Abstract
Background:
The spectrum of liver dysfunction in children with dengue infection is wide and has been associated with disease severity.
Aims:
This study was undertaken to estimate the range of hepatic involvement in dengue infection in children.
Materials and Methods:
This study assessed the biochemical and clinical profile of hepatic involvement by dengue virus in 120 children with serologically positive dengue fever (DF), aged 2 months to 14 years.
Results:
All cases were grouped into DF without warning signs (Group 1), DF with warning signs (Group 2) and severe dengue (Group 3) according to revised World Health Organization 2009 criteria. The spectrum of hepatic manifestations included hepatomegaly (80.8%), hepatic tenderness (46.3%), jaundice (60%), raised aspartate transaminase (AST), alanine transaminase (ALT) and prolonged prothrombin time (41.7%) and reduced serum albumin (56%).
Conclusions:
Hepatic dysfunction was observed more in Groups 2 and 3. There was 84.4% and 93.75% ALT and AST elevation respectively in Group 2 and 94.5% and 95.9% ALT and AST elevation respectively in Group 3 and fulminant hepatic failure was observed in Group 3. Therefore in a child with fever, jaundice, hepatomegaly and altered liver function tests, the diagnosis of dengue infection should be strongly considered in areas where dengue infection is endemic.
Keywords: Liver function tests, Severe dengue
Introduction
Dengue infection, an arthropod - borne viral hemorrhagic fever, continues to be a major challenge to public health in South-East Asia.[1] Estimates suggest that annually over 50 million cases of severe dengue occur in Asian countries with a case fatality rate of lesser than 5%.[2] Of these, at least 90% are children younger than 15 years old.[2]
Although dengue virus is a non-hepatotropic virus, hepatomegaly is commonly seen in dengue along with a rise in serum aminotransferases. The degree of liver dysfunction varies from mild injury with elevation of aminotransferases to even fulminant hepatic failure.[3,4] Hepatic dysfunction in dengue infection may be attributed to direct viral effect on liver cells or as a consequence of dysregulated host immune responses against the virus.[3]
Jaundice in dengue infection has been associated with fulminant liver failure and by itself is a poor prognostic factor.[5] However, there are only a few studies concerning liver dysfunction in children with liver dysfunction.
This study was conducted to assess the profile of liver involvement in children with dengue fever (DF) admitted in the Department of Pediatrics of a Tertiary Care Hospital of Eastern India.
Materials and Methods
Ethical Committee clearance was taken from the institution and informed consent was taken from the guardian of every patient who took part in this study. This prospective, hospital based study was conducted in the Department of Pediatrics, of a Tertiary Care Hospital of Eastern India from January 2011 to January 2013. All clinically suspected dengue infection as per the revised World Health Organization (WHO) guidelines 2009 in children of age between 2 months and 14 years were screened. Patients included were serologically confirmed (immunoglobulin M [IgM] positive), by dengue IgM capture enzyme-linked immunosorbent assay (ELISA), DF patients admitted to the Department of Pediatrics, all patients between 2 months and 14 year, patients giving informed consent for the study. Those excluded were IgM negative dengue like illness; children aged >2 months and <14 years of age, children with pre-existing liver diseases, other concomitant infections affecting the liver such as malaria, typhoid, hepatitis A and B, those patients who refused to be included in this study.
A detailed history and thorough clinical examination were performed in all cases. Data was collected in a predesigned, pretested proforma. All cases were subjected to the following investigations: Dengue IgM capture ELISA, hemoglobin (Hb), total count, differential leukocyte count, platelet count (PLC), hematocrit (HCT), peripheral blood smear, serum bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), serum albumin, serum globulin, total proteins, prothrombin time (PT), Activated partial thromboplastin time (APTT), ultrasound abdomen and thorax. Other causes of hepatitis such as malaria, viral hepatitis, enteric fever, malignancies were excluded by clinical and laboratory investigations.
Sample size of this study came to be 120 patients after excluding 10 patients as they met the exclusion criteria. As per dengue update: WHO 2009 guideline, probable dengue was defined as live in/travel to dengue endemic area with two of the following: Nausea, vomiting; rash; aches and pains; tourniquet test positive; leucopenia; any warning sign.[2] Warning signs were abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleed, lethargy and restlessness, liver enlargement <2 cm, laboratory: Increase in HCT with concurrent rapid decrease in PLC. Criteria for severe dengue was severe plasma leakage leading to dengue shock syndrome, fluid accumulation with respiratory distress; severe bleeding; severe organ involvement (liver: AST or ALT <1000, impaired consciousness, heart and other organs involvement).[2] The study population was subcategorized into dengue without warning signs (n = 15) (Group 1); dengue (with warning signs) (n = 32) (Group 2) and severe dengue (n = 73) (Group 3).
