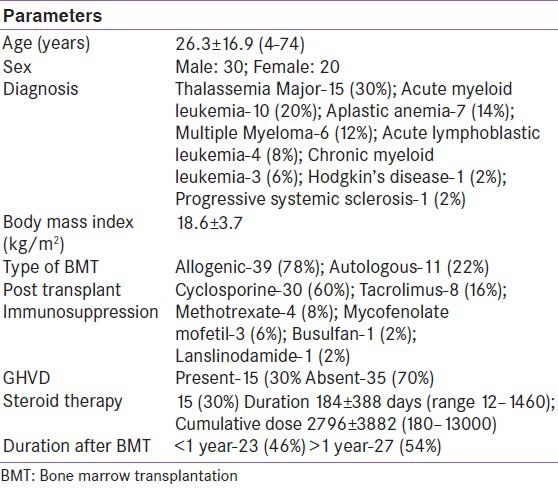
Table 2.
Endocrinal abnormalities according to age groups
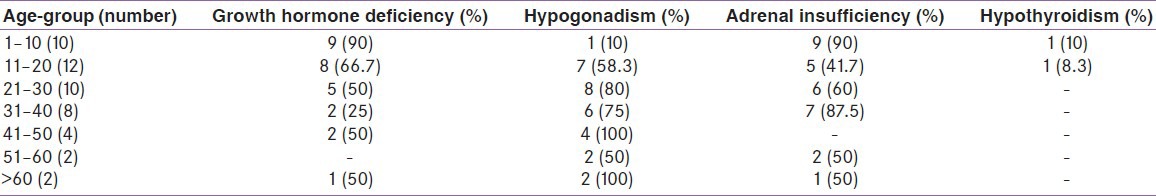
Figure 1.
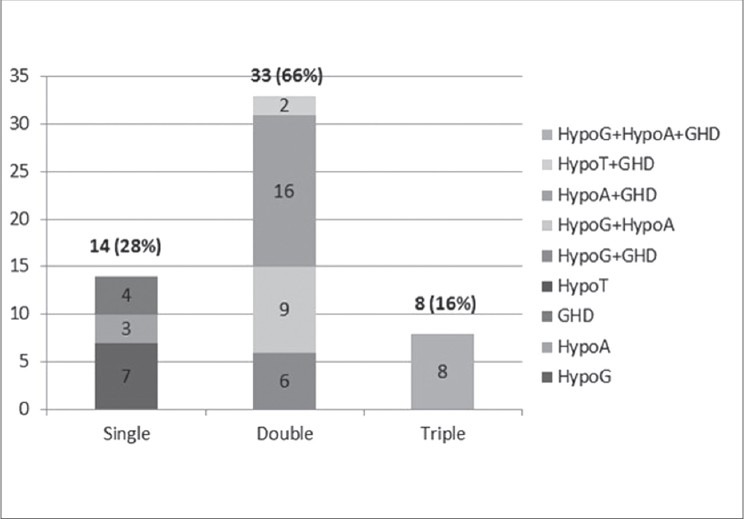
Spectrum of endocrine deficiency in the study group
Effect on growth hormone
Growth velocity was evaluated in group-I patients who had successfully completed 1 year of transplant (11 patients). Eight were transplanted for thalassemia major (TM). Two of them had attained final adult height. All except one had GHD. Growth velocity was 5.0 ± 3.1 cm/year in this subset of patients. Only two of these patients had growth velocity of less than 4 cm and were started on growth hormone therapy [Table 3]. The mean height standard deviation score (SDS) was not statistically significant in those who had GHD (0.46 ± 0.85) when compared with subjects without GHD (0.87 ± 0.98, P value 0.42). Seventy six percent allo-HSCT recipients were diagnosed to have GHD and the only patient who had an auto-HSCT also had GHD. Those who had GHD, mean peak GH value was 3.76 ± 3.09 ng/mL [Figure 2]. Twelve of the 17 children had severe GHD (peak GH <5 ng/mL), out of these 8 were TM and the other 2 were ALL who had received prophylactic cranial irradiation. Patients with TM had increased risk for GHD (risk ratio 2.86, 95% CI: 1.0-8.18; P = 0.016). Ninety-one percent, who had received HSCT more than a year ago, had GHD compared to 64% than those whose HSCT duration was less than 1 year (risk ratio 1.94, 95% CI: 0.95-3.98; P = 0.15). Eighty-three percent of patients who had GVHD had GHD when compared to those without GVHD (75%), this was not statistically significant (P = 0.58) [Table 4].
Table 3.
Growth parameters in group-I who had successfully completed 1 year of transplant
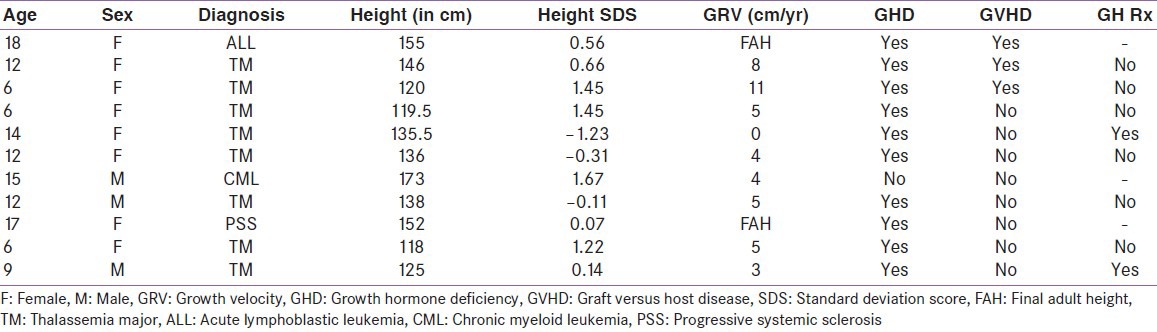
Figure 2.
