Abstract
Remote sensing technique is useful for monitoring large crop area at a single time point, which is otherwise not possible by visual observation alone. Yellow mosaic disease (YMD) is a serious constraint in soybean production in India. However, hardly any basic information is available for monitoring YMD by remote sensing. Present study examines spectral reflectance of soybean leaves due to Mungbean yellow mosaic India virus (MYMIV) infection in order to identify YMD sensitive spectral ratio or reflectance. Spectral reflectance measurement indicated significant (p < 0.001) change in reflectance in the infected soybean canopy as compared to the healthy one. In the infected canopy, reflectance increased in visible region and decreased in near infra-red region of spectrum. Reflectance sensitivity analysis indicated wavelength ~642, ~686 and ~750 nm were sensitive to YMD infection. Whereas, in yellow leaves induced due to nitrogen deficiency, the sensitive wavelength was ~589 nm. Due to viral infection, a shift occurred in red and infra-red slope (called red edge) on the left in comparison to healthy one. Red edge shift was a good indicator to discriminate yellow mosaic as chlorophyll gets degraded due to MYMIV infection. Correlation of reflectance at 688 nm (R688) and spectral reflectance ratio at 750 and 445 nm (R750/R445) with the weighted mosaic index indicated that detection of yellow mosaic is possible based on these sensitive bands. Our study for the first time identifies the yellow mosaic sensitive band as R688 and R750/R445, which could be utilized to scan satellite data for monitoring YMD affected soybean cropping regions.
Keywords: Soybean yellow mosaic, Mungbean yellow India mosaic virus, Spectral indices, Red edge, Remote sensing
Introduction
Soybean is one of the most important grain legumes cultivated worldwide. In the Indian subcontinent, soybean production is seriously affected due to yellow mosaic disease (YMD). Two begomovirus species known to cause YMD of soybean are Mungbean yellow mosaic India virus (MYMIV) and Mungbean yellow mosaic virus [10, 18, 36]. The affected plants initially develop yellow specks followed by bright yellow mosaic on leaves. The leaves of severely affected plants turn completely yellow and eventually become almost unproductive. The YMD of soybean spreads rapidly by whitefly (Bemisia tabaci) and resulting into an epidemic situation. The yield loss due to YMD in soybean has been estimated in the tune of 30–70 % [19]. Ability to identify diseases in early infection stage and to accurately quantify the severity of infection is crucial for plant disease assessment and management [37]. Assessment of extent of disease occurrence is essential for estimating economic impact and application of control measures. Traditionally proportion of diseases and pest damage in a large plant population is assessed based on visual observation of symptomatic plants. However, there are several limitations in assessment of viral diseases with traditional approach that it is often time consuming, labour intensive and erroneous as induction of similar symptoms due to several other reasons [3, 6]. Remote sensing data especially reflectance found to be capable of detecting changes in the biophysical properties of plant and canopy associated with pathogens [13, 21, 22]. Additionally, remote sensing may provide a better means to objectively quantify disease stress than visual assessment methods, and it can be used to repeatedly collect sample measurements non-destructively and non-invasively [21, 25].
Viral infections are known to cause changes in leaf pigments, biochemical components and metabolic alterations in infected leaves [2, 12, 35]. Characteristic changes in reflectance spectrum has been observed due to Grapevine leafroll associated virus-3 in grape [23], Sugarcane yellow leaf virus in sugarcane [8], Potato yellow vein virus infection in potato [7] and viral infection in Nicotiana debneyi [26]. Though, commonly used broadband have been shown to detect differences between healthy and diseased plants [16, 24, 32], but discrimination of healthy plants from those showing mild symptom is not very sharp. However, measurement of reflectance contiguously (hyperspectral remote sensing) as a series of narrow wavelength band provides pertinent information for discrimination of disease and other plant stresses. Use of hyerspectral remote sensing techniques and studies [21, 39] has indicated the immense potentiality of hyperspectral imaging for discrimination of disease and other plant stresses and early their detection [3, 38]. A few studies have demonstrated the feasibility of using remote sensing data for detection of virus diseases like grapevine leaf roll [23], tobacco mosaic [26], sugarcane yellow leaf [8], barley yellow dwarf and wheat streak mosaic [29, 40], potato yellow vein [7] and mungbean yellow mosaic [27]. For remote sensing detection specific spectral reflectance or ratio associated with yellow mosaic infection is required for large scale assessment and monitoring of yellow mosaic disease in soybean field especially for strategic or tactical crop management decision and yield loss prediction. However, such studies are not available with reference to YMD in soybean. Objective of the present investigation was to determine spectral ratio and/or reflectance at a particular wavelength associated with MYMIV infection in soybean for detection of the disease in large area based on remote sensing data.
Materials and methods
Inoculation of soybean plants through whiteflies
The susceptible test plants of soybean cv. JS 335 were raised during July–September on pots containing mixture of compost, garden soil and fertilizer NPK (10:30:30). A naturally infected trifoliate leaf of soybean cv. JS 335 showing typical YMD symptoms was used as a source of inoculation. Presence of MYMIV was confirmed by polymerase chain reaction (PCR) using specific primer (AVI forward: 5′-GCATGATATTGCCCACACAG-3′ and reverse: 5′-TCACGCAGATCGTTCTTCAC-3′) to coat protein (AV1) gene of MYMIV [10, 18, 37]. Whitefly (Bemisia tabaci) culture and inoculation standard protocol [31] was followed. Fifty aviruliferous whiteflies were allowed on the infected soybean leaves for acquisition access period of about 18 h. A group of six pots each containing four test seedlings was placed in an inoculation cage and viruliferous whiteflies were released into those cages for 24 h of inoculation access period. The inoculated plants were then transferred to a greenhouse and maintained for symptoms expression. A group of 30 soybean seedlings were maintained as healthy control in a separate chamber of the greenhouse. The MYMIV infection in the inoculated plants was confirmed by PCR using the same specific primer as mentioned.
