Abstract
Background:
Growth hormone deficiency (GHD) in adults is associated with a cluster of cardiovascular risk factors that may contribute to an increased mortality for cardiovascular disease. In children, relatively few studies have investigated the effect of GHD and replacement therapy on cardiac performance and metabolic abnormalities that may place them at a higher risk of cardiovascular disease (CVD) at an early age.
Aim:
This study was aimed to assess the left ventricular function, lipid profile, and degree of insulin resistance in Egyptian children with GHD before and after 1 year of GH replacement therapy.
Settings and Design:
Prospective case-control study, single-center study.
Materials and Methods:
Thirty children with short stature due to GHD were studied in comparison to 20 healthy age- and sex-matched children. All subjects were subjected to history, clinical examination, auxological assessment, and echocardiography to assess the left ventricular function. Blood samples were collected for measuring IGF-1, lipid profile (Total, LDL, HDL cholesterol, triglyceride, and atherogenic index (AI), fasting blood sugar, and fasting insulin levels. In addition, basal and stimulated GH levels were measured in children with suspected GHD.
Statistical Analysis Used:
Student's t-test was used for parametric data, and the Mann-Whitney U-test was used for non-parametric data.
Results:
Total, LDL cholesterol, triglyceride, AI, and insulin were significantly higher in children with GHD than in healthy controls at baseline. After 12 months of GH replacement therapy, total, LDL cholesterol, triglyceride, AI and insulin were significantly decreased, while homeostatic model assessment for insulin resistance index (HOMA-IR) was significantly increased compared to both pre-treatment and control values. At baseline, the left ventricular mass (LVM) and left ventricular mass index (LVMi) were significantly lower in GHD children than in controls. After 12 months of GH replacement therapy, LVM and LVMi in GHD patients were significantly increased compared to pre-treatment values.
Conclusions:
GHD in children is associated with a significantly reduced cardiac mass and impairment of lipid profile. GH replacement therapy exerts beneficial effects both on cardiac mass and lipid metabolism by normalizing cardiac size and improving the lipid profile. On the contrary, an increase in insulin resistance is observed after 12 months GH treatment. The study suggests that children with GH deficiency should have echocardiography and lipid profile monitoring before and during treatment with GH.
Keywords: Growth hormone, insulin resistance, left ventricular mass, lipid profile
INTRODUCTION
It is now established that adults with growth hormone deficiency (GHD) may develop a cluster of cardiovascular risk factors, including unfavorable lipid profile, increased body fat, premature atherosclerosis, decreased fibrinolytic activity, increased peripheral insulin resistance,[1] as well as reduced cardiac performance,[2] all of which may contribute to a reduced life expectancy with an increased mortality for cardiovascular disease (CVD).[3] The existing evidence indicates that atherosclerotic CVD begins in childhood.[4] In children, obesity occurs with other risk factors for CVD, such as increased blood pressure, adverse changes in serum lipoproteins, and hyperinsulinemia, leading to acceleration of atherosclerotic lesion; therefore, the primary prevention of atherosclerotic CVD should begin in childhood. The major therapeutic role of GH in children is to promote linear growth. GH has, however, other important physiological functions in the human body influencing several key metabolic processes, body composition, muscle strength, bone mineral density, and reproductive capacity.[5] In children with GHD, echocardiographic studies of systolic function have yielded contrasting results on the effect of both GHD and GH therapy on cardiac performance.[6] On the other hand, relatively few studies have investigated the effect of GHD and GH replacement therapy on cardiac performance and metabolic abnormalities that may place them at a higher risk of CVD at an early age.[7,8] The aim of the present study is to assess the left ventricular function, lipid profile, and degree of insulin resistance in Egyptian children with GHD before and after 1 year of GH replacement therapy.
MATERIALS AND METHODS
This is prospective case-control study. It included 30 children with GHD. GHD was diagnosed according to clinical and auxological criteria[9] and by peak concentration less than 10 ug/liter after two stimulation tests. In addition, 20 apparently healthy age, sex- and BMI-matched children were studied as a control. Both patients and controls were recruited from Pediatric Endocrinocriology Outpatients Clinic in Assiut University Children Hospital and the Pediatric Health insurance clinics in Assiut Governorate –Egypt. The study protocol was approved by the Ethical Committees of Assiut University Children Hospital, Egypt. Written informed consents were obtained from the parents of both patients and controls.