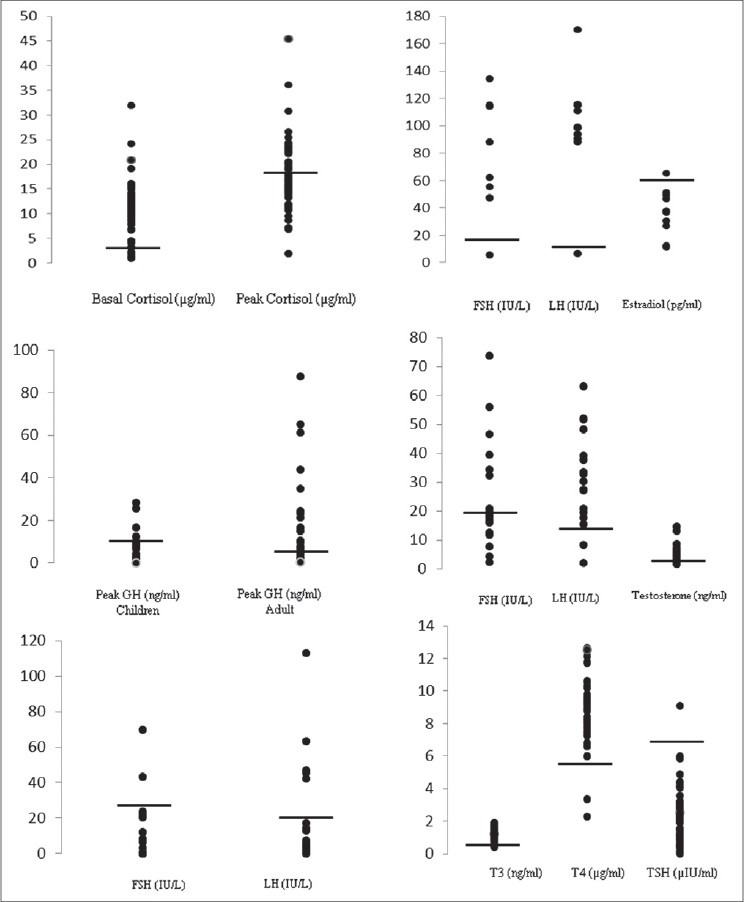
Hormonal values in the study group. A: Basal and Peak cortisol after insulin tolerance test (ITT); B: Peak growth hormone after ITT in children and adults; C: Gonadal hormone pattern in Children; D: Gonadal hormone pattern in females; E: Gonadal hormone pattern in males; E: Thyroid function test
Table 4.
Characteristics of the hormonal defi ciency in the study group
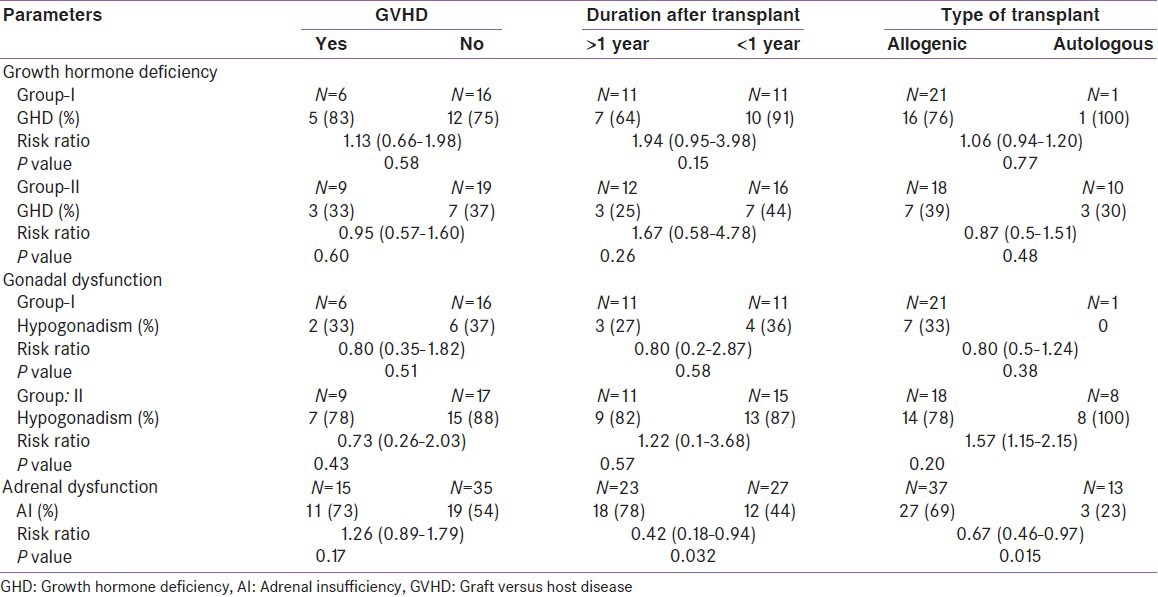
In group-II (n = 28), 36% had GHD. Those who had GHD, mean peak GH response was 2.14 ± 1.78 ng/mL. The type of transplant (P = 0.48) and GVHD (P = 0.60) had no bearing on the occurrence of GHD. Those who had been transplanted more than a year back had higher risk of developing GHD (risk ratio 1.67, 95% CI: 0.58-4.78; P = 0.26).
