Abstract
Pseudoexfoliation syndrome is a primarily ophthalmological disorder caused by deposition of whitish-gray protein on the lens, iris, and multiple other eye tissues. There is increasing evidence over the previous years that pseudoexfoliation syndrome is a systemic disorder with various extraocular manifestations and has recently been linked to several cardiovascular disorders. The present article aims to summarize the current knowledge on cardiovascular implications of this well-described clinical entity.
Keywords: Cardiovascular, Coronary disease, Pseudoexfoliation syndrome
Introduction
Pseudoexfoliation (PEX) syndrome is a well-recognized age-related fibrillopathy that has been linked to multiple pathological entities of the eye; including glaucoma, cataract, and perioperative complications during eye surgery. Over the previous decades, PEX has been associated with various other conditions, such as Alzheimer’s type dementia, sensory-neural hearing loss, and cardiovascular disease.[1,2,3] Moreover, recent studies have demonstrated the presence of PEX material in a variety of tissues; such as lung, heart, brain, and vessels.[4,5,6] These findings have led to the hypothesis that PEX is more likely a systemic disorder with multiple clinical manifestations. We systematically reviewed all full text articles indexed in PubMed related to cardiovascular implications of the PEX syndrome.
Ocular Manifestations
PEX is a degenerative disease of the eye caused by production of abnormal fibrillar material that accumulates in multiple ocular structures.[7] It is the most common clinical precursor of open-angle glaucoma (PEX glaucoma) and a significant cause of intraoperative complications during eye surgery due to zonular instability, poor papillary dilatation, and increased permeability of vessels.[8]
Posterior synecchiae, pseudouveitis, keratopathy, loss of normal radial iris vessels, and acceleration of cataract formation have also been described in patients with PEX material deposits.[9] The diagnosis of PEX is based on slit lamp observations of the typical gray-white flakes at the papillary margin or deposits on the central area of the anterior surface of lens capsule, separated from a peripheral griddle of deposits by a clear zone.[9] Although it was previously believed that PEX deposits can be seen unilaterally or bilaterally, recent studies based on electron microscopy support the concept that PEX syndrome is a generalized bilateral disorder with clinically marked asymmetric manifestation.[10]
Epidemiology
The reported prevalence of PEX varies extensively among different populations and tends to increase with latitude in the Northern Hemisphere. Although Scandinavian populations show high prevalence of the disease, the condition has been described in all human races. The prevalence is as high as 20% in Finland and over 25% in Iceland.[11] Interestingly, though in parts of Denmark and in Greenland Eskimos the prevalence of PEX has been estimated as low as 5% and 0, respectively.[12] In western European countries, such as England and Germany, a prevalence of 4.0 and 4.7% has been reported, respectively.[11]
It has been previously shown that the incidence of PEX increases with age. In a study of a Greek population, it was found that the prevalence increases from 1.2% in the 6th decade of life to about 34% in patients older than 80 years.[13] A population-based study in the United States found a prevalence of 0.67% in people aged 52-64 years, 2.6% in people aged 65-74 years, and 5% in people aged 78-85 years.[14] Moreover, in the Reykjavik Eye Study; the prevalence of PEX in Iceland increased from 2.5% in those aged 50-59 years to 40.6% in those aged <80 years.[15]
Genetics
PEX deposits have been found in a variety of extraocular locations. Abnormal elastin fibers have been identified by electron microscopy in the heart, lung, liver, kidney, gallbladder, and meninges.[4,5,6] Although the clinical significance of these findings is not always clear, multiple correlations between the presence of PEX deposits and system disorders have been demonstrated. Association of PEX with various diseases is shown in Figure 1.
Figure 1.