Inclusion criteria
Age 4-10 years
Height <–2 standard deviation score (SDS) (<3rd percentile)
Idiopathic, isolated GH deficiency
Normal thyroid function tests.
Exclusion criteria
Any acute severe illness during the previous 6 months
Evidence or current cardiovascular disease, respiratory, renal, liver, or endocrine disease
Family history of atherosclerosis and cardiovascular disease
Children with dysmorphic phenotypes, such as skeletal dysplasias or Turner syndrome
Children on GH replacement
Prematurity or intrauterine growth retardation
Multiple pituitary hormone deficiency
Children with midline defect.
Methodology
All cases were subjected to
Full history and clinical examination including, heart rate, systolic blood pressure (SBP), and diastolic blood pressure (DPB)
Standing height of the patients was measured using a Harpenden fixed stadiometer (Holtain Ltd, Crosswell, UK) with a sensitivity of 0.1 cm, and body weight was measured using a balance scale (SECA, Hamburg, Germany) with a sensitivity of 0.1 kg. The weight of the each subject was measured with all of the clothing removed except undergarments. Body mass index (BMI) was calculated as weight (kg) divided by square of the height (m). Target height was calculated by the method of Tanner et al., taking the average of mother's and father's height after addition of 13 cm in boys or subtractions of them in girls, while mid-parental height is calculated as before ±6.5 cm.[10]
Radiological examination
Bone age (BA) was done by performing plain X-ray left hand and wrist together with calculation of bone age SDS according to the standards of Greulich and Pyle[11]
Magnetic resonance imaging (MRI) of the hypothalamus-pituitary region.
Laboratory tests
All patients underwent the following tests (following not less than 12 hours fasting period):
Routine general laboratory tests, if needed, which include complete blood picture, renal, and liver function tests
Total cholesterol (normal range 100-200 mg/dl), high-density lipoprotein cholesterol (HDL-c) (normal range 30-70 mg/dl), low-density lipoprotein cholesterol (LDL-c) (normal value less than 130 mg/dl), TG (normal range 35-160 mg/dl), and fasting blood sugar (FBS) (normal range 65-100 mg/dl). Measurement was carried out using an auto-analyzer (Synchron-clinical system-CX5). The atherogenic index (AI) was calculated as the ratio of total to HDL cholesterol, considered as an index of severe cardiovascular risk.[12] Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula. Fasting serum insulin measurement was done by an auto-analyzer (DDC/immulite). IR was calculated using the following equation: The homeostasis model assessment method: HOMA-IR = fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5.[13] In both children with GHD and the controls, the evaluation of these parameters was also repeated after 12 months of GH replacement therapy
Thyroid profile: Thyroid stimulating hormone (TSH) was estimated by immunoradiometric assay (IRMA), while FT3 and FT4 were estimated by radioimmunoassay kits from Diagnosis Product Corporation, (Los angeles, CA, USA.)
Stimulation of GH secretion by 2 provocation tests (clonidine and insulin tolerance test separated by 1 week interval was done. Growth hormone was analyzed by immunoradiometric assay (IRMA). The dose of clonidine given before the test was 0.15 mg/m2 orally while that of insulin was 0.1 IU/kg i.v. In the ITT test, the blood glucose should decrease by 50% or more of the basal value or decrease to 40 mg/dl. If no hypoglycemia occurred, another dose of insulin (0.05 IU/kg) was given. With adequate hypoglycemia, peak GH levels less than 10 ng/ml indicated GHD
Insulin-like growth factor-1 (IGF-1) was determined at diagnosis using solid phase immunoradiometric assay (IRMA)[14] using kits from Diagnostic System Laboratories Inc (DSL)(Texas, USA).
