Abstract
Influenza is a serious respiratory illness which can be debilitating and cause complications that lead to hospitalization and death. Although influenza vaccine can prevent influenza virus infection, the only therapeutic options to treat influenza virus infection are antiviral agents. Given temporal and geographic changes and the shifts in antiviral drug resistance among influenza viruses, it is time to consider natural antiviral agents against influenza virus. Jatropha curcas is known for various medicinal uses. Its antimicrobial, anti-cancer and anti-HIV activity has been well recognized. Because of its broad-spectrum activity, we investigated aqueous and methanol leaf extracts for cytotoxicity and its potential to inhibit hemagglutinin protein of influenza virus. The bioactive compounds from leaf extracts were characterized by high-performance thinlayer chromatography which revealed the presence of major phytochemicals including flavonoids, saponins and tannins. The cytotoxic concentration 50 for aqueous and methanol extracts were determined using trypan blue dye exclusion assay. Inhibition of hemagglutinin protein was assessed using minimal cytotoxic concentrations of the extracts and 102.5 TCID50 (64 HA titre) of the Influenza A (H1N1) virus with different exposure studies using hemagglutination assay. Aqueous and methanol extracts were found to be non toxic to Madin darby canine kidney cells below concentration of 15.57 and 33.62 mg/mL for respectively. Inhibition of hemagglutinin was studied using reducing hemagglutination titre which confirmed that the J. curcas extracts have direct effect on the process of virus adsorption leading to its inhibition. Our results provide the information which shows the potential of Jatropha extracts in the treatment of influenza A (H1N1) virus infection. With an established reduced toxicity and prevention of infection by inhibiting hemagglutinin protein, these extracts and its derivatives may be further developed as broad spectrum anti-influenza drugs for prevention and treatment of infections by different types of influenza viruses with further mechanistic studies on anti-influenza.
Keywords: Influenza, Antiviral, Jatropha curcas, Cytotoxicity, Hemagglutination
Introduction
Influenza is historically known to be a scourge to mankind, which still continues. The infection is caused by a variety of strains of influenza viruses. In any given year, some strains of influenza virus can cause seasonal disease while others may cause epidemics, with a potential to cause pandemics. During the past decade the world has witnessed the global occurrence of seasonal influenza, the manifestation of avian influenza due to the influenza A (H5N1) virus with a high mortality rate in many countries, and the wave of a pandemic in 2009 due to a novel strain of influenza (pH1N1). While seasonal influenza occurs every year, epidemics may occur after several years and pandemics strike once in several decades [7].
The recent pandemic and the emergence of influenza viruses represent enormous challenges to worldwide control by vaccination and currently available antivirals. Antiviral therapy and vaccination are the only options for controlling an influenza virus infection. Vaccination represents the best protection against influenza, but an appropriate vaccine cannot be developed before a new virus strain emerges. For instance, influenza viruses rapidly develop resistance to antivirals when they are used extensively. These examples emphasize the importance of investigating all sources for potential antiviral drugs and particularly the largely unexplored resource of natural substances. An excellent outcome would be the identification of materials that are active against influenza virus, in contrast to vaccines and some antiviral drugs. Also the effectiveness of current drugs and vaccines is limited, and there is concern that they may be insufficient for the next influenza pandemic. Hence, new anti-influenza virus agents are required to address this problem.
Plants have a long evolutionary history of developing resistance against viruses and have increasingly drawn attention as potential sources of antiviral drugs. Jatropha curcas belongs to the family Euphorbiaceae, which is known for various medicinal uses. Preparations of all parts of the plant, including seeds, leaves and bark, fresh or as a decoction, are used in traditional medicine and for veterinary purposes. The oil has a strong purgative action and is also widely used for skin diseases and to soothe pain such as that caused by rheumatism. A decoction of leaves is used against cough and as an antiseptic after birth. Branches are used as a chewing stick in Nigeria [2, 10].