Effect on gonadal functions
In group-I, 36% had hypogonadism. All had HH. The mean FSH and LH values were 54.75 ± 36.25 IU/L and 33.05 ± 18.83 IU/L, respectively, in those with HH [Figure 2]. In those who were ≤10 years when they were transplanted only one had definitive evidence of hypogonadism and in the rest no comment can be made due to immaturity of gonadal axis. In those who were >10 years, 58% had HH. Seven patients had normal pubertal development. One female patient had secondary amenorrhea and one delayed puberty. Among male patients, two had arrested and one had delayed puberty.
In contrast to group-I, 79% in group-II had gonadal dysfunction. HH was seen in all female recipients, both FSH and LH were elevated in all and none of them had recovered ovarian function. The mean FSH, LH, and estradiol values were 109.69 ± 28.63 IU/L, 92.85 ± 30.93 IU/L, and 35.19 ± 16.79 pg/mL, respectively. Seventy-nine percent of males had GC dysfunction, and all of them had azoospermia. LC dysfunction was present in 89% of males, and 79% of them had compensated LC dysfunction. The mean FSH, LH, and testosterone values were 33.78 ± 15.60 IU/L, 27.44 ± 17.63 IU/L, and 5.66 ± 3.66 pg/mL, respectively. Only two males with LC dysfunction had low testosterone. The duration after HSCT (P = 0.57), type of transplant (P = 0.20), and GVHD (P = 0.43) had no impact on development gonadal dysfunction [Table 4].
Effects on adrenal functions
AI was present in 60% of subjects. Those who underwent allo-HSCT had a statistically significant risk of developing AI (P = 0.015), so were patients whose transplant duration was less than 1 year (P = 0.032). History of steroid intake in past was not associated with increased risk of AI (risk ratio 1.07, 95% CI: 0.76-1.51, P = 0.94). Though patients with GVHD had higher risk for development of AI (risk ratio 1.26, 95% CI: 0.89-1.79), this was not statistically significant (73% vs. 80%; P = 0.47). The mean peak cortisol value was 12.75 ± 4.16 μg/dL [Figure 2]. Mean peak cortisol was not significantly different according to duration of HSCT (12.18 ± 4.01 vs. 13.62 ± 4.40 μg/dL (P = 0.36). Patients with AI had significantly lower high-density lipoprotein cholesterol (HDL-C) than those had normal adrenal functions (30.9 ± 7.48 mg/dL v/s 40.30 ± 9.45; P value 0.0003), but low density lipoprotein cholesterol (LDL-C) levels were similar (93.11 ± 31.15 vs. 84.20 ± 22.62 mg/dL; P = 0.27).
Effects on thyroid functions
One patient had overt hypothyroidism and one had sub-clinical hypothyroidism. The levels of T3, T4, and TSH are shown in Figure 2. One patient who had received auto-HSCT for progressive systemic sclerosis developed acute reversible autoimmune hypothyroidism five months after transplant. A four and half year old TM patient developed subclinical hypothyroidism with mild elevation of anti-TPO Ab.
Effect on glucose homeostasis
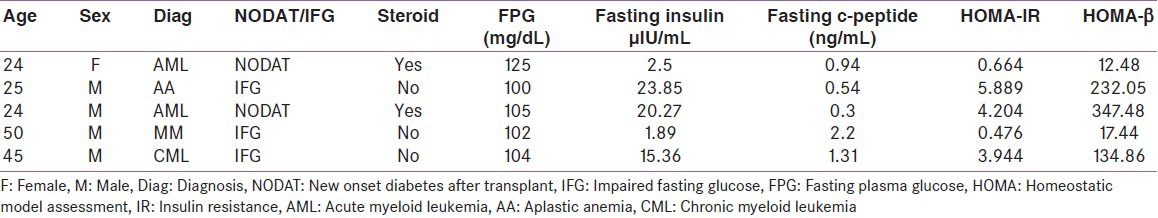
Two patients had NODAT and three had IFG. The mean body mass index (BMI), HOMA-IR, and HOMA-β in our cohort was 18.2 ± 3.6 kg/m2, 1.07 ± 1.67, and 77.81 ± 116.15, respectively. The mean BMI (19.7 ± 1.3 vs. 18.6 ± 3.8 kg/m2 ; P = 0.52) and HOMA-β (148.87 ± 143.56 vs. 69.92 ± 111.85; P = 0.15) were comparable in patients with or without dysglycemia. HOMA-IR was significantly higher (3.04 ± 2.37 vs. 0.85 ± 1.44; P = 0.004) in patients with dysglycemia than those with normoglycemia. The various parameters concerning glucose metabolism in patients with dysglycemia are shown in Table 5.
Table 5.