Echocardiography
All cases and controls underwent echocardiographic studies at the time of diagnosis and 1 year after GH treatment using M-mode, two-dimensional, and Doppler techniques, using commercially available phased array system employing a 4 and 7 MHZ transducer, respectively (Magic bright 2, Vivid 3, Vingmed–Tech). Measurements were performed using the machine's incorporated analysis package. The records were made by two investigators blinded to the subjects’ status. All patients were studied according to the recommendations of the American Society of Echocardiography.[15] The following measurements were taken on all patients and controls:
- Left ventricular systolic functions:
- Left ventricular end diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular posterior wall thickness LVPWT, and interventricular septal wall thickness IVSWT
-
Percentage of fractional shortening (FS): LVFS% was calculated using the following formula 8: FS = EDD-ESD/EDD × 100.Where, EDD is the end diastolic diameter of the left ventricle and ESD is the end systolic diameter of the left ventricle
-
Ejection fraction (EF) was measured from the “cubed equation:EF = (EDD)3−(ESD)3/(EDD)3 × 100
- Left ventricular mass (LV mass) and left ventricular mass index. It is performed by using LVMI calculator. The LV mass (LVM) was calculated by using Devereux's formula according to Penn's convention with the regression-corrected cube formula LVM = 1.04 [(ISV + LVEDD + PWT)3 − (LVEDD)3] −13.8 g, and expressed by LVM index (LVMi) after correction for BSA
Left ventricle diastolic function: Doppler studies provided indexes of ventricular filling, which were derived from the mitral flow velocity curves, i.e., maximal early diastolic flow velocity (E in cm/s), maximal late diastolic flow velocity (A in cm/s), the ratio between E and A curves (E/A, normal value >1); the isovolumetric relaxation time (IRT), which represents the interval between the end of aortic valve closure and the onset of mitral valve opening, was also evaluated.
In both children with GHD and the controls, the evaluation of these echocardiographic parameters was also repeated after 12 months of GH replacement therapy.
Treatment protocol
The Egyptian health insurance institute covers all aspect of investigation, diagnosis, and treatment of GHD in children. All patients received rhGH with a standard dose of 20 IU/m2/week.[9] The calculated dose per week was divided for 6 days and given subcutaneously at night. The study group was followed every 3 months for anthropometric assessment, to assure compliance to therapy, to observe side-effects, and to renew the GH prescription. Compliance to therapy is continuously verified by more than one parameter e.g. height velocity, asking the parents about mode of injection and dosing, counting the empty vials, and sometimes by analysis of serum IGF-1.
Statistical analysis
Analysis was carried out using SPSS (version 16). The numerical data were represented as mean ± SD. For comparison of the two groups, Student's t-test was used for parametric data and the Mann-Whitney U-test was used for non-parametric data. P value less than 0.05 was considered statistically significant.
RESULTS
Table 1 shows demographic, anthropometric, clinical, and hormonal parameters in GHD children and in controls at study entry. Height and serum IGF-I were significantly lower, as expected, in GHD subjects than in healthy children (P < 0.001), while heart rate, SBP, and DBP were similar in the two groups.
Table 1.
Demographic, anthropometric, clinical, and hormonal parameters in GHD children and in controls
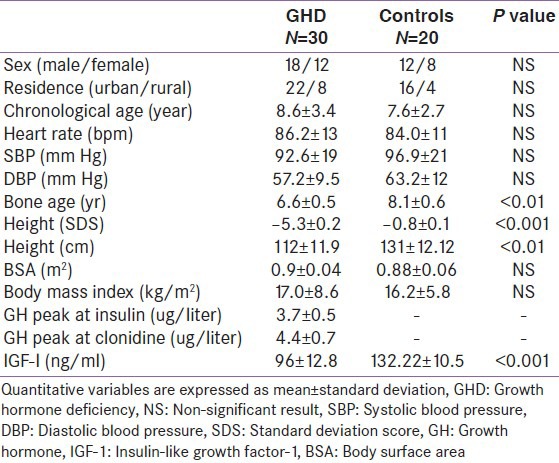
Table 2 shows metabolic parameters in children with GHD at baseline and 12 months of GH replacement therapy and in controls at baseline and after 12 months follow-up. Total, LDL cholesterol, triglyceride, AI, and insulin were significantly higher in children with GHD than in healthy controls at baseline. After 12 months of GH replacement therapy, total, LDL cholesterol, triglyceride, AI, and insulin were significantly decreased, while HOMA-IR was significantly increased compared to both pre-treatment and control values.