Statistical analysis
Statistical analysis was carried out with IBM Statistical Package for the Social Sciences 19.0, (SPSS. Inc., IBM Copyright 1989, U.S.A. 2010 SPSS) using descriptive statistics, crosstabs, Chi-square test for categorical outcomes and t-test and Mann-Whitney U test for comparison of means.
Results
The study group included 120 children of age between 2 months and 14 years during the 2 years study period satisfying the revised WHO 2009 criteria for DF after excluding malaria, enteric fever, hepatitis A and hepatitis B. Out of these, 59 (49.2%) patients were male and 61 (50.8%) were females. 77 (64.2%) patients belonged to rural area while 43 (35.8%) came from urban locality. Minimum age of patient was 2 months and maximum age was 13 years with mean age of 7.1 ± 1.1. The epidemiological profile and clinical features have been summarized in Table 1.
Table 1.
The epidemiological and clinical features of study population (n=120)
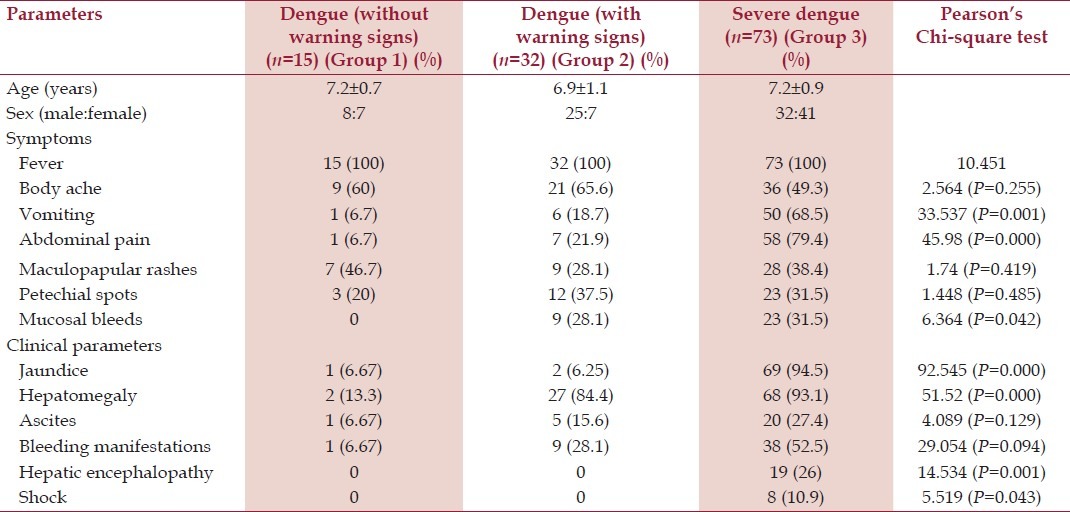
All patients suffered from fever (100%), body aches was present in 63.3% patients. Majority patients presented with fever of short duration, but 16 (13.3%) patients had prolonged history of fever <15 days. Abdominal pain was present in 6.7%, 18.7% and 68.5% of Group 1, Group 2 and Group 3 patients (P > 0.001). Vomiting was also significantly present in severe dengue (Group 3) in comparison with Groups 1 and 2 (P = 0.001). Maculopapular rash and petechial spots were not statistically significant between the three groups. However, mucosal bleeding was present in 31.5% of Group 3 patients and 28.1% of Group 2 patients, but not in Group 1 (P = 0.042).
Out of 120 patients, 94.5% of severe dengue (Group 3) clinically had icterus, whereas only 6% of Groups 1 and 2 had icterus (P > 0.001). Hepatomegaly was present in 93.1% of Group 3 and 84.4% of Group 2, but only 13% of Group 1 (P > 0.001). Hepatic tenderness was observed in 46.3% of children, which was more in Group 1 (63.8%), Group 2 (52.8%) compared with Group 3 (22.3%) group (P = 0.001). Splenomegaly was present in only 42.3% patients with severe dengue, but none in Groups 1 and 2. Hepatic encephalopathy and shock were significantly more in severe dengue (19 [26%] and 8 [10.9%] respectively, P > 0.001 and P = 0.053 respectively).
