Abstract
Background:
Cryptococcal meningoencephalitis (CM) kills about half a million human immunodeficiency virus (HIV) patients per year, mostly in Africa.
Aim:
The aim of this study was to determine the prevalence, clinical presentation and in-hospital outcome of CM among HIV-infected patients in Douala.
Materials and Methods:
A cross-sectional clinical note review of 672 HIV-1 patients’ files admitted from January 1 st 2004 to December 31 st 2009 at the Internal Medicine unit of the Douala General Hospital, Cameroon was performed. Only patients diagnosed of CM by microscopy of Indian ink stained cerebrospinal fluid (CSF) were studied.
Results:
The prevalence of CM in the study was 11.2%. Mean age of patients was 36.9 12.7 years. Median cluster of differentiation 4 (CD4) cell count was 23 cells/μL, (interquartile range [IQR]: 10-61) and 62.7% of CD4 cell counts were >50 cells/μL. The most prevalent symptom was headache in 97.3% of patients. In CSF, median proteins was 0.9 g/L (IQR: 0.6-1); median glucose 0.2 g/L (IQR: 0.1-0.3) and median leucocyte count 54 cells/μL (IQR: 34-76) mostly of mixed cellularity. The case fatality rate was 52% and low CD4 cell count was strongly associated with death, odd ratio 4.6 (95% confidence interval: 2.6-8.0, P > 0.001).
Conclusion:
The high case fatality of CM in Douala warrants adequate diagnostic measures and optimization of standardized treatment to reduce mortality.
Keywords: Cerebrospinal fluid, Cluster of differentiation 4, Cryptococcal meningoencephalitis, Headaches, Human immunodeficiency virus/acquired immunodeficiency syndrome
Introduction
Cryptococcosis, an environmentally acquired fungal infection, remains a major cause of opportunistic infection in human immunodeficiency virus (HIV) infection in sub-Saharan Africa.[1] Cryptococcal meningoencephalitis (CM) the most common form of cryptococcosis is the most severe and life-threatening fungal infection in HIV even in high income settings.[2,3] Its incidence in HIV-infected patients varies considerably across settings from 5% to 11% in the USA and Europe to 25.8% and 33% in South East Asia and Africa respectively.[4] Recently, in South Africa, CM accounted for 63% of microbiological diagnoses in all adult patients with meningitis 99% of which occurred in HIV-infected patients.[5] In addition, CM is among the leading causes of mortality in patients initiating antiretroviral therapy (ART) in resource limited settings.[6]
Recent analysis of data from the center for disease control shows that cryptococcal disease is associated with an estimated half a million deaths per year with a 3 month mortality exceeding 70% in Africa.[1] Until date, the combination high incidence and existence of known unsatisfactory treatment for cryptococcosis implies it still remains a major cause of death among patients with acquired immunodeficiency syndrome (AIDS).[7,8]
In high income settings, the widespread use of ART has been associated with a decrease in incidence of cryptococcosis, but this is not the case in low income settings where incidence and mortality remain extremely high[1] due to uncontrolled HIV disease and limited access to ART or health-care. As such, CM is most common among patients with advanced HIV disease, those unaware of their HIV serostatus and those failing or not compliant with treatment.[9]
In Cameroon, where the prevalence of HIV among adults has been estimated at 5.3%[10] data on CM in HIV-infected patients is sparingly available.[11,12] More so, the burden of CM as a cause of mortality among HIV-infected patients has not been fully elucidated. We therefore, opted to carry out a clinical case note analysis of HIV-infected patients admitted to the Douala General Hospital so as to determine the prevalence of CM, its clinical features as well as the in-hospital outcome.
Materials and Methods
Prior to the study, local institutional ethical clearance from the Douala General Hospital was sought and obtained.
A cross-sectional study was carried out in the internal medicine unit of the Douala General Hospital, a tertiary hospital situated in Douala, the largest city and economic capital of Cameroon. The study population comprised of all adult (age < 18 years) HIV-1-infected patients with a confirmatory diagnosis of CM admitted to this hospital between January 1 st 2004 and December 31 st 2009. In order to identify eligible patients for the study, the admission register of the internal medicine unit was used. This register, which is held by the consulting physicians of the unit, contains patients’ diagnoses and their in-hospital outcome: Discharged or dead. From this register, all HIV-infected patients were sorted and those with CM identified. From the archives, clinical case files of CM patients were obtained for abstraction of information relevant to the study. At the Douala General Hospital, diagnosis of central nervous system (CNS) disease is made following an algorithm.