Induction of yellowing due to nitrogen deficiency
To induce nitrogen deficiency in soil, sorghum (exhaust crop for nitrogen) was grown in 10 inch diameter earthen pots containing soil:sand mixture (50:50). The pots showing visible nitrogen deficiency in sorghum were selected for sowing susceptible soybean cv. JS 335. The experiment was designed in randomized block fashion with five replicates of pots each containing 20 seedlings. The seedlings were maintained in the glasshouse with a weekly spray 0.01 % imidacloprid to prevent infection.
Field plot experiment for creation of gradient of mosaic severity
The cv. JS 335 susceptible to MYMIV was sown during first week of July 2009–2010 in the IARI experimental field with complete randomized block design. Few plots (4 m × 4 m) were sprayed with water as control. To create various levels of incidence of mosaic symptoms in different plots imidacloprid @0.01 % spraying was given at weekly interval but skipping few plots each time so that gradation of sprays like 10, 9, 8, … 2 and 1 were maintained.
Assessment of yellow mosaic severity and yellowing induced due to nitrogen deficiency
Weighted mosaic index (WMI) was calculated for a group of plants. Selected plants were assessed visually following a modified 0–5 scale, where 0: no symptoms or apparently healthy; 1: initiation of yellow specks; 2: few yellow patches; 3: yellow patches coalescing; 4: larger yellow patches with small green island and 5: completely yellow leaves. Disease grades were ranked according to their relative frequencies [34] and WMI was calculated as [∑(rank × infection type)]/∑rank. Assessment of yellowing of leaves due to nitrogen deficiency was also performed following the similar method but with a modified 0–5 scale, where 0: no yellowing leaves; 1: 1–10 % leaves yellowing; 2: 11–20 % leaves yellowing; 3: 21–50 % leaves yellowing; 4: 51–75 % leaves yellowing; and 5: >75 % yellowing. The weighted yellowing index (WYI) due to deficiency of nitrogen was calculated as described for yellow mosaic.
Measurements of spectral reflectance
ASD spectroradiometer (Field Spec®, Analytical Spectral Devices) was standardized based on the reflectance readings on the BaSO4 coated sheet in sunlight in the open field conditions. Reflectance was measured over 350–2,500 nm wavelength. Infected leaves from the inoculated and non-inoculated plants of nitrogen deficiency were collected and reflectance was measured using probe attached to the spectroradiometer. Reflectance of five leaves (n = 5) was measured on adaxial surface and the average reflectance was considered for spectral analysis. For field readings, a probe system was placed on the canopy with 25° field of view and the average of five readings was considered for spectral analysis.
Analysis of reflectance sensitivity
Reflectance difference was computed by subtracting mean (n = 5) reflectance of healthy leaves from the mean reflectance (n = 5) of MYMIV infected leaves. Reflectance sensitivity to MYMIV infection was computed by dividing the reflectance difference by mean reflectance of healthy leaves. The significance of reflectance sensitivities for infected and healthy leaves was determined by ANOVA and Dunnett’s means test [4].
Spectral derivative and red edge analysis
First derivative of mean reflectance was calculated and appropriate order of polynomial fitting was performed through least squares method [30]. Red edge shifts and shapes of the red peak in the first derivative curve have been studied under various levels of MYMIV in comparison to the nitrogen deficit condition. Wavelength (λre) and amplitude (drre) of the red peak for each infection level were estimated through linear interpolation technique by fitting a second order polynomial equation to the red-infrared slope [9]. Weighted mosaic index (WMI) was explained in relation to the following red edge parameters;
λre the wavelength of this red edge peak, drre the maximum amplitude of the red edge peak in the first derivative reflectance curve and ∑(dr 680–780) sum of the first derivative reflectance amplitudes between 680 and 780 nm.
Development of ratios or indices
Reflectance in particular wavelengths is selected for calculation of ratios or indices. Reflectances at wavelengths 622, 688, 750 nm where significant change occurred due to MYMIV infection and not influenced by chlorophyll content were selected. Each ratio included a waveband in which reflectance changed significantly with infection, and a waveband in which reflectance did not change significantly with infection. Reflectance at wavelength 445 nm was taken as denominator as no change occurred due to infection. Reflectance in a given waveband was assigned the numerator or denominator so that the resultant ratio was greater for infected than healthy leaves. Data used in ratio analyses were limited to visible and infrared spectrum (400–800 nm). Reflectance for estimating MYMIV infection in soybean was R622, R688 and R750 and reflectance ratio R622/R445, R688/R445, R750/R445 were used along with normalized ratios (R622 − R445)/(R622 + R445), (R688 − R445)/(R688 + R445) and (R750 − R445)/(R750 + R445).