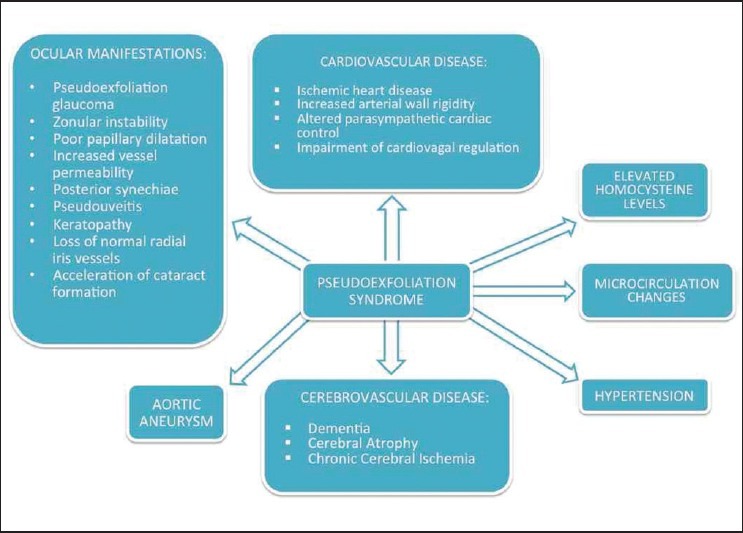
Association of pseudoexfoliation syndrome with various diseases
Previous studies have suggested that PEX syndrome is inherited as an autosomal dominant trait with late onset and incomplete penetrance.[16] A breakthrough in understanding the disease was the identification of the lysyl-oxidase-like-1 (LOXL1) gene on chromosome 15q24 in an Icelandic population with the PEX phenotype.[17] Further studies in other populations confirmed the genetic predisposition of LOXL1 carriers to PEX.[18,19] LOXL1 is known to bind to tropoelastin monomers and assists in the formation of elastin fibers in the extracellular matrix. Available data suggest that upregulation of LOXL1 during the early stages of PEX fibrogenesis results in the aggregation of abnormal fiber deposits, while the decreased expression of LOXL1 during the advanced stages of the disease can affect elastin metabolism and promote elastotic processes that predispose to the development of glaucoma and other disorders.[20] In a recent study, atomic force microscopy (AFM) antibody recognition imaging for localization of the LOXL1 protein on PXF-affected lens capsules was employed. LOXL1 was detected on lens capsules affected by PXF syndrome by three different AFM antibody recognition techniques. The protein was found in localized regions and was associated with fibers on the surface, supporting an important role in the formation of the filamentous protein aggregates. In particular, it is conceivable that given its amino oxidase function, LOXL1 may remain attached to the elastic fibers, which eventually form part of the PEX material after performing cross-linking of elastin.[21]
Pseudoexfoliation Syndrome and Cardiovascular Disease
Several studies have demonstrated a link between PEX and coronary artery disease (CAD). In the Blue Mountains Eye Study, a population-based study in Australia, it was found that PEX was significantly associated with the history of angina or hypertension or a combined history of angina, acute myocardial infarction or stroke, after multivariate adjustment; including age, sex, glaucoma, and vascular risk factors.[22] More recently, Citirik et al., showed significant difference in the prevalence of PEX in patients with CAD and also in the prevalence of CAD in PEX individuals.[23] In another study designed to investigate the prevalence of glaucoma and CAD in patients with cataract and PEX, Andrikopoulos et al., also demonstrated that PEX was positively associated with the risk for CAD in patients of 50 years or older.[13] Similar conclusions were drawn by other studies after analyzing the prevalence of cardiovascular disorders in large populations with PEX.[24,25] Moreover, silent myocardial ischemia, demonstrated by means of tissue Doppler echocardiography, has also been shown in individuals with PEX.[26]
Despite accumulating evidence that PEX may be an independent risk factor for cardiovascular disease, other studies have failed to demonstrate any significant association with CAD or increased cardiovascular mortality.[27,28,29] In a study by Shrum et al., that included 472 patients with diagnosed PEX syndrome, no association was found between ocular PEX and cardiovascular or cerebrovascular mortality.[28] Interestingly, Brajkovic et al., examined the association between PEX and various cardiovascular diseases such as hypertension, arrhythmias, CAD, diabetes, and cerebrovascular events; and only the prevalence of arrhythmia was found to be higher in patients with PEX syndrome.[30] Furthermore, a more recent study by Emiroglu et al., failed to show any significant relationship between PEX and CAD, aortic aneurysm, or peripheral artery disease.[31]
Several pathophysiological mechanisms that could explain the increased incidence of cardiovascular disorders in patients with PEX have been described. Homocysteine has been proven to play an important role in cardiovascular disease. Multiple studies have shown that patients with PEX have significantly higher plasma homocysteine levels compared to healthy controls.[32,33,34,35] Elevated homocysteine levels in PEX patients have been identified in plasma, aqueous humor, and tears. These findings could account for the increased cardiovascular risk among patients with PEX, since high plasma homocysteine levels have been linked to a variety of vascular disorders including stroke, myocardial infarction, and veno-occlusive disease.[36] In addition to high homocysteine concentrations, patients with PEX have lower folate, vitamin B12, and B6 levels.[37] This could partially explain the higher prevalence of vitamin B deficiency in other diseases that have been linked to PEX, such as Alzheimer’s disease.