Table 2.
Metabolic parameters in children with GHD at baseline and 12 months of GH replacement therapy and in controls at study entry and after 12 months follow-up
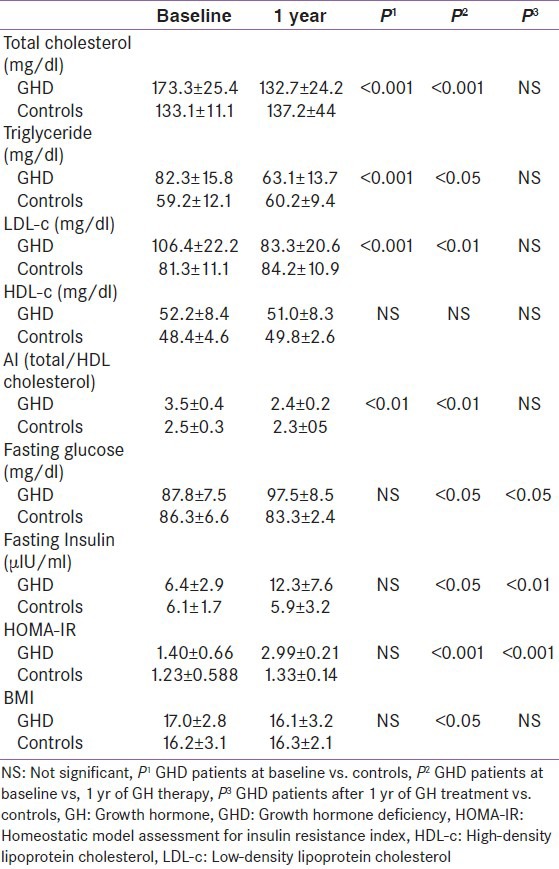
After 12 months of GH replacement therapy, BMI was significantly decreased compared to pre-treatment value (16.1 ± 3.2 vs. 17.0 ± 2.8, P < 0.05).
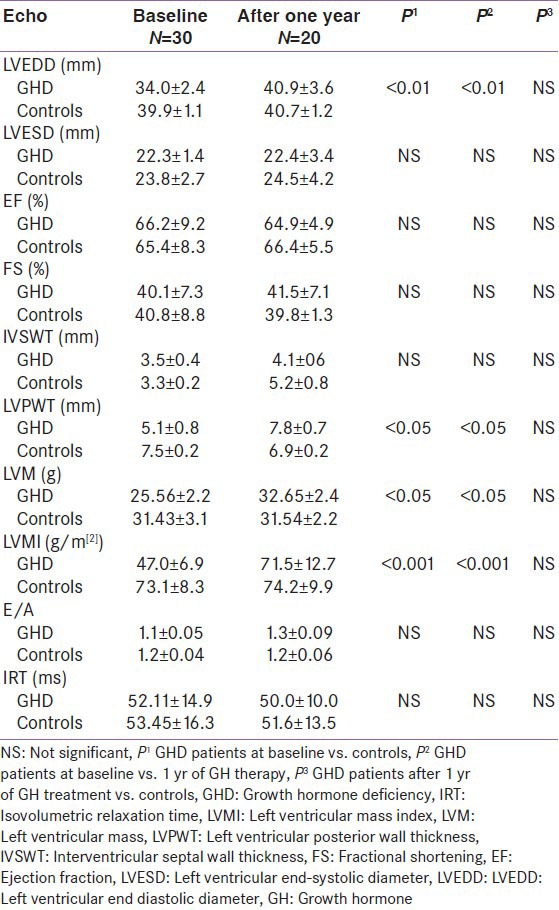
Table 3 shows echocardiographic characteristics of children with GHD at baseline and 12 months of GH replacement therapy and in controls at baseline and after 12 months follow-up. LV systolic function, measured by FS and LVEF, and diastolic function, measured by E/A ratio and IRT, did not change significantly during treatment. No significant changes in cardiac size and function were observed in controls when retested after 1 year. LVM and LVMi in GHD patients were significantly increased after 1 year of replacement therapy, compared to pre-treatment values, but not significantly different compared to LVM and LVMi of controls evaluated after 1 year of follow-up.