Parameters of glucose metabolism in patients with dysglycemia
DISCUSSION
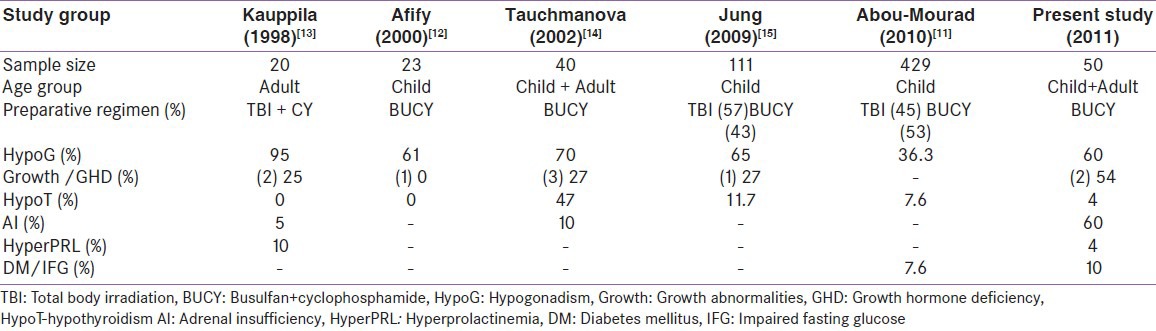
HSCT has come a long way in curing many hematological and non-hematological diseases. With increasing survival more long-term complications are being recognized. Many studies have evaluated endocrine complications,[11,12,13,14,15] but these studies reported endocrine dysfunctions as a part of all post HSCT complications[11] or had not used the prevailing gold standards to document endocrine deficiencies.[13,14] In this study, BUCY regimen was used uniformly in HSCT protocol and this study is among the largest cohort to evaluate endocrine functions in this subset of HSCT patients [Table 6].
Table 6.
Comparison of the endocrine dysfunction of few prominent studies with the present study
GH deficiency
In the present study, though most of the children had GHD, only two required GH therapy, based on growth velocity. Important factors for adequate growth in our cohort were steroid-free transplant regimen and the absence of TBI in the protocol. This in coherence with an earlier study which showed that chemotherapy-only based regimen doesn’t disturb growth in children, in fact they noted spurt in growth after HSCT.[16] However, another study had shown comparable growth deceleration after HSCT when either BUCY or TBI/CY protocols were used.[17] There was no significant difference in mean height standard deviation score (SDS) for patients with and without GHD. The interval between the onset of a hematological disorder and the transplant is less as compared to other end stage disease for which transplant is performed. This might explain the comparable height SDS in group-I. The high prevalence of GHD in this group can be attributed to the fact that 68% of the patients in this group had TM. Eighty percent of TM had GHD. Those who had TM had higher risk of developing GHD (odds ratio 5.33, 95% CI: 1.27-22.32; P = 0.016) than other patients. Whether GHD was present before transplant, as is expected in TM due to hemosiderin deposition in pituitary,[18] can’t be commented due to unavailability of pre-transplant endocrine work-up. Brauner et al.[19] reported that those who had received TBI had a decrease in growth velocity in spite of normal peak GH, and those who had received chemotherapy alone had catch up growth. This they explained by the skeletal effects of TBI. The absence of TBI in our regimen had a favorable height outcome. Interestingly, there wasn’t a single case of TM in their cohort.
One study showed that GVHD-prevented catch up growth.[20] However, in our cohort two patients who were successfully treated for GVHD had normal growth velocity, which has also been reported by Cohen et al.[21] Bakker et al.[22] found unexplained growth disturbance in 35% of children without growth limiting disorder, who had received busulfan-based preparative regimen. Busulfan crosses blood brain barrier and accordingly might have negative outcome on growth.[23]
There have not been many studies which have evaluated the growth hormone secretion pattern in adults post HSCT. The prevalence of GHD was as high as 36% in our cohort. We also speculate that GHD was a late complication after HSCT and busulfan preconditioning can be a major factor. Kaupilla et al. studied the growth hormone secretion pattern in 20 adults. They found 20% of them had GHD.[13] However, they used GH increment after (<5 μg/dL) GHRH stimulation test as the basis for diagnosis of GHD. In our study, we have used ITT, the gold standard for diagnosing GHD in adults.[6,7] The high prevalence of GHD requires attention due to increased morbidity and poor quality of life associated with it in adults.[6] These patients probably require further follow-up and re-evaluation to recommend growth hormone (GH) therapy. None of them have received GH therapy.
Hypogonadism
In group-1, 36% and in group-II 79% had HH. Neither the sex, duration after transplant, nor GVHD had any significant effect on the occurrence of HH. HH is most likely due the BUCY regimen.[12,24,25] Jung et al.[15] noted gonadal functions in long-term survivors of childhood HSCT—65.5% of females and 64.3% of males had gonadal dysfunction, which is similar to observed in the present study. They observed that risk of gonadal dysfunction was significantly higher with BUCY preconditioning in females. Mayer et al. prospectively followed gonadal functions in survivors of childhood HSCT. FSH and LH elevated in 100% and 89% of boys aged more than 14 years and 75% and 75% in females aged more than 13 years.[26] However, all but three of these had normal pubertal development. In the present study, among three hypogonadal girls, one had secondary amenorrhea, one had delayed puberty, and another had entered puberty. Of the four hypogonadal boys, two had arrested puberty and one delayed puberty, and another had entered puberty. De Sanctis et al.[27] noted that in prepubertal TM patients receiving HSCT, 80% of girls went on to develop gonadal failure, but all boys had FSH and LH in the normal range. In the present study, of the 15 TM, 6 children were in the pubertal group and out of these, 2 girls and 1 boy had definite evidence of HH. Thibaud et al.[25] studied ovarian function in 31 girls who had received HSCT, 81% had ovarian failure, and FSH was elevated in 100%, and LH in 79% of the recipients. Most of them had received either busulfan-based regimen or TBI as the preparative regimen.