The majority of patients had Hb lesser than 10 g%. The difference in mean Hb between Groups 1 and 3 was statistically significant (P = 0.002). Thrombocytopenia was present in the majority (90%) with a minimum count of 12,000/cumm and a maximum of 180,000/cumm. The difference in mean PLCs between Groups 1 and 2 and Groups 1 and 3 was statistically significant (P = 0.008 and P = 0.001, respectively) as shown in Table 2.
Table 2.
The liver function tests and other laboratory parameters of our study subjects (n=120)

Abnormal liver functions were significantly more in Groups 2 and 3. Table 2 shows the comparison of PLCs, serum bilirubin, ALT, AST, ALP, serum albumin, globulin, total protein, PT, APTT levels between the groups. The rise in the levels of bilirubin was statistically significant between Groups 1 and 3 (U = 63, P > 0.001) and Groups 2 and 3 (U = 146.5, P > 0.001). Mean ALT elevation was statistically significant between Groups 1 and 3 (97, P > 0.001) and Groups 2 and 3 (U = 424.5, P > 0.001). Mean AST, ALP, serum albumin and serum globulin elevations were also statistically significant between Groups 1 and 3 and Groups 2 and 3. More than 10-fold increase in the levels of both ALT were observed mainly in Groups 2 (10.7%) and 3 (21.3%). There was no significant difference in the liver function tests (LFT’s) in children with or without hepatomegaly. Among those with hepatomegaly also there was no significant difference in the LFT’s with/without hepatic tenderness. Fulminant hepatic failure was observed in 7 (9.7%) patients with severe dengue (Group 3), but not in Groups 2 and 3.
Ultra sound revealed gall bladder thickening, ascites and pleural effusion more in the Group 3 (64.4%, 27.4% and 59.6%) and Group 2 (21.9%, 15.6%, 48%) compared with Group 1 (6.7%, 6.7% and 6.7%) (between Groups 1 and 3, U = 231.5, P ≤ 0.001).
Out of 120 dengue patients, three children (aged between 2 months and 1 year) with deranged LFT, two with pulmonary edema, two patients with coagulopathy and two patients with multi organ dysfunction expired, all of them belonging to severe dengue group (Group 3) (P > 0.001 between Groups 1 and 3).
Follow-up PLCs were performed, 67.8% of patients had improvements in their counts in 10 days while remaining improved in 3 weeks.
Discussion
Liver involvement in dengue is usually manifested by hepatomegaly (clinically) or increase in liver enzymes (biochemically). Presentation with jaundice can simulate acute hepatitis. Severe dengue can manifest with fulminant hepatic failure and has been the cause of death in many children with dengue infection.[6,7]
Out of 120 cases in our study, 80.8% had hepatomegaly, which was more common in severe dengue (93.1%) and dengue with warning signs (84.4%) group than in dengue without warning signs group (13.3%). Similar association of hepatomegaly in dengue has been reported in 43-100% of cases in children.[4,5,8,9] In fact, Petdachai reported hepatomegaly in all children with Dengue Shock Syndrome.[10]
Jaundice was observed in 72 children (60%), most common in severe dengue (94.5%); thus, making jaundice an important predictor of severe dengue. Jaundice has been reported in 2-25% of cases by several authors.[4,11,12] Nimmannitya et al. reported jaundice and encephalopathy in 18 cases of dengue hemorrhagic fever of whom 10 died.[9]
Minor manifestations such as fever (100%), body ache (66.3%) and rashes (36.7%) were all more common in severe dengue, but the result was not statistically significant.
Among the other warning signs, abdominal pain (especially hepatic tenderness) was the dominant finding in 66 (55%) of the children, it also being more prominent in 58 children with severe dengue (79.4%). This is very similar to the observations made in a study from Thailand.[13] Persistent vomiting was present in 57 children (47.5%) with 87.7% of them in the severe dengue group. However, bleeding manifestations in the form of skin bleed/mucosal bleed was not very common (31.5% each) with equal number of children being affected (23 children each) in the severe dengue group mandating the need to search for evidences of mucosal bleed in children presenting solely with skin bleed.