Functional changes in systemic macrocirculation and microcirculation have been found in PEX patients.[38] PEX deposits have been associated with many endothelial cell markers, collagen, fibroblasts, and elastin.[39] Moreover, it has been shown that patients with PEX demonstrate impaired endothelial function. Atalar et al., used high-resolution ultrasound to assess brachial artery endothelial function by vascular response to reactive hyperemia (flow-mediated) and sublingual nitroglycerin.[40] They found that both parameters were significantly lower compared to healthy subjects, indicating that systemic endothelial dysfunction is impaired in PEX individuals. With the use of a high precision automated ultrasonic wall-tracking increased arterial wall rigidity and decreased elasticity in the common carotid artery of PEX patients has been shown. These findings are consistent with previous studies that histologically demonstrated the presence of pseudoexfoliative deposits in the adventitia and subendothelium of the aorta and smaller arteries, accompanied by extensive fibrosis and elastosis of the tunica media.[5,6] In support of the above, PEX patients have been found to have raised inflammatory markers, cytokines, and markers of endothelial dysfunction such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), high sensitivity C-reactive protein (hsCRP) and YKL-40.[41,42,43] However, the fact these findings have not been consistent in all studies suggests that the pathogenesis of PEX is more complex and likely multifactorial.[44,45]
Although the effects of PEX material on autonomic dysfunction have not been thoroughly addressed, there is evidence to support that the parasympathetic system is also affected in these patients. Hollo et al., identified decreased cutaneous capillary blood flow and diminished cutaneous flow reactions to cold and warmth provocation, without any change in plasma endothelin-1 levels.[46] The same research group showed that baroreflex sensitivity was significantly reduced in patients with PEX, suggesting a pathologically altered parasympathetic vascular control.[47] The above findings are supported by another study by the same group which reported statistically significant impairment of cardiovagal regulation and increased pulse wave velocity in PEX.[48]
Hypertension
Several studies have correlated PEX syndrome with arterial hypertension. However, the majority of those findings were based on a small number of patients and therefore the prevalence of hypertension among patients with PEX could be distorted. However, the Australian Blue Mountains Eye Study, a large study including a total of 3,654 patients, showed that PEX is significantly associated with a history of hypertension, angina, or both.[22] In another large cross-sectional study in Japan, which included 1,884 patients; the prevalence of arterial hypertension was high and significantly associated with PEX.[49] Endothelial dysfunction, oxidative stress, elastosis, and impaired autonomic regulation are some of the proposed mechanisms that may account for the high prevalence of arterial hypertension in patients with PEX syndrome. Another possible cause has recently been described by Gonen et al., who depicted a high incidence of renal artery stenosis in PEX individuals.[50] Nevertheless, a small number of studies have not demonstrated a clear correlation between PEX and arterial hypertension.[51]
Cerebrovascular disease
Blood flow velocities of the middle cerebral artery have been found to be decreased in patients with PEX-related glaucoma and this could predispose to cerebrovascular disease.[52] In addition, total cerebral perfusion in these patients is lower than normal and previous imaging studies have demonstrated diffuse cerebral ischemic changes and cerebral infarcts.[53,54,55] Another study showed that chronic cerebral diseases such as senile dementia, cerebral atrophy, and chronic cerebral ischemia are more common in patients with PEX-glaucoma. In the same study, patients with PEX-glaucoma had higher probability of developing acute cerebrovascular events compared to patients with non-PEX related glaucoma.[56] Alzheimer’s disease has also been linked to PEX syndrome.[1,57]
Aortic aneurysm and peripheral vascular disease
A high frequency of abdominal aortic aneurysm (AAA) has been reported in patients with PEX syndrome.[50,58] In a prospective study by Schumacher et al., 55 patients that required surgery for aneurysm of the abdominal were examined and 44% of them demonstrated ocular manifestations of PEX.[6] Diffuse fibrosis and elastosis of the tunica intima and accumulation of PEX deposits in the adventitial and subendothelial connective tissue are the most common histopatholgical findings in patients with coexistence of AAA and PEX.