Most parts of the plant are used for the treatment of various human and veterinary ailments. Traditionally this plant is used for treating dysentery and diarrhea. J. curcas has been known for its antibacterial activity against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. The latex of Jatropha contains an alkaloid known as “Jatrophine” which is believed to have anti-cancer properties. It is also used for skin diseases, rheumatism and for sores on domestic livestock [2]. The white latex serves as a disinfectant in mouth infections in children. The leaves of J. curcas also contain apigenin, vitexin and isovitexin, which, along with other factors enable them to be used against malaria, rheumatic and muscular pains [18]. Antibiotic activity of Jatropha has been observed against organisms including S. aureus and E. coli [8]. Crude stem bark extracts of J. curcas Linn. was reported to inhibit the growth of pathogenic bacteria and fungi [6]. In addition, crude extract of J. curcas Linn. has been found to inhibit HIV induced cytopathic effects with low cytotoxicity [17].
Based on the above facts and since J. curcas has not been explored for its potential anti-influenza activity, the present study was carried out to investigate and assess the preliminary effect of aqueous and methanol fraction of J. curcas leaves for its cytotoxicity and potential to inhibit hemagglutinin of influenza virus.
Materials and methods
Plant collection
Fresh mature healthy leaves were collected from fully grown J. curcas Linn. plant from outskirts of Rajaramnagar, Islampur, Sangali District, Maharashtra, India. The plant materials were identified and authenticated using the flora of Islampur, Sangali District at Department of Botany, St. Xavier’s college, Mumbai, India. Voucher specimen was deposited in Herbarium. (Specimen no. K.V.S 2298)
Extraction of plant material
The shadow air-dried leaves were pulverized into fine powder using a mixer grinder. About 30 g of powdered material was extracted in Soxhlet extraction apparatus (continuous hot percolation) with 600 mL of each of the following solvents; methanol and water. Methanol used for the extraction was of analytical grade. The separated extracts were then filtered through Whatman’s No. 1 filter paper and the aqueous and methanol filtrate were separately concentrated to dryness using a rotary evaporator to remove the water and methanol. The sticky greenish and brown substances were obtained and stored in refrigerator prior to use [3].
Screening of phytochemicals using HPTLC finger printing
The condensed extracts were used for screening of secondary metabolites using HPTLC on a third-party commercial basis (M/s Anchrom Test Lab (I) Pvt. Ltd., Mumbai, India). HPTLC was performed using the standard protocol. Chromatography was performed on pre-activated (at 110 °C) silica gel MERCK 60F254 HPTLC plates using toluene: ethyl acetate: formic acid (7:2:1) mobile phase. Standard compounds and samples (5 μL) were applied to the layer as 8 mm wide bands, using an automated CamagLinomat IV spotter. Each TLC plate was developed to a height of about 10 cm under the laboratory conditions of 25–30 °C with 40–50 % relative humidity in developing chamber. Developed plates were dried in a stream of air and then sprayed with a solution of sulphuric acid: ethanol (5:95 v/v). After drying, the plates were heated at 110 °C for 25 min to develop the color of the spots. For quantitative determination, the corresponding spots were scanned using a CAMAG TLC scanner at 254 and 366 nm.
Cell culture
Continuous MDCK cell lines were maintained in complete modified eagles medium (MEM) containing 100 IU/mL penicillin, 100 mg/mL streptomycin, 2 mM l-glutamine and 1.5 g/L sodium bicarbonate, supplemented with 10 % fetal bovine serum (FBS) at 37 °C in a humidified 5 % CO2 incubator.
Virus and viral titration
Experiments were performed using Influenza Virus A/H1N1/PUNE/2009; standard virus obtained from National Institute of Virology, Pune, India.
Influenza virus stocks were grown in cell culture using Madin Darby canine kidney (MDCK) cells and titre of infectious virus were measured by 50 % tissue culture infective dose (TCID50) titration. MDCK cells for viral titration were grown in complete MEM. Confluent MDCK cell monolayer in 96-well tissue culture plates were washed once with serum-free-MEM before use. Serial 10-fold dilutions of virus in serum-free-MEM containing 0.3 % BSA and 2 μg/mL l-(toslyamido 2-phenyl) ethyl chloromethyl ketone (TPCK)-treated trypsin and Nystatin (50 U/mL) were incubated in replicate wells (200 μL/well) for 4 days at 37 °C with 5 % CO2. Wells positive for virus growth were identified by staining the cells with 0.1 % trypan blue and TCID50 titre were calculated by the method of Reed and Muench [13].
