Abstract
Aim:
We aimed to compare the International Association of Diabetes and Pregnancy Study Groups (IADPSG) and the World Health Organization (WHO) criteria to diagnose gestational diabetes mellitus (GDM) in Chennai, India.
Materials and Methods:
We reviewed the retrospective data of 1351 pregnant women who underwent screening for GDM at four selected diabetes centers at Chennai (three private and one government). All women underwent an oral glucose tolerance test using 75g glucose load and fasting, 1-h, and 2-h samples were collected. The IADPSG and WHO criteria were compared for diagnosis of GDM.
Results:
A total of 839 women had GDM by either the IADPSG or the WHO criteria, of whom the IADPSG criteria identified 699 and the WHO criteria also identified 699 women as having GDM. However, only 599/839 women (66.6%) were identified by both criteria. Thus, 140/839 women (16.7%) were missed by both the IADPSG and the WHO criteria. 687/699 (98.2%) of the women with GDM were identified by the WHO criteria. In contrast, each value of IADPSG criteria i.e., fasting, 1 h, and 2 h identified only 12.5%, 14%, and 22%, respectively.
Conclusions:
A single WHO cut-point of 2 h > 140 mg/dl appears to be suitable for large-scale screening for GDM in India and other developing countries.
Keywords: Asian Indians, gestational diabetes, international association of diabetes and pregnancy study groups, south indians, world health organization
INTRODUCTION
Along with the rising tide of the current epidemic of diabetes in India,[1,2] the prevalence of gestational diabetes mellitus (GDM) is also rising in India.[3] However, the diagnosis of GDM has always been beset with problems related to differing diagnostic criteria with conflicting evidence regarding the maternal and fetal outcomes.[4]
Till recently, the GDM diagnostic criteria proposed by World Health Organization (WHO)[5] or the American Diabetes Association (ADA)[6] were followed in most countries. Though the WHO recommendation was not based on studies with maternal and fetal outcomes, the 2-h cut-off value of > 140 mg/dl for diagnosis of GDM was found to predict the neonatal outcomes in a fairly robust manner.[7,8,9,10,11] Recently, the International Association of Diabetes and pregnancy study groups (IADPSG) based on the hyperglycemia and adverse pregnancy outcome study has introduced new GDM criteria in an attempt to unify the GDM criteria throughout the world.[12] The IADPSG criteria require three samples i.e., fasting, 1 h, and 2 h after 75 g glucose, whereas the WHO criteria need two samples namely the fasting and 2 h, although in practice, only the 2 h is used.[13] In this paper, we have applied the WHO and the IADPSG cut-off values and compared the two criteria with respect to their impact on diagnosing GDM among pregnant women seen at four diabetic clinics in Chennai city in Southern India.
MATERIALS AND METHODS
We reviewed the retrospective data of 1351 pregnant women who underwent screening for GDM at four selected diabetes centers at Chennai (three private and one government). At diabetes centers, pregnant women with a high index of suspension with elevated glucose levels are referred for a confirmation of GDM. Hence, the prevalence of GDM at such center would be very high and hence they do not reflect the prevalence of GDM in the community. All women underwent an oral glucose tolerance test (OGTT) using 75 g glucose load and fasting, 1-h, and 2-h samples were collected. The IADPSG and WHO criteria were compared for diagnosis of GDM. According to the IADPSG criteria, any one of the following criteria was used for diagnosis of GDM, i.e., fasting ≥ 92 mg/dl (5.1 mmol/L), 1 h ≥ 180 mg/dl (10.0 mmol/L), or 2 h ≥ 153 mg/dl (8.5 mmol/L). According to the WHO criteria, either fasting ≥ 126 mg/dl (7.0 mmol/L) or 2-h value ≥ 140 mg/dl (7.8 mmol/L) was classified as GDM.
RESULTS
Out of a total of 1351 pregnant women who underwent screening for GDM at four diabetes clinics in Chennai, 839 women were diagnosed to have GDM either by the IADPSG or by the WHO criteria.