Results
Reflectance pattern in MYMIV infected and nitrogen deficient soybean
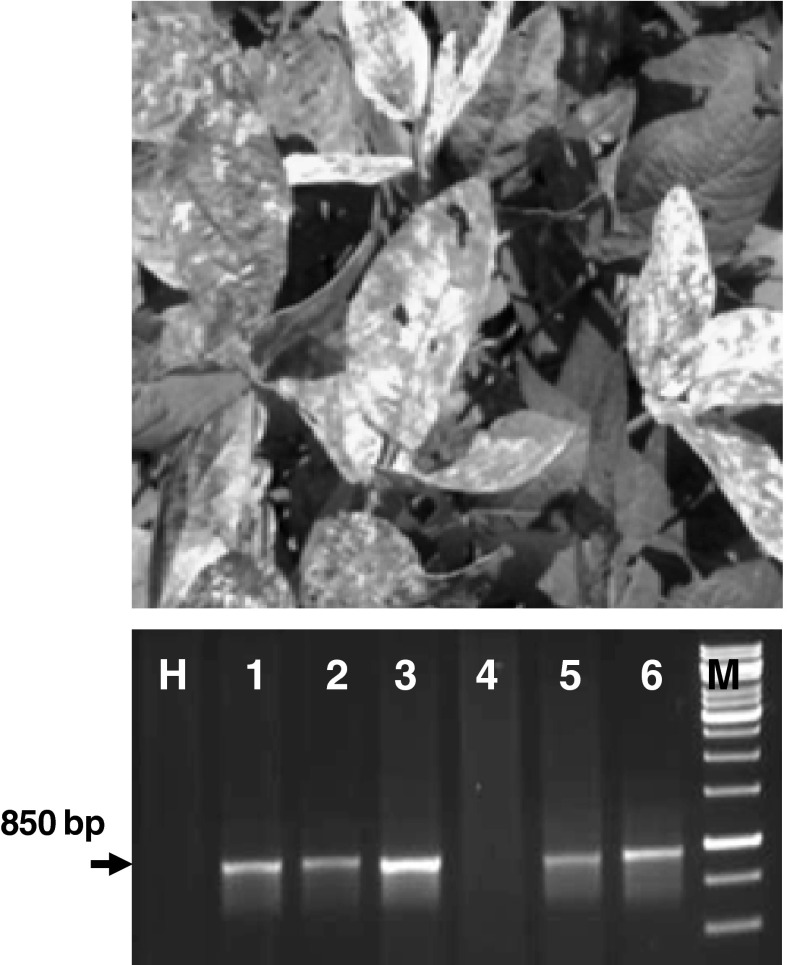
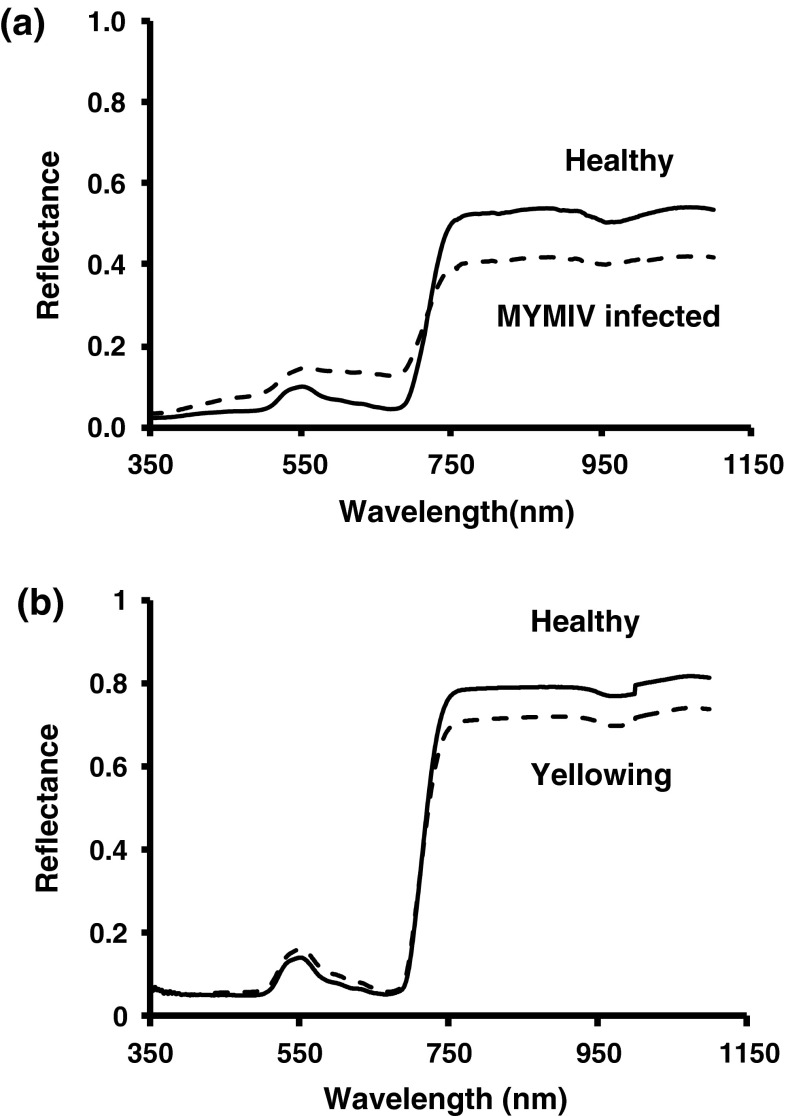
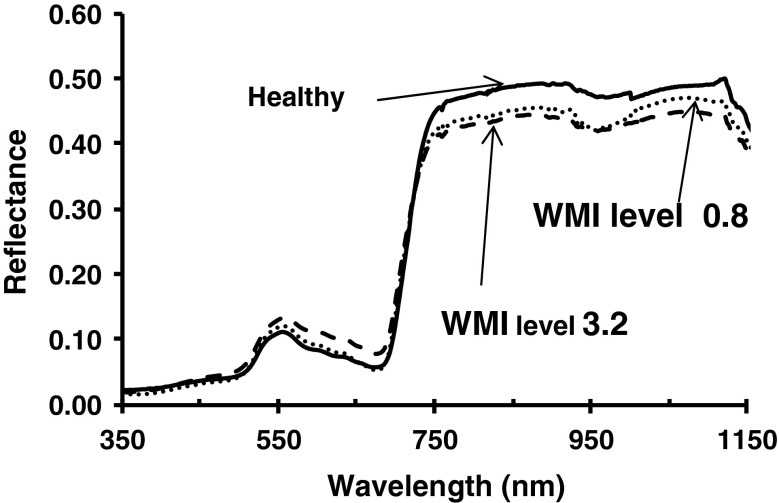
In inoculated plants, symptoms appeared in the form of mild scattered yellow specks or spots were developed at about 20 days post inoculation (dpi). PCR amplification of the desired band of 850 bp in the symptomatic plants has confirmed MYMIV infection (Fig. 1). At 20 dpi, spectral reflectance in infected leaves increased significantly (p < 0.01) in visible (420–696 nm) and decreased in near infra-red region (NIR, 750–1,400 nm) wavelength in comparison to healthy soybean leaves (Fig. 2a). Infected leaves with passage of time progressively turned into yellow and ultimately into bright yellow covering maximum area of the leaves. Plants grown under deficit nitrogen conditions showed uniform yellowing of leaves without mosaic pattern. Reflectance in nitrogen deficiency induced leaves showed different pattern characterized by marginal increase in visible region (607–656 nm) and decrease in NIR region (Fig. 2b). Reflectance increase in visible range and decrease in NIR range was associated with increase in weighted mosaic indices (WMI) (Fig. 3). It was evident that reflectance pattern change in MYMIV infected soybean canopy was due to change in reflectance in visible and NIR wavelengths.