Among post-pubertal patients, all males with HH had azoospermia and all females had HH with secondary amenorrhea. The pattern of development of HH is characteristic in males, the GC are predominantly involved, and LC damage is minimal as evidenced by low testosterone in only two patients. This is similar to those observed previously.[27,28,29,30] Anserini et al.[31] demonstrated a high incidence of azoospermia (70%), but they also interestingly showed reversal of azoospermia in a significant proportion. Tauchmanovà et al.[14] studied 40 allo-HSCT patients of whom 95% of women had ovarian insufficiency and 47% male had abnormal spermatogenesis.
Adrenal insufficiency
AI was present in 60% of recipients. Interestingly we found that history of steroid intake didn’t increase the risk of AI and so was history of GVHD. AI was present in 78% patients with HSCT duration <1 year than 44% in those with HSCT duration >1 year. This suggest that the occurrence of AI is an early phenomenon. Mean peak cortisol also increased with increasing interval after HSCT (12.17 v/s 13.62 μg/dL) and more number of subjects had cortisol <10 μg/dL with duration <1 year (5 vs. 1). The ACTH stimulation test might not be an appropriate test to detect secondary AI in acute setting.[32] None of the studies have commented the type of AI post-HSCT and it has been assumed to be due to steroids. Assuming that chances of secondary AI is high, an ITT would be the most ideal test in this situation. Unfortunately, we were unable to measure plasma ACTH.
In one of the earliest studies, Sanders et al.[33] noted AI in 24% of patients, but the methodology used remains questionable. Ogilvy-Stuart et al.[34] used ITT in TBI-based HSCT to demonstrate AI in 2 of the 31 evaluated patients. Tauchmanová et al.[14] found AI in 4 of the 40 patients evaluated had secondary adrenal insufficiency and all of them were on steroids. However, they diagnosed AI on the basis of basal cortisol values which can be erroneous. Bakker et al. reported AI in 6 of the 64 childhood HSCT recipients.[13] However, four patients had X-linked adrenoleukodystrophy and two had received steroids. Tauchmanová and colleagues found AI in 30% of auto-HSCT recipients when evaluated within 1 year of transplant.[35] They hadn’t performed any stimulation test and all patients had received steroids in past.
All these patients had no symptoms related to adrenal insufficiency, hence early assessment post-HSCT is important to start supplement and prevent acute adrenal crisis at the time of stress like infections, which they are predisposed to being immune compromised status. The mean HDL-C value in AI group was significantly lower compared to non-AI group. AI patients also had a HDL-C values lower than mean for Indians i.e., ~44 mg/dL.[36] The association of HDL-C with AI needs to be studied further.
Thyroid dysfunction
All except two had normal thyroid functions. The low incidence of thyroid dysfunction in our study may be due to the absence of TBI in our preparative regimen. In one of the largest studies till date, Sanders et al. observed hypothyroidism in 30% of 791 HSCT recipients.[37] Subclinical hypothyroidism was seen in 15%, overt hypothyroidism in 1.3%, central hypothyroidism in 9.3%, and hyperthyroidism in 2.9%. AITD as described in our patient has been previously been reported.[38,39,40,41,42] Of the 147 allo-HSCT recipients, Ishiguro et al.[43] found overt hypothyroidism in 0.03%, subclinical hypothyroidism in 26.5%, and hyperthyroidism in one subject. Siekierska-Hellmann et al.[44] found that at 1 year none of patients developed overt hypothyroidism in HSCT recipients prepared with chemotherapy. Somali et al.[45] in non-TBI-based HSCT protocol found overt hypothyroidism in 6% of male patients and 5% of female patients and subclinical hypothyroidism in 13% of males and 5% of females.
Dysglycemia
In the present study, two patients developed NODAT and three had IFG [Table 5]. Both the patients of NODAT had history of steroid intake and their glycemic control was initially maintained by basal bolus insulin regimen and subsequently on metformin. Majhail et al.[46] reported a very high incidence of NODAT-30% with 2 years of transplant and also showed reversal in 70% of them. Exposure to steroid increased the risk of NODAT.[47] However, low prevalence of NODAT in the present study could be due to steroid-free immunosuppression and inclusion of children in the total cohort.
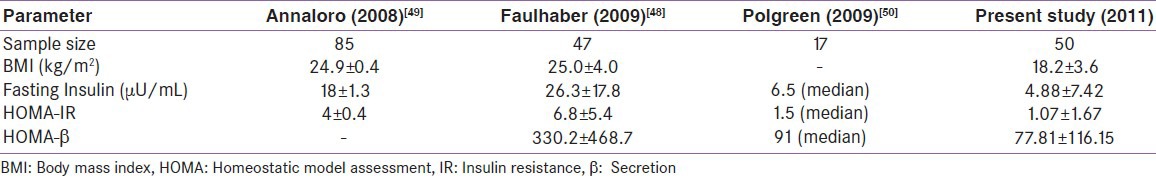
There have been very few studies which have addressed insulin resistance (IR) and secretion in post-HSCT patients. One of the early studies had utilized the intravenous glucose tolerance test to show that after HSCT, IR is common.[47] Another study when evaluating bone health in post-HSCT patients found high HOMA-IR and HOMA-β.[48] An Italian group had also shown elevated HOMA-IR in their post-HSCT cohort.[49] The mean HOMA-IR in our group was less compared to others. This could be possible due to lower BMI in our group. These results indicate that post-HSCT recipients are insulin sensitive; however, those who develop dysglycemia tend to have IR as a pathophysiologic event. However, we were unable to compare these values with age- and sex-matched controls. Table 7 compares metabolic parameters of few relevant studies.