Trypan blue dye exclusion assay for cytotoxicity
The MDCK cells were grown in 96-well plate for 24 h. The medium was replaced with that containing serially diluted plant extracts (100 μL/well) in triplicates. Then the plate was further incubated for 18 h. To determine the extents of cell death, cells were trypsinized to obtain a single cell suspension. After the addition of MEM containing 10 % FBS to the suspension to neutralize the trypsin and to stabilize the cells, the number of the living or dead cells were determined by a dye exclusion method with trypan blue as described previously [5]. The percent viability was then calculated by using formula: % viability = (live cell count/total cell count) × 100. The cytotoxicity of the compound against MDCK cells was evaluated in term of cytotoxic concentration 50 (CC50) which was determined by statistical analyses using GraphPad Prism software (GraphPad Software, Inc., California, USA).
Study on inhibition of Influenza hemagglutinin protein
Dried plant extracts were dissolved in DMEM without phenol red and required dilutions were prepared. Confluent MDCK cell cultures were treated with different dilutions of methanol and aqueous extract in three sets of experiments as follows:
Pre-penetration exposure
Virus stock of 102.5 TCID50 (64 HA titre) was exposed to effective minimal cytotoxic concentrations of plant extracts for 1 h at 37 °C. Then 100 μL of the mixture was added to the cells cultured fluently in 96-well flat-bottom microtitre plate. Following 1 h incubation at 37 °C, the supernatants were removed and the cells were washed with phosphate buffered saline (PBS). Then 100 μL of the TPCK-containing medium was added to each well.
Simultaneous exposure
Virus stock of 102.5 TCID50 (64 HA titre) and effective minimal cytotoxic concentrations of plant extracts were mixed together in equal proportions using TPCK-containing medium. The mixture was added to the cells and left on the cells throughout.
Post-penetration exposure
Virus stock of 102.5 TCID50 (64 HA titre) (100 μL/well) was added to each well. After 1 h incubation at 37 °C the unabsorbed viruses were removed and cells were washed and 100 μL of plant extracts diluted with TPCK-containing medium was added to each well.
All the plates including MDCK cells (Cell control) and influenza virus inoculated MDCK cells were incubated at 37 °C in CO2 incubator for 48 h and the virus titration was carried out by Hemagglutination assay.
Hemagglutination assay
In order to assess the presence of the virus in cell culture, serial dilutions of the cell culture media (50 μL/well) were added in 96-well U-shaped microtitre plate. Guinea pig red blood cells (gRBCs) (0.75 %) were added to each well (50 μL/well). Following incubation at least for 1 h at room temperature, the virus titre of each well was recorded. A diffused sheet of agglutinated erythrocytes covering the bottom was regarded as 100 % hemagglutination, while a well circumscribed button of non-agglutinated erythrocytes was regarded as non-hemagglutination. The HA titre of the supernatant was defined as the reciprocal of the highest dilution showing complete hemagglutination. Complete settling of the RBCs demonstrated absence of the virus while hemagglutination indicated the presence of the virus.
Results
HPTLC finger print analysis
The aqueous and methanol extracts of J. curcas were subjected to HPTLC analysis by specific solvent system toluene: ethyl acetate: formic acid (7:2:1) and detected under UV at 254 and 366 nm. The HPTLC fingerprint shown in Fig. 1 indicates that all sample constituents were clearly separated without any tailing and diffuseness. The plant extracts showed the presence of tannins, flavonoids and saponins in the aqueous extract and flavonoids and saponins in the methanol extract
Fig. 1.