Table 3.
Echocardiographic characteristics of children with GHD at baseline an d 12 months of GH replacement therapy and in controls at study entry and after 12 months follow-up
DISCUSSION
Our study demonstrated that LV mass and LV mass index were significantly lower at baseline in the GH-deficient group than in the control group, and that these parameters increased significantly after 1 year of GH treatment reaching values comparable to those of controls. These findings are in agreement with those reported by Shulman et al.,[16] in a prospective, uncontrolled, study enrolling 10 children with GHD documented a reduced LVM that significantly increased after 1 year of GH therapy. The major drawback of the study by Shulman et al. was the lack of a control group. In a previous short-term case-control study, Salerno M, et al.[17] demonstrated that heart size was significantly reduced in 30 GHD children and increased significantly after 1 year of GH replacement. Altogether, these results indicate that GH, directly or indirectly through IGF-I, is not only involved in the regulation of somatic growth in children but also in cardiac growth, probably through the modulation of the size of cardiomyocytes.[18] On the other hand, Radetti et al.[19] reported that LVM and systolic and diastolic functions did not differ from a control group after 1 year of GH therapy. The difference between our result and Radetti et al. results may be attributed to different GH dosages, duration of GH treatment, and degree of GHD severity. The difsferent degree of GHD may result in a different response to GH replacement, which might ultimately result in a varied increase in cardiac size.
In the present study, no abnormalities of LV systolic or diastolic function were identified in these GH-deficient children at baseline or after 1 year of rhGH therapy. Cardiac dysfunction and subsequent GH treatment amelioration may require a longer duration of GH deficiency.[16] The age of children included in the study ranged between 4 and 10 years, i.e. pre-pubertal, to rule out the possible influence of changes in insulin and other metabolic parameters as a result of pubertal development. In the present study, total, LDL cholesterol, AI, and insulin were significantly higher in children with GHD than in healthy controls at study entry. After 12 months of GH replacement therapy, total, LDL cholesterol, AI, and insulin were significantly decreased. This beneficial effect of GH treatment on lipid profile and AI has been reported in other short- and long-term studies evaluating the efficacy of GH therapy on lipid profile in GHD children.[20,21] In a 6-yr follow-up study, Van Der Sluis et al.[22] documented a long-term beneficial effect of GH therapy on AI, as well as on HDL cholesterol in GHD children. The same beneficial effect on lipid metabolism has been observed in children with short stature, born small for gestational age after 6 years of GH treatment.[23] It is well-known that abnormalities in lipid profile may severely increase the coronary risk of GHD patients;[24] thus, the decrease in the total/HDL cholesterol ratio during GH therapy can be clinically relevant to the prevention of CVD in midlife, because it represents one of the most efficient predictors of coronary heart disease in adults.[25] The exact mechanisms that underlie these changes are not fully understood, but GH may act through the regulation of both the activity of the cholesterol 17-alpha -hydroxylase enzyme and the regulation of LDL cholesterol receptor numbers.[26] On the other hand, Gleeson et al.[27] reported no beneficial effect of GH treatment on lipid profile. This difference may be explained by the variety of etiologies of GH deficiency, differences in severity and duration of this deficit, and the association with other pituitary hormone deficiencies. In the present study, no difference was seen between GH deficiency group and normal controls for HDL-C levels. This finding is in line with results of previous reports suggesting that normal HDLc is a feature of childhood onset GH deficiency as opposed to adult onset disease, which is associated with a reduced HDL-C.[28]