Table 7.
Comparison of metabolic parameters in our group with few of those reported in literature
Following were the limitations of our study. Firstly, the present study was a cross-sectional study without the control group. Secondly, pre-transplant endocrine status of the cohort was not available, and hence the findings cannot be completely attributed to HSCT. The large number of TM patients in group-I can bias the effect of HSCT, as TM by itself is known to be associated with many endocrine dysfunction. Finally, AI couldn’t be classified as primary or secondary as plasma ACTH couldn’t be measured due to local constraints.
CONCLUSIONS
Endocrine manifestations are common after HSCT; they can occur as early or late complications. All patients undergoing HSCT should have endocrine evaluation as per prevalent guidelines. However, this is hindered by non-availability of trained endocrinologist. By this study we were able to emphasize the need for pre- and post-transplant endocrine evaluation of all patients requiring HSCT at our center. AI, GHD, and hypogonadism are equally prevalent (~60%) after BUCY-based HSCT. Multiple hormone deficiencies are common. All children with GHD might not require therapy, as growth velocity can be normal after HSCT even in the presence of GHD. Hypogonadism is universal in adult females. The most common pattern of gonadal dysfunction in adult males is germ cell failure with compensated Leydig cell failure. All recipients in reproductive age should be considered for preservation of gonadal functions prior to HSCT.[50]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Thomas ED. A history of haemopoietic cell transplantation. Br J Haematol. 1999;105:330–9. doi: 10.1111/j.1365-2141.1999.01337.x. [DOI] [PubMed] [Google Scholar]
- 2.Hows JM, Passweg JR, Tichelli A, Locasciulli A, Szydlo R, Bacigalupo A, et al. Comparison of long-term outcomes after allogeneic hematopoietic stem cell transplantation from matched sibling and unrelated donors. Bone Marrow Transplant. 2006;38:799–805. doi: 10.1038/sj.bmt.1705531. [DOI] [PubMed] [Google Scholar]
- 3.Brennan BM, Shalet SM. Endocrine late effects after bone marrow transplant. Br J Haematol. 2002;118:58–66. doi: 10.1046/j.1365-2141.2002.03527.x. [DOI] [PubMed] [Google Scholar]
- 4.Chandy M. Stem cell transplantation in India. Bone Marrow Transplant. 2008;42(Suppl 1):S81–4. doi: 10.1038/bmt.2008.124. [DOI] [PubMed] [Google Scholar]
- 5.Maghnie M, Uga E, Temporini F, Di Iorgi N, Secco A, Tinelli C, et al. Evaluation of adrenal function in patients with growth hormone deficiency and hypothalamic-pituitary disorders: Comparison between insulin-induced hypoglycemia, low-dose ACTH, standard ACTH and CRH stimulation tests. Eur J Endocrinol. 2005;152:735–41. doi: 10.1530/eje.1.01911. [DOI] [PubMed] [Google Scholar]
- 6.Cook DM, Yeun KC, Biller BM, Kemp SF, Lee Vance M. American association of clinical endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients-2009 update. Endocr Pract. 2009;15(Suppl 2):S1–29. doi: 10.4158/EP.15.S2.1. [DOI] [PubMed] [Google Scholar]
- 7.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Endocrine Society. Evaluation and treatment of adult growth hormone deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–609. doi: 10.1210/jc.2011-0179. [DOI] [PubMed] [Google Scholar]
- 8.Basevi V, Di Mario S, Morciano C, Nonino F, Magrini N. Comment on: American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Mourad YR, Lau BC, Barnett MJ, Forrest DL, Hogge DE, Nantel SH, et al. Long-term outcome after allo-SCT: Close follow-up on a large cohort treated with myeloablative regimens. Bone Marrow Transplant. 2010;45:295–302. doi: 10.1038/bmt.2009.128. [DOI] [PubMed] [Google Scholar]
- 12.Afify Z, Shaw PJ, Clavano-Harding A, Cowell CT. Growth and endocrine function in children with acute myeloid leukaemia after bone marrow transplantation using busulfan/cyclophosphamide. Bone Marrow Transplant. 2000;25:1087–92. doi: 10.1038/sj.bmt.1702384. [DOI] [PubMed] [Google Scholar]
- 13.Kauppila M, Koskinen P, Irjala K, Remes K, Viikari J. Long-term effects of allogeneic bone marrow transplantation (BMT) on pituitary, gonad, thyroid and adrenal function in adults. Bone Marrow Transplant. 1998;22:331–7. doi: 10.1038/sj.bmt.1701337. [DOI] [PubMed] [Google Scholar]
- 14.Tauchmanovà L, Selleri C, Rosa GD, Pagano L, Orio F, Lombardi, et al. High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer. 2002;95:1076–84. doi: 10.1002/cncr.10773. [DOI] [PubMed] [Google Scholar]