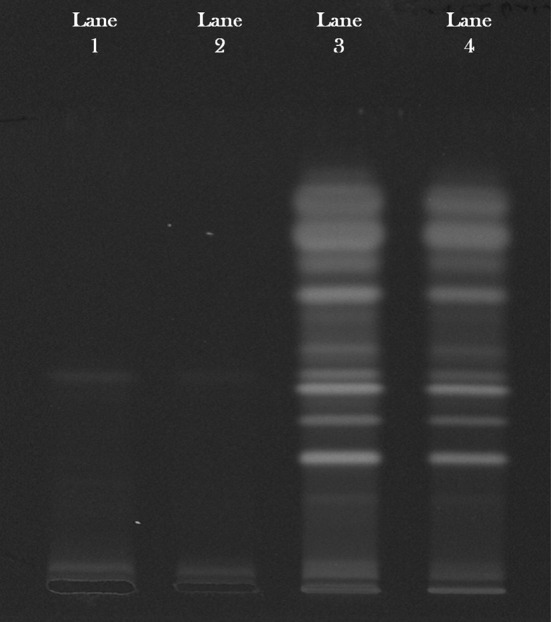
HPTLC fingerprint of aqueous (lane 1, 2) and methanol (lane 3, 4) extracts of Jatropha curcas Linn.
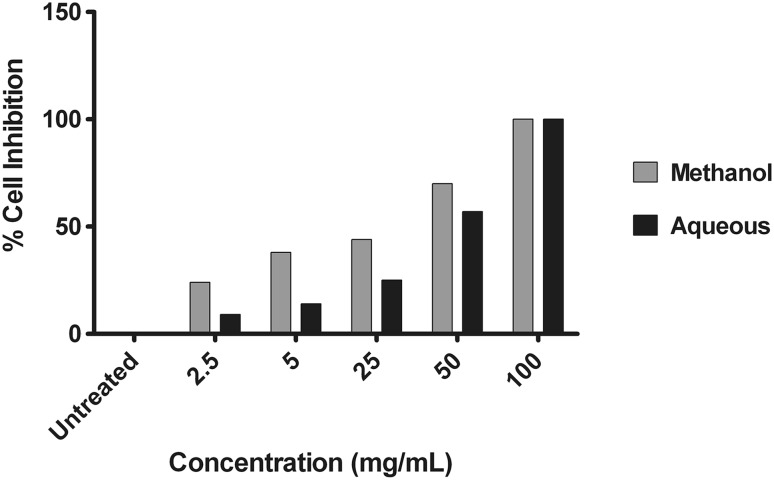
Cytotoxic effects of methanol and aqueous extract on MDCK cells
A major prerequisite for an antiviral agent is safety. Thus, we tested whether therapeutic concentrations of J. curcas extracts would have any harmful effects on healthy cells. Initially, cells treated with J. curcas extracts at the indicated concentrations were examined for changes in morphology. No differences in cell shape or cell number could be observed compared with untreated control cells. Then we further evaluated the cytotoxicity of methanol and aqueous extracts of J. curcas on MDCK cells using trypan blue dye exclusion assay. The results show that both the extracts have no cytotoxic effect on the cells at concentrations of 5 mg/mL (Fig. 2). Interestingly, more than 50 % cell viability was noted when extracts were used at a concentration of 25 mg/mL. The concentrations estimated to reduce cell viability by 50 % or the CC50 of the methanol and aqueous extracts were 15.57 and 33.62 mg/mL respectively.
Fig. 2.
Cytotoxicity assay for methanol and aqueous extracts of Jatropha curcas MDCK cells were left untreated (negative control) and treated with the different concentration (2.5, 5, 25, 50 and 100 mg/mL) of both extracts (100 μL/well) and incubated for 18 h at 37 °C. The cell viability was measured using trypan blue dye exclusion assay
Hemagglutinin protein inhibition activity
Viral hemagglutinin protein inhibition activity was determined using three different sets of experiment; pre-penetration exposure, simultaneous exposure and post-penetration exposure using methanol and aqueous leaf extracts.