Fig. 1.
Yellow mosaic symptom on soybean leaves, cv JS335 (upper) and genomic DNA amplification based on coat protein AV1 specific primers from infected leaves (H healthy leaves, Lane1, 2, 3, 5 and 6- infected leaves)
Fig. 2.
Spectral reflectance pattern in soybean leaves infected by Mungbean yellow mosaic India virus (MYMIV) through whitefly transmission under artificial conditions and yellowing induced by nitrogen deficiency in pots. Reflectance of a five infected and healthy leaves (n = 5) and b five leaves showing yellowness (n = 5) were measured on adaxial surface and the average reflectance was plotted. The significance of reflectance for infected/yellowness and healthy leaves was determined by ANOVA and Dunnett’s means test
Fig. 3.
Spectral reflectance pattern of weighted mosaic index (WMI) levels of MYMIV infection in soybean created artificially spraying Imidacloprid. WMI calculated as [∑(rank × infection type)]/∑rank for a group of plants graded visually following a modified 0–5 scale and grades were ranked according to their relative frequencies
Reflectance sensitivity in MYMIV infected and nitrogen deficient soybean
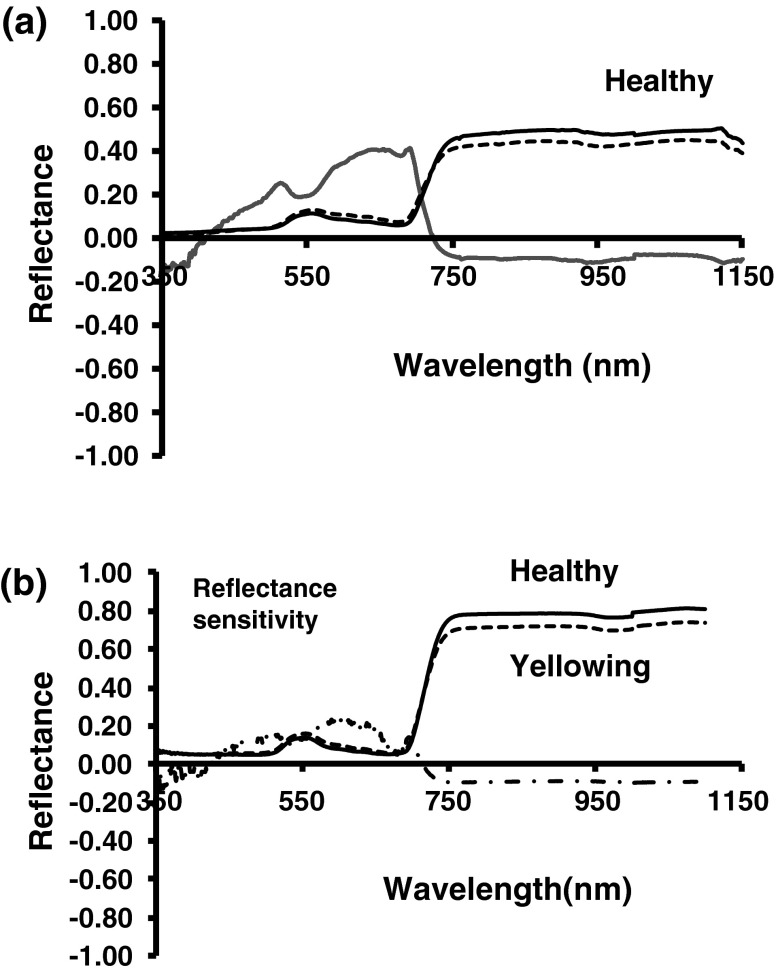
For infected soybean canopy, reflectance sensitivity analysis indicated maximum peak in the wavelength 642 and 688 nm in red region and 750 nm in NIR region (Fig. 4a). Wavelength 420 and 720 nm was the least sensitive wavelength not affected by MYMIV infection. In green and red region (560–691 nm) the sensitivity was significantly higher (p < 0.001). Reflectance sensitivity in nitrogen deficit leaves had significant variation in wavelengths 589 and 750 nm and least variation at 420 and 711 nm (Fig. 4b). It was evident that visible and infra red reflectance responded most consistently to MYMIV infection as well as in nitrogen deficit leaves but differed with respect to wavelength.
Fig. 4.