In the present study, insulin sensitivity was markedly affected, with a significant increase in HOMA-IR, related to increased levels of both insulin and fasting glycemia. Other studies have reported even increased insulin sensitivity in young pre- or early pubertal children, and that there is progressive deterioration in this insulin sensitivity with advancing age and pubertal development.[29,30]
Most short-term studies have reported a deterioration of insulin sensitivity, whereas long-term studies suggested that, after an initial worsening, insulin sensitivity returned toward baseline values.[31] Previous studies in short children have shown that short-term GH replacement was associated with development of insulin resistance and peripheral hyperinsulinemia, as measured by the hyperglycemic clamp technique or using oral glucose tolerance testing, even if insulin levels remained within the physiological range of normal control children.[32,33] In short small-for-gestational-age children, GH replacement induces high fasting insulin levels with normal glucose. In addition, concern has been expressed that GH administration in children and adolescents may cause or exacerbate, in predisposed individuals, type 2 diabetes mellitus.[34] Growth hormone has antagonistic effects to that of insulin, and a decrease in insulin sensitivity has been reported in acromegaly, in puberty, or during growth hormone replacement therapy. Children with GHD have a larger tendency to present with hypoglycemia both fasting and induced, possibly due to an alteration in the regulation of counter regulatory hormones and an increase in insulin sensitivity. This susceptibility to hypoglycemia tends to diminish with age, and adults with GHD present with insulin resistance even before growth hormone administration; this could be due to changes in body composition, metabolic responses to growth hormone or to the interaction with sexual hormones. Growth hormone replacement therapy increases lypolysis with an increment in the concentrations of free fatty acids, which could diminish the uptake of glucose into skeletal muscle.[35] Studies using acipimox, a free fatty acid blocker, have confirmed the inverse relation that exists between circulating free fatty acid concentrations and insulin sensitivity in adults with GHD.[36] However, additional follow-up is necessary to evaluate whether insulin sensitivity will continue to worsen as an effect of GH therapy, or this mild increase may instead represent a component of the anabolic process of somatic development.
CONCLUSIONS
The results of the present study demonstrate that GHD in children is associated with a significantly reduced cardiac mass and impairment of lipid profile. GH replacement therapy exerts beneficial effects both on cardiac mass and lipid metabolism by normalizing cardiac size and reducing the AI. On the contrary, an increase in insulin resistance is observed after 1 year of GH treatment.
Recommendations
Children with GH deficiency should have echocardiography to observe the dynamics of the left ventricle functional disorders
Lipid profile monitoring is mandatory in children with GHD.
Limitations of the study
Small sample size
Possible confounders, including the potential effects of body mass index, change in the body composition (increased skeletal muscle), and physical activity
Echocardiography might not be sensitive enough to reveal minimal abnormalities in cardiac performance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med. 2000;133:111–22. doi: 10.7326/0003-4819-133-2-200007180-00010. [DOI] [PubMed] [Google Scholar]
- 2.McCallum RW, Petrie JR, Dominiczak AF, Connell JM. Growth hormone deficiency and vascular risk. Clin Endocrinol (Oxf) 2002;57:11–24. doi: 10.1046/j.1365-2265.2002.01559.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–8. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 4.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107:1562–6. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