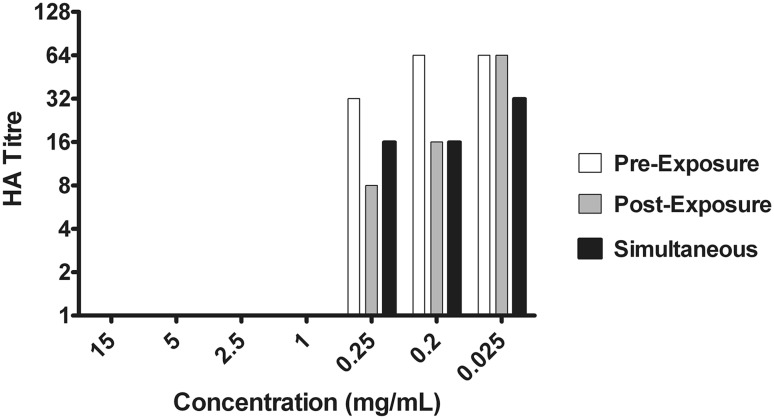
Inhibitory effect of methanol extracts
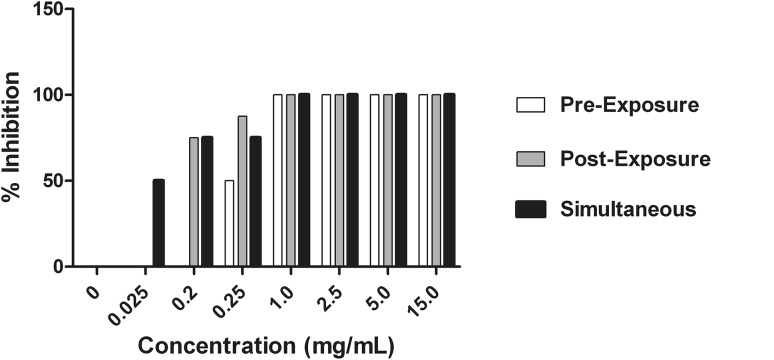
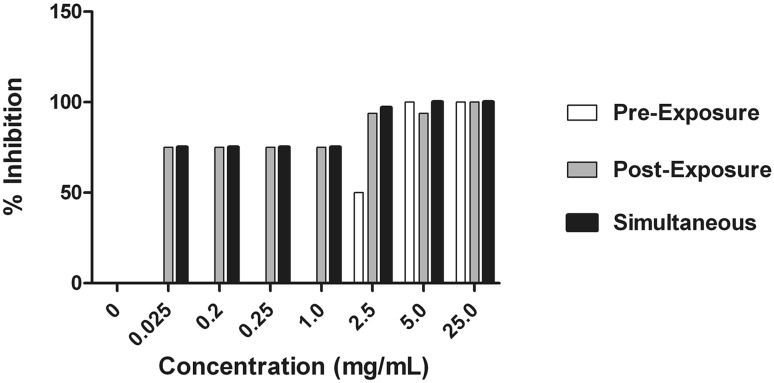
Cell culture of the virus in the presence of methanol extracts in different sets of experiments was assessed by hemagglutination assay. The inhibitory effect of the extract is shown by reducing HA titre (Fig. 3). While assessing the percent virus inhibition using reducing HA titre, it was found that Methanol extracts showed 100 % inhibition of the virus in all three experiments with concentration ranging from 15 mg to 1 mg/mL. To determine the effect the concentration was further lowered down below 1 mg/mL. The results revealed that, pre-penetration exposure did not show any significant result at lower concentration. Post-penetration exposure showed 87.5 and 75 % inhibition of the virus with concentration 0.25 and 0.2 mg/mL respectively. However, simultaneous exposure showed best inhibitory effect of 75 % at 0.25 and 0.2 mg/mL concentration and 50 % at an even lesser concentration of 0.025 mg/mL (Fig. 4).
Fig. 3.
Methanol extract inhibits hemagglutinating activity of influenza virus. Cell culture with virus and different concentrations of the extract from pre exposure, simultaneous exposure and post exposure experiments were added in 96-well plate with equal amount of guinea pig erythrocytes (0.75 %) and incubated for 1 h. We observed that at concentration of 15–1 mg/mL of methanol extract reduces HA activity of virus from 64 to no HA titre, indicating an interaction of methanol extract with the viral HA
Fig. 4.