Reflectance sensitivity in soybean leaves infected by Mungbean yellow mosaic India virus (MYMIV) a yellowing induced by nitrogen deficiency b reflectance sensitivity to MYMIV infection was computed by dividing the reflectance difference by mean reflectance of healthy leaves and reflectance difference was computed by subtracting mean (n = 5) reflectance of healthy leaves from the mean reflectance (n = 5) of MYMIV infected leaves. The significance of reflectance sensitivities for infected and healthy leaves was determined by ANOVA and Dunnett’s means test
First derivative analysis and red edge (red-infra-red slope) features
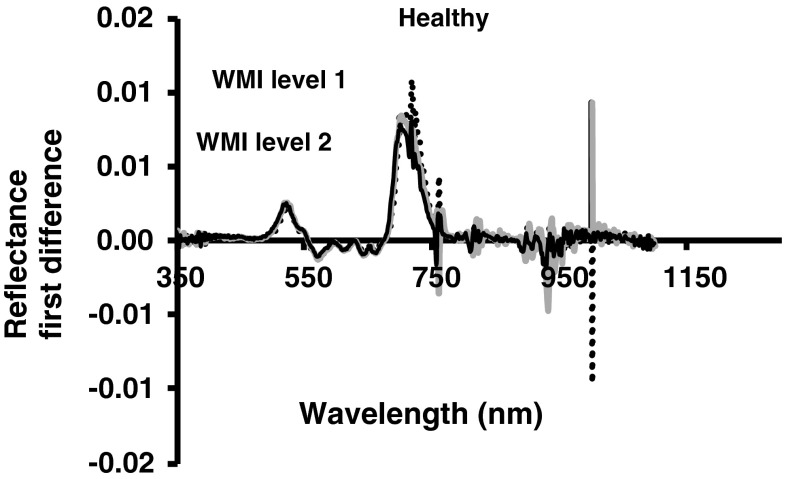
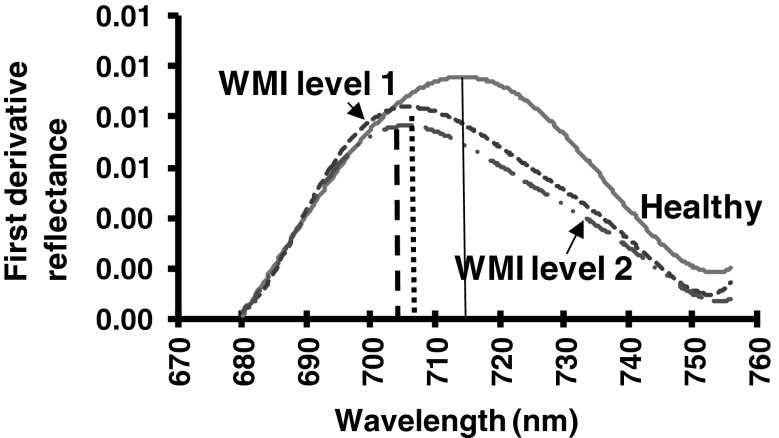
By plotting the relative change in reflectance with respect to the change in wavelength (first derivative) revealed the pattern in the wavelength range between red edge slopes (680–750 nm). There was characteristics change in red edge parameters in this wavelength (Fig. 5). Relationship between weighted mosaic index and red edge parameters (Table 1) were significant (p < 0.067). In healthy soybean leaves, the red-infrared slopes (red edge) showed a shift towards longer wavelengths and increased in amplitude in the red edge peak in the first derivative curve (Fig. 6). Wavelength (λre) of red edge curve for healthy canopy was in around 714–718 nm, whereas it was around 705 to 708 nm in infected plant canopy. Lower red edge peak amplitudes in infected plant were in consonance with their higher mosaic severity. Amplitude (drre) of red edge peak in healthy leaves was 0.009, whereas in infected it is between 0.007 to 0.008. At about 520 and 720 nm peaks in first derivative of infected canopy showed progressive decrease as compared to healthy canopy. In nitrogen deficient canopy red edge position shifted to shorter wavelength. However, no obvious difference was observed for first difference reflectance after 760 nm. Whereas, infected leaves whose chlorophyll content was low have shown shift shorter wavelengths with a decrease in red edge amplitude.
Fig. 5.
First derivative reflectance spectra in soybean leaves showing yellow mosaic disease due to Mungbean yellow mosaic India virus infection along with healthy leaves
Table 1.
Relationships between red edge parameters in the first derivative reflectance curve and weighted mosaic index (WMI) on soybean leaves infected by Mungbean yellow mosaic India virus through whitefly transmission under artificial conditions
| Relationshipsa | R2 | Significance level |
|---|---|---|
| WMI = 714.298 (λre)−0.007 | 0.974 | 0.001 |
| WMI = 1/(1/0.009 + 477.05 dr0.776re) | 0.960 | 0.005 |
| WMI = 0.012 + 0.002 (∑(dr 680–780)) | 0.736 | 0.067 |
aInfected leaves were graded visually following a modified 0–5 scale and grades were ranked according to their relative frequencies and weighted mosaic index (WMI) was calculated as [∑(rank × infection type)]/∑rank for a group of leaves. Wavelength (λre) and amplitude (drre) of the red peak for each infection level were estimated through linear interpolation technique by fitting polynomial equation to the red-infrared slope
Fig. 6.
Red edge peak and amplitude in soybean leaves showing yellow mosaic disease due to Mungbean yellow mosaic India virus infection. Wavelength and amplitude of the red peak for each infection level were estimated through linear interpolation technique by fitting a second order polynomial equation to the red-infrared slope. (Color figure online)
Ratio for detection of MYMIV infection in soybean
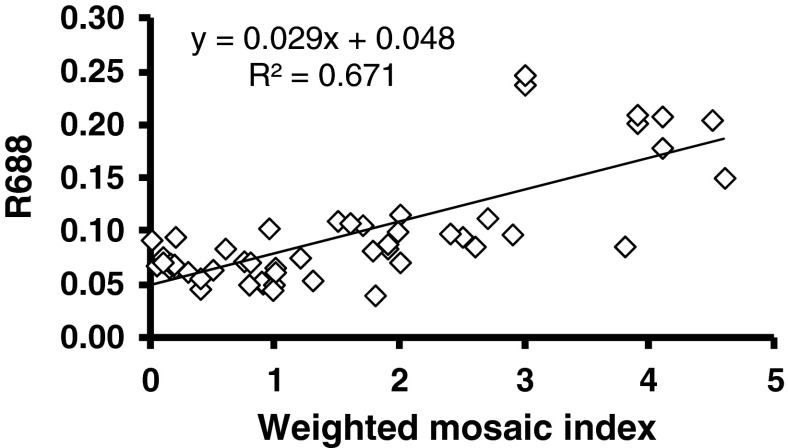
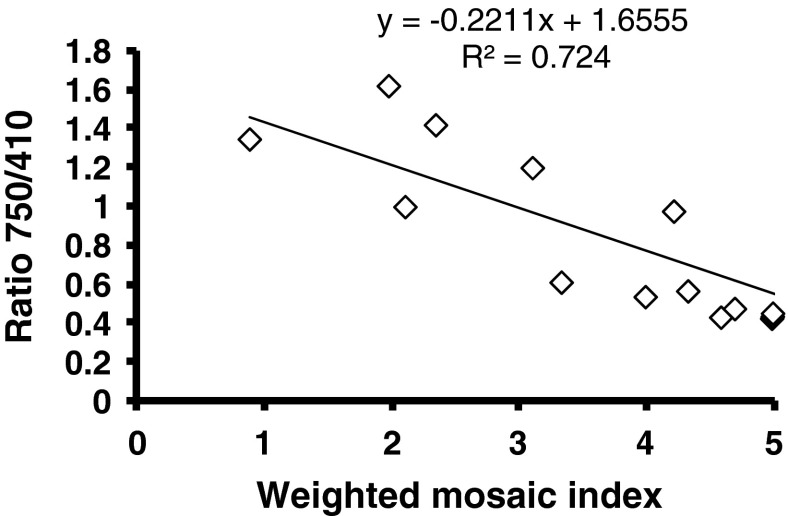
Reflectance at 642, 688 and 750 nm was found to increase as the mosaic severity increased. However, linear regression of reflectance values (R688) with the mosaic severity (MYMIV) were the most significant (p < 0.001) based on slope coefficients and coefficient of determination (Fig. 7). Ratios calculated as R642/R410, R686/R410 and R750/410 did not show relationship with the mosaic severity as the ratios did not contribute to the slope coefficients with mosaic severity. Spectral reflectance ratio R750/R410 has shown significant trend with MYIMV level (Fig. 8). Therefore, spectral reflectance R688 and ratio R750/R410 may be used for sensitive detection and monitoring of yellow mosaic infection in soybean. Spectral ratio R520/R720 has been shown to decrease with increase in relative water content.