Although a clear association between PEX and peripheral vascular disease has not been demonstrated, a recent study including a total of 160 patients showed that subjects with PEX and cataract had significantly lower ankle branchial index (ABI) compared to the control group.[59]
Conclusions
Although most of current experience with PEX syndrome exists in the field of ophthalmology, there is accumulating clinical and laboratory evidence that this condition may occur as part of a general systemic disorder with major cardiovascular implications. Individuals with PEX are susceptible to increased cardiovascular risk; therefore, physicians should be alerted in early recognition of symptoms, prompt diagnosis, and prevention strategies. Nevertheless, more data are still required with regards to the optimal treatment strategies, such as the role of early statin treatment in patient presenting with features of the PEX syndrome and need for routine noninvasive assessment, in order to diagnose promptly any coexistent cardiovascular comorbidities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Cumurcu T, Dorak F, Cumurcu BE, Erbay LG, Ozsoy E. Is there any relation between pseudoexfoliation syndrome and alzheimer’s type dementia? Semin Ophthalmol. 2013;28:224–9. doi: 10.3109/08820538.2013.793726. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos TA, Charalabopoulou M, Vathylakis I, Goumas P, Gartaganis S, Naxakis S. Prevalence and severity of sensorineural hearing loss in patients with exfoliation syndrome. Eur Rev Med Pharmacol Sci. 2012;16:902–7. [PubMed] [Google Scholar]
- 3.Alpaslan M, Karalezli A, Borazan M, Köktekir BE, Müderrisoðlu IH. Decreased aortic root elasticity-as a novel systemic manifestation of the pseudoexfoliation syndrome: An observational study. Anadolu Kardiyol Derg. 2012;12:483–7. doi: 10.5152/akd.2012.155. [DOI] [PubMed] [Google Scholar]
- 4.Schlotzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome. Ocular mani-festation of a systemic disorder? Arch Ophthalmol. 1992;110:1752–6. doi: 10.1001/archopht.1992.01080240092038. [DOI] [PubMed] [Google Scholar]
- 5.Streenten BW, Li ZY, Wallace RN, Eagle RC, Jr, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol. 1992;110:1757–62. doi: 10.1001/archopht.1992.01080240097039. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher S, Schlotzer-Schrehardt U, Martus P, Lang W, Naumann GO. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet. 2001;357:359–60. doi: 10.1016/s0140-6736(00)03645-x. [DOI] [PubMed] [Google Scholar]
- 7.Braunsmann C, Hammer CM, Rheinlaender J, Kruse FE, Schäffer TE, Schlötzer-Schrehardt U. Evaluation of lamina cribrosa and peripapillary sclera stiffness in pseudoexfoliation and normal eyes by atomic force microscopy. Invest Ophthalmol Vis Sci. 2012;53:2960–7. doi: 10.1167/iovs.11-8409. [DOI] [PubMed] [Google Scholar]
- 8.Musch DC, Shimizu T, Niziol LM, Gillespie BW, Cashwell LF, Lichter PR. Clinical characteristics of newly diagnosed primary, pigmentary and pseudoexfoliative open-angle glaucoma in the Collaborative Initial Glaucoma Treatment Study. Br J Ophthalmol. 2012;96:1180–4. doi: 10.1136/bjophthalmol-2012-301820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naumann GO, Schlötzer-Schrehardt U, Küchle M. Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocular and systemic manifestations. Ophthalmology. 1998;105:951–68. doi: 10.1016/S0161-6420(98)96020-1. [DOI] [PubMed] [Google Scholar]
- 10.Hammer T, Schlotzer-Schrehardt U, Naumann GO. Unilateral or asymmetric pseudoexfoliation syndrome. An ultrastructural study? Arch Ophthalmol. 2001;119:1023–31. doi: 10.1001/archopht.119.7.1023. [DOI] [PubMed] [Google Scholar]
- 11.Aasved H. Prevalence of fibrillopathia epitheliocapsularis [pseudoexfoliation] and capsular glaucoma. Trans Ophthalmol Soc U K. 1979;99:293–5. [PubMed] [Google Scholar]