Inhibitory effect of methanol extract of Jatropha curcas was studied at different concentrations (15, 5, 2.5, 1, 0.25, 0.2 and 0.025 mg/mL) followed by determination of percent viral inhibition using hemagglutination assay
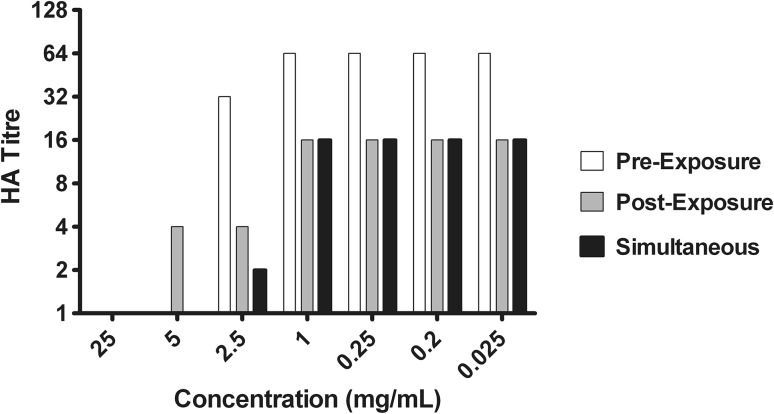
Inhibitory effect of aqueous extracts
Cell culture of the virus in the presence of aqueous extracts in different sets of experiments was assessed by hemagglutination assay. The inhibitory effect of the extract is shown by reducing HA titre (Fig. 5). Virus inhibition activity of the aqueous extracts showed 100 % inhibition of the virus in all three experiments at a concentration of 25 and 5 mg/mL except post-penetration exposure analysis which showed 93.75 % inhibition at 5 mg/mL. There was >50 % antiviral activity seen with all experiments at concentration of 2.5 mg/mL. However, when the concentration was lowered; it has been found that post-penetration and simultaneous exposure showed 75 % inhibition of the virus at concentration ranging from 1 to 0.025 mg/mL. There has been no antiviral activity seen with pre-penetration exposure analysis with these lower concentrations (Fig. 6).
Fig. 5.
Aqueous extract inhibits hemagglutinating activity of influenza virus. Cell culture with virus and different concentrations of the extract from different experiments pre exposure, simultaneous exposure and post exposure were added in 96-well plate with equal amount of guinea pig erythrocytes (0.75 %) and incubated for 1 h. We observed that at a concentration of 25 mg/mL; aqueous extract reduces HA activity of virus from 64 to no HA titre. When the concentration was further reduced to 0.025 mg/mL in simultaneous and post exposure analysis; HA activity reduced to 16 as compared to pre exposure where it was 64 HA titre
Fig. 6.
Inhibitory effect of aqueous extract of Jatropha curcas was studied at different concentrations (25, 5, 2.5, 1, 0.25, 0.2 and 0.025 mg/mL) followed by determination of percent viral inhibition using hemagglutination assay
Discussion
Influenza remains a serious problem in many countries, coupled with a long history of outbreaks, epidemics and recent pandemic. Almost all antiviral are subject to drug resistance as the virus mutate over time, becoming less susceptible to the treatment. In addition, influenza viruses rapidly develop resistance to antiviral when they are used extensively. It has become imperative to develop effective medical strategies for the management of influenza, which can assume pandemic proportions and become a major threat to humanity. Complementary and alternative medicines have been used effectively by humans over several centuries for treating various diseases and can be effectively employed to target the host response during influenza outbreaks.
Traditional medicine and medicinal plants, in general, continue to be a powerful source of new drugs, now contributing about 90 % of the newly discovered pharmaceuticals. Traditional medicine continues to provide health coverage for over 80 % of the world population, especially in the developing world. The past and the present are all full of living examples of discoveries of drugs, ranging from anti-cancer, anti-asthma, anti-diabetic, anti-hypertensive’s and many others which owe their origin to traditional medicine [9].