Fig. 7.
Yellow mosaic disease severity grade (WMI) in relation to spectral reflectance at 688 nm wavelength. WMI calculated as [∑(rank × infection type)]/∑rank for a group of plants graded visually following a modified 0–5 scale and grades were ranked according to their relative frequencies
Fig. 8.
Yellow mosaic disease severity grade (WMI) in relation to spectral ratio R750/R410. WMI calculated as [∑(rank × infection type)]/∑rank for a group of plants graded visually following a modified 0–5 scale and grades were ranked according to their relative frequencies
Discussion
Characteristics spectral reflectance pattern associated with MYMIV infection in soybean leaf canopy has been identified. Sensitive wavelength for reflectance or ratio specific to MYMIV infection in comparison to healthy canopy is a basic criterion for remote sensing detection especially when large scale disease monitoring is required. Therefore, based on satellite data for larger geographic area, remote sensing detection of MYMIV infection is possible as spectral indices or ratio has been used for satellite remote sensing of wheat streak mosaic [20] as well as late blight in tomato [41].
Spectral reflectance pattern has been characterized as viral infection causes decrease in photosynthesis because of a reduction in the amount of chlorophyll in leaf area per plant [8, 28, 29]. In addition, in the infected tissues soluble nitrogen and carbohydrate levels go down drastically during rapid virus replication [12, 33, 36]. MYMIV infection in bean caused significant metabolic changes such as reduction in the total dry matter of plants, chlorophylls and carotenoids in the leaf tissues [36]. Therefore, increase in reflectance in visible band especially in blue and red region due to reduction of pigments like chlorophyll and carotenoids in soybean is expected and also progressive increase in canopy reflectance with increase in mosaic severity is justified. Nitrogen deficiency expressed as yellowness index of leaves produced different reflectance pattern. Yellowness index is an approximation of spectral second derivative, and provides a measure of the change in shape of the reflectance spectra between the maximum near 550 nm and the minimum near 650 nm [1]. In soybean however, second derivative did not show any significant change. Yellowness is also induced by water-logging as well as mineral deficiencies which have not been considered in the study [15].
MYMIV infection in soybean has shown to decrease reflectance in NIR region. In addition to reduction or degradation of chlorophyll, virus infection very often induces histological changes due to reduction/enlargement of cell size or necrosis [6]. The cytological effects of virus infection have been associated with, hypertrophy of the nucleolus, deposition of electron-dense particles in the nucleus, and accumulation of fibrillar rings in the nucleus [14]. In healthy leaves due to compactness of the cellular structures, total internal reflection in NIR region is normally higher than the leaves under stress [1, 5, 38]. Therefore, comparing the spectrum difference of plants in stress from the healthy one, it is evident that decrease in leaf reflectance in NIR region could be associated with alteration in cellular structure primarily due to destruction of mesophyll cells and loss of air spaces [15].
In healthy leaves reflectance normally remains low because of absorption in red band due to pigments and increase in reflectance in NIR due to total internal reflection in normal cellular structures. This abrupt increase in reflectance between red and NIR band was characterized by taking difference in reflectance and plotting the difference against wavelength. Sharp increase or decrease could be detected by the peaks called as red edge peaks and based on shifts left or right plant stresses are detected (9). In soybean due to MYMIV infection, red edge peak (λre) shift occurred on the left in comparison to healthy plant. Red edge analysis showed that it could also be an indicator for YMD detection. The findings highlighted the need to establish that VIS–NIR spectral regions (rather than the full spectral range) seemed sufficient to detect chlorotic and necrotic leaves caused by MYMIV infection. For fungal diseases it was shown that wavelength range 400–1,100 nm is the most affected region as significant differences in reflectance of winter wheat infected with Septoria tritici in the visible and infrared spectral range [24]. Similarly, in field bean (Vicia faba) spectral reflectance properties in leaf infected by Botrytis fabae were found to be significantly affected by changes in the degree of infection in four spectral bands over the wavelength range 400–1,100 nm [17]. Reflectance sensitivity analysis indicated maximum peak in the wavelength 642 and 688 nm in red region and 750 nm in NIR region. Sensitive bands for yellow mosaic infection in mungbean, however, has been reported to be 505, 510 and 669 nm [27]. Although, for soybean yellow mosaic there were increase in green band but maximum sensitivity was in red band. Comparatively, low difference in green band sensitivity for the same virus in soybean might be due to interaction from the host. For soybean, spectral reflectance at R642 and R688 indicated maximum sensitivity in red band. The band at 669 nm reported in mungbean is also in the range of red band indicating maximum sensitivity of red region to YMD particularly in soybean and mungbean, if not in all legumes. Therefore, for remote sensing detection of YMD in legumes using red band reflectance might give a considerable amount of disease signature both in soybean and mungbean. However, lower slope coefficient values between weighted mosaic severity and the reflectance (R688) or spectral ratio (R750/R410) indicated only qualitative separation between healthy and infected canopy rather than quantitative separation between gradations of mosaic severity. It is expected that spectral indices or ratio would be able to distinguish between healthy and infected soybean field at higher levels of mosaic severity. Therefore, the present study first time has identified yellow mosaic sensitive band as R688 and a spectral ratio R750/R445, which could be utilized for satellite remote sensing of YMD affected areas of soybean.