Patients, who present with symptoms and signs suggestive to CNS disease, first of all have a head computerized tomographic (CT) scan performed so as to exclude space occupying lesions and/or signs of raised intracranial pressure. In the absence of these and no other contraindication for a lumbar tap (LP), cerebrospinal fluid (CSF) is obtained for microbiological and biochemical analysis. Etiological diagnosis of CNS disease is therefore made using an aggregate of clinical, CSF and CT scan features. For CM, the diagnosis is only confirmed when Cryptococcus neoformans is identified in CSF following Indian ink staining. In this study, only patients with confirmed CM were included in the analysis. Until date, CM is managed using high dose fluconazole at 1200 mg/day: Amphotericin B and flucytosine are not used because they are expensive and unavailable.
In this institution, diagnosis of HIV is made according to the guidelines of the Cameroon National Aids Control Committee[13] by antibody detection on two successive samples using a third generation enzyme-linked immunosorbent assay test BIOREX® (Biorex Diagnostics Limited, Antrim, United Kingdom). If both the samples are positive, third sample is collected and tested using Genie® III HIV-1/HIV-2 assay (Bio-Rad Diagnostics, Marnes la Coquette, France) to specify whether HIV-1 or HIV-2. Patient will be declared positive for HIV if these three tests are positive. In case of any discordance, testing is performed using a Western blot assay (New LAV blot, Diagnostics, Pasteur, Marnes la Coquette, France).
Statistical analysis
Data analysis was performed using the STATA 11.2 statistical package (Stata Corporation, College Station, Texas, USA). Continuous variables were expressed as means and standard deviations or as medians and interquartile ranges (IQR) where necessary. Comparisons in contingency tables were done using Pearson Chi-squared test or Fisher’s exact test where appropriate. Categorical variables were defined as either present or absent. The main outcome of interest was in-hospital mortality expressed as a percentage of the study population. Mantel-Haenszel analysis method was used to establish the association between the main outcome and other variables and this association was expressed as odd ratio (OR) together with its 95% confidence interval (CI). However, logistic regression was not done because of the relatively small sample size, which rendered certain cells of contingency tables empty; hence, making such analysis impossible. Evidence of association was considered for a two-tailed P > .05. The study period represents the era when ART was being introduced in Cameroon and had to be obtained by out-of-pocket payment by patients, most of whom could not afford; therefore, information on ART management was not obtainable from the files, hence not included in the analysis.
Results
Characteristics of the study population
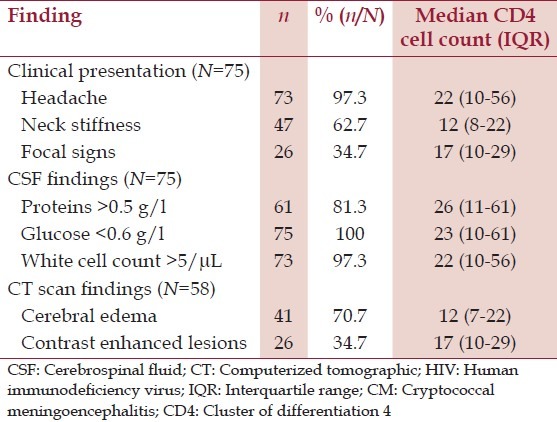
During the study, period out of the 672 HIV-infected patients admitted to the wards 11.2% (75/672) were diagnosed with CM. Their mean age was 36.9 ± 12.7 years. The median cluster of differentiation 4 (CD4) cell count was 23 cells/μL (IQR: 10-61) and 62.7% (47/75) of them had CD4 cell counts of less than 50 cells/μL [Table 1]. Men had a relatively lower median CD4 cell count than women (12 cells/μL, IQR: 7-23 vs. 65 cells/μL, IQR: 51-101, P > 0.001). The most common clinical symptom was headache in 97.3% (73/75) of patients [Table 2]. All patients had an LP done for CSF analysis. Pre-LP head CT scan was done in 77.3% (58/75) of patients, 70.7% (41/58) of whom had cerebral edema [Table 2]. Macroscopically, all CSF were clear. On CSF analysis median proteins level was 0.9 g/L (IQR: 0.6-1), median glucose level was 0.2 g/L (IQR: 0.1-0.3) and median leucocyte count was 54 cells/μL (IQR: 34-76) most of which were mixed cell types. In all 75 CSF, C. neoformans was identified by Indian ink staining.