Over the past decades, many compounds isolated from medicinal plants have been found to possess inhibitory activity against influenza [4, 11, 14–16, 19, 20].The use of J. curcas in traditional medicine is well known. Our earlier study on J. curcas leaf extracts showed effective anti-viral and probable entry inhibition activity against potentially drug-resistant HIV isolates [1]. The significant result from this study and established medicinal value of J. curcas encouraged us to investigate the potential of methanol and aqueous leaf extracts for evaluation of its cytotoxicity and inhibition of hemagglutinin of influenza virus.
In this study the cytotoxic potential of methanol and aqueous extracts was investigated. Trypan blue dye exclusion assay was used to determine the CC50 of the test compounds to eliminate any possible cytotoxic or anti-cell proliferation effect of the phytochemical on the host cells. These compounds with minimal cytotoxic concentrations found to prevent binding of hemagglutinin to cell receptors leading to inhibition of infection of influenza virus. Thus it can be good potential as lead compounds in the future development of anti-influenza drugs.
Investigations on the phytochemical screening of J. curcas leaf extracts revealed the presence of saponins, tannins and flavonoids. The similar results were mentioned in earlier work during a screening of secondary metabolites from J. curcas Linn. [12].
In this study inhibition of influenza virus adsorption to MDCK cells and red blood cells by methanol and aqueous extract was investigated by hemagglutination assay. High percent protection following exposure of plant extracts to the virus cell culture was determined by reduction of HA titer of the virus cell culture.
In the present study, we have evaluated the virus inhibitory potential of methanol and aqueous extract by means of in vitro system. The different exposure studies revealed that the anti-influenza activity of aqueous and methanol extract was higher in simultaneous and post-penetration exposure experiment than pre-penetration exposure experiment. From this study it appears that during simultaneous exposure, J. curcas extracts probably exert their effect by preventing viral adsorption onto the cells since viral hemagglutination was inhibited, which is mediated by the sialic acid-specific hemagglutinin protein. Moreover, its effect on the infectivity of the virus was more pronounced when added several times post-infection, suggesting that it prevented the adsorption of viral progeny released by infected cells into new cells. These results suggest that both the extracts may interfere with the early stage of viral replication, such as the adsorption and penetration of the virus. Furthermore, it was observed that in the single cycle growth condition, the J. curcas Linn. extracts inhibited virus release when the MDCK cells were exposed to extracts only after the viral adsorption phase suggesting that independent to virucidal effect, the extracts also inhibits influenza virus replication. In addition, pre-treatment of RBC’s with the extracts followed by its removal did not affect the hemagglutination activity of influenza virus, demonstrating that the membranes of RBC’s remain intact. This result indicates that the HA inhibitory effect is primarily due to the interaction of the extracts with virus particles, not via an effect on RBC’s. To our knowledge, this is the first report demonstrating that J. curcas leaf extracts inhibits hemagglutination activity of influenza virus and the anti-influenza effect via a mechanism that interferes with virus-cell attachment.
In conclusion, this study explored the potential anti influenza activity of J. curcas leaf extracts by means of hemagglutinin inhibition, which will be useful in verifying the antiviral therapeutic efficacy of different parts of J. curcas against influenza infection and helpful in encouraging development of anti-influenza virus medicines. Further studies are expected to assess its efficacy in animal models, the mechanism of action of active moiety and the activity spectrum of these extracts against other RNA viruses.
Acknowledgments
We are grateful to Dr. (Mrs). M. S. Chadha, Assistant Director, Influenza group, National Institute of Virology, Pune, Maharashtra, India for providing us Influenza virus strain.