Acknowledgments
First author is thankful to PG School, IARI, New Delhi for providing support during this thesis work.
References
- 1.Adams ML, Philpot WD, Norvell WA. Yellowing index: an application of spectral second derivatives to estimate chlorosis of leaves in stressed vegetation. Int J Rem Sen. 1999;20:3663–3675. doi: 10.1080/014311699211264. [DOI] [Google Scholar]
- 2.Barón M, Flexas J, DeLucia EH. Photosynthesis responses to biotic stress. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in changing environments. A molecular, physiological and ecological approach. Cambridge: Cambridge Press; 2012. pp. 331–350. [Google Scholar]
- 3.Bravo C, Moshou D, West J, McCartney A, Ramon H. Early disease detection in wheat fields using spectral reflectance. Biosys Eng. 2003;84:37–45. doi: 10.1016/S1537-5110(02)00269-6. [DOI] [Google Scholar]
- 4.Carter GA. Response of leaf spectral reflectance to plant stress. Am J Bot. 1993;80:239–243. doi: 10.2307/2445346. [DOI] [Google Scholar]
- 5.Carter GA, Knaap AK. Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am J Bot. 2001;88:677–684. doi: 10.2307/2657068. [DOI] [PubMed] [Google Scholar]
- 6.Chaerle L, Hagenbeek D, Vanrobaeys X, Van Der Straeten D, Jones, Schofield. Early detection of nutrient and biotic stress in Phaseolus vulgaris. Int J Rem Sens. 2007;28:3479–3492.
- 7.Chavez P, Zorogastua P, Chuquillanqui C, Salazar LF, Mares V, Quiroz R. Assessing potato yellow vein virus (PYVV) infection using remotely sensed data. Int J Pest Manag. 2011;55:251–256. doi: 10.1080/09670870902862685. [DOI] [Google Scholar]
- 8.Grisham MP, Johnson RM, Zimba PV. Detecting sugarcane yellow leaf virus infection in asymptomatic leaves with hyperspectral remote sensing and associated leaf pigment changes. J Virol Methods. 2010;167:140–145. doi: 10.1016/j.jviromet.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Guyot G, Baret F, Major DJ. High spectral resolution: determination of spectral shifts between the red and the near infrared. Int Arch Photogramm Rem Sens. 1988;11:750–760. [Google Scholar]
- 10.Haq QMI, Jyotsna P, Ali A, Malathi VG. Coat protein deletion mutation of Mungbean yellow mosaic India virus (MYMIV) J Plant Biochem Biotechnol. 2011;20:182–189. doi: 10.1007/s13562-011-0044-7. [DOI] [Google Scholar]
- 11.Hatfield JL, Pinter PJ. Remote sensing for crop protection. Crop Prot. 1993;12:403–413. doi: 10.1016/0261-2194(93)90001-Y. [DOI] [Google Scholar]
- 12.Hofius DK, Herbers M, Melzer SA, Omid E, Wolf S, Sonnewald U. Evidence of expression level dependent modulation of carbohydrate status and viral resistance by potato leaf roll virus movement protein in transgenic potato plants. Plant J. 2001;28:529–543. doi: 10.1046/j.1365-313X.2001.01179.x. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JR. Remote sensing of the environment: an earth resources perspective. 2. New Jersey: Pearson Education, Inc.; 2007. pp. 12–56. [Google Scholar]
- 14.Kim KS, Flores EM. Nuclear changes associated with euphorbia mosaic virus transmitted by whiteflies. Phytopathology. 1979;69:980. doi: 10.1094/Phyto-69-980. [DOI] [Google Scholar]
- 15.Krezova D, Iliev I, Yanev T, Kirova E. Assessment of the effect of salinity on the early growth stage of soybean plants (Glycine max L.). Int Conf Recent Adv Space Technol IEEE Proc. 2009;397–402.