Table 1.
General characteristics of 75 HIV-infected patients diagnosed with CM
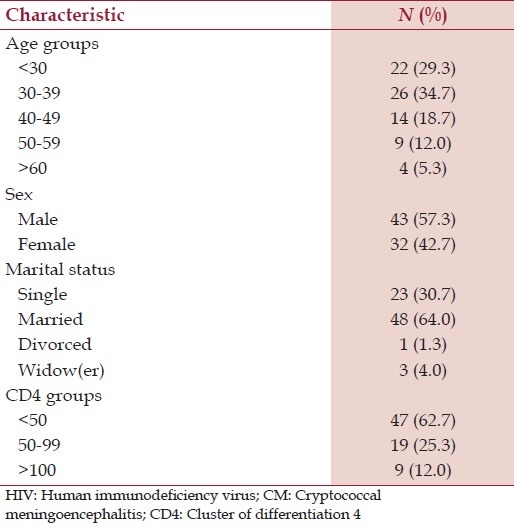
Table 2.
Clinical, CSF and CT scan findings and median CD4 cell counts in HIV patients with CM
Case fatality and its related factors
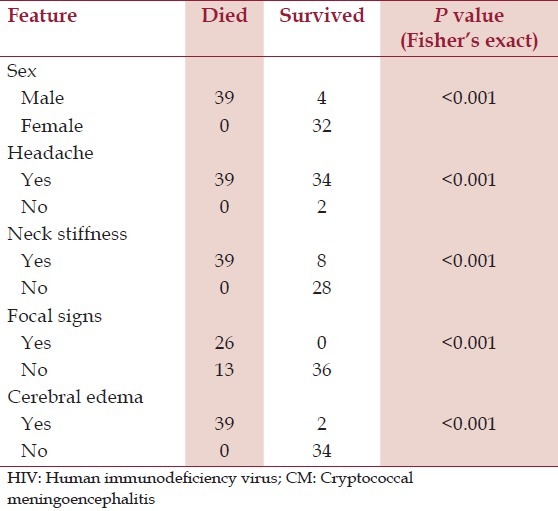
Out of the 75 patients, 52% (39/75) died. Those who died were relatively younger, with a mean age of 33.5 ± 10.7 years and older age was weakly associated with survival (OR 0.4, 95% CI: 0.1-1, P = 0.05). The median CD4 cell count of those who died was 12 cells/μL (IQR: 12-23) and low CD4 cell count was a factor strongly associated with death, OR 4.6 (95% CI: 2.6-8.0, P > 0.001). All those who died were men [Table 3].
Table 3.
Contingency table showing fatality rate and its related factors in 75 HIV patients with CM
Discussion
In our study, CM was found in 11.2% of HIV patients admitted in the medical ward of the Douala General Hospital. This finding was similar to that of other studies[14,15,16] though in settings with lower HIV and cryptococcal disease burden, but lower than what was found in Nairobi in 2007,[17] a setting similar to ours. This difference in prevalence might reflect the difference in the methods of ascertainment of CM. In our study, the confirmatory diagnosis of CM was only made when C. neoformans was identified in CSF by Indian ink stain. During the study period, the search for cryptococcal antigen in CSF as well as CSF culture for Cryptococcus was not common practice. This might have led to an underestimation of the prevalence of CM in our study population explaining the difference in prevalence in settings with a similar burden to ours. Therefore, in order to increase the diagnostic sensitivity of CM in our setting, routine CSF fungal cultures have to be done, a practice which until date is still not yet commonly done. To the best of our knowledge, the search of cryptococcal antigen in CSF and/or blood, which has been shown to have high positive and high negative predictive values of CM in HIV-infected patients[7] is not routinely practiced in our milieu.