- 15.Jung MH, Cho KS, Lee JW, Chung NG, Cho B, Suh BK, et al. Endocrine complications after hematopoietic stem cell transplantation during childhood and adolescence. J Korean Med Sci. 2009;24:1071–7. doi: 10.3346/jkms.2009.24.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoshniat M, Ghavamzadeh A, Larijani B, Bahar B, Tabatabaei O. Effect on growth parameters of bone marrow transplantation with a chemotherapy-only conditioning regimen. Transplant Proc. 2003;35:3085–8. doi: 10.1016/j.transproceed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Wingard JR, Plotnick LP, Freemer CS, Zahurak M, Piantadosi S, Miller DF, et al. Growth in children after bone marrow transplantation: Busulfan plus cyclophosphamide versus cyclophosphamide plus total body irradiation. Blood. 1992;79:1068–73. [PubMed] [Google Scholar]
- 18.De Simone M, Verrotti A, Iughetti L, Palumbo M, Di Bartolomeo P, Olioso P, et al. Final height of thalassemic patients who underwent bone marrow transplantation during childhood. Bone Marrow Transplant. 2001;40:29–35. doi: 10.1038/sj.bmt.1703123. [DOI] [PubMed] [Google Scholar]
- 19.Brauner R, Fontoura M, Zucker JM, Devergie A, Souberbielle JC, Prevot-Saucet C, et al. Growth and growth hormone secretion after bone marrow transplantation. Arch Dis Child. 1993;68:458–63. doi: 10.1136/adc.68.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adan L, de Lanversin ML, Thalassinos C, Souberbielle JC, Fischer A, Brauner R. Growth after bone marrow transplantation in young children conditioned with chemotherapy alone. Bone Marrow Transplant. 1997;19:253–6. doi: 10.1038/sj.bmt.1700643. [DOI] [PubMed] [Google Scholar]
- 21.Cohen A, Rovelli A, Bakker B, Uderzo C, van Lint MT, Esperou H, et al. Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: A study by the Working Party for Late Effects-EBMT. Blood. 1999;93:4109–15. [PubMed] [Google Scholar]
- 22.Bakker B, Oostdijk W, Bresters D, Walenkamp MJ, Vossen JM, Wit JM. Disturbances of growth and endocrine function after busulphan-based conditioning for haematopoietic stem cell transplantation during infancy and childhood. Bone Marrow Transplant. 2004;33:1049–56. doi: 10.1038/sj.bmt.1704481. [DOI] [PubMed] [Google Scholar]
- 23.Dix SP, Yee GC. Pharmacologic and biologic agents. In: Whedon MK, Wujcik D, editors. Blood and Marrow Stem Cell Transplantation: Principle, Practice and Nursing Insights. 2nd ed. Sudbury, MA: Jones and Barrlett Publishers; 1997. pp. 100–50. [Google Scholar]
- 24.Teinturier C, Hartmann O, Valteau-Couanet D, Benhamou E, Bougneres PF. Ovarian function after autologous bone marrow transplantation in childhood: High-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant. 1998;22:989–94. doi: 10.1038/sj.bmt.1701483. [DOI] [PubMed] [Google Scholar]
- 25.Thibaud E, Rodriguez-Macias K, Trivin C, Espérou H, Michon J, Brauner R. Ovarian function after bone marrow transplantation during childhood. Bone Marrow Transplant. 1998;21:287–90. doi: 10.1038/sj.bmt.1701075. [DOI] [PubMed] [Google Scholar]
- 26.Mayer EI, Dopfer RE, Klingebiel T, Scheel-Walter H, Ranke MB, Niethammer D. Longitudinal gonadal function after bone marrow transplantation for acute lymphoblastic leukemia during childhood. Pediatr Transplant. 1999;3:38–44. doi: 10.1034/j.1399-3046.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 27.De Sanctis V, Galimberti M, Lucarelli G, Polchi P, Ruggiero L, Vullo C. Gonadal function after allogenic bone marrow transplantation for thalassaemia. Arch Dis Child. 1991;66:517–20. doi: 10.1136/adc.66.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee R, Kottaridis PD, McGarrigle HH, Eliahoo J, McKeag N, Mackinnon S, et al. Patterns of Leydig cell insufficiency in adult males following bone marrow transplantation for haematological malignancies. Bone Marrow Transplant. 2001;28:497–502. doi: 10.1038/sj.bmt.1703160. [DOI] [PubMed] [Google Scholar]
- 29.Grigg AP, McLachlan R, Zaja J, Szer J. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg) Bone Marrow Transplant. 2000;26:1089–95. doi: 10.1038/sj.bmt.1702695. [DOI] [PubMed] [Google Scholar]
- 30.Somali M, Mpatakoias V, Avramides A, Sakellari I, Kaloyannidis P, Smias C, et al. Function of the hypothalamic-pituitary-gonadal axis in long-term survivors of hematopoietic stem cell transplantation for hematological diseases. Gynecol Endocrinol. 2005;21:18–26. doi: 10.1080/09513590500099255. [DOI] [PubMed] [Google Scholar]
- 31.Anserini P, Chiodi S, Spinelli S, Costa M, Conte N, Copello F, et al. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30:447–51. doi: 10.1038/sj.bmt.1703651. [DOI] [PubMed] [Google Scholar]
- 32.Dökmetaº HS, Colak R, Keleºtimur F, Selçuklu A, Unlühizarci K, Bayram F. A comparison between the 1-microg adrenocorticotropin (ACTH) test, the short ACTH (250 microg) test, and the insulin tolerance test in the assessment of hypothalamo-pituitary-adrenal axis immediately after pituitary surgery. J Clin Endocrinol Metab. 2000;85:3713–19. doi: 10.1210/jcem.85.10.6879. [DOI] [PubMed] [Google Scholar]