Abnormal hepatic enzymes in dengue infection varies from 36.4% to 96% both in children and adults in different articles.[14,15] The hepatic enzymes were elevated significantly in Groups 2 and 3, especially 10-fold rises when compared with Group 1, which is similar to other studies.[12,15] In a large study from Brazil, out of 1585 dengue cases, elevation in AST and ALT were seen in 63.4% and 45% of patients respectively, with 3.8% of cases having 10-fold increase in transaminase levels.[15] In our study, more than 10-fold increase in the levels of ALT and AST were observed mainly in Group 2 (10.7%) and Group 3 (21.3%). This may indicate that children are at higher risk of hepatic involvement compared with adults. Detection of abnormally high transaminase enzymes may indicate the possibility of consequent hepatic encephalopathy.[3] Elevation of AST was more compared with ALT in the present study similar to other observations may be due to involvement of myocytes.[5,16] This differs from the pattern seen in viral hepatitis, in which ALT levels are usually higher than or equal to AST levels.[16]
Other warning signs like fluid accumulation were evident in 26 children (21.7%) with 20 of them being in severe dengue infection and thrombocytopenia was present in the majority (90%) with a minimum count of 12,000/cumm and a maximum of 180,000/cumm.
Prolonged PT (INR < 1.5) values in 41.7% of the cases (54.2% of Group 3 and 31.2% of Group 2 (P = 0.001). Hyperglobulinemia was observed in 56% of the cases. Hyperglobulinemia was observed more in Group 3 (59%) and Group 2 (52.3%) compared to Group 1 (12.3%). Wong and Shen reported low globulin level in 14.2% and low albumin level in 16.5%, derangements in PT and APTT in 42.5% of his adult cases.[12] However, Itha et al. noticed hypoalbuminemia in 76%, deranged PT and APTT in 7% of adult cases.[17] The reduction of serum globulin may be a significant factor in fluid loss into third space, which is indicative of severity of dengue infection.
CNS manifestation in the form of hepatic encephalopathy was prominent in 19 children (15.8% of 120 patients) with 8 of them (6.7%) presenting with shock and multiple organ involvement. All these children with fulminant hepatic failure were the major group with the mortality among the 120 children studied. In recent studies from India and Thailand, dengue infection is the most important cause of acute hepatic failure in children contributing to 18.5% and 34.3% of the cases respectively.[10,11] This is somewhat different from our findings as well as a study from India, which state that hepatic dysfunction was more severe in children with prolonged shock.[3] Of the patients with dengue without warning signs (Group 1), 100% were cured while 31 patients (96.9%) were cured in the dengue with warning signs group (Group 2) and 63 patients (87.5%) were cured in the severe dengue group (Group 3). Mortality amounted to one patient (3.1%) and nine patients (12.5%) in the dengue with warning signs and the severe dengue group respectively (P > 0.001 between Groups 1 and 3). Therefore, mortality from dengue does appear to be important, which can only be minimized by increasing the awareness about dengue specific signs and symptoms, like bleeding, rash and even shock, which is low and mass media like television appears to play a very important role for increasing the knowledge about dengue among people.[18]
Mechanisms of liver injury in dengue may be due to the direct effects of the virus or host immune response on liver cells, circulatory compromise, metabolic acidosis and/or hypoxia caused by hypotension or localized vascular leakage inside the liver.[17] A study from Mexico in the mice and humans established the correlation between liver damage and dengue based on the AST activity.[3] An Indian study reported correlation between mortality and severe liver dysfunction in children with dengue infection.[19] Elevated transaminase levels have been suggested as a potential marker to help differentiate dengue from other viral infections during the early febrile phase.[12]
Our study is more powerful than previous studies as some are retrospective, study group included seronegative dengue cases and they have not excluded common illnesses such as malaria, enteric fever, viral hepatitis pertinent to tropical region. Our study included only serologically confirmed children of dengue infection not having concomitant infection. However, the limitation of our study was that liver biopsy was not performed on humanitarian grounds in any child for confirmation of diagnosis.
Conclusion
Hepatic involvement in dengue varies from jaundice to more than 10-folds elevation of liver enzymes. Hepatomegaly is a most important clinical sign, but alteration of LFT’s can occur with or without hepatomegaly. Significant rise of liver enzymes signifies severe dengue. Presence of fever, jaundice and hepatomegaly in endemic areas should arouse the suspicion of dengue hepatitis in addition to malaria, enteric fever and viral hepatitis.