The IADPSG criteria identified 699/839 (83.3%) of the total number of women classified as GDM. The WHO criteria, again, identified 699/839 (83.3%) of the total number of women with GDM. However, as shown in the Venn diagram [Figure 1], only 559 women of the total 839 women with GDM (66.6%) were identified by both the IADPSG and WHO criteria. Thus, 140/839 (16.7%) of the GDM women would have been missed if IADPSG criteria alone were used. Conversely, 140/839 (16.6%) of the GDM women would have been missed if WHO criteria alone was used.
Figure 1.
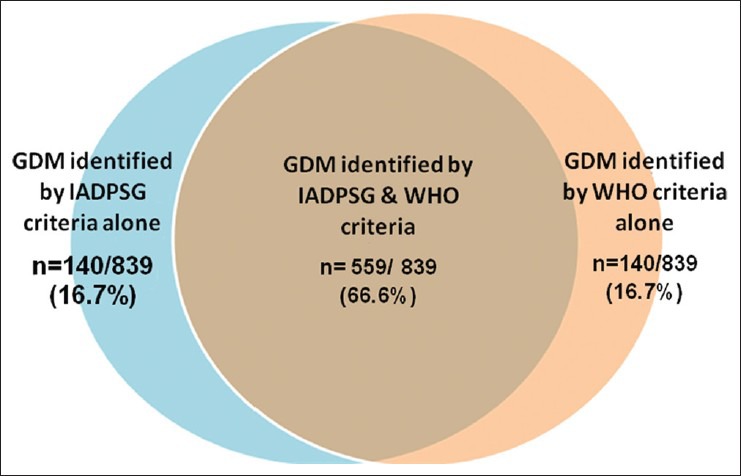
Venn diagram showing the gestational diabetes cases identified by both international association of diabetes and pregnancy study Groups and World Health Organization criteria and by either criteria
We next compared the usefulness of the WHO 2-h criteria alone in comparison to the full WHO criteria namely 2 h and fasting WHO criteria and the results are shown in Table 1. Use of the WHO 2-h criteria was found to identify 687/699 (98.2%) of the GDM cases identified by the full WHO criteria.
Table 1.
Comparison of the components of the international association of diabetes and pregnancy study groups and world health organization criteria to identify gestational diabetes mellitus
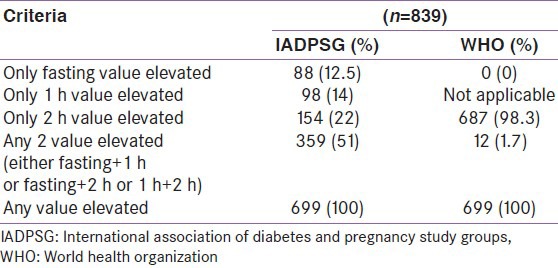
Table 1 also compares the percentage of GDM women identified by the fasting, 1 h and 2 h IADPSG compared to the full IADPSG criteria i.e., any 1 elevated value.
It can be seen that in contrast to the 2-h WHO criteria where one value could pick up the majority (>98%) of GDM cases, each value of the IADPSG criteria identified much lower percentages of GDM cases compared to using all three values. Using the IADPSG criteria, the fasting value alone identified 12.5%, 1-h alone identified 14% and the 2-h alone identified 22% cases and using any two values identified only 51% of the GDM cases. Hence, it is clear that all three values are needed to identify GDM cases by the IADPSG criteria.
Of the 88 women with fasting value alone elevated according to the IADPSG criteria, only 30 (34%) were picked up by WHO 2-h value. Among the 98 individuals who had elevated 1-h value on IADPSG, only 47 (48%) were picked up by WHO 2-h value.
Comparing the 2-h cut-points of 140 mg/dl (WHO) and 153 mg/dl (IADPSG), it is seen that 113 women had a 2-h cut-point between 140 and 153 mg/dl. These 113 would have been missed using the higher 2-h IADPSG cut-point.
DISCUSSION
This study shows that the number of GDM cases identified at four selected diabetes centers in Chennai is the same by WHO criteria and the IADPSG criteria. However, only 66.6% of the GDM cases identified by the WHO and IADPSG criteria are the same individuals, whereas 16.7% each of the GDM women identified by IADPSG and WHO criteria are different individuals.