Clinically, the onset of CM is usually insidious, symptoms and signs may be subtle and non-specific.[18] Headache, which is a symptom of overt brain dysfunction and meningitis[19] was the most prominent clinical presentation, present in 97.3% of our patients. This finding was in accordance with other reports[17,20,21,22] implying that headache in HIV-infected patients should be thoroughly investigated as it could be the only symptom of a life-threatening disease. In fact, evidence shows that in an ambulatory African HIV-infected population presenting with new onset of headache, the diagnosis of CM is second to bacterial sinusitis as a cause in 28% of patients.[23]
This is to be highly considered by caregivers to HIV-infected patients, especially in very busy HIV out-patient clinics where some symptoms might be overlooked and not appropriately investigated. Thus, in settings where minimal CSF analysis could be done, clinicians should not hesitate to perform LPs in HIV patients with symptoms of CNS disease if no contraindication.
In our setting, the main available diagnostic modalities of CM are head CT scan and CSF analysis. Though all our patients had their CSF analyzed, head CT scan was done in only 77.3% (58/75) of patients albeit our CNS disease diagnostic algorithm, which defines an indication for head CT scans to all patients who present with symptoms of CNS disease. Despite the fact that most of our patients presented with headache and that the primary diagnostic procedure for headache in HIV positive subjects is neuroimaging,[24,25] CT scanning still presents as a real challenge in sub-Saharan Africa where costs, access to diagnostic expertise and equipment are still limiting factors.[23] However, among those who could afford, the most common CT scan finding was cerebral edema. In CSF analysis, routine biochemical (proteins and glucose) and cell counts findings revealed results comparable with those of other studies.[5,21] India ink stains found Cryptococcus in all our patients meanwhile some other authors found positivity in 74-88% of patients.[21,26] This difference is simply because Indian ink identification of Cryptococcus in CSF of patients was a strict inclusion criterion for our study population. This creates a caveat in our findings because many cases of CM could have been missed given our sole reliance on India ink stain for diagnosis. This therefore, implies we need more rapid, less invasive and highly sensitive diagnostic tests like cryptococcal antigen detection in serum, some of which are sensitive enough to detect antigenemia, many days before the onset of symptoms.[7] Given that positive antigenemia, especially in the presence of symptoms has a high positive predictive value for CM,[7] this will ameliorate our timely diagnosis and facilitate the institution of appropriate treatment so as to reduce mortality.
CM is a fatal disease as could be shown in a study in Malawi in 1994 where mortality was 81% with a median survival of 4 days for patients who did not receive antifungal chemotherapy because they could not afford.[20] In our study, the case fatality rate of 52%, though high it was in line with findings of other studies.[22,27,28] This high fatality rate could be related first to the natural history of CM and secondly to the inappropriate management issues in our milieu, which need to be addressed.[29] During our study period, the only available treatment (and until date) was generic fluconazole, whose availability was not sustained. In high income settings, the preferred regimen is induction therapy with amphotericin B intravenously plus flucytosine orally for 2 weeks followed by consolidation therapy with fluconazole after significant clinical improvement and negative CSF culture.[30] The use of flucytosine in this regimen is associated with more rapid mycological response and relapses may be less likely to occur.[31] In resource limited settings, these drugs are not available because of their high price. More so, most centers cannot handle amphotericin B administration due to its high toxicity, which requires close laboratory monitoring. The sustained introduction and safe use of these drugs in such settings are therefore indispensable in reducing case fatality associated with CM. Adjuvant to appropriate antifungal chemotherapy is the management of increased intracranial pressure, one of the most critical determinants of outcome of CM.[32] In patients with suspected CM, CSF opening pressure measurement during the LP should be done because it has been shown to be an indicator of more severe symptoms and increased mortality.[32] In our study population, CSF opening pressure was never measured, a factor which could in part explain our high case fatality. Consequently, clinicians caring for HIV patients should routinely measure CSF opening pressure during LP and consider aggressive repeated high-volume CSF drainage in CM patients when this pressure exceeds 25 cm H 2 O.[32] More so our high case fatality was observed mostly in men. This could be simply because in Cameroon, women are more easily accessible to screening for HIV than men because it is a routine practice to screen all pregnant women for HIV. As such, early diagnosis and hence treatment could prevent severe immune depression. The fact that men were dying more could be because they present to hospitals late in a state of advanced immune depression most especially as very low CD4 cell count was a factor in this study, strongly associated with death. Another study similarly found that most patients with CM had less 100 CD4 cells.[33]
This study had certain limitations. Firstly, its retrospective nature involving data abstraction from clinical case notes was a source of bias due to missing data most especially as patient findings are not always recorded the same way. Secondly, choosing to study only patients with a confirmed diagnosis of CM might have led to a gross underestimation of the burden of CM in AIDS patients admitted to the Douala General hospital during the study period. Thirdly, the relatively small sample size, though reflecting the reality of confirmed CM during the study period, limited statistical analysis and did not allow the measurement of the magnitude of association between CM and other covariates. Fourthly, the study period was when ART was still being introduced in Cameroon, with very few patients being inconsistently on HIV treatment due to a low scale up at the time. The fact that the effects of ART were not included in the analysis, might have confounded the high case fatality we found. Finally, being a hospital based study in an institution where access to care is not affordable to many, the findings could not be generalized to the Cameroonian setting. However, the study has permitted the identification of the flaws in the diagnosis and appropriate management of CM, which if properly addressed, would go a long way to decrease the high mortality associated with CM in our setting.