References
- 1.Dahake RV, Roy SS, Deepak Patil DY, Chowdhary AS, Deshmukh RA. Evaluation of anti-viral activity of Jatropha curcas leaf extracts against potentially drug-resistant HIV isolates. BMC Infect Dis. 2012;12(Suppl 1):P14. doi: 10.1186/1471-2334-12-S1-P14. [DOI] [Google Scholar]
- 2.Das Gupta D, Md Enamul Haque, Md Nahidul Islam, Shakhinur Islam Md, BA Shibib. Antimicrobial and cytotoxic activities of Jatropha curcas (Euphorbiaceae) Dhaka Univ J Pharm Sci. 2010;9(2):139–142. [Google Scholar]
- 3.Dhale DA, Birari AR. Preliminary screening of antimicrobial and phytochemical studies of Jatropha gossypifolia Linn. Recent Res Sci Technol. 2010;2(7):24–28. [Google Scholar]
- 4.Ehrhardt C, Hrincius ER, Korte V. A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antivir Res. 2007;76(1):38–47. doi: 10.1016/j.antiviral.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Giuseppe SA, Saydam G, Banerjee D, Bertino JR. Cytotoxicity and cell growth assays. Cell Biol. 2006;38:315–324. [Google Scholar]
- 6.Igbinosa OO, Igbinosa EO, Aiyegoro OA. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn.) Afr J Pharmacol. 2009;3:58–62. [Google Scholar]
- 7.Kamps BS, Teran GR. Influenza. In: Kamps BS, Hoffmann C, Preiser W, editors. Influenza report 2006. Wuppertal: Flying Publisher; 2006. pp. 17–38. [Google Scholar]
- 8.Matsuse IT, Lim YA, Hattori M, Correa M, Gupta MP. A search for anti-viral properties in Panamanian medicinal plants: the effects on HIV and its essential enzymes. J Ethnopharmacol. 1998;64:15–22. doi: 10.1016/S0378-8741(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 9.Moshi MJ. Current and future prospects of integrating traditional and alternative medicine in the management of diseases in Tanzania. Tanzan Health Res Bull. 2005;7:159–167. doi: 10.4314/thrb.v7i3.14254. [DOI] [PubMed] [Google Scholar]
- 10.Nayak BS, Patel KN. Pharmacognostic studies of the Jatropha curcas leaves. Int J Pharm Tech Res. 2010;2(1):140–143. [Google Scholar]
- 11.Rajbhandari M, Wegner U, Schopke T, Lindequist U, Mentel R. Inhibitory effect of Bergenia ligulata on influenza virus A. Pharmazie. 2003;58(4):268–271. [PubMed] [Google Scholar]
- 12.Reddy Prasad DM, Izam A, Khan MR. Jatropha curcas: plant of medical benefits. J Med Plants Res. 2012;6(14):2691–2699. doi: 10.5897/JMPR10.977. [DOI] [Google Scholar]
- 13.Reed LJ, Muench HA. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 14.Ryu YB, Curtis-Long MJ, Kim JH. Pterocarpans and flavanones from Sophora flavescens displaying potent neuraminidase inhibition. Bioorg Med Chem Lett. 2008;18(23):6046–6049. doi: 10.1016/j.bmcl.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Serkedjieva J, Manolova N. Plant polyphenolic complex inhibits the reproduction of influenza and herpes simplex viruses. Phytother Res. 1999;10(5):441–443. doi: 10.1002/(SICI)1099-1573(199608)10:5<441::AID-PTR867>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antivir Res. 2005;68:66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Sriprang S, Sriprang N, Sumpradit T, Shimbhu D. Antibacterial activities of crude extracts from physic nut (Jatropha curcas) seed residues. Sci Asia. 2010;36:346–348. doi: 10.2306/scienceasia1513-1874.2010.36.346. [DOI] [Google Scholar]
- 18.Thomas R, Sah NK, Sharma PB. Therapeutic biology of Jatropha curcas: a mini review. Curr Pharm Biotechnol. 2008;9:315–324. doi: 10.2174/138920108785161505. [DOI] [PubMed] [Google Scholar]
- 19.Wirotesangthong M, Nagai T, Yamada H, Amnuoypol S, Mungmee C. Effects of Clinacanthus siamensis leaf extract on influenza virus infection. Microbiol Immunol. 2009;53(2):66–74. doi: 10.1111/j.1348-0421.2008.00095.x. [DOI] [PubMed] [Google Scholar]
- 20.Yingsakmongkon S, Miyamoto D, Sriwilaijaroen N. In vitro inhibition of human influenza A virus infection by fruit-juice concentrate of Japanese plum (Prunus mume SIEB.et ZUCC) Biol Pharm Bull. 2008;31(3):511–515. doi: 10.1248/bpb.31.511. [DOI] [PubMed] [Google Scholar]