The WHO first proposed criteria for GDM using a 75 g OGTT in the 1980s.[14,15] In its technical report published in 1994, it defined GDM as diabetes mellitus (DM) first recognized during pregnancy, and gestational impaired glucose tolerance (GIGT) as impaired glucose tolerance (IGT) first recognized during pregnancy.[16] In 1998, WHO recommended new criteria.[5,17] With regard to GDM, pregnant women who met the WHO criteria for DM or IGT were classified as having GDM and, therefore, the term GIGT disappeared. Some studies have been published taking fasting plasma glucose (FPG) >126 mg/dl as the criteria for GDM.[18] However, the more recent studies have altogether ignored the FPG criteria and have used only the 2-h > 140 mg/dl criteria of the WHO.[3,13] When the ADA lowered the FPG to 100 mg/dl from the previous 110 mg/dl for diagnosis of impaired fasting glucose in non-pregnant adults, the FPG level of 126 mg/dl in pregnancy started looking too high and most people just chose to ignore the FPG level for the diagnosis of GDM. However, till date, there is no official recommendation from WHO to drop FPG criteria and to follow only the 2-h value of 140 mg/dl.
It appears an anomaly that in the WHO criteria, the fasting cut-off had been set at 126 mg/dl which is diagnostic of diabetes in non-pregnant adults, whereas the 2-h cut-off was set at 140 mg/dl, which is the diagnostic cut-point for IGT in non-pregnant adults. Probably because of this inherent contradiction in the diagnostic criteria, the fasting values in the WHO criteria are not particularly useful to diagnose GDM and this might explain why the WHO 2-h value alone picked up over 98% of all cases diagnosed by both fasting and 2-h WHO criteria in this study. Another point to be noted is that if a pregnant woman has a FPG ≥ 126 mg/dl, it is considered overt diabetes complicating pregnancy, and not as GDM, by the IADPSG criteria.[8,12]
Another issue of concern is whether too many women would get diagnosed as GDM because of the low FPG cut-off in the IADPSG criteria. Indeed, of the 88 women who were diagnosed as GDM by virtue of their FPG abnormality alone using IADPSG criteria, only 30 (34%) were classified as GDM by the WHO criteria. A similar comparison with those with GDM according to the IADPSG 1-h cut-off value showed that only 47/98 (48%) had GDM by WHO criteria. It is thus possible that by reducing the FPG cut-point to 92 mg/dl, we could be over-diagnosing GDM in normal pregnant women.[19] This could lead to overloading of the health systems in many countries.[20,21]
We have earlier reported that the sensitivity of the 2-h value in the glucose tolerance test (GTT) is much higher than the fasting plasma glucose among non-pregnant Indian adults.[22] Thus, it is reasonable to assume that since the IADPSG has raised the 2-h value in the IADPSG to 153 mg/dl, many cases of GDM could be missed.
One of the limitations of the study is that with our present data, we cannot conclude whether IADPSG or WHO criteria is better for Indian pregnant women as we do not have data on the maternal and fetal outcomes. In the absence of the outcome data, which however, was beyond the purview of the study, it was not possible to comment on the suitability of diagnosing GDM by either of the two criteria in this population. Nonetheless, this study compared the ease of use of two criteria in the population studied. Future studies should compare the outcomes of the GDM cases diagnosed by IADPSG and WHO criteria as this would provide the final answer as to which criteria is more suitable for Indians.
The second limitation is that as the cases were selected from diabetes centers where women had been referred with suspected diabetes, this study cannot be used to study the prevalence rates of GDM. This should be done in a maternity clinic or a general population where the entire population of pregnant women is screened to study the prevalence rates of GDM. This study however reflects the usefulness of the WHO and IADPSG criteria at a diabetes centers.
However, the strength of the study is that it is the first to our knowledge, to directly compare the IADPSG and the WHO criteria, especially in an Asian Indian population. Another strength is the relatively large numbers of subjects studied.
In summary, the WHO 2-h criteria of > 140 mg/dl alone appears to be sufficient to diagnose GDM, as it picks up the majority of GDM cases diagnosed by both the whole WHO criteria as well as the same number of cases as the three sample IADPSG criteria. This could have great benefit especially in rural areas in India where obtaining three blood samples as required by the IADPSG criteria, could be a major challenge.