Conclusion
CM is common among HIV-infected patients in Douala. Up-to-date, cheap, easy to use and highly sensitive diagnostic methods for CM are indispensable for the proper estimation of the burden of CM in Cameroon. The introduction and optimization of standard antifungal chemotherapy, an aggressive adjuvant management of high CSF opening pressure as well as increased use of ART are important factors to be considered in order to reduce the morbidity and mortality associated with CM in Cameroon.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Bhagwan S, Naidoo K. Aetiology, clinical presentation, and outcome of meningitis in patients coinfected with human immunodeficiency virus and tuberculosis. AIDS Res Treat. 2011;2011:180352. doi: 10.1155/2011/180352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powderly WG. Cryptococcal meningitis and AIDS. Clin Infect Dis. 1993;17:837–42. doi: 10.1093/clinids/17.5.837. [DOI] [PubMed] [Google Scholar]
- 4.Levy RM, Bredesen DE, Rosenblum ML. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): Experience at UCSF and review of the literature. J Neurosurg. 1985;62:475–95. doi: 10.3171/jns.1985.62.4.0475. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: Findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 8.Okongo M, Morgan D, Mayanja B, Ross A, Whitworth J. Causes of death in a rural, population-based human immunodeficiency virus type 1 (HIV-1) natural history cohort in Uganda. Int J Epidemiol. 1998;27:698–702. doi: 10.1093/ije/27.4.698. [DOI] [PubMed] [Google Scholar]
- 9.Antinori S, Ridolfo A, Fasan M, Magni C, Galimberti L, Milazzo L, et al. AIDS-associated cryptococcosis: A comparison of epidemiology, clinical features and outcome in the pre-and post-HAART eras. Experience of a single centre in Italy. HIV Med. 2009;10:6–11. doi: 10.1111/j.1468-1293.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS. Joint United Nations Programme on HIV/AIDS. Geneva: Switzerland; 2011. Report on the Global HIV/AIDS Epidemic. [Google Scholar]
- 11.Dzoyem JP, Kechia FA, Ngaba GP, Lunga PK, Lohoue PJ. Prevalence of cryptococcosis among HIV-infected patients in Yaounde, Cameroon. Afr Health Sci. 2012;12:129–33. doi: 10.4314/ahs.v12i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertout S, Drakulovski P, Kouanfack C, Krasteva D, Ngouana T, Dunyach-Remy C, et al. , Genotyping and antifungal susceptibility testing of Cryptococcus neoformans isolates from Cameroonian HIV-positive adult patients. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 2013;19:763–9. doi: 10.1111/1469-0691.12019. [DOI] [PubMed] [Google Scholar]
- 13.Plan strategique national de lutte contre le vih, le sida et les ist. 2011. [Accessed August 31, 2012]. at http://www.circbcom/doc/PSN%202011-2015.pdf .