- 5.Salomon F, Cuneo RC, Hesp R, Sonksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolismin adults with growth hormone deficiency. N Engl J Med. 1989;321 doi: 10.1056/NEJM198912283212605. 1797803. [DOI] [PubMed] [Google Scholar]
- 6.Colao A, Di Somma C, Salerno M, Spinelli L, Orio F, Lombardi G. The cardiovascular risk of GH-Deficient adolescents. Clin Endocrinol Metab. 2002;87:3650–5. doi: 10.1210/jcem.87.8.8777. [DOI] [PubMed] [Google Scholar]
- 7.Feinberg MS, Scheinowitz M, Laron Z. Cardiac dimension and function in patients with childhood onset growth hormone deficiency, before and after growth hormone retreatment in adult age. Am Heart J. 2003;145:549–53. doi: 10.1067/mhj.2003.175. [DOI] [PubMed] [Google Scholar]
- 8.Heuschmann D, Butenandt O, Vogel M. Left ventricular Volume and mass in children on growth hormone therapy compared with untreated children. Eur J Pediatr. 1996;155:77–80. doi: 10.1007/BF02075754. [DOI] [PubMed] [Google Scholar]
- 9.GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary of the GH research society. J Clin Endocrinol Metab. 2000;85:3990–3. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 10.Tanner JM, Goldstein H, Whitehouse RH. Standard for children's height at ages 2 to 9 years allowing for height of parents. Arch Dis Child. 1970;45:755. doi: 10.1136/adc.45.244.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greulich WW, Pyle SI. Stanford, California: Stanford University Press; 1959. Radiographic atlas of skeletal development of the hand and wrist. [Google Scholar]
- 12.Castelli WP. Lipid, risk factors and ischaemic heart disease. Atherosclerosis. 1996;124:S1–9. doi: 10.1016/0021-9150(96)05851-0. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Ranke MB, Blum WF, Bierich JR. Clinical relevance of serum measurements of IGFs and IGF binding proteins. Acta Paediatr Scand Suppl. 1988;347:114–26. [PubMed] [Google Scholar]
- 15.Sahn DJ, De Maria A, Kisslo J, Weyman A. The Committee on M-mode Standardization of the American Society of Echocardiography. Recommendations regarding quantitation in M-mode echocardiography: Results of asurvey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 16.Shulman DL, Root AW, Diamond FB, Bercu BB, Martinez R, Boucek RJ. Effect of 1 year of recombinant human growth hormone (GH) therapy on cardiac mass and function in children with classical GH deficiency. J Clin Endocrinol Metab. 2003;88:4095–9. doi: 10.1210/jc.2003-030030. [DOI] [PubMed] [Google Scholar]
- 17.Salerno M, Esposito V, Spinelli L, Di Somma C, Farina V, Muzzica S, et al. Left ventricular mass and function in children with GH deficiency before and during 12 months GH replacement therapy. Clin Endocrinol. 2004;60:630–6. doi: 10.1111/j.1365-2265.2004.02026.x. [DOI] [PubMed] [Google Scholar]
- 18.Cittadini A, Stromer H, Katz SE, Clark R, Moses AC, Morgan JP, et al. Differential cardiac effects of growth hormone and insulin-like growth factor-1 in the rat. A combined in vivo and in vitro evaluation. Circulation. 1996;93:800–9. doi: 10.1161/01.cir.93.4.800. [DOI] [PubMed] [Google Scholar]
- 19.Radetti G, Crepaz R, Paganini C, Gentili L, Pitscheider W. Medium-term cardiovascular effects of high-dose growth hormone treatment in growth hormone-deficient children. Hormone Res. 1999;52:247–52. doi: 10.1159/000023469. [DOI] [PubMed] [Google Scholar]
- 20.Kuromaru R, Kohno H, Ueyama N, Hassan HM, Honda S, Hara T. Long-term prospective study of body composition and lipid profiles during and after growth hormone (GH) treatment in children with GH deficiency: Gender-specific metabolic effects. J Clin Endocrinol Metab. 1998;83:3890–6. doi: 10.1210/jcem.83.11.5261. [DOI] [PubMed] [Google Scholar]
- 21.Esposito V, Di Biase S, Lettiero T, Labella D, Simeone R, Salerno M. Serum homocysteine concentrations in children with growth hormone (GH) deficiency before and after 12 months GH replacement. Clin Endocrinol (Oxf) 2004;61:607–11. doi: 10.1111/j.1365-2265.2004.02142.x. [DOI] [PubMed] [Google Scholar]