Acknowledgements
The authors would like to thank the Departments of Biochemistry & Microbiology, Medical College and Hospitals, Kolkata.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Halstead SB. Dengue. Curr Opin Infect Dis. 2002;15:471–6. doi: 10.1097/00001432-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Dengue: Guideline for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. [Google Scholar]
- 3.Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg. 2006;100:608–14. doi: 10.1016/j.trstmh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Lum LC, Lam SK, George R, Devi S. Fulminant hepatitis in dengue infection. Southeast Asian J Trop Med Public Health. 1993;24:467–71. [PubMed] [Google Scholar]
- 5.Chhina RS, Goyal O, Chhina DK, Goyal P, Kumar R, Puri S. Liver function tests in patients with dengue viral infection. Dengue Bull. 2008;32:110–7. [Google Scholar]
- 6.Wiwanitkit V. Liver dysfunction in dengue infection: An analysis of the previously published Thai cases. J Ayub Med Coll Abbottabad. 2007;19:10–2. [PubMed] [Google Scholar]
- 7.Kumar R, Tripathi P, Tripathi S, Kanodia A, Venkatesh V. Prevalence of dengue infection in north Indian children with acute hepatic failure. Ann Hepatol. 2008;7:59–62. [PubMed] [Google Scholar]
- 8.Mohan B, Patwari AK, Anand VK. Hepatic dysfunction in childhood dengue infection. J Trop Pediatr. 2000;46:40–3. doi: 10.1093/tropej/46.1.40. [DOI] [PubMed] [Google Scholar]
- 9.Nimmannitya S, Thisyakorn U, Hemsrichart V. Dengue haemorrhagic fever with unusual manifestations. Southeast Asian J Trop Med Public Health. 1987;18:398–406. [PubMed] [Google Scholar]
- 10.Petdachai W. Hepatic dysfunction in children with dengue shock syndrome. Dengue Bull. 2005;29:112–7. [Google Scholar]
- 11.Wahid SF, Sanusi S, Zawawi MM, Ali RA. A comparison of the pattern of liver involvement in dengue hemorrhagic fever with classic dengue fever. Southeast Asian J Trop Med Public Health. 2000;31:259–63. [PubMed] [Google Scholar]
- 12.Wong M, Shen E. The utility of liver function tests in dengue. Ann Acad Med Singapore. 2008;37:82–3. [PubMed] [Google Scholar]
- 13.Burke T. Dengue haemorrhagic fever: A pathological study. Trans R Soc Trop Med Hyg. 1968;62:682–92. doi: 10.1016/0035-9203(68)90120-x. [DOI] [PubMed] [Google Scholar]
- 14.Kamath SR, Ranjit S. Clinical features, complications and atypical manifestations of children with severe forms of dengue hemorrhagic fever in South India. Indian J Pediatr. 2006;73:889–95. doi: 10.1007/BF02859281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souza LJ, Alves JG, Nogueira RM, Gicovate Neto C, Bastos DA, Siqueira EW, et al. Aminotransferase changes and acute hepatitis in patients with dengue fever: Analysis of 1,585 cases. Braz J Infect Dis. 2004;8:156–63. doi: 10.1590/s1413-86702004000200006. [DOI] [PubMed] [Google Scholar]
- 16.Chairulfatah A, Setiabudi D, Ridad A, Colebunders R. Clinical manifestations of dengue haemorrhagic fever in children in Bandung, Indonesia. Ann Soc Belg Med Trop. 1995;75:291–5. [PubMed] [Google Scholar]
- 17.Itha S, Kashyap R, Krishnani N, Saraswat VA, Choudhuri G, Aggarwal R. Profile of liver involvement in dengue virus infection. Natl Med J India. 2005;18:127–30. [PubMed] [Google Scholar]
- 18.Chinnakali P, Gurnani N, Upadhyay RP, Parmar K, Suri TM, Yadav K. High level of awareness but poor practices regarding dengue fever control: A cross-sectional study from North India. N Am J Med Sci. 2012;4:278–82. doi: 10.4103/1947-2714.97210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhooria GS, Bhat D, Bains HS. Clinical profile and outcome in children of dengue hemorrhagic fever in North India. Iran J Pediatr. 2008;18:222–8. [Google Scholar]