- 14.Bolokadze N, Gabunia P, Ezugbaia M, Gatserelia L, Khechiashvili G. Neurological complications in patients with HIV/AIDS. Georgian Med News. 2008;165:34–8. [PubMed] [Google Scholar]
- 15.Trujillo JR, Garcìa-Ramos G, Novak IS, Rivera VM, Huerta E, Essex M. Neurologic manifestations of AIDS: A comparative study of two populations from Mexico and the United States. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:23–9. [PubMed] [Google Scholar]
- 16.Espié E, Pinoges L, Balkan S, Chanchhaya N, Molfino L, Narom P, et al. Cryptococcal meningitis in HIV-infected patients: A longitudinal study in Cambodia. Trop Med Int Health. 2010;15:1375–81. doi: 10.1111/j.1365-3156.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- 17.Jowi JO, Mativo PM, Musoke SS. Clinical and laboratory characteristics of hospitalised patients with neurological manifestations of HIV/AIDS at the Nairobi Hospital. East Afr Med J. 2007;84:67–76. doi: 10.4314/eamj.v84i2.9506. [DOI] [PubMed] [Google Scholar]
- 18.Shankar SK, Mahadevan A, Satishchandra P, Kumar RU, Yasha TC, Santosh V, et al. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J Med Res. 2005;121:468–88. [PubMed] [Google Scholar]
- 19.Price RW. Neurological complications of HIV infection. Lancet. 1996;348:445–52. doi: 10.1016/S0140-6736(95)11035-6. [DOI] [PubMed] [Google Scholar]
- 20.Maher D, Mwandumba H. Cryptococcal meningitis in Lilongwe and Blantyre, Malawi. J Infect. 1994;28:59–64. doi: 10.1016/s0163-4453(94)94161-0. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian S, Mathai D. Clinical manifestations and management of cryptococcal infection. J Postgrad Med. 2005;51(Suppl 1):S21–6. [PubMed] [Google Scholar]
- 22.Millogo A, Ki-Zerbo GA, Andonaba JB, Lankoandé D, Sawadogo A, Yaméogo I, et al. Cryptococcal meningitis in HIV-infected patients at Bobo-Dioulasso Hospital (Burkina Faso) Bull Soc Pathol Exot. 2004;97:119–21. [PubMed] [Google Scholar]
- 23.Katwere M, Kambugu A, Piloya T, Wong M, Hendel-Paterson B, Sande MA, et al. Clinical presentation and aetiologies of acute or complicated headache among HIV-seropositive patients in a Ugandan clinic. J Int AIDS Soc. 2009;12:21. doi: 10.1186/1758-2652-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham CB, 3rd, Wippold FJ., 3rd Headache in the HIV patient: A review with special attention to the role of imaging. Cephalalgia. 2001;21:169–74. doi: 10.1046/j.1468-2982.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 25.Tso EL, Todd WC, Groleau GA, Hooper FJ. Cranial computed tomography in the emergency department evaluation of HIV-infected patients with neurologic complaints. Ann Emerg Med. 1993;22:1169–76. doi: 10.1016/s0196-0644(05)80984-9. [DOI] [PubMed] [Google Scholar]
- 26.Schaars CF, Meintjes GA, Morroni C, Post FA, Maartens G. Outcome of AIDS-associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazole. BMC Infect Dis. 2006;6:118. doi: 10.1186/1471-2334-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imwidthaya P, Poungvarin N. Cryptococcosis in AIDS. Postgrad Med J. 2000;76:85–8. doi: 10.1136/pmj.76.892.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chhin S, Rozycki G, Pugatch D, Harwell JI. Aetiology of meningitis in HIV-infected patients in a referral hospital in Phnom Penh, Cambodia. Int J STD AIDS. 2004;15:48–50. doi: 10.1258/095646204322637263. [DOI] [PubMed] [Google Scholar]
- 29.Leligdowicz A, Katwere M, Piloya T, Ronald A, Kambugu A, Katabira E. Challenges in diagnosis, treatment and follow-up of patients presenting with central nervous system infections in a resource-limited setting. Mcgill J Med. 2006;9:39–48. [PMC free article] [PubMed] [Google Scholar]
- 30.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer A, Loyse A, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: A randomized trial. Clin Infect Dis. 2008;47:123–30. doi: 10.1086/588792. [DOI] [PubMed] [Google Scholar]
- 32.Graybill JR, Sobel J, Saag M, van Der Horst C, Powderly W, Cloud G, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID mycoses study group and AIDS Cooperative treatment groups. Clin Infect Dis. 2000;30:47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 33.Sonkar SK, Gupta A, Atam V, Chaudhary SC, Tripathi AK, Sonkar GK. Clinical profile of neurological manifestation in human immunodeficiency virus - Positive patients. N Am J Med Sci. 2012;4:596–9. doi: 10.4103/1947-2714.103329. [DOI] [PMC free article] [PubMed] [Google Scholar]