- 12.Forsius H. Prevalence of pseudoexfoliation of the lens in Finns, Lapps, Icelanders, Eskimos and Russians. Trans Ophthalmol Soc U K. 1979;99:296–8. [PubMed] [Google Scholar]
- 13.Andrikopoulos GK, Mela EK, Georgakopoulos CD, Papadopoulos GE, Damelou AN, Alexopoulos DK, et al. Pseudoexfoliation syndrome prevalence in Greek patients with cataract and its association to glaucoma and coronary artery disease. Eye (Lond) 2009;23:442–7. doi: 10.1038/sj.eye.6702992. [DOI] [PubMed] [Google Scholar]
- 14.Hiller R, Sperduto RD, Krueger DE. Pseudoexfoliation, intraocular pressure and senile lens changes in a population-based survey. Arch Ophthalmol. 1982;100:1080–2. doi: 10.1001/archopht.1982.01030040058007. [DOI] [PubMed] [Google Scholar]
- 15.Arnarsson A, Damji KF, Sverrisson T, Sasaki H, Jonasson F. Pseudoexfoliation in the Reykjavik Eye Study: Prevalences and related ophthalmological variables. Acta Ophthalmol Scand. 2007;85:822–7. doi: 10.1111/j.1600-0420.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 16.Forsman E, Cantor RM, Lu A, Eriksson A, Fellman J, Järvelä I, et al. Exfoliation syndrome: Prevalence and inheritance in a subisolate of the Finnish population. Acta Ophthalmol Scand. 2007;85:500–7. doi: 10.1111/j.1600-0420.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- 17.Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 18.Aragon-Martin JA, Ritch R, Liebmann J, O’Brien C, Blaaow K, Mercieca F, et al. Evaluation of LOXL1 gene polymorphism in exfoliation syndrome and exfoliation glaucoma. Mol Vis. 2008;14:533–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Mossbock TG, Renner W, Faschinger C. LOXL1 gene polymorphism and exfoliation glaucoma in a Central European population. Mol Vis. 2008;14:857–61. [PMC free article] [PubMed] [Google Scholar]
- 20.Schlötzer-Schrehardt U. Genetics and genomics of pseudoexfoliation syndrome/glaucoma. Middle East Afr J Ophthalmol. 2011;18:30–6. doi: 10.4103/0974-9233.75882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creasey R, Sharma S, Gibson CT, Craig JE, Ebner A, Becker T, et al. Atomic force microscopy-based antibody recognition imaging of proteins in the pathological deposits in pseudoexfoliation syndrome. Ultramicroscopy. 2011;111:1055–61. doi: 10.1016/j.ultramic.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997;124:685–7. doi: 10.1016/s0002-9394(14)70908-0. [DOI] [PubMed] [Google Scholar]
- 23.Citirik M, Acaroglu G, Batman C, Yildiran L, Zilelioglu O. A possible link between the pseudoexfoliation syndrome and coronary heart disease. Eye (Lond) 2007;21:11–5. doi: 10.1038/sj.eye.6702177. [DOI] [PubMed] [Google Scholar]
- 24.French DD, Margo CE, Harman LE. Ocular pseudoexfoliation and cardiovascular disease: A national cross-section comparison study. N Am J Med Sci. 2012;4:468–73. doi: 10.4103/1947-2714.101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekeroglu MA, Bozkurt B, Irkec M, Ustunel S, Orhan M, Saracbasi O. Systemic associations and prevalence of exfoliation syndrome in patients scheduled for cataract surgery. Eur J Ophthalmol. 2008;18:551–5. doi: 10.1177/112067210801800408. [DOI] [PubMed] [Google Scholar]
- 26.Demir N, Ulus T, Yucel OE, Kumral ET, Singar E, Tanboga HI. Assessment of myocardial ischaemia using tissue Doppler imaging in pseudoexfoliation syndrome. Eye (Lond) 2011;25:1177–80. doi: 10.1038/eye.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringvold A, Blika S, Sandvik L. Pseudoexfoliation and mortality. Acta Ophthalmol Scand. 1997;75:255–6. doi: 10.1111/j.1600-0420.1997.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 28.Shrum KR, Hattenhauer MG, Hodge D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol. 2000;129:83–6. doi: 10.1016/s0002-9394(99)00255-x. [DOI] [PubMed] [Google Scholar]