ACKNOWLEDGEMENT
The following centers contributed data for the study: Swamy Diabetes and Prashanthi Infertility Research Center, Chennai, Geetika Diabetes Center, Chennai and the Institute of Diabetology, Madras Medical College and Government General Hospital, Chennai.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V, Deepa M, Deepa R, Shanthirani CS, Farooq S, Ganesan A, et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India: The Chennai Urban Rural Epidemiology Study (CURES-17) Diabetologia. 2006;49:1175–8. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 3.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu): A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 4.Ferrara A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care. 2007;30:S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 7.Deerochanawong C, Putiyanun C, Wongsuryrat M, Serirat S, Jinayon P. Comparison of national diabetes data group and World Health Organization criteria for detecting gestational diabetes mellitus. Diabetologia. 1996;39:1070–3. doi: 10.1007/BF00400656. [DOI] [PubMed] [Google Scholar]
- 8.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes: A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt MI, Duncan BB, Reichelt AJ, Branchtein L, Matos MC, Costa e Forti A, et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. 2001;24:1151–5. doi: 10.2337/diacare.24.7.1151. [DOI] [PubMed] [Google Scholar]
- 10.Gayle C, Germain S, Marsh MS, Rajasingham D, Brackenridge A, Carroll P, et al. Comparing pregnancy outcomes for intensive versus routine antenatal treatment of GDM based on a 75 g OGTT 2-h blood glucose (>140 mg/dl) Diabetologia. 2010;53:S435. [Google Scholar]
- 11.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus (GDM) and its outcomes in Jammu region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 12.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A. Gestational diabetes mellitus in India. J Assoc Physicians India. 2004;52:707–11. [PubMed] [Google Scholar]
- 14.WHO expert committee on diabetes mellitus: Second report. World Health Organ Tech Rep Ser. 1980;646:1–80. [PubMed] [Google Scholar]
- 15.Report of a WHO Study Group. Technical Report Series, no. 727. Geneva: World Health Organization; 1985. World Health Organization. Diabetes mellitus. [PubMed] [Google Scholar]
- 16.World Health Organization. Prevention of diabetes mellitus. World Health Organ Tech Rep Ser. 1994;844:11–35. [PubMed] [Google Scholar]
- 17.Report of a WHO consultation. Geneva: WHO Department of Noncommunicable Disease Surveillance; 1999. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. [Google Scholar]
- 18.Sugaya A, Sugiyama T, Nagata M, Toyoda N. Comparison of the validity of the criteria for gestational diabetes mellitus by WHO and by the Japan Society of Obstetrics and Gynecology by the outcomes of pregnancy. Diabetes Res Clin Pract. 2000;50:57–63. doi: 10.1016/s0168-8227(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Muñoz E, Parra A, Castillo-Mora A, Ortega-González C. Effect of the diagnostic criteria of the international association of diabetes and pregnancy study groups on the prevalence of gestational diabetes mellitus in urban Mexican women: A cross-sectional study. Endocr Pract. 2012;18:146–51. doi: 10.4158/EP11167.OR. [DOI] [PubMed] [Google Scholar]
- 20.Bodmer-Roy S, Morin L, Cousineau J, Rey E. Pregnancy outcomes in women with and without gestational diabetes mellitus according to the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstet Gynecol. 2012;120:746–52. doi: 10.1097/AOG.0b013e31826994ec. [DOI] [PubMed] [Google Scholar]
- 21.Kendrick JM. Screening and diagnosing gestational diabetes mellitus revisited: Implications from HAPO. J Perinat Neonatal Nurs. 2011;25:226–32. doi: 10.1097/JPN.0b013e318222dded. [DOI] [PubMed] [Google Scholar]
- 22.Deepa R, Shanthi Rani S, Premalatha G, Mohan V. Comparison of ADA 1997 and WHO 1985 criteria for diabetes in south Indians: The Chennai Urban Population Study. American Diabetes Association. Diabet Med. 2000;17:872–4. doi: 10.1046/j.1464-5491.2000.00385.x. [DOI] [PubMed] [Google Scholar]