- 22.Van der Sluis IM, Boot AM, Hop WC, De Rijke YB, Krenning EP, de Muinck Keizer-Schrama SM. Long-term effects of growth hormone therapy onbone mineral density, body composition, and serum lipid levels in growth hormone deficient children: A 6-year follow-up study. Horm Res. 2002;58:20714. doi: 10.1159/000066262. [DOI] [PubMed] [Google Scholar]
- 23.Sas T, Mulder P, Hokken-Koelega A. Body composition, blood pressure, and lipid metabolism before and during long term growth hormone (GH) treatment in children with short stature born small for gestational age either with or without GH deficiency. J Clin Endocrinol Metab. 2000;85:3786–92. doi: 10.1210/jcem.85.10.6917. [DOI] [PubMed] [Google Scholar]
- 24.Abdu TA, Neary R, Elhadd TA, Akber M, Clayton RN. Coronary risk in growth hormone deficient hypopituitary adults: Increased predicted risk is due largely to lipid profile abnormalities. Clin Endocrinol (Oxf) 2001;55:209–16. doi: 10.1046/j.1365-2265.2001.01320.x. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Wilson PW. Efficacy of lipid profiles in prediction of coronary disease. Am Heart J. 1992;124:76874. doi: 10.1016/0002-8703(92)90288-7. [DOI] [PubMed] [Google Scholar]
- 26.Rudling M, Parini P, Angelin B. Growth hormone and bile acid synthesis. Key role for the activity of hepatic microsomal cholesterol 7-hydroxylase in the rat. J Clin Invest. 1997;99:2239–45. doi: 10.1172/JCI119398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleeson HK, Souza AH, Gill MS, Wieringa GE, Barretto ES, Barretto-Filho JA, et al. Lipid profiles in untreated severe congenital isolated growth hormone deficiency through the lifespan. Clin Endocrinol. 2002:5789–95. doi: 10.1046/j.1365-2265.2002.01568.x. [DOI] [PubMed] [Google Scholar]
- 28.Gleeson H, Barreto ES, Salvatori R, Costa L, Oliveira CR, Pereira RM, et al. Metabolic effects of growth hormone (GH) replacement in children and adolescents with severe isolated GH deficiency due to a GHRH receptor mutation. Clin Endocrinol. 2007:66466–74. doi: 10.1111/j.1365-2265.2007.02753.x. [DOI] [PubMed] [Google Scholar]
- 29.Husbands S, Ong KK, Gilbert J, Wass JA, Dunger DB. Increased insulinsensitivity in young, growth hormone deficient children. Clin Endocrinol. 2001;55:87–92. doi: 10.1046/j.1365-2265.2001.01298.x. [DOI] [PubMed] [Google Scholar]
- 30.Cañete R, Valle M, Martos R, Sánchez-Carrión A, Cañete MD, van Donkelaar EL. Short-term effects of GH treatment on coagulation, fibrinolysis, inflammation biomarkers, and insulin resistance status in prepubertal children with GH deficiency. Eur J Endocrinol. 2012;167:255–60. doi: 10.1530/EJE-12-0214. [DOI] [PubMed] [Google Scholar]
- 31.Giavoli C, Porretti S, Ronchi CL, Cappiello V, Ferrante E, Orsi E, et al. Long-term monitoring of insulin sensitivity in growth hormone-deficient adults on substitutive recombinant human growth hormone therapy. Metabolism. 2004;53:7403. doi: 10.1016/j.metabol.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Heptulla RA, Boulware SD, Caprio S, Silver D, Sherwin RS, Tamborlane WV. Decreased insulin sensitivity and compensatory hyperinsulinemia after hormone treatment in children with short stature. J Clin Endocrinol Metab. 1997;82:3234–8. doi: 10.1210/jcem.82.10.4302. [DOI] [PubMed] [Google Scholar]
- 33.Walker J, Chaussain JL, Bougneres PF. Growth hormone treatment of children with short stature increases insulin secretion but does not impair glucose disposal. J Clin Endocrinol Metab. 1989;69:253–8. doi: 10.1210/jcem-69-2-253. [DOI] [PubMed] [Google Scholar]
- 34.Cutfield WS, Wilton P, Bennmarker H, Albertsson-Wikland K, Chatelain P, Ranke MB, et al. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet. 2000;355:610–13. doi: 10.1016/S0140-6736(99)04055-6. [DOI] [PubMed] [Google Scholar]
- 35.Husbands S, Ong K, Gilbert J, Wass J, Dunger D. Increased insulin sensitivity in young, growth hormone deficient children. Clin Endocrinol. 2001;55:87–92. doi: 10.1046/j.1365-2265.2001.01298.x. [DOI] [PubMed] [Google Scholar]
- 36.Bramnert M, Segerlantz M, Luarila E, Daugaard JR, Manhe P, Groop L. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab. 2003:1455–63. doi: 10.1210/jc.2002-020542. [DOI] [PubMed] [Google Scholar]