- 29.Gradum K, Heijl A, Bengtssan B. Glaucoma and mortality. Graefes Arch Clin Exp Ophthalmol. 2004;242:397–401. doi: 10.1007/s00417-004-0858-2. [DOI] [PubMed] [Google Scholar]
- 30.Ercegoviæ A, Brajkoviæ J, Suraæ IK, Haluzan MB. Prevalence, distribution and types of corneal astigmatism in cataract surgery patients in Sibenik County. Acta Clin Croat. 2012;51:275–8. [PubMed] [Google Scholar]
- 31.Emiroglu MY, Coskun E, Karapinar H, Capkýn M, Kaya Z, Kaya H, et al. Is pseudoexfoliation syndrome associated with coronary artery disease? N Am J Med Sci. 2010;2:487–90. doi: 10.4297/najms.2010.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bleich S, Roedl J, Von Ahsen N, Schlötzer-Schrehardt U, Reulbach U, Beck G, et al. Elevated homocysteine levels in aqueous humor of patients with pseudoexfoliation glaucoma. Am J Ophthalmol. 2004;138:162–4. doi: 10.1016/j.ajo.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Roedl JB, Bleich S, Reulbach U, Rejdak R, Kornhuber J, Kruse FE, et al. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma. 2007;16:234–9. doi: 10.1097/IJG.0b013e31802d6942. [DOI] [PubMed] [Google Scholar]
- 34.Puustjarvi T, Blomster H, Kontkanen M, Punnonen K, Teräsvirta M. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol. 2004;242:749–54. doi: 10.1007/s00417-004-0918-7. [DOI] [PubMed] [Google Scholar]
- 35.Tranchina L, Centofanti M, Oddone F, Tanga L, Roberti G, Liberatoscioli L, et al. Levels of plasma homocysteine in pseudoexfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:443–8. doi: 10.1007/s00417-010-1487-6. [DOI] [PubMed] [Google Scholar]
- 36.Liao D, Yang X, Wang H. Hyperhomocysteinemia and high-density lipoprotein metabolism in cardiovascular disease. Clin Chem Lab Med. 2007;45:1652–9. doi: 10.1515/CCLM.2007.358. [DOI] [PubMed] [Google Scholar]
- 37.Roedl JB, Bleich S, Reulbach U, Rejdak R, Naumann GO, Kruse FE, et al. Vitamin deficiency and hyperhomocysteinemia in pseudoexfoliation glaucoma. J Neural Transm. 2007;114:571–5. doi: 10.1007/s00702-006-0598-z. [DOI] [PubMed] [Google Scholar]
- 38.Brooks AM, Gillies WE. The development of microneovascular changes in the iris in pseudoexfoliation of the lens capsule. Ophthalmology. 1987;94:1090–7. doi: 10.1016/s0161-6420(87)33329-9. [DOI] [PubMed] [Google Scholar]
- 39.Schlotzer-Schrehardt U, Kuchle M, Naumann GO. Electron-microscopic identification of pseudoexfoliation material in extrabulbar tissue. Arch Ophthalmol. 1991;109:565–70. doi: 10.1001/archopht.1991.01080040133044. [DOI] [PubMed] [Google Scholar]
- 40.Atalar PT, Atalar E, Kilic H, Abbasoglu OE, Ozer N, Aksöyek S, et al. Impaired systemic endothelial function in patients with pseudoexfoliation syndrome. Int Heart J. 2006;47:77–84. doi: 10.1536/ihj.47.77. [DOI] [PubMed] [Google Scholar]
- 41.Yildirim Z, Yildirim F, Uçgun NI, Sepici-Dinçel A. The role of the cytokines in the pathogenesis of pseudoexfoliation syndrome. Int J Ophthalmol. 2013;6:50–3. doi: 10.3980/j.issn.2222-3959.2013.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorkhabi R, Ghorbanihaghjo A, Ahoor M, Nahaei M, Rashtchizadeh N. High-sensitivity C-reactive Protein and Tumor Necrosis Factor Alpha in Pseudoexfoliation Syndrome. Oman Med J. 2013;28:16–9. doi: 10.5001/omj.2013.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Türkyýlmaz K, Oner V, Kýrbas A, Sevim MS, Sekeryapan B, Ozgür G, et al. Serum YKL-40 levels as a novel marker of inflammation and endothelial dysfunction in patients with pseudoexfoliation syndrome. Eye (Lond) 2013 doi: 10.1038/eye.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stafiej J, Malukiewicz G, Lesiewska-Junk H, Rosæ D, KaŸmierczak K. Endothelial cell markers in patients with pseudoexfoliation syndrome? Scientific World Journal. 2012;2012:863949. doi: 10.1100/2012/863949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yüksel N, Pirhan D, Altintaþ O, Caðlar Y. Systemic high-sensitivity C-reactive protein level in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Glaucoma. 2010;19:373–6. doi: 10.1097/IJG.0b013e3181bdb570. [DOI] [PubMed] [Google Scholar]