- 33.Sanders JE, Pritchard S, Mahoney P, Amos D, Buckner CD, Witherspoon RP, et al. Growth and development following marrow transplantation for leukemia. Blood. 1986;68:1129–35. [PubMed] [Google Scholar]
- 34.Ogilvy-Stuart AL, Clark DJ, Wallace WH, Gibson BE, Stevens RF, Shalet SM, et al. Endocrine deficit after fractionated total body irradiation. Arch Dis Child. 1992;67:1107–10. doi: 10.1136/adc.67.9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tauchmanovà L, Selleri C, De Rosa G, Esposito M, Di Somma C, Orio F, et al. Endocrine disorders during the first year after autologous stem-cell transplant. Am J Med. 2005;118:664–70. doi: 10.1016/j.amjmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Radhika G, Ganesan A, Sathya RM, Sudha V, Mohan V. Dietary carbohydrates, glycemic load and serum high-density lipoprotein cholesterol concentrations among South Indian adults. Eur J Clin Nutr. 2009;63:413–20. doi: 10.1038/sj.ejcn.1602951. [DOI] [PubMed] [Google Scholar]
- 37.Sanders JE, Hoffmeister PA, Woolfrey AE, Carpenter PA, Storer BE, Storb RF, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years′ experience. Blood. 2009;113:306–8. doi: 10.1182/blood-2008-08-173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldouri MA, Ruggier R, Epstein O, Prentice HG. Adoptive transfer of hyperthyroidism and autoimmune thyroiditis following allogeneic bone marrow transplantation for chronic myeloid leukaemia. Br J Haematol. 1990;74:118–9. doi: 10.1111/j.1365-2141.1990.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 39.Holland FJ, McConnon JK, Volpé R, Saunders EF. Concordant Graves′ disease after bone marrow transplantation: Implications for pathogenesis. J Clin Endocrinol Metab. 1991;72:837–40. doi: 10.1210/jcem-72-4-837. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt DT, Lum LG, Casper J, Hunter J, Camitta B. Autoimmune thyroiditis after bone marrow transplantation. Bone Marrow Transplant. 1990;5:357–61. [PubMed] [Google Scholar]
- 41.Karthaus M, Gabrysiak T, Brabant G, Prahst A, Link H, Soudah B, et al. Immune thyroiditis after transplantation of allogeneic CD34+selected peripheral blood cells. Bone Marrow Transplant. 1997;20:697–99. doi: 10.1038/sj.bmt.1700955. [DOI] [PubMed] [Google Scholar]
- 42.Vialettes B, Maraninchi D, San Marco MP, Birg F, Stoppa AM, Mattei-Zevaco C, et al. Autoimmune polyendocrine failure-type 1 (insulin-dependent) diabetes mellitus and hypothyroidism-after allogeneic bone marrow transplantation in a patient with lymphoblastic leukaemia. Diabetologia. 1993;36:541–46. doi: 10.1007/BF02743271. [DOI] [PubMed] [Google Scholar]
- 43.Ishiguro H, Yasuda Y, Tomita Y, Shinagawa T, Shimizu T, Morimoto T, et al. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. 2004;89:5981–6. doi: 10.1210/jc.2004-0836. [DOI] [PubMed] [Google Scholar]
- 44.Siekierska-Hellmann M, Babiñska A, Oboloñczyk L, Sworczak K, Hellmann A. One-year follow-up of TSH level and thyroid volume in patients with bone marrow or peripheral blood hematopoietic stem cell transplantation following chemotherapy. Pol Merkur Lekarski. 2007;23:170–3. [PubMed] [Google Scholar]
- 45.Somali M, Mpatakoias V, Avramides A, Sakellari I, Smias CH, Anagnostopoulos A, et al. Thyroid dysfunction in adult long-term survivors after hemapoeitic stem-cell transplantation (HSCT) Horm Metab Res. 2005;37:494–99. doi: 10.1055/s-2005-870308. [DOI] [PubMed] [Google Scholar]
- 46.Majhail NS, Challa TR, Mulrooney DA, Baker KS, Burns LJ. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1100–7. doi: 10.1016/j.bbmt.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Lorini R, Cortona L, Scaramuzza A, De Stefano P, Locatelli F, Bonetti F, et al. Hyperinsulinemia in children and adolescents after bone marrow transplantation. Bone Marrow Transplant. 1995;15:873–7. [PubMed] [Google Scholar]
- 48.Faulhaber GA, Premaor MO, Moser Filho HL, Silla LM, Furlanetto TW. Low bone mineral density is associated with insulin resistance in bone marrow transplant subjects. Bone Marrow Transplant. 2009;43:953–7. doi: 10.1038/bmt.2009.70. [DOI] [PubMed] [Google Scholar]
- 49.Annaloro C, Usardi P, Airaghi L, Giunta V, Forti S, Orsatti A, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 50.Polgreen LE, Thomas W, MacMillan ML, Wagner JE, Moran A, Petryk A. First phase insulin release and glucose tolerance in children with Fanconi anemia after hematopoietic cell transplantation. Pediatr Blood Cancer. 2009;53:191–196. doi: 10.1002/pbc.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]