- 16.Lorenzen B, Jensen A. Change in leaf spectral properties induces in barley by cereal powdery mildew. Rem Sens Environ. 1989;27:201–209. doi: 10.1016/0034-4257(89)90018-7. [DOI] [Google Scholar]
- 17.Malthus TJ, Madeira AC. High resolution spectroradiometry: spectral reflectance of field beans infected by Botrytis fabae. Rem Sens Environ. 1993;45:107–116. doi: 10.1016/0034-4257(93)90086-D. [DOI] [Google Scholar]
- 18.Mandal B, Varma A, Malathi VG. Systemic infection of Vigna mungo using the cloned DNAs of the blackgram isolate of Mungbean yellow mosaic geminivirus through agroinoculation and transmission of the progeny virus by whiteflies. J Phytopathol. 2008;145:505–510. doi: 10.1111/j.1439-0434.1997.tb00358.x. [DOI] [Google Scholar]
- 19.Masuda T, Goldsmith PD. World soybean production: area harvested, yield, and long term projections. Int Food Agribus Manag Rev. 2009;12:143–162. [Google Scholar]
- 20.Mirik M, Jones DC, Price JA, Workneh F, Ansley RJ, Rush CM. Satellite remote sensing of wheat infected by wheat streak mosaic virus. Plant Dis. 2011;95:4–12. doi: 10.1094/PDIS-04-10-0256. [DOI] [PubMed] [Google Scholar]
- 21.Moran S, Inoues Y, Barnes EM. Opportunities and limitation for image-based remote sensing in precision farming. Rem Sens Environ. 1997;61:319–346. doi: 10.1016/S0034-4257(97)00045-X. [DOI] [Google Scholar]
- 22.Moshou D, Bravo C, Oberti R, West J, Bodria L, McCartney A, Ramon H. Plant disease detection based on data fusion of hyper-spectral and multi-spectral fluorescence imaging using Kohonen maps. Real Time Imaging. 2005;11:75–83. doi: 10.1016/j.rti.2005.03.003. [DOI] [Google Scholar]
- 23.Naido RA, Perry EM, Pierce FJ, Mekuria T. The potential spectral reflectance techniques for the detection of Grapevine leafroll-associated virus-3 in two red-berried wine grape cultivars. Comp Electron Agric. 2009;66:38–45. doi: 10.1016/j.compag.2008.11.007. [DOI] [Google Scholar]
- 24.Nicolas H. Using remote sensing to determine of the date of a fungicide application on winter wheat. Crop Prot. 2004;23:853–863. doi: 10.1016/j.cropro.2004.01.008. [DOI] [Google Scholar]
- 25.Nillson HE. Remote sensing and image analysis in plant pathology. Annu Rev Phytopathol. 1995;15:489–527. doi: 10.1146/annurev.py.33.090195.002421. [DOI] [PubMed] [Google Scholar]
- 26.Polischuk VP, Shadchina TM, Kampanetz TI. Changes in reflectance spectrum characteristic of Nicotiana debneyi plant under the influence of viral infection. Arch Phytopathol Plant Prot. 1997;31:115–119. doi: 10.1080/03235409709383221. [DOI] [Google Scholar]
- 27.Prabhakar M, Prasad YG, Desai S, Thirupathi M, Gopika K, Rao G, Venkateswarlu RB. Hyperspectral remote sensing of yellow mosaic severity and associated pigment losses in Vigna mungo using multinomial logistic regression models. Crop Prot. 2013;45:132–140. doi: 10.1016/j.cropro.2012.12.003. [DOI] [Google Scholar]
- 28.Ram RS, Chenelu VV, Sastry LVS. Metabolic changes in blackgram infected with mung yellow mosaic virus. Indian Phytopathol. 1984;88:446–449. [Google Scholar]
- 29.Riedell WE, Osborne SL, Langham MA, Hesler LS. Remote sensing of barley yellow dwarf and wheat streak diseases in winter wheat canopies. Proc Int Conf Precis Agric. 2003;6:160–166. [Google Scholar]
- 30.Savitzky A, Golay MJE. Smoothing and differentiation of data by simplified least squares procedures. Ann Chem. 1964;36:1627–1639. doi: 10.1021/ac60214a047. [DOI] [Google Scholar]
- 31.Senanayake DMJB, Varma A, Mandal B. Virus–vector relationships, host range, detection and sequence comparison of chilli leaf curl virus associated with an epidemic of leaf curl disease of chilli in Jodhpur, India. J Phytopathol. 2012;160:146–155. doi: 10.1111/j.1439-0434.2011.01876.x. [DOI] [Google Scholar]
- 32.Sharp EL, Perry CR, Scharen AL. Monitoring cereal rust development with a spectral radiometer. Phytopathology. 1985;75:936–939. doi: 10.1094/Phyto-75-936. [DOI] [Google Scholar]
- 33.Shivaparasad PV, Akbergenov R, Trinks R, Rajeswaran R, Veluthambi K, Hohn T, Pooggin MM. Promoters, transcripts and regulatory proteins of Mungbean yellow mosaic geminivirus. J Virol. 2005;79:8149–8163. doi: 10.1128/JVI.79.13.8149-8163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steffenson BJ, Wilcoxson RD, Roelfs AP. Resistance of barley to Puccinia graminis f. sp. tritici and Puccinia graminis f. sp. secalis. Phytopathology. 1985;75:1108–1111. doi: 10.1094/Phyto-75-1108. [DOI] [Google Scholar]
- 35.Suarez L, Zarco-Tejada PJ, Sepulcre-Canto O, Miller JR, Jimenez-Munoz JC, Sobrino J. Assessing canopy PRI for water stress detection with diurnal airborne imagery. Rem Sens Environ. 2008;112:560–575. doi: 10.1016/j.rse.2007.05.009. [DOI] [Google Scholar]
- 36.Szczepanski M, Redolfi P. Changes in the proteins of bean leaves infected with tobacco necrosis of Alfalfa mosaic viruses. J Phytopathol. 2008;113:57–65. doi: 10.1111/j.1439-0434.1985.tb00826.x. [DOI] [Google Scholar]
- 37.Usharani KS, Surendranath B, Haq QMR, Malathi VG. Yellow mosaic virus infecting soybean in northern India is distinct from the species infecting soybean in southern and western India. Cur Sci. 2004;86:845–850. [Google Scholar]
- 38.West JS, Bravo C, Oberti R, Lemaire D, Moshou D, McCartney HA. The potential of optical canopy measurement for targeted control of field crop diseases. Annu Rev Phytopathol. 2003;41:593–614. doi: 10.1146/annurev.phyto.41.121702.103726. [DOI] [PubMed] [Google Scholar]
- 39.Workneh F, Jones DC, Rush CM. Quantifying wheat yield across the field as a function of wheat streak mosaic intensity: a state space approach. Phytopathology. 2009;99:432–440. doi: 10.1094/PHYTO-99-4-0432. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf BL, Yong H. Application of hyperspectral imaging sensor to differentiate between the moisture and reflectance of healthy and infected tobacco leaves. Afr J Agric Res. 2011;6:6267–6280. [Google Scholar]
- 41.Zhang M, Qin Z, Liu X, Ustin SL. Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. Int J Appl Earth Obs Geoinf. 2003;4:295–310. doi: 10.1016/S0303-2434(03)00008-4. [DOI] [Google Scholar]