- 46.Hollo G, Lakatos P, Farkas K. Cold pressor test and plasma endothelin-1 concentration in primary oper-angle and capsular glaucoma. J Glaucoma. 1998;7:105–10. [PubMed] [Google Scholar]
- 47.Visontai Z, Merisch B, Kollai M, Hollo G. Increase in carotid artery stiffness and decrease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br J Ophthalmol. 2006;90:563–7. doi: 10.1136/bjo.2005.087908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visontai Z, Horváth T, Kollai M, Holló G. Decreased cardiovagal regulation in exfoliation syndrome. J Glaucoma. 2008;17:133–8. doi: 10.1097/IJG.0b013e3181379d67. [DOI] [PubMed] [Google Scholar]
- 49.Miyazaki M, Kubota T, Kubo M, Kiyohara Y, Iida M, Nose Y, et al. The prevalence of pseudoexfoliation syndrome in a Japanese population: The hisayama study. J Glaucoma. 2005;14:482–4. doi: 10.1097/01.ijg.0000185436.15675.b3. [DOI] [PubMed] [Google Scholar]
- 50.Gonen KA, Gonen T, Gumus B. Renal artery stenosis and abdominal aorta aneurysm in patients with pseudoexfoliation syndrome. Eye (Lond) 2013;27:735–41. doi: 10.1038/eye.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Speèkauskas M, Tamoðiûnas A, Jaðinskas V. Association of ocular pseudoexfoliation syndrome with ischaemic heart disease, arterial hypertension and diabetes mellitus. Acta Ophthalmol. 2012;90:e470–5. doi: 10.1111/j.1755-3768.2012.02439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akarsu C, Unal B. Cerebral haemodynamics in patients with pseudoexfoliation glaucoma. Eye (Lond) 2005;19:1297–300. doi: 10.1038/sj.eye.6701776. [DOI] [PubMed] [Google Scholar]
- 53.Harris A, Zarfati D, Zalish M, Biller J, Sheets CW, Rechtman E, et al. Reduced cerebrovascular blood flow velocities and vasoreactivity in open-angle glaucoma. Am J Ophthalmol. 2003;135:144–7. doi: 10.1016/s0002-9394(02)01927-x. [DOI] [PubMed] [Google Scholar]
- 54.Stroman GA, Stewart WC, Golnik KC, Cure JK, Olinger RE. Magnetic resonance imaging in patients with low-tension glaucoma. Arch Ophthalmol. 1995;113:168–72. doi: 10.1001/archopht.1995.01100020050027. [DOI] [PubMed] [Google Scholar]
- 55.Ong K, Farinelli A, Billson F, Houang M, Stern M. Comparative study of brain magnetic resonance imaging findings in patients with low-tension glaucoma and control subjects. Ophthalmology. 1995;102:1632–8. doi: 10.1016/s0161-6420(95)30816-0. [DOI] [PubMed] [Google Scholar]
- 56.Ritland JS, Egge K, Lydersen S, Juul R, Semb SO. Exfoliative glaucoma and primary open-angle glaucoma: Associations with death causes and comorbidity. Acta Ophthalmol Scand. 2004;82:401–4. doi: 10.1111/j.1395-3907.2004.00297.x. [DOI] [PubMed] [Google Scholar]
- 57.Linnér E, Popovic V, Gottfries CG, Jonsson M, Sjögren M, Wallin A. The exfoliation syndrome in cognitive impairment of cerebrovascular or Alzheimer’s type. Acta Ophthalmol Scand. 2001;79:283–5. doi: 10.1034/j.1600-0420.2001.790314.x. [DOI] [PubMed] [Google Scholar]
- 58.Djordjevic-Jocic J, Jovanovic P, Bozic M, Tasic A, Rancic Z. Prevalence and early detection of abdominal aortic aneurysm in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Curr Eye Res. 2012;37:617–23. doi: 10.3109/02713683.2012.665120. [DOI] [PubMed] [Google Scholar]
- 59.Praveen MR, Shah SK, Vasavada AR, Diwan RP, Shah SM, Zumkhawala BR, et al. Pseudoexfoliation as a risk factor for peripheral vascular disease: A case-control study. Eye (Lond) 2011;25:174–9. doi: 10.1038/eye.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


